Abstract
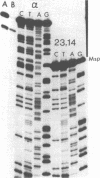
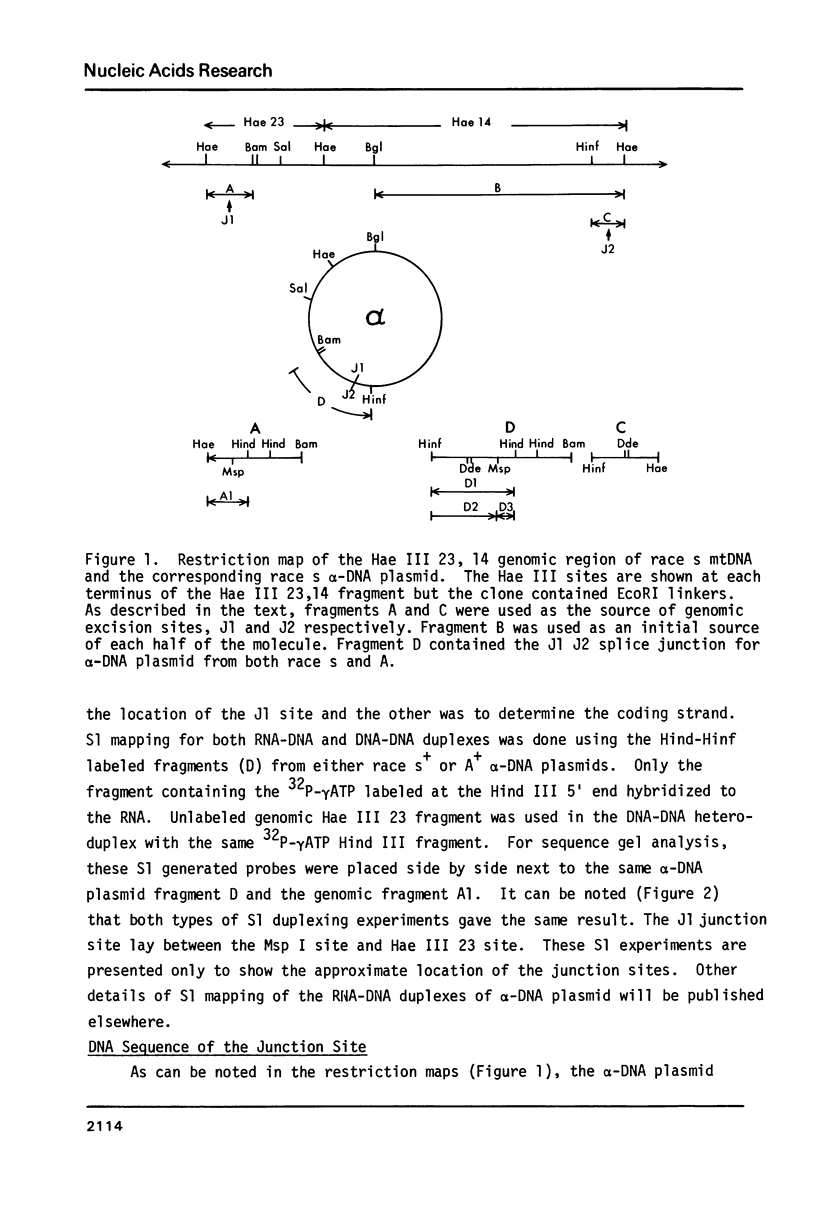
During senescence in Podospora anserina, specific gene regions of the mitochondrial genome are excised and amplified. The most prevalent, termed alpha-event senDNA, is a 2600 bp circular molecule which is excised from the contiguous Hae III fragments 23,14 region of the mitochondrial DNA restriction map. We have cloned alpha-DNA plasmid from races s+ and A+ as well as the genomic fragments Hae III 23,14 and have sequenced those regions which constitute the alpha-junction sites. We have found that one excision site (J1) is located 24 bp from the proximal Hae III 23 restriction site and the other (J2) 172 bp from the distal Hae III 14 site. Flanking the alpha-DNA sequences on the mitochondrial genome, there are 10 bp palindromic sequences: CAATATATTG, ending 3 bases from the J1 site, and ATTATATAAT which starts 8 bases from the J2 site. Neither of these 10 bp palindromes are present on the alpha-DNA plasmid. Abutting the J1 site on the alpha-DNA there is a 5 bp sequence (GTGCT) which is repeated 8 bp downstream. In joining the two distal J1 and J2 sites, a 7 bp repeat (ACGTGCG) is produced. These results are discussed within the context of site-specific recombination.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Calabretta B., Robberson D. L., Barrera-Saldaña H. A., Lambrou T. P., Saunders G. F. Genome instability in a region of human DNA enriched in Alu repeat sequences. Nature. 1982 Mar 18;296(5854):219–225. doi: 10.1038/296219a0. [DOI] [PubMed] [Google Scholar]
- Calos M. P., Miller J. H. Transposable elements. Cell. 1980 Jul;20(3):579–595. doi: 10.1016/0092-8674(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Belcour L., Grandchamp C. Etude au microscope électronique du DNA mitochondrial de Podospora anserina et présence d'une série multimérique de molécules circulaires de DNA dans des cultures sénescentes. C R Acad Sci Hebd Seances Acad Sci D. 1978 Jul 17;287(3):157–160. [PubMed] [Google Scholar]
- Cummings D. J., Belcour L., Grandchamp C. Mitochondrial DNA from Podospora anserina. I. Isolation and characterization. Mol Gen Genet. 1979 Mar 27;171(3):229–238. doi: 10.1007/BF00267577. [DOI] [PubMed] [Google Scholar]
- Cummings D. J., Belcour L., Grandchamp C. Mitochondrial DNA from Podospora anserina. II. Properties of mutant DNA and multimeric circular DNA from senescent cultures. Mol Gen Genet. 1979 Mar 27;171(3):239–250. doi: 10.1007/BF00267578. [DOI] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Jamet-Vierny C., Begel O., Belcour L. Senescence in Podospora anserina: amplification of a mitochondrial DNA sequence. Cell. 1980 Aug;21(1):189–194. doi: 10.1016/0092-8674(80)90126-9. [DOI] [PubMed] [Google Scholar]
- Kleckner N. Translocatable elements in procaryotes. Cell. 1977 May;11(1):11–23. doi: 10.1016/0092-8674(77)90313-0. [DOI] [PubMed] [Google Scholar]
- Landy A., Ross W. Viral integration and excision: structure of the lambda att sites. Science. 1977 Sep 16;197(4309):1147–1160. doi: 10.1126/science.331474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- RIZET G. Les modifications qui conduisent à la sénescence chez Podosproa sontelles de nature cytoplasmique? C R Hebd Seances Acad Sci. 1957 Jan 28;244(5):663–665. [PubMed] [Google Scholar]
- RIZET G. Sur l'impossibilité d'obtenir la multiplication végétative ininterrompue et illimitée de l'ascomycète Podospora anserina. C R Hebd Seances Acad Sci. 1953 Oct 12;237(15):838–840. [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Stahl U., Kück U., Tudzynski P., Esser K. Characterization and cloning of plasmid like DNA of the ascomycete Podospora anserina. Mol Gen Genet. 1980;178(3):639–646. doi: 10.1007/BF00337872. [DOI] [PubMed] [Google Scholar]
- Wang J. C. Helical repeat of DNA in solution. Proc Natl Acad Sci U S A. 1979 Jan;76(1):200–203. doi: 10.1073/pnas.76.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R. M., Horrum M. A., Cummings D. J. Are mitochondrial structural genes selectively amplified during senescence in Podospora anserina? Cell. 1982 Jun;29(2):505–515. doi: 10.1016/0092-8674(82)90167-2. [DOI] [PubMed] [Google Scholar]