Abstract
Aims
Hypoxia causes protein kinase C epsilon (PKCɛ) gene repression in foetal hearts, resulting in heightened cardiac susceptibility to ischaemic injury in offspring. We tested the hypothesis that hypoxia inducible factor 1 (HIF-1) and/or reactive oxygen species (ROS) mediate hypoxia-induced PKCɛ gene repression.
Methods and results
Hypoxia induced in vivo to pregnant rats, ex vivo to isolated foetal rat hearts, and in vitro in the rat embryonic ventricular myocyte cell line H9c2 resulted in a comparable decrease in PKCɛ protein and mRNA abundance in foetal hearts and H9c2 cells, which was associated with a significant increase in CpG methylation of the SP1-binding sites at the PKCɛ promoter. In H9c2 cells and foetal hearts, hypoxia caused nuclear accumulation of HIF-1α, which was inhibited by 3-(5′-hydroxymethyl-2′-furyl)-1-benzylindazole and 2-methoxy estradiol. The HIF-1α inhibitors had no significant effect on hypoxia-induced PKCɛ mRNA repression. Hypoxia produced a time-dependent increase in ROS production in H9c2 cells and foetal hearts that was blocked by ROS scavengers N-acetyl-cysteine or tempol. In accordance, N-acetyl-cysteine and tempol, but not apocynin, inhibited the hypoxic effect and restored PKCɛ protein and mRNA expression to the control values in foetal hearts and H9c2 cells. The ROS scavengers blocked hypoxia-induced CpG methylation of the SP1-binding sites, restored SP1 binding to the PKCɛ promoter, and abrogated the hypoxia-induced increase in the susceptibility of the heart to ischaemic injury in offspring.
Conclusions
The results demonstrate that hypoxia induces epigenetic repression of the PKCɛ gene through a NADPH oxidase-independent ROS-mediated pathway in the foetal heart, leading to heightened heart vulnerability to ischaemic injury in offspring.
Keywords: Hypoxia, Heart, Protein kinase C, Epigenetic, Oxidative stress
1. Introduction
A clear association between adverse intrauterine environment and increased risk of heart disease later in life has been demonstrated in recent epidemiological and animal studies.1–4 A common form of foetal stress in utero is hypoxia, which may occur under many conditions, including pregnancy at high altitude, maternal anaemia, pre-eclampsia, placental insufficiency, cord compression, maternal heart, lung and kidney disease, or haemoglobinopathy. Our recent studies in rats have demonstrated that maternal hypoxia causes an increase in promoter methylation and epigenetic repression of protein kinase C epsilon (PKCɛ) gene expression pattern in the developing heart, resulting in the heightened susceptibility of the heart to ischaemia and reperfusion injury in male offspring in a sex-dependent manner.5–7
The mechanisms underlying hypoxia-mediated PKCɛ gene repression remain unknown. In addition to hypoxia inducible factor 1 (HIF-1) that regulates many genes involved in external and internal adaptation to hypoxic stress,8 intracellular reactive oxygen species (ROS) paradoxically increases under hypoxic conditions.9 The main site for ROS production is the electron transport system (ETS) located in the inner membrane of mitochondria. Uncoupling of the ETS caused by hypoxia slows the electron flow, thereby increasing the probability of molecular oxygen interacting with free radicals to produce superoxide ion.9,10 Cardiomyocytes are major producers of ROS due to their high metabolic demand. Increased ROS can significantly alter gene expression patterns through the induction of integrated stress response that involves PERK activation, eIFα phosphorylation, and ATF4-mediated stress gene induction.11 Recent studies have suggested a link between prolonged oxidative stress and aberrant DNA methylation patterns.12–14
The present study tested the hypothesis that HIF-1 and/or ROS may mediate the hypoxia-induced epigenetic repression of PKCɛ gene expression pattern in foetal rat hearts and rat embryonic ventricular H9c2 cells. Our recent study has demonstrated a congruent underlying mechanism in foetal hearts and H9c2 cells in the epigenetic regulation of PKCɛ gene repression.7 Herein, we present evidence that blockade of hypoxia-derived ROS, but not HIF-1, inhibits the hypoxia-induced increase in methylation of the SP1-binding sites, reverses the decreased SP1 binding to the PKCɛ promoter, restores PKCɛ mRNA and protein abundance to the control levels, and abrogates hypoxia-induced increase in susceptibility of the heart to ischaemic injury in offspring.
2. Methods
An expanded ‘Methods’ section is available in the Supplementary material online.
2.1. Experimental animals
Time-dated pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Portage, MI, USA) and were randomly divided into two groups: (i) normoxic control, and (ii) hypoxic treatment of 10.5% oxygen from gestational Day 15 to Day 21, as described previously.6,7 To examine the effect of antioxidant, the rats were treated in the absence or presence of N-acetyl-cysteine (NAC, Sigma) in the drinking water (500 mg/kg/day). Hearts were isolated from Day 17 and Day 21 foetuses, and from 3-month-old offspring. To isolate hearts, rats were anaesthetized with 75 mg/kg ketamine and 5 mg/kg xylazine injected intramuscularly. The adequacy of anaesthesia was determined by the loss of a pedal withdrawal reflex and any other reaction from the animal in response to pinching the toe, tail, or ear of the animal. Additionally, even the respiration rate of the animal under anaesthesia was closely monitored, and an increased respiration rate was used as a sign that anaesthesia is too light. After removing foetuses, pregnant rats were killed by removing the hearts. Foetuses were sacrificed by decapitation, and hearts were collected for the studies. For ex vivo hypoxic treatment, hearts isolated from Day 17 foetuses were cultured in M199 medium (Hyclone, Logan, UT, USA) supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C in 95% air/5% CO2, as reported previously.7 Hearts were given 24 h of recovery time before being placed in a hypoxic chamber with 1% O2 for 48 h in the absence or presence of NAC (1 mM). All procedures and protocols were approved by the Institutional Animal Care and Use Committee guidelines, and followed the guidelines by US National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Cell culture
Rat embryonic ventricular myocyte cell line H9c2 was obtained from ATCC (Rockville, MD, USA). Cells were maintained in DMEM and supplemented with 10% FBS and 1% penicillin/streptomycin at 37°C in 95% air/5% CO2. Cells were grown and sub-cultured in six-well plates with experiments performed between 70 and 80% confluent. For hypoxic studies, cells were treated with 1% or 20.5% O2, respectively, for 24 h.7
2.3. Western blot analysis
Protein was isolated from foetal hearts and H9c2 cells. Protein abundance of PKCɛ and HIF-1α was measured with western blot analysis, and was normalized to β-actin, as described previously.7,15
2.4. Real-time RT–PCR
RNA was extracted from foetal hearts and H9c2 cells and PKCɛ mRNA abundance was determined by real-time RT–PCR and was normalized to GAPDH.7,15
2.5. Quantitative methylation-specific PCR
DNA was collected from foetal hearts and H9c2 cells and was treated with sodium bisulfite at 55°C for 16 h. Bisulfite-treated DNA was used as a template for real-time fluorogenic methylation-specific PCR using primers created to amplify promoter-binding sites containing possible methylation sites based on our previous sequencing of rat PKCɛ promoter.7,15,16
2.6. Measurement of intracellular ROS
The fluorescent indicator 2′7′-dichlorofluorescein (DCF) diacetate was used to measure intracellular ROS in H9c2 cells, as described previously.17 Total ROS in foetal hearts were measured with the Oxiselect™ in vitro ROS/RNS assay kit, following the manufacturer's instruction. Dihydroethidium fluorescence was determined to image ROS in foetal hearts using a confocal microscope.18 Additionally, MitoTracker® Red CM-H2XRos was used to measure mitochondrial ROS in H9c2 cells.19
2.7. Chromatin immunoprecipitation (ChIP)
Chromatin extracts were prepared from H9c2 cells, and ChIP assays were performed for the two SP1-binding sites at the PKCɛ promoter in DNA sequences pulled down by an SP1 antibody, as described previously.7,15
2.8. Hearts subjected to ischaemia and reperfusion
Isolated hearts from 3-month-old male offspring were subjected to 20 min of global ischaemia followed by 45 min of reperfusion in a Langendorff preparation, as previously described.6,7 Post-ischaemic recovery of left ventricular function and lactate dehydrogenase (LDH) release was determined.6,7
2.9. Statistical analysis
Data are expressed as mean ± SEM. Experimental number (n) represents the hearts of foetuses from different dams. Statistical significance (P < 0.05) was determined by the analysis of variance followed by Neuman–Keuls post hoc testing or Student's t-test, where appropriate.
3. Results
3.1. The effect of HIF-1α inhibitors on hypoxia-induced decrease in PKCɛ expression
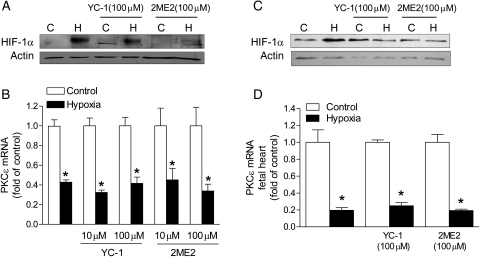
To assess the role of HIF-1α in hypoxia-induced decrease in PKCɛ expression, YC-1 and 2-methoxyestradiol (2-ME) were used to block HIF-1α nuclear accumulation. YC-1 blocks HIF-1α protein by enhancing degradation,20 and 2-ME blocks HIF-1α through an oxygen- and proteasome-independent pathway that involves disruption of microtubules.21 H9c2 cells were treated with 1% O2 for 24 h in the absence or presence of YC-1 (10 or 100 μM) or 2-ME (10 or 100 μM). Nuclear extracts were collected for determining HIF-1α nuclear accumulation. Figure 1A shows that HIF-1α protein accumulated in the nuclear compartment under the hypoxic treatment. The addition of YC-1 or 2-ME significantly reduced HIF-1α nuclear accumulation (Figure 1A). However, neither YC-1 nor 2-ME had significant effects on the hypoxia-induced decrease in PKCɛ mRNA expression (Figure 1B), suggesting a minimal role of HIF-1α in regulating PKCɛ gene transcription under hypoxic conditions. Similar results were obtained in isolated foetal hearts treated ex vivo with 1% O2, showing the lack of effect of HIF-1α inhibitors in regulating PKCɛ gene transcription under hypoxic conditions (Figure 1C and D).
Figure 1.
HIF-1α inhibitors have no effect on hypoxia-mediated PKCɛ gene repression. H9c2 cells (A and B) and isolated foetal hearts (C and D) were treated with 21% O2 (control, C) or 1% O2 (hypoxia, H) for 24 or 48 h, respectively, in the absence or presence of YC-1 or 2-ME2. HIF-1α protein abundance in nuclear extracts was measured by western blots. PKCɛ mRNA abundance was determined by real-time RT–PCR. Data are means ± SEM. *P < 0.05, hypoxia vs. control; n = 5–8.
3.2. Hypoxia increased ROS production in H9c2 cells and foetal hearts
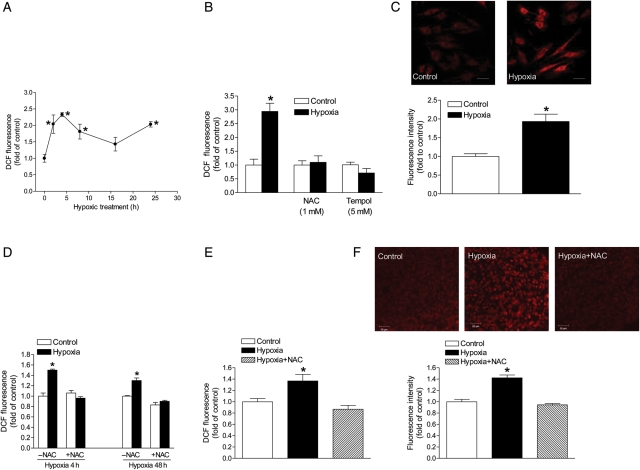
To determine whether hypoxia significantly alters ROS production in H9c2 cells, we performed a time course experiment using 2′7′-DCF diacetate to measure intracellular ROS production. H9c2 cells were treated with 1% O2 for 2, 4, 8, 16, and 24 h. Fluorescence of DCF was measured using a microplate reader and normalized to the cell count. As shown in Figure 2A, ROS levels were significantly elevated at the 2 h treatment. At the 4 h time point, ROS levels peaked and gradually declined afterwards until the 16 h mark, when it continued to increase again (Figure 2A). We further assessed the effect of ROS scavengers, NAC, and tempol on hypoxia-induced ROS production at the 4 h time point. As shown in Figure 2B, in the presence of NAC or tempol, the hypoxia-induced ROS production was blocked. Figure 2C shows a significant increase in mitochondrial ROS production under the hypoxic condition in H9c2 cells. In agreement with the findings in H9c2 cells, isolated foetal hearts treated ex vivo with 1% O2 showed a significant increase in ROS production, which was blocked by NAC (Figure 2D). Additionally, the in vivo treatment of maternal hypoxia resulted in a comparable increase in ROS in the foetal heart, which was abrogated by NAC (Figure 2E and F).
Figure 2.
Hypoxia increases ROS production. ROS were measured in H9c2 cells (A, B, and C) and isolated foetal hearts (D) treated with 21% O2 (control) or 1% O2 (hypoxia), and in the hearts isolated from near-term foetuses of pregnant rats treated with normoxic control and hypoxia (E and F). (A) Time course of hypoxia-induced ROS production in H9c2 cells; (B) ROS production in H9c2 cells at the 4 h hypoxic-treatment in the absence or presence of NAC or tempol; (C) mitochondrial ROS production in H9c2 cells at the 4 h hypoxic-treatment; scale bar: 20 µm (D) ROS production in isolated foetal hearts at 4 and 48 h hypoxic treatments in the absence or presence of 1 mM NAC; (E) ROS production in the foetal hearts from pregnant rats treated with normoxic control and hypoxia in the absence or presence of NAC; (F) ROS production, measured with confocal microscopy, in the foetal hearts from pregnant rats treated with normoxic control and hypoxia in the absence or presence of NAC. Scale bar: 50 µm Data are means ± SEM. *P < 0.05, hypoxia vs. control; n = 4–8.
3.3. ROS scavengers abrogated hypoxia-induced decrease in PKCɛ expression
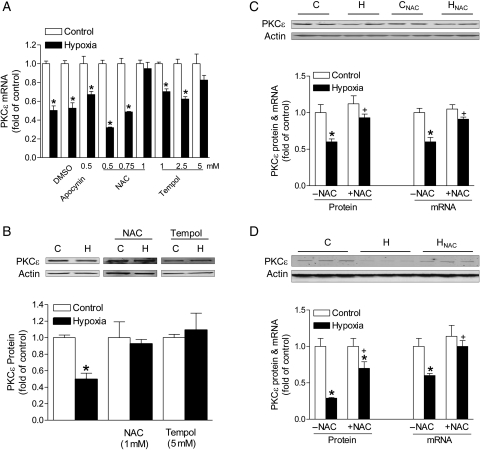
To determine the role of ROS in hypoxia-induced decrease in PKCɛ expression, H9c2 cells were treated with 1% O2 for 24 h in the absence or presence of NAC (0.5, 0.75, 1 mM) or tempol (1, 2.5, 5 mM). Additionally, apocynin (0.5 mM) was used to determine the role of NADPH oxidase in the hypoxic effect. Hypoxia significantly decreased PKCɛ protein and mRNA abundance (Figure 3A and B). NAC produced a dose-dependent inhibition of the hypoxic effect on PKCɛ mRNA repression (Figure 3A). Similar findings of blockade of the hypoxic effect were obtained with tempol (Figure 3A). In contrast, apocynin had no significant effect on the hypoxia-mediated down-regulation of PKCɛ expression (Figure 3A). Consistent with the results of mRNA, both NAC and tempol blocked the hypoxia-induced reduction in PKCɛ protein expression in H9c2 cells (Figure 3B). Similar results were obtained in isolated foetal hearts treated ex vivo with 1% O2, showing the reversal of hypoxia-induced down-regulation of PKCɛ gene expression by NAC (Figure 3C). In agreement with the findings in H9c2 cells and ex vivo foetal hearts, maternal hypoxia-mediated down-regulation of PKCɛ mRNA and protein expression in the foetal heart was inhibited by NAC (Figure 3D).
Figure 3.
ROS scavengers abrogate hypoxia-mediated PKCɛ gene repression. (A and B) H9c2 cells were treated with 21% O2 (control, C) or 1% O2 (hypoxia, H) in the absence or presence of apocynin, NAC, or tempol for 24 h. (C) Isolated foetal hearts were treated ex vivo with control (C) and hypoxia (H) in the absence or presence of 1 mM NAC. (D) Hearts were isolated from near-term foetuses of pregnant rats treated with control (C) and hypoxia (H) in the absence or presence of NAC. PKCɛ mRNA and protein abundance was determined by real-time RT–PCR and western blots, respectively. Data are means ± SEM. *P < 0.05, hypoxia vs. control; †P < 0.05, +NAC vs. –NAC; n = 4–9.
3.4. ROS scavengers abolished hypoxia-induced methylation of PKCɛ promoter
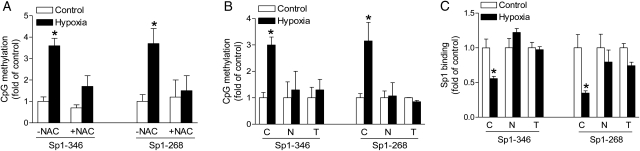
Previous studies have demonstrated that prolonged hypoxic treatment significantly increases methylation of the SP1-binding sites −346 and −268 at the PKCɛ promoter in foetal hearts and H9c2 cells.7 We, therefore, determined whether the inhibition of ROS significantly altered the methylation status of SP1-binding sites at the PKCɛ promoter. Genomic DNA was isolated and methylation-specific PCR was performed using primers that had been previously designed for the SP1 sites −346 and −268.7,16 Consistent with previous findings, maternal hypoxia significantly increased the methylation status of both SP1-binding sites in foetal hearts in the absence of ROS scavengers (Figure 4A). This hypoxia-induced promoter methylation was abrogated in the presence of NAC (Figure 4A). Similar results were obtained in H9c2 cells. In the absence of NAC or tempol, hypoxia significantly increased the methylation status of both SP1-binding sites in H9c2 cells (Figure 4B). NAC and tempol blocked the hypoxia-induced increase in CpG methylation of both SP1-binding sites −346 and −268 at the PKCɛ promoter (Figure 4B).
Figure 4.
ROS scavengers inhibit hypoxia-induced methylation and restore SP1 binding to PKCɛ promoter. (A) Hearts were isolated from near-term foetuses of pregnant rats treated with control and hypoxia in the absence or presence of NAC. (B and C) H9c2 cells were treated with 21% O2 (control) or 1% O2 (hypoxia) in the absence (C) or presence of 1 mM NAC (N) or 5 mM tempol (T) for 24 h. Methylation of the SP1-binding sites at −346 and −268 was determined by methylation-specific PCR. SP1 binding to the PKCɛ promoter at −346 and −268 in the context of intact chromatin was determined by ChIP assays. Data are means ± SEM. *P < 0.05, hypoxia vs. control; n = 4–9.
3.5. ROS scavengers restored SP1 binding to PKCɛ promoter
Previous studies have demonstrated that methylation of the SP1-binding sites −346 and −268 decreases SP1 binding to the PKCɛ promoter resulting in the reduced transcription activity.7,15 To evaluate further whether the inhibition of ROS restored the binding of SP1 protein to the SP1-binding sites at the proximal PKCɛ promoter in the context of intact chromatin, ChIP assays were performed using the SP1 antibody. Figure 4C shows the binding of SP1 to both SP1 elements at −346 and −268 at the PKCɛ promoter in intact chromatin in H9c2 cells. Hypoxia significantly decreased SP1 binding to both SP1-binding sites (Figure 4C). In the presence of NAC or tempol, the SP1 binding was restored to the control values for both the SP1-binding sites (Figure 4C).
3.6. ROS scavenger reversed the hypoxia-mediated increase in susceptibility of the heart to ischaemic injury in offspring
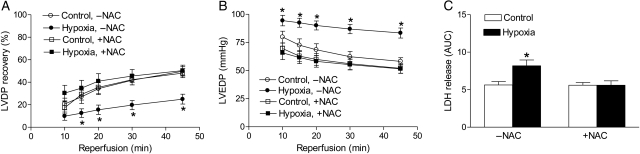
The causal role of PKCɛ gene repression in hypoxia-mediated and sex-dependent programming of increased susceptibility of the heart to ischaemia and reperfusion injury in adult male offspring has been previously demonstrated.6,7 To determine further the cause-and-effect relation between hypoxia-mediated ROS production in the developing heart and the heightened susceptibility of the heart to ischaemic injury in offspring, hearts were isolated from adult male offspring treated with hypoxia before birth in the absence or presence of NAC, and were studied in a Langendorff preparation. There were no significant differences in the left ventricle developed pressure (LVDP), heart rate (HR), dP/dtmax, dP/dtmin, and coronary flow rate at the baseline among all groups (Table 1). In the absence of NAC, foetal hypoxia caused a significant decrease in post-ischaemic recovery of LVDP and increases in the left ventricle end-diastolic pressure (LVEDP) and release of LDH (Figure 5), as previously reported.6,7 NAC had no significant effect on post-ischaemic recovery of left ventricular function in the control animals, but abolished the effect of hypoxia (Figure 5).
Table 1.
Pre-ischaemic left ventricle functional parameters
| HR (b.p.m.) | LVEDP (mmHg) | LVDP (mmHg) | dP/dtmax (mmHg/s) | dP/dtmin (mmHg/s) | CF (mL/min) | |
|---|---|---|---|---|---|---|
| Control, −NAC | 281 ± 5 | 9 ± 1 | 101 ± 2 | 2538 ± 129 | 1909 ± 80 | 16 ± 1 |
| Hypoxia, −NAC | 287 ± 6 | 11 ± 1 | 100 ± 2 | 2396 ± 126 | 1768 ± 75 | 14 ± 1 |
| Control, +NAC | 280 ± 4 | 9 ± 1 | 101 ± 3 | 2558 ± 166 | 1848 ± 123 | 14 ± 1 |
| Hypoxia, +NAC | 286 ± 5 | 9 ± 0 | 102 ± 3 | 2724 ± 156 | 1925 ± 123 | 14 ± 0 |
HR, heart rate; LVDP, left ventricular developed pressure; LVEDP, left ventricular end-diastolic pressure; dP/dtmax, maximal rate of contraction; dP/dtmin, maximal rate of relaxation; CF, coronary flow. n = 7–12 rats.
Figure 5.
ROS scavenger abrogates hypoxia-induced increase in susceptibility of the heart to ischaemia and reperfusion injury in offspring. Hearts were isolated from 3-month-old male offspring that had been exposed to normoxia (control) or hypoxia before birth in the absence or presence of NAC, and were subjected to 20 min of ischaemia and 45 min of reperfusion in a Langendorff preparation. Post-ischaemic recovery of the left ventricular developed pressure (LVDP, A) and the left ventricular end-diastolic pressure (LVEDP, B) were determined. Lactate dehydrogenase (LDH, C) release over 45 min of reperfusion was measured as the area under the curve (AUC). Data are means ± SEM. *P < 0.05, hypoxia vs. control; n = 7–2.
4. Discussion
The present study presents evidence for the first time that prolonged hypoxic treatment mediates epigenetic repression of the PKCɛ gene in the foetal heart through an ROS, but not HIF-1α, dependent pathway. The studies demonstrate that the inhibition of hypoxia-derived ROS restores PKCɛ protein and mRNA expression by blocking CpG methylation of the SP1-binding sites and restoring SP1 binding to the PKCɛ promoter. In contrast, the inhibition of HIF-1α did not affect the hypoxic effect on re-pression of the PKCɛ gene. Previous studies have demonstrated the cause-and-effect relation between foetal hypoxia-mediated PKCɛ gene repression and increased susceptibility of the heart to ischaemia and reperfusion injury in adult male offspring in a sex-dependent manner.6,7 The present finding that ROS scavenger NAC abrogated the hypoxia-mediated increase in susceptibility of the heart to ischaemic injury provides further functional evidence of a causal role of ROS in hypoxia-mediated programming of heightened heart ischaemic vulnerability in offspring.
The previous study demonstrated the heightened susceptibility of the heart to ischaemia and reperfusion injury in offspring that had been experienced prolonged hypoxia before birth.5,6 Interestingly, the ventricles of these offspring showed significantly less PKCɛ abundance compared with the control animals.6 It has been shown that acute exposure to hypoxia increases the activity of PKCɛ in the adult heart.22 We have demonstrated that chronic gestational hypoxia decreases the expression of PKCɛ in the foetal heart, suggesting that prolonged in utero hypoxia suppresses PKCɛ gene activity.7 Further investigation has revealed that chronic hypoxia directly regulates PKCɛ gene expression through increased methylation of two SP1-binding sites at the PKCɛ promoter.7 This pattern of increased promoter methylation presents in the foetal heart and persists into adulthood.6,7 Consistent with increased methylation, SP1 binding to the PKCɛ promoter in the context of intact chromatin was significantly decreased.7,15,16 Site-directed methylation of PKCɛ promoter-luciferase constructs for both SP1 sites, but not either site alone, caused a significant decrease in the promoter activity in H9c2 cells, demonstrating an important epigenetic mechanism involving the two SP1-binding sites in regulating PKCɛ gene transcription activity.7,15 Furthermore, the causal role of DNA methylation in the hypoxia-induced PKCɛ gene repression was demonstrated using a methylation inhibitor, 5-aza-2-deoxycytdine that blocked the hypoxic effect on down-regulation of PKCɛ gene expression, thereby restoring PKCɛ protein and mRNA to the control values.7
The present findings add new insights into the hypoxia-mediated regulation of PKCɛ expression in cardiomyocytes and demonstrate that hypoxia-derived ROS mediates the epigenetic repression of PKCɛ gene in the foetal heart. The finding that NAC and tempol, but not apocynin, blocked the hypoxic effect on PKCɛ repression is intriguing and suggests a role of NADPH oxidase-independent ROS in the hypoxia-mediated effect in the foetal heart. While NADPH oxidase has been shown to play a role in regulating the ROS production under chronic hypoxic conditions in some cell types, particularly in the pulmonary vasculature and carotid body, its involvement in hypoxia-mediated ROS production and hypoxia-related gene regulation appears to be tissue and organ selective. Consistent with the finding of a minimal role of NADPH oxidase in the hypoxia-mediated effect in the present study, previous studies in guinea pig ventricular myocytes demonstrated that NADPH oxidase did not appear to contribute substantially in the hypoxia-induced ROS production and myocyte dysfunction.23 The present findings that ROS scavengers NAC and tempol inhibited hypoxia-mediated methylation of the SP1-binding sites and restored SP1 binding to the PKCɛ promoter indicate that hypoxia-derived ROS plays a vital role in causing DNA methylation of the PKCɛ promoter in the foetal heart. Similar findings showed that NAC significantly reduced global DNA methylation during anchorage blockade in murine non-tumourigenic melanocyte, supporting the notion that ROS plays an important role in regulating DNA methylation.24 Consistent with these findings, previous studies demonstrated that prolonged exposure to ROS caused significant hypermethylation of the E-cadherin promoter.14 ROS-mediated methylation of E-cadherin promoter involved up-regulation of Snail, which recruited epigenetic effectors (i.e. DNA methyltransferase 1) to suppress gene transcription. Interestingly, Snail over-expression alone was sufficient to induce hypermethylation of E-cadherin promoter, suggesting that Snail regulation was a key factor in mediating epigenetic modification of gene promoters.14 Determining whether Snail activity is important in hypoxia-induced increase in methylation of CpG dinucleotides at transcription factor-binding sites of the PKCɛ promoter deserves further investigation. Furthermore, understanding whether the mechanisms by which hypoxia via ROS mediates methylation of the SP1-binding sites is a broad event (occurring in many genes) or selective (occurring in a few genes) warrants future research.
Previous studies in myocardial and non-myocardial tissues and H9c2 cell line have found that hypoxia increases ROS.11,25,26 Consistent with these findings, we demonstrate that hypoxia significantly increases ROS in H9c2 cells using 2′7′-DCF diacetate. Time course studies revealed a biphasic production of ROS in H9c2 cells with an initial peak at the 4 h treatment, which declined to the 16 h mark, followed by a significant increase at 24 h. Time course studies by Chen et al.27 showed a similar biphasic elevation of ROS in human embryonic kidney and glioma cell lines treated with mitochondria complex inhibitor I rotenone or mitochondria complex II inhibitor TTFA. The present study demonstrates that mitochondria are an important source of hypoxia-induced ROS production in H9c2 cells. The prolonged hypoxic treatment for 24 h maintained significantly higher levels of ROS and produced heightened and prolonged oxidative stress. Similar findings of hypoxia-mediated increase in ROS in foetal hearts in vivo and ex vivo as that observed in H9c2 cells in the present study demonstrate a comparable model of H9c2 cells in the study of hypoxia-induced ROS production in the foetal heart. Although acute exposure to ROS increases the activity of PKCɛ and promotes a cardioprotective phenotype often observed in the acute ischaemia and reperfusion setting in the adult heart,22 the present study demonstrates that prolonged hypoxia causes a sustained increase in ROS, which results in the down-regulation of PKCɛ gene expression in H9c2 cells. These findings suggest differential regulations of the PKCɛ activity and gene expression patterns in response to acute or chronic hypoxia. This difference may represent a negative feedback loop in which short-term hypoxia enhances the PKCɛ activity to promote a cardioprotective phenotype, whereas long-term exposure to hypoxia-derived ROS promotes adaptive changes that include the down-regulation of PKCɛ gene expression.
It has been demonstrated that the treatment of pregnant rats with an ambient oxygen level of 10.5% lowers maternal arterial pO2 by half to ∼50 mmHg.28 This maternal hypoxia resulted in foetal hypoxia and increased HIF-1α protein levels in the foetal heart,29 albeit normal foetal arterial blood pO2 is substantially lower than that of the mother and is close to ∼20 mmHg or 3% O2. In in vitro studies of foetal tissues or cells, it is common to use 1% O2 (∼7 mmHg) to induce hypoxia.30–33 Our previous study in H9c2 cells demonstrated that exposure to 21, 10.5, or 3% O2 had no significantly different effects on PKCɛ expression, but 1% O2 down-regulated PKCɛ expression, suggesting modification in gene expression patterns as a mode of adaptation to oxygen insufficiency in cardiomyocytes.7 Consistent with the in vivo study of maternal hypoxia showing increased HIF-1α protein abundance in the foetal heart,29 the present study found that H9c2 cells exposed to 1% O2 for 24 h resulted in significant nuclear accumulation of HIF-1α that is a marker of hypoxia. Other studies have demonstrated that similar oxygen levels and exposure are sufficient to induce HIF-1α stabilization and nuclear accumulation.34 YC-1 and 2-ME have been widely used to inhibit HIF-1α nuclear accumulation. Previous studies suggested that YC-1 inhibited HIF-1α protein by enhancing its degradation through FIH-dependent COOH-terminal transactivation domain inactivation.20 2-Methoxyestradiol inhibits HIF-1α independent of oxygen and proteasome pathways by disrupting microtubules and the translocation of HIF-1α into the nuclear compartment, thus preventing the HIF-1 activity.21 Interestingly, both YC-1 and 2-ME have been shown to block HIF-2α nuclear accumulation as well, thereby inhibiting the HIF-2 activity. Although HIF-2α is not the focus of this study, the use of YC-1 and 2-ME may also provide clues as to the role of HIF-2α in hypoxia-induced PKCɛ gene repression.
Little is known concerning the role of HIF-1α in the methylation of specific gene promoters.35 HIF-1α regulates the expression of epigenetic effectors, namely histone deacetylases and demethylase,36 but it is unclear whether HIF-1α directly or indirectly regulates DNA methyltransferase. HIF-1α can regulate the c-myc activity and c-myc has been shown to recruit DNA methyltransferase resulting in promoter hypermethylation for some genes,37 suggesting a possible mechanism whereby HIF-1α may influence methylation of promoter regions. Interestingly, Watson et al.38 reported that chronic hypoxia increased global methylation patterns and expression of DNA methyltransferase 3b in prostate cell lines absent of HIF-1α protein expression, suggesting that chronic hypoxia can influence DNA methylation independent of HIF-1α. In the present study, we found that the inhibition of HIF-1α with YC-1 or 2-ME had no significant effect on hypoxia-induced repression of PKCɛ mRNA, suggesting that HIF-1α does not play a significant role in altering PKCɛ promoter methylation. Importantly, it has been demonstrated that hypoxia induces the stabilization of HIF-1α protein through alterations in the redox state. The mechanism is thought to be through mitochondrial-derived ROS from complex III that regulates the prolyl hydroxylase activity.13,39 Other studies contend that oxygen availability instead of ROS production is the main stimulus altering prolyl hydroxylase activity and therefore HIF-1α stabilization.40 In the present study, we demonstrate that attenuation of ROS, but not HIF-1α, plays a major role in hypoxia-induced reduction in PKCɛ expression.
A model used in the present study was the embryonic rat ventricular myocyte cell line H9c2, which is a widely used system for studying cardiomyocytes, including cell death, differentiation, and foetal programming.7,25,34 Electrophysically, H9c2 cells are similar to primary cardiomyocytes, but differ phenotypically.41,42 Although differences exist, recent studies using the H9c2 cell line to study the effects of hypoxia on PKCɛ abundance have found consistent results with freshly isolated foetal cardiomyocytes and intact hearts.7 Thus, H9c2 cells exposed to 1% O2 for 24 h displayed a similar pattern of decreased PKCɛ protein and mRNA as those seen in freshly isolated foetal rat cardiomyocytes and intact foetal hearts exposed to 1% O2.7 Furthermore, both models found increased methylation of CpG dinucleotides at SP1-binding sites at the PKCɛ promoter. This suggests that the underlying mechanisms for hypoxia-induced decrease in PKCɛ gene expression are similar in both foetal rat hearts and H9c2 cells. Other studies also demonstrated that prolonged hypoxia in the presence of low or high glucose significantly decreased PKCɛ protein abundance in H9c2 cells.43,44 Consistent with these findings, the present study demonstrates a congruent underlying mechanism of the heightened ROS in hypoxia-mediated PKCɛ gene repression in foetal hearts and H9c2 cells, further supporting the use of H9c2 cells in investigating the epigenetic mechanisms of PKCɛ gene expression patterns.
In addition to PKCɛ, our previous study demonstrated that PKCδ was also significantly decreased in the hearts of male offspring that had been exposed to hypoxia before birth.6 Unlike PKCɛ whose active form of p-PKCɛ was significantly decreased, the active form of PKCδ, p-PKCδ was not changed.6 The role of PKCδ in ischaemia and reperfusion injury is less clear and is somewhat controversial. Inhibition of PKCδ during reperfusion has been shown to decrease reperfusion-induced injury.45 Other studies demonstrated the cardioprotective effects of PKCδ.46–48 The role of ROS in epigenetic regulation of PKCδ gene expression in the heart remains to be investigated.
In summary, the present study identifies a novel mechanism of hypoxia-derived ROS in inducing CpG methylation of sequence-specific transcription factor-binding sites at the PKCɛ promoter and its gene repression in the foetal heart, leading to the increased susceptibility of the heart to ischaemic injury in offspring. Although it is difficult to translate directly these findings into humans, linking chronic exposure to hypoxia-derived ROS with epigenetic repression of a cardioprotective gene in the developing heart and the heightened susceptibility of the heart to ischaemic injury has the significant in clinical implications. This is because hypoxia is a common form of foetal stress and large epidemiological studies have indicated a clear link between foetal stress and increased risk of ischaemic heart disease in offspring. Elevated levels of ROS have been implicated in numerous disease models and, thus, may initiate epigenetic modification of cardioprotective genes in the long term leading to increased susceptibility to ischaemic heart disease. Potentially, this knowledge may lead to interventions involving antioxidants defence during gestation, which may prevent the long-term adverse effects of chronic intrauterine hypoxia.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This study was supported by the National Institutes of Health grants HL82779 (L.Z.), HL83966 (L.Z.), HL89012 (L.Z.), HL110125 (L.Z.), DA032510 (D.X.), and California Tobacco-Related Disease Research Program Award 18KT-0024 (D.X.). Andrew Patterson was supported by NIH fellowship award 5R25GM060507.
Supplementary Material
References
- 1.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 2.Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 3.Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 5.Li GXY, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig. 2003;10:265–274. doi: 10.1016/s1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- 6.Xue Q, Zhang L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: role of PKCɛ. J Pharmacol Exp Ther. 2009;330:624–632. doi: 10.1124/jpet.109.153239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKCɛ gene repression in rat hearts. Circ Res. 2010;107:365–373. doi: 10.1161/CIRCRESAHA.110.221259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Semenza GL. Hypoxia-inducible factor 1: master regulator of O2 homeostasis. Curr Opin Genet Dev. 1998;8:588–594. doi: 10.1016/s0959-437x(98)80016-6. [DOI] [PubMed] [Google Scholar]
- 9.Poyton RO, Ball KA, Castello PR. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol Metab. 2009;20:332–340. doi: 10.1016/j.tem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Hervouet E, Simonnet H, Godinot C. Mitochondria and reactive oxygen species in renal cancer. Biochimie. 2007;89:1080–1088. doi: 10.1016/j.biochi.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 11.Duranteau J, Chandel NS, Kulisz A, Shao Z, Schumacker PT. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J Biol Chem. 1998;273:11619–11624. doi: 10.1074/jbc.273.19.11619. [DOI] [PubMed] [Google Scholar]
- 12.Liu L, Wise DR, Diehl JA, Simon MC. Hypoxic reactive oxygen species regulate the integrated stress response and cell survival. J Biol Chem. 2008;283:31153–31162. doi: 10.1074/jbc.M805056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galanis A, Pappa A, Giannakakis A, Lanitis E, Dangaj D, Sandaltzopoulos R. Reactive oxygen species and HIF-1 signalling in cancer. Cancer Lett. 2008;266:12–20. doi: 10.1016/j.canlet.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 14.Lim SO, Gu JM, Kim MS, Kim HS, Park YN, Park CK, et al. Epigenetic changes induced by reactive oxygen species in hepatocellular carcinoma: methylation of the E-cadherin promoter. Gastroenterology. 2008;135:2128–2140. doi: 10.1053/j.gastro.2008.07.027. 40 e1–8. [DOI] [PubMed] [Google Scholar]
- 15.Meyer K, Zhang H, Zhang L. Direct effect of cocaine on epigenetic regulation of PKCepsilon gene repression in the fetal rat heart. J Mol Cell Cardiol. 2009;47:504–511. doi: 10.1016/j.yjmcc.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang H, Meyer KD, Zhang L. Fetal exposure to cocaine causes programming of Prkce gene repression in the left ventricle of adult rat offspring. Biol Reprod. 2009;80:440–448. doi: 10.1095/biolreprod.108.072983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Joseph JA. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic Biol Med. 1999;27:612–616. doi: 10.1016/s0891-5849(99)00107-0. [DOI] [PubMed] [Google Scholar]
- 18.Csont T, Bereczki E, Bencsik P, Fodor G, Gorbe A, Zvara A, et al. Hypercholesterolemia increases myocardial oxidative and nitrosative stress thereby leading to cardiac dysfunction in apoB-100 transgenic mice. Cardiovasc Res. 2007;76:100–109. doi: 10.1016/j.cardiores.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Kuznetsov AV, Kehrer I, Kozlov AV, Haller M, Redl H, Hermann M, et al. Mitochondrial ROS production under cellular stress: comparison of different detection methods. Anal Bioanal Chem. 2011;400:2383–2390. doi: 10.1007/s00216-011-4764-2. [DOI] [PubMed] [Google Scholar]
- 20.Li SH, Shin DH, Chun YS, Lee MK, Kim MS, Park JW. A novel mode of action of YC-1 in HIF inhibition: stimulation of FIH-dependent p300 dissociation from HIF-1α. Mol Cancer Ther. 2008;7:3729–3738. doi: 10.1158/1535-7163.MCT-08-0074. [DOI] [PubMed] [Google Scholar]
- 21.Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, et al. 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer Cell. 2003;3:363–375. doi: 10.1016/s1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- 22.Kabir AM, Clark JE, Tanno M, Cao X, Hothersall JS, Dashnyam S, et al. Cardioprotection initiated by reactive oxygen species is dependent on activation of PKCɛ. Am J Physiol Heart Circ Physiol. 2006;291:H1893–H1899. doi: 10.1152/ajpheart.00798.2005. [DOI] [PubMed] [Google Scholar]
- 23.Hool LC, Di Maria CA, Viola HM, Arthur PG. Role of NAD(P)H oxidase in the regulation of cardiac L-type Ca2+ channel function during acute hypoxia. Cardiovasc Res. 2005;67:624–635. doi: 10.1016/j.cardiores.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 24.Campos AC, Molognoni F, Melo FH, Galdieri LC, Carneiro CR, D'Almeida V, et al. Oxidative stress modulates DNA methylation during melanocyte anchorage blockade associated with malignant transformation. Neoplasia. 2007;9:1111–1121. doi: 10.1593/neo.07712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kumar S, Sitasawad SL. N-acetylcysteine prevents glucose/glucose oxidase-induced oxidative stress, mitochondrial damage and apoptosis in H9c2 cells. Life Sci. 2009;84:328–336. doi: 10.1016/j.lfs.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 26.Ishida I, Kubo H, Suzuki S, Suzuki T, Akashi S, Inoue K, et al. Hypoxia diminishes toll-like receptor 4 expression through reactive oxygen species generated by mitochondria in endothelial cells. J Immunol. 2002;169:2069–2075. doi: 10.4049/jimmunol.169.4.2069. [DOI] [PubMed] [Google Scholar]
- 27.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J Cell Sci. 2007;120:4155–4166. doi: 10.1242/jcs.011163. [DOI] [PubMed] [Google Scholar]
- 28.Rhee JW, Zhang L, Ducsay CA. Suppression of myometrial contractile responses to oxytocin after different durations of chronic hypoxia in the near-term pregnant rat. Am J Obstet Gynecol. 1997;177:639–644. doi: 10.1016/s0002-9378(97)70158-3. [DOI] [PubMed] [Google Scholar]
- 29.Bae S, Xiao Y, Li G, Casiano CA, Zhang L. Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am J Physiol Heart Circ Physiol. 2003;285:983–990. doi: 10.1152/ajpheart.00005.2003. [DOI] [PubMed] [Google Scholar]
- 30.Tang Y, Jackson M, Qian K, Phillips MI. Hypoxia inducible double plasmid system for myocardial ischemia gene therapy. Hypertension. 2002;39:695–698. doi: 10.1161/hy0202.103784. [DOI] [PubMed] [Google Scholar]
- 31.Mizukami Y, Ono K, Du CK, Aki T, Hatano N, Okamoto Y, et al. Identification and physiological activity of survival factor released from cardiomyocytes during ischaemia and reperfusion. Cardiovasc Res. 2008;79:589–599. doi: 10.1093/cvr/cvn148. [DOI] [PubMed] [Google Scholar]
- 32.Uetani T, Nakayama H, Okayama H, Okura T, Higaki J, Inoue H, et al. Insufficiency of pro-heparin-binding epidermal growth factor-like growth factor shedding enhances hypoxic cell death in H9c2 cardiomyoblasts via the activation of caspase-3 and c-Jun N-terminal kinase. J Biol Chem. 2009;284:12399–12409. doi: 10.1074/jbc.M900463200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shin EJ, Schram K, Zheng XL, Sweeney G. Leptin attenuates hypoxia/reoxygenation-induced activation of the intrinsic pathway of apoptosis in rat H9c2 cells. J Cell Physiol. 2009;221:490–497. doi: 10.1002/jcp.21883. [DOI] [PubMed] [Google Scholar]
- 34.Hwang JM, Weng YJ, Lin JA, Bau DT, Ko FY, Tsai FJ, et al. Hypoxia-induced compensatory effect as related to Shh and HIF-1alpha in ischemia embryo rat heart. Mol Cell Biochem. 2008;311:179–187. doi: 10.1007/s11010-008-9708-6. [DOI] [PubMed] [Google Scholar]
- 35.Shaw RJ, Omar MM, Rokadiya S, Kogera FA, Lowe D, Hall GL, et al. Cytoglobin is upregulated by tumour hypoxia and silenced by promoter hypermethylation in head and neck cancer. Br J Cancer. 2009;101:139–144. doi: 10.1038/sj.bjc.6605121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beyer S, Kristensen MM, Jensen KS, Johansen JV, Staller P. The histone demethylases JMJD1A and JMJD2B are transcriptional targets of hypoxia-inducible factor HIF. J Biol Chem. 2008;283:36542–36552. doi: 10.1074/jbc.M804578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Benanti JA, Wang ML, Myers HE, Robinson KL, Grandori C, Galloway DA. Epigenetic down-regulation of ARF expression is a selection step in immortalization of human fibroblasts by c-Myc. Mol Cancer Res. 2007;5:1181–1189. doi: 10.1158/1541-7786.MCR-06-0372. [DOI] [PubMed] [Google Scholar]
- 38.Watson JA, Watson CJ, McCrohan AM, Woodfine K, Tosetto M, McDaid J, et al. Generation of an epigenetic signature by chronic hypoxia in prostate cells. Hum Mol Genet. 2009;18:3594–3604. doi: 10.1093/hmg/ddp307. [DOI] [PubMed] [Google Scholar]
- 39.Chandel NS, McClintock DS, Feliciano CE, Wood TM, Melendez JA, Rodriguez AM, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275:25130–25138. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 40.Chua YL, Dufour E, Dassa EP, Rustin P, Jacobs HT, Taylor CT, et al. Stabilization of hypoxia-inducible factor-1alpha protein in hypoxia occurs independently of mitochondrial reactive oxygen species production. J Biol Chem. 2010;285:31277–31284. doi: 10.1074/jbc.M110.158485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res. 1991;69:1476–1486. doi: 10.1161/01.res.69.6.1476. [DOI] [PubMed] [Google Scholar]
- 42.Kimes BW, Brandt BL. Properties of a clonal muscle cell line from rat heart. Exp Cell Res. 1976;98:367–381. doi: 10.1016/0014-4827(76)90447-x. [DOI] [PubMed] [Google Scholar]
- 43.Kim MH, Jung YS, Moon CH, Jeong EM, Lee SH, Baik EJ, et al. Isoform-specific induction of PKCɛ by high glucose protects heart-derived H9c2 cells against hypoxic injury. Biochem Biophys Res Commun. 2003;309:1–6. doi: 10.1016/s0006-291x(03)01525-0. [DOI] [PubMed] [Google Scholar]
- 44.Kim MJ, Moon CH, Kim MY, Kim MH, Lee SH, Baik EJ, et al. Role of PKCδ during hypoxia in heart-derived H9c2 cells. Jpn J Physiol. 2004;54:405–414. doi: 10.2170/jjphysiol.54.405. [DOI] [PubMed] [Google Scholar]
- 45.Murriel CL, Mochly-Rosen D. Opposing roles of delta and epsilon pkc in cardiac ischemia and reperfusion: targeting the apoptotic machinery. Arch Biochem Biophys. 2003;420:246–254. doi: 10.1016/j.abb.2003.08.038. [DOI] [PubMed] [Google Scholar]
- 46.Bouwman RA, Salic K, Padding FG, Eringa EC, van Beek-Harmsen BJ, Matsuda T, et al. Cardioprotection via activation of protein kinase C-delta depends on modulation of the reverse mode of the Na+/Ca2+ exchanger. Circulation. 2006;114:226–232. doi: 10.1161/CIRCULATIONAHA.105.000570. [DOI] [PubMed] [Google Scholar]
- 47.Kawamura S, Yoshida K, Miura T, Mizukami Y, Matsuzaki M. Ischemic preconditioning translocates PKC-delta and -epsilon, which mediate functional protection in isolated rat heart. Am J Physiol. 1998;275:2266–2271. doi: 10.1152/ajpheart.1998.275.6.H2266. [DOI] [PubMed] [Google Scholar]
- 48.Zhao J, Renner O, Wightman L, Sugden PH, Stewart L, Miller AD, et al. The expression of constitutively active isotypes of protein kinase C to investigate preconditioning. J Biol Chem. 1998;273:23072–23079. doi: 10.1074/jbc.273.36.23072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.