Abstract
Introduction
Alliumplants are an important part of the diet of many populations and there is a long-held belief in their health-enhancing properties such as cancer prevention. In this study, the anticancer and anti-inflammatory activities of the aqueous extract of the Allium ascalonicum bulbs have been studied.
Material and methods
The antiproliferative and anti-growth activity of the aqueous extract of A. ascalonicum was examined in vitro on different tumor cell lines. Furthermore, the acetic acid-induced vascular permeability as an in vivo assay was used for studying anti-inflammatory activity of the extract.
Results
The aqueous extract of A. ascalonicum had the most anti-growth activity on the cancer cell lines; Jurkat and K562 against Wehi 164 with lower cytotoxic preference. The extract also showed much less cytotoxicity against the normal cell (HUVEC) line and significant anti-inflammatory activity in vivo.
Conclusions
It is of interest that the extract of this plant has shown much less cytotoxicity against the normal cell line, and, if this also occurs in vivo, the use of this plant clinically for the treatment of cancer patients would have some scientific support. The results of these assays indicated that A. ascalonicum can be a candidate for prevention and treatment of many diseases related to inflammation and malignancy.
Keywords: Allium ascalonicum, anti-inflammatory, anticancer, LDH assay
Introduction
Cancer is one of the most prevalent diseases worldwide, which is a major public health issue in both developed and developing countries. Because of the high demand for cancer drugs, the need to less side effects products from natural sources and the constraints of present production technologies for pharmaceuticals, attention has recently been focused on investigation and screening of pharmaceutical anticancer compounds in plants. Indeed, plants have a long history of use in the treatment of cancer [1]. However, the undeniable role of diet containing plants should not be disregarded in the prevention of many diseases such as cancer.
Until recently, a number of biological active phytochemicals have been identified in plant foods [2]. Plant-derived compounds have been also an important source of several clinically useful anticancer agents, which include vinblastine, vincristine, the camptothecin derivatives, topotecan and irinotecan, etoposide, derived from epipodophyllotoxin, and paclitaxel (taxol). Moreover, several promising new agents, including flavopiridol and combretastatin A4 phosphate, are in clinical development based on selective activity against cancer-related molecular targets while some agents which failed in earlier clinical studies have been noticed again [3].
Shallot (Allium ascalonicum) is a member of the Liliaceae family, which has been used mainly as a spice traditionally from the ancient times. It has many different benefits including antibacterial and anti-fungal properties [4, 5], beneficial hematological influences [6], antioxidant properties [7], anti-Helicobacter pyloripotential effect [8] and peroxynitrite-scavenging capacity [9] which have been studied until now. Besides, the chemical composition of A. ascalonicum was analyzed by other researchers, and some effective components such as a mannose-specific lectin [10], an antifungal peptide [11], new furostanol saponins [12], selenium and sulfur species [13], and various flavonol glucosides [14], inhibit proliferation and growth of tumor cell lines as HeLa and MCF-7 cell lines extract [15]. Cytotoxic effect of selenized odorless garlic and shallot against human leukemia cells (HL-60) [16], arresting cell cycle progression and inducing apoptosis in human cervical carcinoma HeLa cells [17] have been identified and isolated from it.
The aim of this study was to investigate anticancer and anti-inflammatory activities of the aqueous extract of A. ascalonicum which were performed using in vitro three cancer cell lines and an in vivo acetic acid-induced vascular permeability assay in mice.
Material and methods
Reagents
DMEM (Dulbeccos modified minimum essential medium), RPMI 1640 (Life Technologies, Grand Island, NY), fetal bovine serum (FBS), Trypan blue 0.4% (Gibco, New York, USA), LDH (Lactate dehydrogenase) cytotoxicity assay kit (Roch Chemical Co.), Penicillin/streptomycin, trypsin /EDTA (Sigma Chemical Co.).
Plant material
The bulbs of A. ascalonicum were prepared from local vegetable markets at Kermanshah (West of Iran), and authenticated by one of the authors (Dr. Ali Mostafaie).
Preparation of plant extract
Preparation of the aqueous extract of A. ascalonicum was performed as previously described [18]. In brief, the bulbs (1 kg) were grounded in a mortar with one liter of distilled water and stirred overnight to complete extraction. Then, it was filtrated through a cheese cloth and centrifuged at 16000 xg and 4°C for 30 min. After complete extracting by distilled water and drying the extract by freeze dryer, it’s the yield of which was about 27.4% as compared to original bulbs weight. Before use, it was weighed and dissolved in sterile phosphate buffered saline (PBS).
Cell culture
The cancer cell lines including Wehi164 (mouse fibrosarcoma cells), Jurkat (human–acute T-cell leukemia) and K562 (human erythroleukemia), and human umbilical vein endothelial cells (HUVEC) as a normal cell line were purchased from the National Cell Bank, Pasteur Institute of Iran.
Cell lines were seeded in 75-cm2 tissue culture flasks and maintained in RPMI 1640 and Dulbecco’s MEM supplemented with 10% heat-inactivated fetal bovine serum, 50 U/ml penicillin and 50 µg/ml streptomycin. The medium was renewed every two days and the cell cultures were incubated at 37°C in a humidified atmosphere (95% air and 5% CO2).
Cell viability inhibition assays
Cytotoxicity effects on cancer cell lines by A. ascalonicumwere evaluated by Trypan blue exclusion assay and activity of lactate dehydrogenase (LDH) method.
Trypan blue assay
Cancer cell lines at a density of 5 × 104 per well were seeded in 24-well plates overnight. The day after, the medium was changed by fresh complete medium which contained increasing concentrations of A. ascalonicum (0, 25, 50, 100, 200 400, 600, 800, 1000, 1500, and 2000 µg/ml). At 24, 48 and 72 h of treatments, the cells were washed with PBS and harvested. Trypan blue dye was further added to the cell suspensions. The Trypan blue-staining cells were examined as damaged or dead cells.
LDH cytotoxicity assay
The cytotoxic effect of the aqueous extract of A. ascalonicum was also examined using LDH assay as described by Linford [19] with some modifications. Briefly, the cell lines were separately plated at a density of 5 × 103 per well in 96-well microplates with RPMI 1640-DMEM medium containing 10% FBS, and allowed to incubate overnight. After 24 h of early cell culture, the fresh medium with extract at concentrations of (0, 25, 50, 100, 200 400, 600, 800, 1000, 1500, and 2000 µg/ml) was renewed. At 72 h of treatments, the plates were centrifuged at 200 xg, and 100 µl of the media from each well was then transferred to new 96-well plates. Thereafter, 100 µl of LDH assay mixture was added to each well and plates were incubated at 37°C for 30 min. A group of wells was treated with 1% Triton X-100 solution for 45 min to maximum LDH release. The LDH release was estimated using a microplatereader at 495 nm according to the manufacturer's instructions. Tests were performed in triplicate. The mean cell viability was expressed as a percentage of the control.
Proliferation assay
The cell lines were plated in 24-well plates at a density of 5 × 104 cells/well in complete medium and incubated at 37°C under a humidified atmosphere containing 5% CO2 for 24 h. The day after, the cells were treated with fresh medium containing 10% FBS and the determined concentrations of the extract (0, 25, 50, 100, 200, 400, 600, 800, 1000, 1500, and 2000 µg/ml). After 72 h, the treated and untreated cells were removed and counted against control wells using a Coulter counter (KX-21 Sysmex Co).
Acetic acid-induced vascular permeability in mice
For acetic acid-induced vascular permeability test, the Whittle’s method [20] was used with somewhat modifications. Briefly, one hour after oral administration of the aqueous extract of A. ascalonicum (50-200 mg/kg), and Indomethacin (INN) (5 mg/ml); 0.1 ml/10 g body weight of 1% Evans blue solution was injected intravenously in each mouse. Thirty minutes later, 0.1 ml/10 g body weight of 0.7% acetic acid in saline was intraperitoneally injected for 30 min. Following the administration of acetic acid, the mice were killed by cervical dislocation and after extravasation of 10 ml of saline into the peritoneal cavity then, the washing solutions were collected in separated test tubes. Concentrations of Evans blue leaked into the peritoneal cavities were measured by the absorbance at 590 nm of the collected washing solutions. The vascular permeability was represented in terms of the absorbance (A590).
Statistical analysis
All measurements were evaluated statically expressed as mean ± standard deviation. Results were analyzed by one way ANOVA. Values of p < 0.05 were considered significant. All experiments were performed in triplicate unless otherwise noted.
Results
Cytotoxicity effects of the aqueous extract of Allium ascalonicumon cancer cell lines and normal cell line
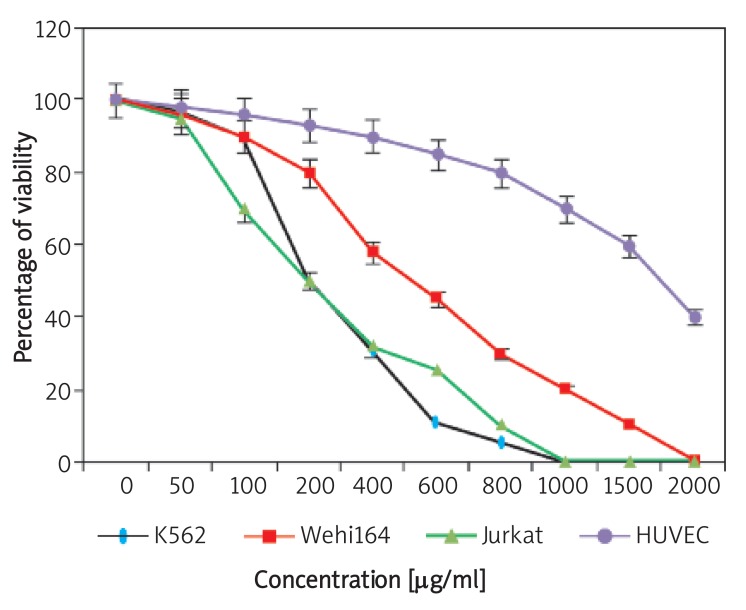
In this study, we evaluated the effects of the aqueous extract of A. ascalonicum on viability of the three cancer cell lines including K562, Wehi164 and Jurkat, and normal cell line (HUVEC) using Trypan blue and LDH assays evaluation. On the basis of obtained results, after 24, 48 and 72 h of incubation, the extract significantly reduced the viability of three cell lines at different concentrations compared with the control group, and these effects were stronger as time increased. On the other hand, the studied cytotoxicity treatments resulted in a dose and time-dependent manner. Furthermore, the observed results from effective concentrations of the extract were accompanied with differences for various cancer cell lines. In fact, and based on data of Figure 1, the cytotoxic effects of A. ascalonicumfor Wehi164 cell line were considerably lower than those on K562 and Jurkat cell lines. As shown in Figure 1, the extract inhibited viability of K562 and Jurkat cell lines at 100 µg/ml significantly, while this effect for Wehi164 cell line occurred at higher concentrations (400 µg/ml). The IC50 values of the A. ascalonicum extract (the concentration required for a 50% viability inhibition) for each cancer cell line have been shown in Table I. Furthermore, treatment of HUVECs as normal cell lines with the aqueous extract of the A. ascalonicum bulbs up to 1000 µg/ml or even higher concentrations for 72 h showed no considerable cytotoxic effect on the HUVECs. In fact, under the concentration of 1000 µg/ml, there was no significant difference in viability between the test and control wells. The IC50 value of the extract for growth inhibition of the cells was determined above 1 mg/ml (Table I). The IC50 values of the A. ascalonicum extract (the concentration required for a 50% viability inhibition) for each cancer cell line have also been shown in Table I.
Figure 1.
The cytotoxic effects of different concentrations of the aqueous extract of A. ascalonicum on K562, Jurkat and Wehi164, and HUVEC cell lines. The viability percentage of cell lines was assayed using Trypan blue 4% exclusion and LDH assays. Each data shown are one representative example of three independent experiments, expressed as a percentage of control and standard deviations were within 5% of all experimental values
Table I.
IC50 and GI50 value data of the aqueous extract of A. ascalonicum, for K562, Jurkat and Wehi164, and HUVEC after 72 h of treatment
| Cell line | GI50 [µg/ml] | IC50 [µg/ml] |
|---|---|---|
| K562 | 100 ±10 | 100 ±8 |
| Jurkat | 100 ±12 | 100 ±10 |
| Wehi164 | 400 ±8 | 400 ±6 |
| HUVEC | 400 ±5 | 1600 ±8 |
Aqueous extract of Allium ascalonicum induced suppression of cancer cell lines proliferation
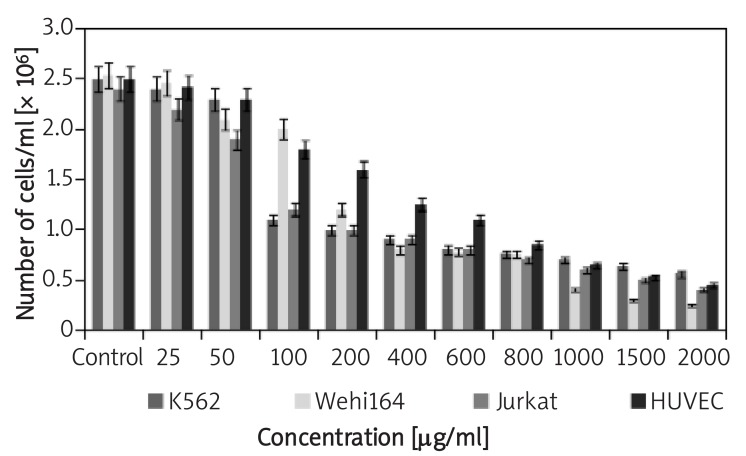
The antiproliferative activity of the A. ascalonicum extract on the cancer cell lines was determined by the Coulter counter, the results of which are shown in Figure 2. After treatment with 25-200 µg/ml and higher concentrations of the extract for 72 h, the proliferation ratio of K562 and Jurkat cell lines decreased gradually as compared with controls. While, the extract could not inhibit the proliferation of Wehi164 cell line significantly at similar concentrations, unless they are higher than 400 µg/ml. These results showed that A. ascalonicum inhibited the proliferation of all cell lines in a dose-dependent manner. The GI50 values of the A. ascalonicum extract (50% proliferation or growth inhibition) tested in these cancer cells were also different after 72 h treatment (Table I). Indeed, K562 and Jurkat cells showed the highest susceptibility (GI50: 100 µg/ml), and Wehi164 cells displayed the lowest susceptibility to A. ascalonicumwith GI50 at 400 µg/ml.
Figure 2.
Inhibition of cancer and normal cell lines by the aqueous extract of A. ascalonicum after 72 h. The cells were counted by the Coulter Counter (Sysmex KX21). Each column represents mean ± S.E. of three independent experiments
*p < 0.04, compared with the control group
We also examined the effects of the aqueous extract of A. ascalonicumon HUVECs proliferation. As the results showed (Figure 2), the extract inhibits proliferation of HUVECs in a dose-dependent manner. At concentrations up to 50 µg/ml the extract did not significantly decrease the number of cells, but at 100 µg/ml and at higher concentrations, it potently inhibited proliferation of HUVECs. Finally, the 50% proliferation inhibition (IC50) of the aqueous extract of A. ascalonicum on HUVECs was estimated at 400 µg/ml.
Anti-inflammatory effect of the Allium ascalonicum extract
In order to assess an anti-inflammatory activity of the aqueous extract of A. ascalonicum, the acetic acid-induced vascular permeability model was used. In this regard, mediators of inflammation released following stimulation, leads to dilation of arterioles and venules and increased vascular permeability [21]. The extract at the oral doses of 25, 50, 100 and 200 mg/kg showed an inhibition in vascular permeability and this activity was increasing in the dose-dependent manner (Table II). On the other hand, the A. ascalonicum extract at the oral doses of 25, 50, 100 and 200 mg/kg showed an inhibition of 10.2%, 21.4%, 38.3% and 80.1% in vascular permeability assay, respectively. In turn, the Indomethacin (INN) could cause 35.4% inhibition of vascular permeability (Table II). From this observation, it is assumed that an anti-inflammatory activity of the A. ascalonicum extract results from its protection on the release of inflammatory mediators at the first stage.
Table II.
Effect of the A. ascalonicum extract on acetic acid-induced vascular permeability in mice. The results are expressed as mean ± S.E. of n = 5. Inhibition percentages were calculated regarding the control treated with saline only. Indomethacin (INN) was chosen as a positive control. P < 0.05 compared with the control group
| Group | Dose [mg/kg, p.o.] | A590 | Inhibition (%) |
|---|---|---|---|
| A. ascalonicum | 50 | 2.56 ±0.14 | 10.2 |
| 100 | 2.24 ±0.18 | 21.4 | |
| 200 | 1.76 ±0.14 | 38.3 | |
| 400 | 0.57 ±0.11 | 80.1 | |
| INN | 5 | 1.84 ±0.04 | 35.4 |
| Control | – | 2.85 ±0.08 | – |
Discussion
Diet plays a major role in cancer etiology and prevention. Although inconsistencies exist across studies that have investigated the relationship between diet and cancer, dietary factors undoubtedly influence cancer risk [22]. There is increasing evidence that fruits and vegetables have chemopreventive properties because of the supplement and synergistic effects of various phytochemicals present in these nourishments [23-26].
From the other point of view, herbs and spices are generally considered safe and proved to be effective against various human ailments and their medicinal uses have increased gradually in many countries. In this regard, to assess the anticancer properties of plants which have been widely consumed in a human diet, the use of vegetable extracts provide an interesting approach, because these extracts contain several bioactive molecules [27-29].
Allium ascalonicum is one of the important Alliumspecies commonly used in many Asian diets and in traditional medicine since ancient times. A lot of evidence also suggested that Allium genus possesses anticancer properties as shown by their ability to suppress tumor proliferation in vivo and in vitro [30-32]. In spite of wide consumption of A. ascalonicum, reports regarding its biological effects are rarely compared to other Allium species such as garlic and onion. A. ascalonicum is usually known for hypocholesterolemic, antimicrobial, antidermatophytic, and anti-angiogenic properties [5, 8, 33-36].
Allium ascalonicum has been also reported to exhibit antioxidative and free radical scavenging capacities. These properties appear to be related to the high contents of flavone, sulfur-containing compounds, and polyphenolic derivatives in the bulb of A. ascalonicum. Furthermore, it is shown that the antioxidant potential of A. ascalonicumcomparing several onion varieties and some garlic preparations is more noticeable [7, 37].
Furthermore, researchers demonstrated that it can be useful to protect against oxidant-induced various illness conditions, including as an adjunct therapy in patients suffering nephrotoxicity from consuming CsA [38]. In previous studies, we could show that A. ascalonicum exhibits significant anti-angiogenic effects on in vitro, ex vivo and also in vivo assays without the toxic effect on the endothelial cells. Finally, we have demonstrated that heat and acid conditions had no effect on its anti-angiogenic activity [18, 39].
The present study was performed to examine the possible potential of the A. ascalonicum extract on anticancer and anti-inflammatory activities. For this purpose, we examined in vitro antiproliferative and cytotoxic effects of the A. ascalonicum extract against three well known cell lines including Wehi164, K562 and Jurkat and HUVEC as a normal cell line. The findings showed that A. ascalonicum, which contains many benefit phytochemicals; significantly affects the viability of all of the cancer cell lines in different IC50 values as shown in Table I.
To examine the influence of the aqueous extract of A. ascalonicumon cancer cell proliferation further; the cells were treated with increasing extract concentrations for 72 h. The obtained data of counting by a Coulter counter showed that A. ascalonicuminduced inhibition of the cell proliferation, resulting in a considerable reduction of cell growth with dose increasing against the control group. The 50% inhibitory concentrations of A. ascalonicum for the above-mentioned cell lines, which significantly resulted in restraint of their growth, were estimated (Table I). In agreement with 50% inhibition of cell proliferation (the concentration of the extract that is required for half-maximal inhibition), the order of sensitivity of the cell lines to extract was K562, Jurkat > Wehi164 with GI50 of 100 and 400 µg/ml, respectively (Table I). Meanwhile, the results showed that the proliferation of HUVEC cells was inhibited by the aqueous extract of A. ascalonicumwith GI50 about 400 µg/ml. In general, K562, Jurkat and Wehi164 cell lines exhibited both decreased viability and proliferation in a dose- and time-dependent manner under experimental conditions. These results further support previous observations concerning inhibition of cell proliferation by shallot on tumor cell lines, which showed that treatment of HeLa and MCF-7 cell lines with different concentrations of the chloroformic extract of shallot resulted in decreased growth in a dose-dependent manner [15]. Finally and on the basis of this investigation, we found that A. ascalonicum had significant antiproliferative effects on Wehi164 representing solid, and K562 and Jurkat cell lines as soft tumor cell lines in comparison with HUVECs as a normal cell.
Inflammation is a healthful response to offense or injury and an important part of innate immunity. Although inflammation acts as an adaptive host defense against infection or injury and is primarily a self-limiting process, inadequate resolution of inflammatory responses often leads to various chronic ailments including cancer [40, 41]. On the other side, chronic or recurrent acute inflammation, as a result of infectious agents or other sources, has potential promotional functions in each of tumor initiation, progression, invasion, and metastasis phases [42, 43]. Besides, most solid tumors are known to exhibit highly enhanced vascular permeability, similar to or more than the inflammatory tissues. Cancer and inflammatory tissues have various common vascular mediators, such as NO, BK and PGs, and their most predominant physiological effect is vascular permeability enhancement. Enhanced vascular permeability of tumor and inflammatory tissue is the key to be considered for future drug development for more selective targeting for the desired tumors or inflammatory lesions, based on enhanced permeability and retention (EPR) effect using macromolecular drugs [44-46].
Acetic acid-induced vascular permeability causes an immediate reaction which is lasting a long period of time over 24 h and arrives to infiltration of fluid rich in plasma proteins [20, 47, 48]. It is therefore used as a well-characterized mouse model of acute inflammation. Thus, inhibition of vascular permeability is considered a major feature for the suppression of the exudative phase of acute inflammation [49]. In the present study, in the well-known in vivo acetic acid-induced vascular permeability model, we observed that the A. ascalonicum extract shows significant anti-inflammatory activity at a dose-response correlation with extract increasing concentrations. However, the aqueous extract of A. ascalonicum at low doses had no considerable effects on prevention of vascular permeability of acetic acid; it showed meaningful differences comparing the inhibitory effect of INN as a common drug in treating the inflammation status. These findings suggest that the significant anti-inflammatory activity of A. ascalonicummay be due to affecting membrane-stabilization to reduce vascular permeability or through inhibition of various inflammatory mediators.
Regarding our and previous findings of beneficial properties of A. ascalonicum, particularly for prevention and treating of cancer and inflammation situations, further studies are needed to clarify the responsible compounds and their possible mechanisms in this regard.
In conclusion, the aqueous extract of A. ascalonicum bulbs had the most cytotoxic activity on cancer cell lines Jurkat and K562 against Wehi164 with lower cytotoxic preference. Treatment of HUVECs with the aqueous extract of the A. ascalonicumbulbs up to 1000 µg/ml or even higher concentrations for 72 h showed no considerable cytotoxic effect on the HUVECs but the extract inhibits proliferation of HUVECs in a dose-dependent manner. It is of interest that the extract of this plant has showed much less cytotoxicity against the normal cell line, and, if this also occurs in vivo, the use of this plant clinically for the treatment of cancer patients would have some scientific support. Moreover, because of anti-inflammatory activity in vivo, it would be regarded as an excellent candidate for the development of an anti-inflammatory drug with fewer side effects, which needs to be more investigated.
Acknowledgment
This study was financially supported by The Research Fund of Medical Biology Research Center.
References
- 1.Hartwell JL. Plants used against cancer. MA: Quarterman, Lawrence; 1982. [Google Scholar]
- 2.Lampe JW. Health effects of vegetables and fruit: assessing mechanisms of action in human experimental studies. Am J Clin Nutr. 1999;70:475S–490S. doi: 10.1093/ajcn/70.3.475s. [DOI] [PubMed] [Google Scholar]
- 3.Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100:72–9. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Yin MC, Tsao SM. Inhibitory effect of seven Allium plants upon three Aspergillus Species. Int J Food Microbiol. 1999;49:49–56. doi: 10.1016/s0168-1605(99)00061-6. [DOI] [PubMed] [Google Scholar]
- 5.Amin M, Kapadnis BP. Heat stable antimicrobial activity of Allium ascalonicum against bacteria and fungi. Indian J Exp Biol. 2005;43:751–4. [PubMed] [Google Scholar]
- 6.Owoyele BV, Alabio T, Adebayo JO, Soladoye AO, Abioye AI, Jimoh SA. Haematological evaluation of ethanolic extract of Allium ascalonicum in male albino rats. Fitoterapia. 2004;75:322–6. doi: 10.1016/j.fitote.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Leelarungrayub N, Rattanapanone V, Chanarat N, Gebicki JM. Quantitative evaluation of the antioxidant properties of garlic and shallot preparations. Nutrition. 2006;22:266–74. doi: 10.1016/j.nut.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Adeniyi BA, Anyiam FM. In vitro anti-Helicobacter pylori potential of methanol extract of Allium ascalonicum Linn. (Liliaceae) leaf: susceptibility and effect on urease activity. Phytotherapy Res. 2004;18:358–61. doi: 10.1002/ptr.1265. [DOI] [PubMed] [Google Scholar]
- 9.Ho SC, Tang YL, Lin SM, Liew YF. Evaluation of peroxynitrite-scavenging capacities of several commonly used fresh spices. Food Chem. 2010;119:1102–7. [Google Scholar]
- 10.Mo HQ, Vandamme EJM, Peumans WJ, Goldstein IJ. Purification and Characterization of a Mannose-Specific Lectin from Shallot (Allium ascalonicum) Bulbs. Arch Biochem Biophys. 1993;306:431–8. doi: 10.1006/abbi.1993.1534. [DOI] [PubMed] [Google Scholar]
- 11.Wang HX, Ng TB. Ascalin, a new anti-fungal peptide with human immunodeficiency virus type 1 reverse transcriptase-inhibiting activity from shallot bulbs. J Peptides. 2002;23:1025–9. doi: 10.1016/s0196-9781(02)00032-3. [DOI] [PubMed] [Google Scholar]
- 12.Fattorusso E, Iorizzi M, Lanzotti V, Taglialatela-Scafati O. Chemical composition of shallot (Allium ascalonicum Hort.) J Agric Food Chem. 2002;50:5686–90. doi: 10.1021/jf020396t. [DOI] [PubMed] [Google Scholar]
- 13.Ogra Y, Ishiwata K, Iwashita Y, Suzuki KT. Simultaneous speciation of selenium and sulfur species in selenized odorless garlic (Allium sativum L. Shiro) and shallot (Allium ascalonicum) by HPLC-inductively coupled plasma-(octopole reaction system)-mass spectrometry and electrospray ionization-tandem mass spectrometry. J Chromatogr A. 2005;1093:118–25. doi: 10.1016/j.chroma.2005.07.081. [DOI] [PubMed] [Google Scholar]
- 14.Bonaccorsi P, Caristi C, Gargiulli C, Leuzzi U. Flavonol glucosides in Allium species: A comparative study by means of HPLC–DAD–ESI-MS–MS. Food Chem. 2008;107:1668–73. [Google Scholar]
- 15.Ghodrati Azadi H, Ghaffari SH, Riazi GH, Ahmadian S, Vahedi F. Antiproliferative activity of chloroformic extract of Persian Shallot, Allium hirtifolium, on tumor cell lines. Cytotechnology. 2008;56:179–85. doi: 10.1007/s10616-008-9145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogra Y, Ishiwata K, Iwashita Y, Suzuki KT. Simultaneous speciation of selenium and sulfur species in selenized odorless garlic (Allium sativum L. Shiro) and shallot (Allium ascalonicum) by HPLC-inductively coupled plasma-(octopole reaction system)-mass spectrometry and electrospray ionization-tandem mass spectrometry. J Chromatogr A. 2005;1093:118–25. doi: 10.1016/j.chroma.2005.07.081. [DOI] [PubMed] [Google Scholar]
- 17.Hsu YL, Chia CC, Chen PJ, Huang SE, Huang SC, Kuo PL. Shallot and licorice constituent isoliquiritigenin arrests cell cycle progression and induces apoptosis through the induction of ATM/p53 and initiation of the mitochondrial system in human cervical carcinoma HeLa cells. Mol Nutr Food Res. 2009;53:826–35. doi: 10.1002/mnfr.200800288. [DOI] [PubMed] [Google Scholar]
- 18.Mohammadi Motlagh HR. MS.c Thesis. Tabriz, IR, Azarbayjan University of Tarbiat Moallem. 2008. The study of anti-angiogenic effects of shallot (Allium hirtifolium) extract and isolation of effective fraction. [Google Scholar]
- 19.Linford NJ, Dorsa DM. 17 β-Estradiol and the phytoestrogen genistein attenuate neuronal apoptosis induced by the endoplasmic reticulum calcium-ATPase inhibitor thapsigargin. J Steroids. 2002;67:1029–40. doi: 10.1016/s0039-128x(02)00062-4. [DOI] [PubMed] [Google Scholar]
- 20.Whittle BA. The use of changes in capillary permeability in mice to distinguish between narcotic and nonnarcotic analgesics. Br J Pharmacol Chemother. 1964;22:246–53. doi: 10.1111/j.1476-5381.1964.tb02030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vogel HG, Vogel WH. Drug Discovery and Evaluations, Pharmacological Assays. Berlin: Springer; 1997. pp. 402–3. [Google Scholar]
- 22.American Institute for Cancer Research. Plant compounds continue to challenge science. Available at: www.aicr.org/site/PageServer .
- 23.Liu RH. Health benefits of fruits and vegetables are from additive and synergistic combination of phytochemicals. Am J Clin Nutr. 2003;78:517S–520S. doi: 10.1093/ajcn/78.3.517S. [DOI] [PubMed] [Google Scholar]
- 24.Okarter N, Liu CS, Sorrells ME, Liu RH. Phytochemical content and antioxidant activity of six diverse varieties of whole wheat. Food Chem. 2009;119:249–57. [Google Scholar]
- 25.Lee HJ, Park SJ, Choi H. High dietary phytochemical intakes lower cancer risk in Korean elderly. Proc Amer Assoc Cancer Res. 2004;45 [Google Scholar]
- 26.Liu RH. McGraw-Hill Yearbook of Science & Technology 2009. Vol. 22. McGraw-Hill, Inc; 2008. Phytochemicals: human disease prevention agents; pp. 281–6. [Google Scholar]
- 27.Johnson I, Williamson G, Musk S. Anticarcinogenic factors in plant foods: a new class of nutrients? Nutr Res Rev. 1994;7:175–204. doi: 10.1079/NRR19940011. [DOI] [PubMed] [Google Scholar]
- 28.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer prevention: a review. J Am Diet Assoc. 1996;96:1027–39. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 29.Liu J, Dong H, Chen B, Zhao P, Liu RH. Fresh apples suppress mammary carcinogenesis, proliferative activity, and induce apoptosis in the mammary tumors of the Sprague-Dawley rat. J Agric Food Chem. 2009;57:297–304. doi: 10.1021/jf801826w. [DOI] [PubMed] [Google Scholar]
- 30.You WC, Sheng CY, Ershow A, et al. Allium vegetables and reduced risk of stomach cancer. J Natl Canc Inst. 1989;81:162–4. doi: 10.1093/jnci/81.2.162. [DOI] [PubMed] [Google Scholar]
- 31.Ahmed N, Laverick L, Sammons J, Zhang H, Maslin DJ. Ajoene, a garlic-derived natural compound, enhances chemotherapy-induced apoptosis in human myeloid leukaemia CD34-positive resistant cells. Anticancer Res. 2001;21:3519–23. [PubMed] [Google Scholar]
- 32.Sengupta A, Ghosh S, Bhattacharjee S. Allium vegetables in cancer prevention: an overview. Asian Pac J Cancer Prev. 2004;5:237–45. [PubMed] [Google Scholar]
- 33.Dankert J, Tromp TF, de Vries H, Klasen HJ. Antimicrobial activity of crude juices of Allium ascalonicum, Allium cepa and Allium sativum. Zentralbl Bakteriol. 1979;245:229–39. [PubMed] [Google Scholar]
- 34.Tappayuthpijarn P, Dejatiwongse Q, Hincheranan T, Suriyant PN. Effect of Allium ascalonicum on erythrocyte shape in induced hypercholesterolemia rabbits. J Med Assoc Thai. 1989;72:448–51. [PubMed] [Google Scholar]
- 35.Seyfi P, Mostafaie A, Mansouri K, Arshadi D, Mohammadi-Motlagh HR, Kiani A. In vitro and in vivo anti-angiogenesis effect of shallot (Allium ascalonicum): A heat-stable and flavonoid-rich fraction of shallot extract potently inhibits angiogenesis. Toxicol In Vitro. 2010;24:1655–61. doi: 10.1016/j.tiv.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 36.Trakranrungsie N, Chatchawanchonteera A, Khunkitti W. Ethnoveterinary study for antidermatophytic activity of Piper betle, Alpinia galanga and Allium ascalonicum extracts in vitro. Res Vet Sci. 2008;84:80–4. doi: 10.1016/j.rvsc.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Yang J, Meyers KJ, van der Heide J, Liu RH. Varietal differences in phenolic content and antioxidant and antiproliferative activities of onions. J Agr Food Chem. 2004;52:6787–93. doi: 10.1021/jf0307144. [DOI] [PubMed] [Google Scholar]
- 38.Wongmekiat O, Leelarugrayub N, Thamprasert K. Beneficial effect of shallot (Allium ascalonicum L.) extract on cyclosporine nephrotoxicity in rats. Food Chem Toxicol. 2008;46:1844–50. doi: 10.1016/j.fct.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 39.Mohammadi Motlagh HR, Mansouri K, Shakiba Y, et al. Anti-angiogenic effect of aqueous extract of shallot (Allium ascalonicum) bulbs in rat aorta ring model. Yakhteh Med J. 2009;11:184–9. [Google Scholar]
- 40.Jackson L, Evers BM. Chronic inflammation and pathogenesis of GI and pancreatic cancers. Cancer Treat Res. 2006;130:39–65. doi: 10.1007/0-387-26283-0_2. [DOI] [PubMed] [Google Scholar]
- 41.Schottenfeld D, Beebe-Dimmer J. Chronic inflammation: a common and important factor in the pathogenesis of neoplasia. CA Cancer J Clin. 2006;56:69–83. doi: 10.3322/canjclin.56.2.69. [DOI] [PubMed] [Google Scholar]
- 42.DeNardo DG, Coussens LM. Inflammation and breast cancer. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grivennikov SI, Greten FR, Karin M. Immunity, Inflammation, and Cancer. Cell. 2010;140:883–99. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sgambato A, Cittadini A. Inflammation and cancer: a multifaceted link. Eur Rev Med Pharmacol Sci. 2010;14:263–8. [PubMed] [Google Scholar]
- 45.Rüegg C. Leukocytes, inflammation, and angiogenesis in cancer: fatal attractions. J Leukocyte Biol. 2006;80:682–4. doi: 10.1189/jlb.0606394. [DOI] [PubMed] [Google Scholar]
- 46.Perwez Hussain S, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer. 2007;121:2373–80. doi: 10.1002/ijc.23173. [DOI] [PubMed] [Google Scholar]
- 47.Choi JH, Jung BH, Kang OH, et al. The anti-inflammatory and anti-nociceptive effects of ethyl acetate fraction of Cynanchi Paniculati Radix. Biol Pharm Bull. 2006;29:971–5. doi: 10.1248/bpb.29.971. [DOI] [PubMed] [Google Scholar]
- 48.Hu XJ, Jin HZ, Xu WZ, et al. Anti-inflammatory and analgesic effects of Daphne retusa Hemsl. J Ethnopharmacol. 2008;120:118–22. doi: 10.1016/j.jep.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 49.Okoli CO, Akah PA, Nwafor SV, Anisiobi AI, Ibegbunam IN, Erojikwe O. Anti-inflammatory activity of hexane leaf extract of Aspilia africana C.D. Adams. J Ethnopharmacol. 2007;109:219–25. doi: 10.1016/j.jep.2006.07.037. [DOI] [PubMed] [Google Scholar]