Abstract
Aims
Cardiac hypertrophy and heart failure are associated with QT prolongation and lethal ventricular arrhythmias resulting from decreased K+ current densities and impaired repolarization. Recent studies in mouse models of physiological cardiac hypertrophy revealed that increased phosphoinositide-3-kinase-α (PI3Kα) signalling results in the up-regulation of K+ channels and the normalization of ventricular repolarization. The experiments here were undertaken to test the hypothesis that increased PI3Kα signalling will counteract the adverse electrophysiological remodelling associated with pathological hypertrophy and heart failure.
Methods and results
In contrast to wild-type mice, left ventricular (LV) hypertrophy, induced by transverse aortic constriction (TAC), did not result in prolongation of ventricular action potentials or QT intervals in mice with cardiac-specific expression of constitutively active PI3Kα (caPI3Kα). Indeed, repolarizing K+ currents and K+ channel subunit transcripts were increased in caPI3Kα + TAC LV myocytes in proportion to the TAC-induced cellular hypertrophy. Congestive heart failure in a transgenic model of dilated cardiomyopathy model is accompanied by prolonged QT intervals and ventricular action potentials, reduced K+ currents and K+ channel transcripts. Increased PI3Kα signalling, but not renin–angiotensin system blockade, in this model also results in increased K+ currents and improved ventricular repolarization.
Conclusion
In the setting of pathological hypertrophy or heart failure, enhanced PI3Kα signalling results in the up-regulation of K+ channel subunits, normalization of K+ current densities and preserved ventricular function. Augmentation of PI3Kα signalling, therefore, may be a useful and unique strategy to protect against the increased risk of ventricular arrhythmias and sudden death associated with cardiomyopathy.
Keywords: PI3Kα signalling, Pathological hypertrophy, Heart failure, Arrhythmia
1. Introduction
Left ventricular (LV) dysfunction is associated with increased risk of life-threatening arrhythmias.1,2 Sudden cardiac death, presumably due to lethal ventricular arrhythmias, accounts for ∼50% of deaths in individuals with heart failure.2 Electrical remodelling in cardiac hypertrophy3,4 and heart failure5 results, at least in part, from reductions in the densities of repolarizing K+ currents,5,6 which can lead to action potential prolongation and increased dispersion of repolarization, both of which are arrhythmogenic. Despite advances in pharmacological and device therapies for LV dysfunction, none purposefully targets fundamental arrhythmia mechanisms at the level of ion channel remodelling.7
It was recently demonstrated that physiological hypertrophy, induced by exercise training or by cardiac-specific expression of constitutively active PI3Kα (caPI3Kα), is associated with transcriptional up-regulation of the subunits encoding repolarizing K+ channels.8 This up-regulation results in increased repolarizing K+ current amplitudes in proportion to the cellular hypertrophy, thereby normalizing ventricular K+ current densities, action potential waveforms, and QT intervals. These observations suggest that activating the PI3Kα signalling pathway could be a therapeutic strategy in pathological cardiac hypertrophy and heart failure to maintain K+ current densities and reduce the risk of life-threatening ventricular arrhythmias.
To test this hypothesis, the impact of increased PI3Kα signalling was examined in: (i) a mouse model of non-failing, pressure overload-induced pathological left ventricular hypertrophy (LVH), produced by transverse aortic constriction (TAC);6 and, (ii) a transgenic mouse model (TG9) of dilated cardiomyopathy and consequent congestive heart failure.9,10 These experiments revealed that enhanced PI3Kα signalling results in transcriptional up-regulation of repolarizing K+ channel subunits, leading to increased K+ current amplitudes, thereby normalizing ventricular K+ current densities, action potential waveforms and functioning.
2. Methods
2.1. Experimental animals
Animals were handled in accordance with the NIH Guide for the Care and Use of Laboratory Animals. All protocols involving animals were approved by the Animal Studies Committee at Washington University Medical School.
2.1.1. Induction of pressure overload-induced LVH
Eight to twelve-week-old male caPI3Kα and wild-type (WT) mice were subjected to TAC to produce pressure overload-induced LVH.6 Animals were anaesthetized with a mixture of xylazine (16 mg/kg, i.p.) and ketamine (80 mg/kg, i.p.). Once deep anaesthesia was confirmed by the absence of toe pinch reflex, the chest was opened and the thoracic aorta was identified. A 7-0 silk suture was placed around the transverse aorta and tied around a 26-gauge needle, which was then removed. Seven days after surgery, caPI3Kα and WT animals, with and without TAC, were analysed.
2.1.2. Transgenic mouse model of heart failure
Experiments were also performed in a TG9 of heart failure.9 Electrophysiological studies were conducted on 10-week male WT, TG9, double transgenic (caPI3KαxTG9) mice as well as on TG9 mice following chronic 4 weeks swim training. As previously described, 6-week-old male TG9 mice were swum in water at 30–32°C (to avoid thermal stress).8,11 Initial swim time was set as 20 min, thereafter increasing by 10 min/day until 90 min sessions were reached. The 90-min training schedule was continued twice a day (separated by 4–5 h), 7 days a week, for 4 weeks. Additional experiments were carried out on 10-week TG9 animals treated with the angiotensin-converting enzyme (ACE) inhibitor, captopril (50 mg/L in the drinking water for 4 weeks).
2.2. Electrocardiogram recordings
Surface electrocardiograms (ECG) were recorded from anaesthetized (Tribromoethanol, 0.25 mg/kg, i.p.) caPI3Kα control, caPI3Kα + TAC, WT, WT + TAC, TG9, and caPI3KαxTG9 animals, using needle electrodes connected to a dual bioamplifier (AD Instrument, PowerLab 26T). Lead II recordings were analysed.8 QT intervals were measured as the time interval between the initiation of the QRS complex and the end of the T wave, and corrected for the heart rate using the formula QTc = QT/(√RR/100).12
2.3. Electrophysioloigcal recordings
Body weights, tibia lengths, and LV weights were measured and recorded at the time of tissue harvesting. Hearts were removed from anaesthetized animals, mounted on a Langendorf-apparatus and perfused retrogradely through the aorta with 25 mL of (0.8 mg/mL) collagenase-containing (type II, Worthington) solution.6,8 Following perfusion, the LV apex was separated, mechanically dispersed, plated on laminin-coated coverslips and maintained in a 95% air-5% CO2 incubator. Whole-cell current- and voltage-clamp recordings were obtained from LV apex myocytes within 24 h of isolation at room temperature (22–24°C). All voltage- and current-clamp experiments were performed using an Axopatch 1B patch clamp amplifier (Molecular Devices) interfaced to a microcomputer with a Digidata 1332 analog/digital interface and the pCLAMP9 software package (Molecular Devices); details are provided in Supplementary material online, Methods.
2.4. Transcript analyses
Total RNA from the LV of individual animals was isolated and treated with DNase using described methods.6,8 Using equal amounts of RNA, transcript analyses of genes encoding K+ channel pore-forming (α) and accessory subunits, the nuclear membrane protein lamin A/C (Lmna) and atrial natriuretic factor (ANF), were carried out using SYBR green RT–PCR in a two-step process.6,8 Data were analysed using the threshold cycle (CT) relative quantification method and normalized to Lmna to reference the transcript expression data to the number of nuclei (i.e. the number of myocytes) in the sample, providing relative differences in transcript expression levels on a per myocyte basis.8 For each transcript, the normalized values were then expressed relative to the mean of the control (caPI3Kα or WT) LV samples.
2.5. Citrate synthase activity
Citrate synthase (CS) activity was measured in gastrocnemius muscles dissected from untrained (n = 4) and swim-trained (n = 4) TG9 animals,13 weighed and frozen in liquid nitrogen. Frozen samples were homogenized on ice in 100 mM Tris–HCl, and protein concentrations were determined using the BCA protein Assay Kit (Pierce). Individual tissue homogenates (5 µL) were then added to a (1 mL) reaction mix containing: 100 mM Tris–HCl, 1.0 mM dithio-bis(2-nitrobenzoic acid), 10 mM oxaloacetate, and 0.2 mM acetyl CoA. The absorbance of each sample at 412 nm was recorded at 25°C every 30 s for 5 min. Mean absorbance change per minute was recorded and CS activity (in µmol*mg protein−1*min−1) was calculated using the extinction coefficient (13.6 mM−1*cm−1) of 5-thio-2-nitrobenzoic acid at 412 nm.13
2.6. Statistical analyses
All averaged electrophysiological and transcript data are presented as means ± SEM. The statistical significance of differences between experimental groups was evaluated by Student's t-test or the Mann–Whitney U test.
3. Results
3.1. Increased PI3Kα signalling prevents the ECG changes associated with pressure overload-induced LVH
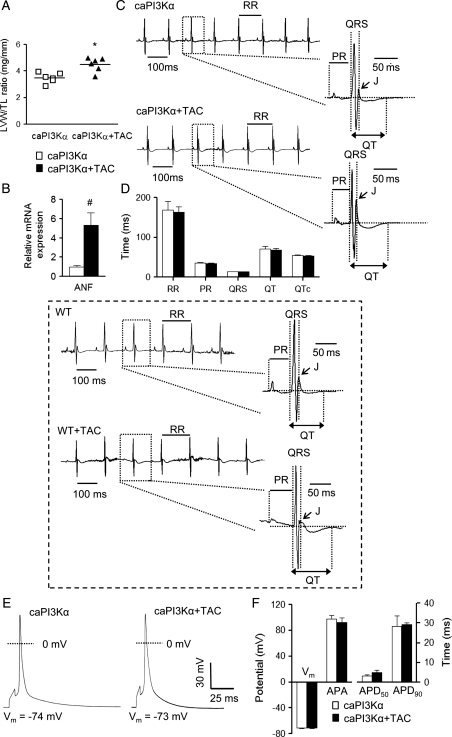
Similar to WT mice,6 TAC produced LVH in caPI3Kα mice.10 The mean ± SEM LV mass to tibia length ratio (LVM/TL), for example, was significantly (P< 0.001) higher (by 29 ± 6%) in caPI3Kα + TAC, than in control caPI3Kα (Figure 1A), animals. In addition, expression of ANF, a marker of pathological hypertrophy, was increased significantly (P< 0.01) in caPI3Kα + TAC, compared with control caPI3Kα, LV (Figure 1B). Interestingly, ECG recordings (Figure 1C) revealed that the morphologies of the QRS complexes, P, J, and T waves (Figure 1C), as well as the durations of the RR, PR, QRS, QT, and corrected QT (QTc) intervals (Figure 1D), were indistinguishable in caPI3Kα control and caPI3Kα + TAC animals. These observations contrast markedly with the ECG abnormalities (prolonged QTc intervals and flattened J waves) observed in WT mice with TAC-induced LVH (Figure 1 inset6). In spite of the marked increases in LV mass and ANF expression, the ECG changes were not observed in caPI3Kα animals with TAC (Figure 1C and D).
Figure 1.
Increased PI3Kα signalling prevents ECG and action potential waveform abnormalities associated with pressure overload-induced LVH following transverse aortic constriction (TAC). (A) LV mass/tibia length (LVM/TL) ratios were determined in caPI3Kα control (n = 6) and caPI3Kα + TAC (n = 6) animals; individual and mean ± SEM values (*P < 0.001) are plotted. (B) Mean ± SEM (n = 6) relative transcript expression level of atrial natriuretic factor (ANF) is significantly (#P < 0.01) higher in caPI3Kα + TAC than in caPI3Kα control LV. (C) Representative ECG (lead II) waveforms from caPI3Kα mice with and without TAC are illustrated; individual beats are shown on an expanded timescale on the right in each panel. (D) Mean ± SEM RR, PR, QRS, QT, and QTc intervals measured in caPI3Kα + TAC (n = 6) and caPI3Kα control (n = 6) animals were not significantly different. Inset: in contrast to the findings in caPI3Kα animals, and as reported previously,6 TAC in WT animals results in J point suppression and QT prolongation. (E) Action potential waveforms in LV myocytes from caPI3Kα control and caPI3Kα + TAC animals are indistinguishable. (F) No significant differences in mean ± SEM resting membrane potentials (Vm), action potential amplitudes (APA) or action potential durations at 50% (APD50) and 90% (APD90) repolarization were observed in caPI3Kα control (n = 10) and caPI3Kα + TAC (n = 9) LV myocytes.
3.2. Increased PI3Kα signalling prevents TAC-induced action potential prolongation
In WT mice, TAC-induced LVH results in impaired repolarization and prolonged action potential durations (APD).6 The observation that ECG waveforms in caPI3Kα animals were not measurably affected by TAC (Figure 1C and D) suggests that augmented PI3Kα signalling abrogates the detrimental effects of pressure overload-induced LVH on ventricular repolarization. To test this hypothesis directly, current clamp recordings were obtained from LV myocytes isolated from caPI3Kα + TAC and caPI3Kα control animals. As shown in Figure 1E, action potential waveforms in caPI3Kα + TAC and caPI3Kα control LV myocytes were indistinguishable. Additionally, no significant differences in mean resting membrane potentials, action potential amplitudes (APA) or action potential durations at 50% (APD50) and 90% (APD90) repolarization in caPI3Kα + TAC and caPI3Kα control LV myocytes were observed (Figure 1F).
3.3. Repolarizing K+ current amplitudes are increased in caPI3Kα + TAC LV myocytes
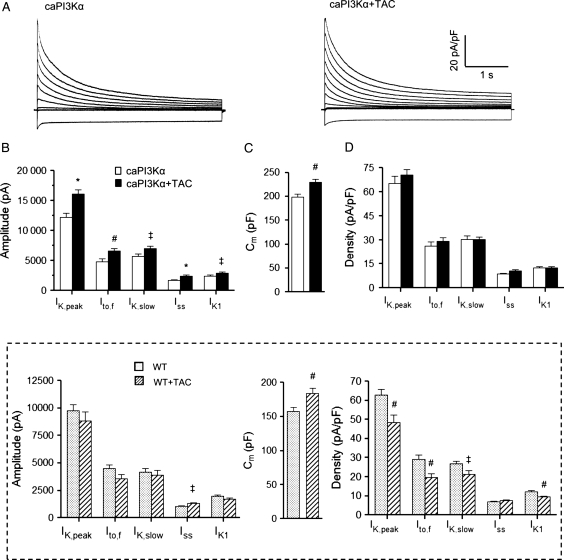
Studies conducted on experimental models of pressure overload-induced LVH have consistently reported reductions in repolarizing LV K+ current densities,6,14 resulting in impaired repolarization (prolonged ventricular APDs and QT/QTc intervals)6 and predisposing to life-threatening ventricular arrhythmias.4 The finding that no electrical abnormalities were evident in caPI3Kα animals with pressure overload-induced LVH suggests that increased PI3Kα signalling results in up-regulation of K+ currents, in parallel with the increase in myocyte size, and the normalization of K+ current densities. To explore this hypothesis, voltage-clamp recordings were obtained from LV apex myocytes isolated from caPI3Kα control and caPI3Kα + TAC animals. As illustrated in Figure 2, these experiments revealed that the amplitudes of the peak (IK,peak) outward voltage-gated (Kv) and the inwardly rectifying (Kir) K+ currents were significantly higher in caPI3Kα + TAC, compared with caPI3Kα control, LV myocytes (P< 0.05; Figure 2B).
Figure 2.
Repolarizing K+ current amplitudes are increased and K+ current densities are normalized in caPI3Kα + TAC LV myocytes. (A) Representative whole-cell K+ currents recorded from myocytes isolated from the LV apex of caPI3Kα control and caPI3Kα + TAC mice. Currents were evoked in response to (4.5 s) voltage steps to test potentials between −120 and +40 mV from a holding potential (HP) of −70 mV. (B) The mean ± SEM amplitudes of the K+ currents are significantly (‡P < 0.05, #P < 0.01,*P < 0.001) higher in caPI3Kα + TAC, compared with caPI3Kα control, LV myocytes. (C) A significant (#P < 0.01) increase in mean ± SEM whole-cell membrane capacitance (Cm), consistent with cellular hypertrophy, is also evident in caPI3K + TAC LV myocytes. (D) Normalizing current amplitudes for differences in cell size (Cm) revealed that mean ± SEM K+ current densities in LV myocytes from caPI3Kα + TAC and caPI3Kα control animals are indistinguishable. Inset: as reported previously,6 IK,peak, Ito,f, IK,slow, and IK1 amplitudes are not increased in WT LV myocytes with TAC and current densities are decreased significantly (‡P < 0.05, #P < 0.01).
Kinetic analyses of the decay phases of the outward K+ currents revealed that the amplitudes of the individual Kv current components, Ito,f, IK,slow, and Iss, were higher in caPI3Kα + TAC LV myocytes (Figure 2B). There were no measurable differences in the time- or the voltage-dependent properties of the Kv currents in cells from caPI3Kα control and caPI3Kα + TAC mice (see Supplementary material online, Table S1). Consistent with TAC-induced LVH, cellular hypertrophy was clearly evident in caPI3Kα + TAC LV myocytes (Figure 2C); the mean whole-cell membrane capacitance (Cm) was significantly (P< 0.01) higher in caPI3Kα + TAC, than in caPI3Kα control, LV cells (Figure 2C). Normalization of the Kv and Kir (IK1) current amplitudes (Figure 2B) to the measured Cm values (in the same cell) revealed that repolarizing K+ current densities in caPI3Kα + TAC and caPI3Kα control LV cells were indistinguishable (Figure 2A and D). These results are in striking contrast to observations in WT animals with TAC-induced LVH.6 Indeed, the amplitudes of the individual K+ currents (except for Iss) were not increased in WT cells in response to TAC and, when normalized to the increase in cell size (Cm), repolarizing K+ current densities were actually decreased significantly in WT LV cells with TAC (Figure 2 inset and Supplementary material online, Table S1).
3.4. Transcriptional up-regulation of K+ channel subunits in caPI3Kα + TAC LV
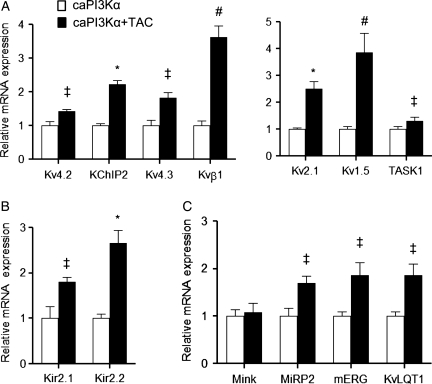
Recent studies demonstrated that the increase in ventricular K+ current amplitudes with enhanced myocardial PI3Kα signalling reflects the up-regulation of the transcripts encoding the underlying K+ channel subunits.8 Subsequent experiments here, therefore, were aimed at determining whether the observed increases in K+ current amplitudes in caPI3Kα LV myocytes with TAC (Figure 2B) might also reflect increased expression of the transcripts encoding the various repolarizing K+ channel subunits.
As illustrated in Figure 3, quantitative RT–PCR revealed that the expression levels of the transcripts encoding Ito,f channel pore-forming (α) subunits, Kcnd2 (Kv4.2)15 and Kcnd3 (Kv4.3),16 as well as the Ito,f channel accessory subunit, Kcnip2 (KChIP2),16,17 were increased significantly (P< 0.05) in caPI3Kα + TAC, compared with caPI3Kα control, LV (Figure 3A). The expression levels of Kcna5 (Kv1.5) and Kcnb1 (Kv2.1), which encode IK,slow118 and IK,slow2,19 respectively, as well as of the K2P channel subunit Kcnk3 (TASK1), which has been suggested to underlie Iss in rat cardiomyocytes,20 were also increased significantly (P< 0.05) in caPI3Kα + TAC LV (Figure 3A). Similarly, expression of the IK1 channel subunits Kcnj2 (Kir2.1) and Kcnj12 (Kir2.2),21 as well as of Kcnh2 (mERG)22 and Kcnq1 (KvLQT1),23 the α subunits encoding the rapid and slow cardiac delayed rectifiers, IKr and IKs, in large mammals, was also increased in caPI3Kα + TAC LV (Figure 3B and C). In contrast with WT animals,6 therefore, the expression levels of a number of K+ channel subunits were actually up-regulated in caPI3Kα ventricles with TAC (see Section 4).
Figure 3.
Transcriptional up-regulation of K+ channel subunits with TAC-induced LVH in caPI3Kα LV. K+ channel subunit transcript expression levels were measured in individual LV samples from caPI3Kα + TAC (n = 6) and caPI3Kα control (n = 6) LV, normalized to lamin A/C and subsequently to the mean value of the caPI3Kα control LV samples (A–C). The mean ± SEM relative expression levels of most of the K+ channel subunit transcripts are significantly (‡P < 0.05, #P < 0.01, *P < 0.001) higher in caPI3Kα + TAC, than in caPI3Kα control, LV.
3.5. Increased PI3Kα signalling attenuates ECG and action potential abnormalities in a mouse model of dilated cardiomyopathy
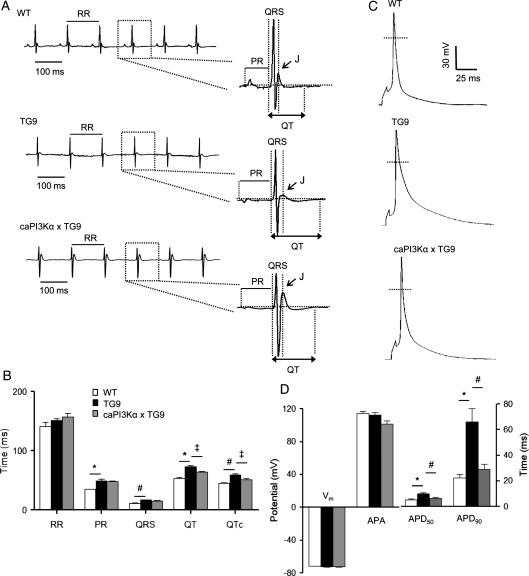
To test the hypothesis that increased PI3Kα signalling might also ameliorate the electrical abnormalities in failing hearts, additional experiments were conducted using a transgenic mouse model of dilated cardiomyopathy (TG9).9 As reported previously,9 TG9 mice develop progressive dilated cardiomyopathy, beginning at 6 weeks of age, and die of overt heart failure at 11–13 weeks. Surface ECG recordings revealed that QT and QTc intervals were significantly (P< 0.001) prolonged in (10 week) TG9, compared with WT, mice (Figure 4A and B), indicating impaired ventricular repolarization, reminiscent of the electrical abnormalities observed in heart failure patients24 and in animal models of heart failure.25,26 Interestingly, PR and QRS intervals were prolonged in TG9, compared with WT, animals (Figure 4A and B) suggesting the presence of conduction abnormalities, which are also prevalent in patients with advanced heart failure.27
Figure 4.
Increased cardiac PI3Kα signalling attenuates the ECG and action potential abnormalities in a transgenic mouse model (TG9) of heart failure. (A) Representative ECG waveforms from WT, TG9, and caPI3KαxTG9 mice. (B) Mean ± SEM PR, QRS, QT, and QTc intervals are significantly (#P < 0.01, *P < 0.001) prolonged in TG9 (n = 6), compared with WT (n = 6), animals. Interestingly, mean QT and QTc intervals in caPI3KαxTG9 mice are significantly (‡P < 0.05) shorter than in TG9 mice. (C) Representative action potential waveforms recorded from LV myocytes isolated from WT, TG9, and caPI3KαxTG9 animals are shown. (D) Mean ± SEM APD50 and APD90 were significantly (*P < 0.001) longer in TG9 (n = 10) than in WT (n = 16) LV myocytes. Increased PI3Kα signalling significantly (#P < 0.01) attenuates the APD prolongation (C and D) seen in TG9 LV myocytes.
To determine the impact of increased PI3Kα signalling on electrical function in heart failure, TG9 and caPI3Kα mice were crossed (caPI3KαxTG9). Surface ECG recordings from (10 week) caPI3KαxTG9 mice revealed that mean ± SEM QT and QTc intervals were abbreviated significantly (P < 0.05) compared with TG9 mice (Figure 4A and B), albeit not identical to WT. In contrast, PR and QRS intervals were prolonged similarly in caPI3KαxTG9 and TG9 animals (see Section 4).
Consistent with the prolonged QT/QTc intervals (Figure 4A and B), current clamp recordings from LV apex myocytes isolated from TG9 animals revealed significantly (P< 0.001) prolonged action potential durations (APD50 and APD90), compared with recordings from WT LV apex myocytes (Figure 4C and D). The action potential prolongation evident in TG9 myocytes (Figure 4C and D), however, was attenuated significantly (P< 0.01) in LV cells isolated from (caPI3KαxTG9) animals with increased caPI3Kα expression.
3.6. Augmentation of PI3Kα signalling results in increased repolarizing K+ currents in failing hearts
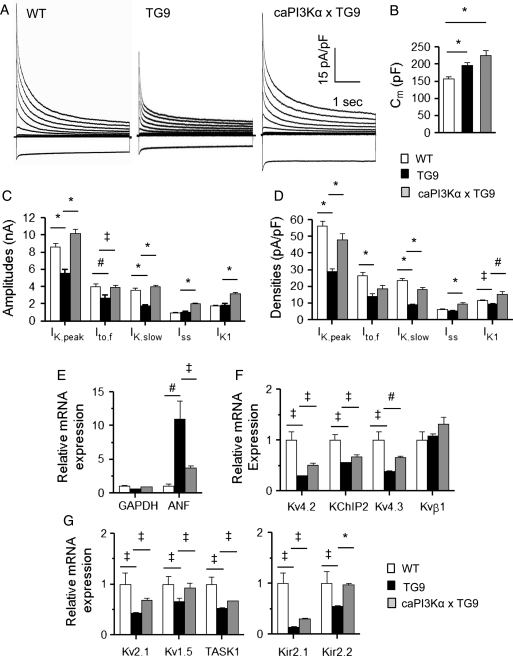
As might be expected based on observations in humans5 and in animal models of heart failure,26,28 voltage-clamp recordings revealed that IK,peak amplitudes were significantly (P < 0.001) lower in TG9, compared with WT, LV myocytes (Figure 5C). The amplitudes of Ito,f and IK,slow were also lower (P < 0.001) in TG9 myocytes (Figure 5C); the time- and voltage-dependent properties of the (Ito,f and IK,slow) currents in TG9 and WT cells, however, were similar (see Supplementary material online, Table S2). The higher mean Cm (Figure 5B) is consistent with marked cellular hypertrophy in TG9, compared with WT, LV cells. Normalization of the K+ current amplitudes (Figure 5C) to myocyte size (Figure 5B) revealed that repolarizing K+ current densities, with the exception of Iss, were significantly lower in TG9, than in WT, LV cells (Figure 5A and D). As also illustrated in Figure 5C, however, increased PI3Kα signalling resulted in significant (P < 0.05) increases in the amplitudes of all of the repolarizing K+ currents, Ito,f, IK,slow, Iss, and IK1, in TG9 LV myocytes. Normalizing the current amplitudes to measured Cm revealed that mean ± SEM IK,peak, IK,slow, Iss, and IK1 densities were actually increased (Figure 5A and D) in caPI3KαxTG9, compared with TG9, cells.
Figure 5.
Increased cardiac PI3Kα signalling in heart failure up-regulates repolarizing K+ currents and the underlying K+ channel subunit transcripts. (A) Representative whole-cell K+ currents were recorded from LV myocytes isolated from WT, TG9, and caPI3KαxTG9 mice. (B) Mean ± SEM Cm values are significantly (P < 0.001) higher in TG9 (n = 21) and in caPI3KαxTG9 (n = 32), than in WT (n = 22), LV myocytes. (C) Mean ± SEM IK,peak, Ito,f, and IK,slow amplitudes in TG9 (n = 21) are markedly reduced compared with WT (n = 22) LV myocytes, whereas mean ± SEM K+ current amplitudes (IK,peak, Ito,f, IK,slow, Iss, and IK1) are higher in caPI3KαxTG9, than in TG9, cells. (D) Normalization for differences in cell size (Cm) revealed that mean ± SEM IK,peak, Ito,f, IK,slow, and IK1 densities are significantly (‡P < 0.05, *P < 0.001) lower in TG9 (n = 21), than in WT (n = 22), LV myocytes, and that increasing PI3Kα signalling in TG9 myocytes results in significant (#P < 0.01, *P < 0.001) increases in IK,peak, IK,slow, Iss, and IK1 densities. Atrial natriuretic factor (ANF) and K+ channel subunit transcript expression levels were determined in individual LV samples from WT, TG9, and caPI3KαxTG9 LV, normalized to lamin A/C in the same sample and to the mean value of the WT control LV samples. As reported previously,9 ANF expression is increased ∼10-fold in TG9 LV. The increase in ANF expression is blunted in the LV of caPI3KαxTG9 animals (E). The expression levels of the transcripts encoding several K+ channel subunits (F, G) are reduced significantly (‡P < 0.05) in TG9, compared with WT, LV; the expression levels of many of these K+ channel subunits, however, are increased (‡P < 0.05, #P < 0.01, *P < 0.001) in caPI3KαxTG9, compared with TG9, LV.
Additional experiments were carried out in TG9 animals treated with the ACE inhibitor, captopril, which has been shown to improve the cardiac function/survival in heart failure patients,29 as well as the survival of TG9 animals.9 These experiments, however, did not reveal measurable effects of renin–angiotensin system blockade on K+ current densities or action potential waveforms (see Supplementary material online, Figure S1) in TG9 LV myocytes (see Section 4).
Quantitative RT–PCR experiments were also conducted to examine K+ channel subunit and ANF transcript expression levels in the LV of WT, TG9, and caPI3KαxTG9 mice. ANF expression was significantly (P < 0.01) increased in TG9, compared with WT, LV, but this increase was attenuated in caPI3KαxTG9 LV (Figure 5E). Consistent with the observed decreases in K+ current amplitudes, the expression levels of Ito,f subunits, Kv4.2, Kv4.3 and KChIP2, IK,slow subunits Kv1.5 and Kv2.1, the putative Iss subunit, TASK1, and both Kir2.1 and Kir2.2 (IK1 subunits) were also lower in TG9, compared with WT (Figures 5F and G), LV. Increased PI3Kα signalling in TG9 LV, however, resulted in marked increases in the expression levels of all of these K+ channel subunit transcripts (Figure 5F and G).
3.7. Exercise training also up-regulates repolarizing K+ currents in heart failure
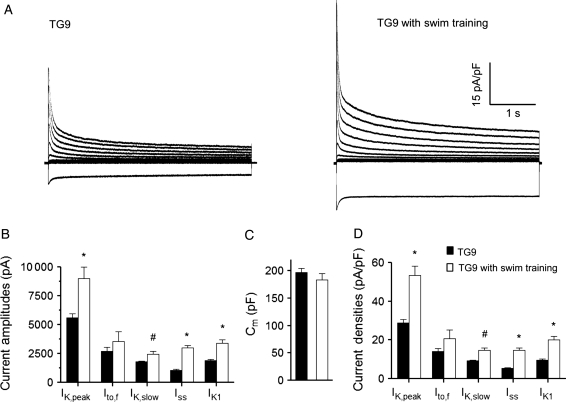
It was recently demonstrated that chronic exercise (swim training)-induced physiological hypertrophy, which results in increased PI3Kα activation,8,11 leads to the up-regulation of repolarizing ventricular K+ currents in parallel with the cellular hypertrophy (increase in myocyte size).8 Additional experiments here, therefore, were designed to determine whether chronic exercise training also results in K+ current up-regulation in the context of heart failure. Following 4-week chronic swim training, voltage-clamp experiments were performed on LV myocytes isolated from (10 week) trained and untrained TG9 mice. Mean ± SEM CS activity was increased significantly (P< 0.05) in the gastrocnemius muscles from swim-trained (0.92 ± 0.11 µmol/mg protein/min; n = 4), compared with untrained (0.49 ± 0.09 µmol/mg protein/min; n = 4), TG9 animals, indicating the robustness of aerobic exercise training.30 The amplitudes of IK,peak and IK1 (P < 0.001), as well as of the Kv current components, IK,slow (P < 0.01) and Iss (P < 0.001), were significantly higher in TG9 LV myocytes from the swim-trained, compared with the untrained, TG9 animals (Figure 6B). The mean ± SEM Cm determined in LV myocytes from trained and untrained animals were similar (Figure 6C). Repolarizing K+ current (IK,peak, IK,slow, Iss and IK1) densities, therefore, were significantly (P < 0.01) higher in TG9 LV myocytes following swim training (Figure 6A and D). Indeed IK,peak and IK1 densities were normalized to the levels found in WT cells (compare values in Supplementary material online, Table S2).
Figure 6.
Exercise training also results in K+ current up-regulation in heart failure. (A) Representative whole-cell K+ currents in myocytes isolated from the LV of TG9 mice with and without swim training are illustrated. (B–D) Mean ± SEM IK,peak, IK,slow, Iss, and IK1 amplitudes and densities are significantly (#P < 0.01,*P < 0.001) higher in LV myocytes from swim-trained (n = 15), compared with untrained (n = 21), TG9 animals. Although mean Ito,f amplitudes/densities are also higher in swim-trained TG9 LV myocytes, the differences did not reach statistical significance.
4. Discussion
The increased incidence of life-threatening ventricular arrhythmias in patients with LV dysfunction is a consequence of complex pathological remodelling in cardiac structural,31 neurohumoral,32 and electrophysiological properties.5,6 Advances in the clinical management of heart failure, specifically pharmacologic,33,34 and device therapies,35 which are aimed at treating neurohumoral and structural abnormalities, have improved patient outcomes.33–35 Targeting the electrophysiological derangements using anti-arrhythmic agents has proven to be much more problematic and, indeed, has been associated with increased, rather than decreased, mortality.36 The results presented here demonstrate that increased PI3Kα signalling in pathological hypertrophy and heart failure helps to normalize ventricular repolarization and maintains electrical functioning through the transcriptional up-regulation of repolarizing K+ channels, a mechanism of action distinct from classic heart failure therapeutics.
Interestingly, PI3Kα signalling has been shown to modulate a number of transcription factors that could potentially affect the expression of cardiac K+ channels. The Forkhead (FOXO) family of transcriptional factors, for example, which are direct targets of the downstream target of PI3Kα, Akt, has been shown to regulate the promoter activity of several K+ channel genes such as Kcnj8 (Kir6.1), Kcnj11 (Kir6.2), and Abcc8 (SUR1).37 Promoter analyses (using web-based program rVista 2.0, http://rvista.decode.org)38 also reveal multiple FOXO transcription factor binding sites in Kcnd2 (Kv4.2), Kcnip2 (KChIP2), Kcnb1 (Kv2.1), and Kcnk3 (TASK1). Glycogen synthase kinase 3 beta (GSK3β), another important downstream target of PI3Kα, also modulates the activity of several transcriptional factors, including NFAT, GATA4, myocardin, c-Myc, c-Jun, and β-catenin, many of which have putative binding sites in the promoter regions of several K+ channel subunit genes and could potentially contribute to the transcriptional regulation of K+ channel expression mediated by increased PI3Kα. Further experiments are needed to explore these hypotheses and delineate PI3Kα-mediated transcriptional regulatory mechanisms directly.
4.1. Increased PI3Kα signalling mitigates impaired repolarization in hypertrophied and failing hearts
Chronic cardiac-specific increases in PI3Kα signalling produce physiological hypertrophy characterized by normal electrical and mechanical function.8,11,39 The results here demonstrate that increased myocardial PI3Kα signalling also eliminates the effects of pressure overload-induced LV (pathological) hypertrophy on ventricular action potential durations. In addition, in caPI3Kα + TAC LV myocytes, the transcripts encoding the subunits that underlie the generation of several repolarizing K+ channels were increased significantly and in parallel with the TAC-induced increase in myocyte size, resulting in normalized K+ current densities. These results contrast strikingly with the effects of TAC-induced LVH in WT animals, in which K+ channel subunit expression levels are not increased in parallel with the cellular hypertrophy, resulting in decreased K+ current densities and impaired repolarization.6 Augmented PI3Kα signalling, therefore, has a clear beneficial effect in the context of pathological LVH.
The experiments here also demonstrated that repolarizing K+ channel subunit transcript expression levels are up-regulated and K+ current amplitudes are increased in failing TG9 hearts with increased PI3Kα signalling. Although the treatment with the ACE inhibitor captopril also improves the survival of TG9 animals,9 there were no detectable effects of captopril treatment on ventricular K+ currents or action potentials in TG9 hearts (see Supplementary material online, Figure S1). Enhanced PI3Kα signalling in the setting of heart failure, therefore, has unique beneficial effects on electrical functioning that are not observed with renin–angiotensin system blockade.
QRS complexes were widened in TG9, compared with WT, mice, suggesting the presence of intracardiac conduction slowing, which is also commonly observed in heart failure patients40 and in animal models of non-ischaemic dilated cardiomyopathy.31 In contrast with the effects on QT intervals, however, increased PI3Kα activity does not normalize the widened QRS complexes in TG9 mice. These observations may reflect low expression of the caPI3Kα transgene in the specialized conduction system, as previous reports suggest that transgenes driven by the α-MHC promoter may not be expressed consistently in nodal and His-Purkinje tissues.41 It is also possible, however, that increased PI3Kα signalling is not sufficient to reverse the structural and molecular changes that underlie the abnormal conduction. Further experiments focused on the conduction system directly are needed to explore these possibilities directly.
4.2. Chronic swim-training normalizes K+ current densities in heart failure
The observation that increased PI3Kα signalling in failing hearts provides beneficial effects in maintaining physiological K+ current expression levels suggests an attractive strategy for alleviating arrhythmogenic electrical remodelling in heart failure.5 A simple and practical approach to increasing cardiac PI3Kα signalling is aerobic exercise training.8,11 Indeed, aerobic exercise training has been shown to improve LV function and prolong survival in heart failure patients,42 as well as in animal models of heart failure.10,43 Various mechanisms have been proposed to explain the beneficial effects of exercise, including improved endothelial function,44 reduced myocardial fibrosis,10 and balanced sympathovagal input.45 The results here demonstrate that chronic exercise training also up-regulates repolarizing K+ currents in the setting of heart failure, an effect that normalizes ventricular repolarizing K+ current densities and action potential durations and reduce arrhythmia susceptibility. In spite of the benefits of exercise, however, it is also clear that adherence to, or compliance with, prescribed exercise regimens can be very difficult for heart failure patients.46 Importantly, the results here suggests the potential of a therapeutic strategy focused on enhancing PI3Kα signalling directly.
4.3. Conclusion
In summary, enhanced cardiac PI3Kα activity is associated with increased expression of myocardial K+ channel subunits in pressure overload-induced LVH and heart failure. The transcriptional up-regulation of K+ channel subunits produced by increased PI3Kα activity leads to increased K+ current amplitudes in proportion to the increase in cell size, thereby normalizing K+ current densities, action potential waveforms, and QT intervals. The experiments here also demonstrate that chronic exercise training, a physiological means to enhance PI3Kα signalling leads to K+ channel up-regulation in the failing heart, whereas the ACE inhibitor captopril does not. These observations suggest that increased PI3Kα signalling, either by exercise or by direct pharmacological intervention, could directly address the electrophysiological basis of life-threatening arrhythmias associated with pathological LVH and heart failure. These results argue for further research focused on developing practical methods to activate PI3Kα signalling in the human heart, as well as on detailing the effects of these manipulations on arrhythmia vulnerability and sudden cardiac death.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by the National Institutes of Health (HL-034161 to J.M.N., NIH K12-HD001487 to P.Y.J.) and the Midwest Affiliate of the American Heart Association (Predoctoral Fellowship to K.C.Y.).
Supplementary Material
References
- 1.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32:1454–1459. doi: 10.1016/s0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 2.Tomaselli GF, Beuckelmann DJ, Calkins HG, Berger RD, Kessler PD, Lawrence JH, et al. Sudden cardiac death in heart failure. The role of abnormal repolarization. Circulation. 1994;90:2534–2539. doi: 10.1161/01.cir.90.5.2534. [DOI] [PubMed] [Google Scholar]
- 3.Mayet J, Shahi M, McGrath K, Poulter NR, Sever PS, Foale RA, et al. Left ventricular hypertrophy and QT dispersion in hypertension. Hypertension. 1996;28:791–796. doi: 10.1161/01.hyp.28.5.791. [DOI] [PubMed] [Google Scholar]
- 4.McIntyre H, Fry CH. Abnormal action potential conduction in isolated human hypertrophied left ventricular myocardium. J Cardiovasc Electrophysiol. 1997;8:887–894. doi: 10.1111/j.1540-8167.1997.tb00850.x. [DOI] [PubMed] [Google Scholar]
- 5.Beuckelmann DJ, Nabauer M, Erdmann E. Alterations of K+ currents in isolated human ventricular myocytes from patients with terminal heart failure. Circ Res. 1993;73:379–385. doi: 10.1161/01.res.73.2.379. [DOI] [PubMed] [Google Scholar]
- 6.Marionneau C, Brunet S, Flagg TP, Pilgram TK, Demolombe S, Nerbonne JM. Distinct cellular and molecular mechanisms underlie functional remodelling of repolarizing K+ currents with left ventricular hypertrophy. Circ Res. 2008;102:1406–1415. doi: 10.1161/CIRCRESAHA.107.170050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jessup M, Brozena S. Heart failure. N Engl J Med. 2003;348:2007–2018. doi: 10.1056/NEJMra021498. [DOI] [PubMed] [Google Scholar]
- 8.Yang KC, Foeger NC, Marionneau C, Jay PY, McMullen JR, Nerbonne JM. Homeostatic regulation of electrical excitability in physiological cardiac hypertrophy. J Physiol. 2010;588:5015–5032. doi: 10.1113/jphysiol.2010.197418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buerger A, Rozhitskaya O, Sherwood MC, Dorfman AL, Bisping E, Abel ED, et al. Dilated cardiomyopathy resulting from high-level myocardial expression of Cre-recombinase. J Card Fail. 2006;12:392–398. doi: 10.1016/j.cardfail.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 10.McMullen JR, Amirahmadi F, Woodcock EA, Schinke-Braun M, Bouwman RD, Hewitt KA, et al. Protective effects of exercise and phosphoinositide 3-kinase(p110alpha) signalling in dilated and hypertrophic cardiomyopathy. Proc Natl Acad Sci USA. 2007;104:612–617. doi: 10.1073/pnas.0606663104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMullen JR, Shioi T, Zhang L, Tarnavski O, Sherwood MC, Kang PM, et al. Phosphoinositide 3-kinase(p110alpha) plays a critical role for the induction of physiological, but not pathological, cardiac hypertrophy. Proc Natl Acad Sci USA. 2003;100:12355–12360. doi: 10.1073/pnas.1934654100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol. 1998;274:H747–H751. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]
- 13.Harrison BC, Bell ML, Allen DL, Byrnes WC, Leinwand LA. Skeletal muscle adaptations in response to voluntary wheel running in myosin heavy chain null mice. J Appl Physiol. 2002;92:313–322. doi: 10.1152/japplphysiol.00832.2001. [DOI] [PubMed] [Google Scholar]
- 14.Volk T, Nguyen THD, Schultz JH, Faulhaber J, Ehmke H. Regional alterations of repolarizing K+ currents among the left ventricular free wall of rats with ascending aortic stenosis. J Physiol Lon. 2001;530:443–455. doi: 10.1111/j.1469-7793.2001.0443k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo W, Jung WE, Marionneau C, Aimond F, Xu H, Yamada KA, et al. Targeted deletion of Kv4.2 eliminates I(to,f) and results in electrical and molecular remodelling, with no evidence of ventricular hypertrophy or myocardial dysfunction. Circ Res. 2005;97:1342–1350. doi: 10.1161/01.RES.0000196559.63223.aa. [DOI] [PubMed] [Google Scholar]
- 16.Guo W, Li H, Aimond F, Johns DC, Rhodes KJ, Trimmer JS, et al. Role of heteromultimers in the generation of myocardial transient outward K+ currents. Circ Res. 2002;90:586–593. doi: 10.1161/01.res.0000012664.05949.e0. [DOI] [PubMed] [Google Scholar]
- 17.Kuo HC, Cheng CF, Clark RB, Lin JJ, Lin JL, Hoshijima M, et al. A defect in the Kv channel-interacting protein 2 (KChIP2) gene leads to a complete loss of I(to) and confers susceptibility to ventricular tachycardia. Cell. 2001;107:801–813. doi: 10.1016/s0092-8674(01)00588-8. [DOI] [PubMed] [Google Scholar]
- 18.London B, Guo W, Pan X, Lee JS, Shusterman V, Rocco CJ, et al. Targeted replacement of Kv1.5 in the mouse leads to loss of the 4-aminopyridine-sensitive component of IK, slow and resistance to drug-induced QT prolongation. Circ Res. 2001;88:940–946. doi: 10.1161/hh0901.090929. [DOI] [PubMed] [Google Scholar]
- 19.Xu H, Barry DM, Li H, Brunet S, Guo W, Nerbonne JM. Attenuation of the slow component of delayed rectification, action potential prolongation, and triggered activity in mice expressing a dominant-negative Kv2 alpha subunit. Circ Res. 1999;85:623–633. doi: 10.1161/01.res.85.7.623. [DOI] [PubMed] [Google Scholar]
- 20.Putzke C, Wemhoner K, Sachse FB, Rinne S, Schlichthorl G, Li XT, et al. The acid-sensitive potassium channel TASK-1 in rat cardiac muscle. Cardiovasc Res. 2007;75:59–68. doi: 10.1016/j.cardiores.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 21.Zaritsky JJ, Redell JB, Tempel BL, Schwarz TL. The consequences of disrupting cardiac inwardly rectifying K(+) current (I(K1)) as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes. J Physiol. 2001;533:697–710. doi: 10.1111/j.1469-7793.2001.t01-1-00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 23.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, et al. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 24.Barr CS, Naas A, Freeman M, Lang CC, Struthers AD. QT dispersion and sudden unexpected death in chronic heart failure. Lancet. 1994;343:327–329. doi: 10.1016/s0140-6736(94)91164-9. [DOI] [PubMed] [Google Scholar]
- 25.Adamson PB, Vanoli E. Early autonomic and repolarization abnormalities contribute to lethal arrhythmias in chronic ischemic heart failure: characteristics of a novel heart failure model in dogs with postmyocardial infarction left ventricular dysfunction. J Am Coll Cardiol. 2001;37:1741–1748. doi: 10.1016/s0735-1097(01)01185-8. [DOI] [PubMed] [Google Scholar]
- 26.Rose J, Armoundas AA, Tian Y, DiSilvestre D, Burysek M, Halperin V, et al. Molecular correlates of altered expression of potassium currents in failing rabbit myocardium. Am J Physiol Heart Circ Physiol. 2005;288:H2077–H2087. doi: 10.1152/ajpheart.00526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akar FG, Tomaselli GF. Conduction abnormalities in nonischemic dilated cardiomyopathy: basic mechanisms and arrhythmic consequences. Trends Cardiovasc Med. 2005;15:259–264. doi: 10.1016/j.tcm.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Petkova-Kirova PS, Gursoy E, Mehdi H, McTiernan CF, London B, Salama G. Electrical remodelling of cardiac myocytes from mice with heart failure due to the overexpression of tumor necrosis factor-alpha. Am J Physiol Heart Circ Physiol. 2006;290:H2098–H2107. doi: 10.1152/ajpheart.00097.2005. [DOI] [PubMed] [Google Scholar]
- 29.Pfeffer MA, Braunwald E, Moye LA, Basta L, Brown EJ, Jr, Cuddy TE, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med. 1992;327:669–677. doi: 10.1056/NEJM199209033271001. [DOI] [PubMed] [Google Scholar]
- 30.Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol. 2001;90:1900–1908. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- 31.Akar FG, Spragg DD, Tunin RS, Kass DA, Tomaselli GF. Mechanisms underlying conduction slowing and arrhythmogenesis in nonischemic dilated cardiomyopathy. Circ Res. 2004;95:717–725. doi: 10.1161/01.RES.0000144125.61927.1c. [DOI] [PubMed] [Google Scholar]
- 32.Vaseghi M, Shivkumar K. The role of the autonomic nervous system in sudden cardiac death. Prog Cardiovasc Dis. 2008;50:404–419. doi: 10.1016/j.pcad.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 34.Kober L, Torp-Pedersen C, Carlsen JE, Bagger H, Eliasen P, Lyngborg K, et al. A clinical trial of the angiotensin-converting-enzyme inhibitor trandolapril in patients with left ventricular dysfunction after myocardial infarction. Trandolapril Cardiac Evaluation (TRACE) Study Group. N Engl J Med. 1995;333:1670–1676. doi: 10.1056/NEJM199512213332503. [DOI] [PubMed] [Google Scholar]
- 35.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 36.The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. N Engl J Med. 1989;321:406–412. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 37.Philip-Couderc P, Tavares NI, Roatti A, Lerch R, Montessuit C, Baertschi AJ. Forkhead transcription factors coordinate expression of myocardial KATP channel subunits and energy metabolism. Circ Res. 2008;102:e20–e35. doi: 10.1161/CIRCRESAHA.107.166744. [DOI] [PubMed] [Google Scholar]
- 38.Loots GG, Ovcharenko I. rVISTA 2.0: evolutionary analysis of transcription factor binding sites. Nucleic Acids Res. 2004;32:W217–W221. doi: 10.1093/nar/gkh383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, et al. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000;19:2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hombach V. Electrocardiogram of the failing heart. Card Electrophysiol Rev. 2002;6:209–214. doi: 10.1023/a:1016316706195. [DOI] [PubMed] [Google Scholar]
- 41.Dobrzynski H, Billeter R, Greener ID, Tellez JO, Chandler NJ, Flagg TP, et al. Expression of Kir2.1 and Kir6.2 transgenes under the control of the alpha-MHC promoter in the sinoatrial and atrioventricular nodes in transgenic mice. J Mol Cell Cardiol. 2006;41:855–867. doi: 10.1016/j.yjmcc.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 42.Coats AJ. Clinical utility of exercise training in chronic systolic heart failure. Nat Rev Cardiol. 2011;8:380–392. doi: 10.1038/nrcardio.2011.47. [DOI] [PubMed] [Google Scholar]
- 43.Lachance D, Plante E, Bouchard-Thomassin AA, Champetier S, Roussel E, Drolet MC, et al. Moderate exercise training improves survival and ventricular remodelling in an animal model of left ventricular volume overload. Circ Heart Fail. 2009;2:437–445. doi: 10.1161/CIRCHEARTFAILURE.108.845487. [DOI] [PubMed] [Google Scholar]
- 44.Hambrecht R, Hilbrich L, Erbs S, Gielen S, Fiehn E, Schoene N, et al. Correction of endothelial dysfunction in chronic heart failure: additional effects of exercise training and oral l-arginine supplementation. J Am Coll Cardiol. 2000;35:706–713. doi: 10.1016/s0735-1097(99)00602-6. [DOI] [PubMed] [Google Scholar]
- 45.Coats AJ, Adamopoulos S, Radaelli A, McCance A, Meyer TE, Bernardi L, et al. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation. 1992;85:2119–2131. doi: 10.1161/01.cir.85.6.2119. [DOI] [PubMed] [Google Scholar]
- 46.O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.