Abstract
Introduction
Two functional single nucleotide polymorphisms (SNPs) in the 5,10-methylenetetrahydrofolate reductase (MTHFR) gene, C677T and A1298C, lead to decreased enzyme activity and affect chemosensitivity of tumour cells.
Material and methods
We evaluated these two common polymorphisms and breast cancer risk association in an Iranian sporadic breast cancer population-based case-control study of 294 breast cancer cases and 306 controls using a PCR-RFLP-based assay.
Results
Analyses of affected and controls show that homozygote genotype MTHFR 677CC has the highest frequency in both groups (28.3% in patients and 25.3% in control group). Genotype MTHFR 677CT and genotype MTHFR 1298AC were found to be statistically significant risk factors in our population (odds ratio: 1.6, 95% CI: 1.019-2.513, p = 0.041; and odds ratio: 2.575, 95% CI: 1.590-4.158, p = 0.001 respectively).
Conclusions
We can conclude based on the results of our study that a significant association between breast cancer and C677T and A1298C polymorphism might exist.
Keywords: MTHFR gene, polymorphism, breast cancer, PCR-RFLP, susceptibility factor
Introduction
MTHFR is a key enzyme in the folate metabolism pathway and regulates the intracellular folate pool for synthesis and methylation of DNA [1, 2]. Two common allele variants of the MTHFR gene have been described, C677T and A1298C, that lead to amino acid substitutions, Ala222Val and Glu429Ala, and to decreased enzyme activity [3-5].
Folate is involved in DNA methylation, synthesis, and repair. Low intake of folate may increase the risk of several cancers, including breast cancer [5, 6].
The enzyme methylenetetrahydrofolate reductase (MTHFR) irreversibly catalyzes 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, the donor for the remethylation of homocysteine to methionine, the precursor for the universal methyl donor S-adenosylmethionine [7, 8]. Two common polymorphisms in the MTHFR gene have been characterized [9, 10]: the 677C →T [9, 11] and the 1298A → C polymorphism codes [10]. Individuals homozygous for the 1298C allele have approximately the same enzyme activity as those heterozygous for the 677T allele [10, 11].
We report here that the allele frequencies of MTHFR 677 and 1298 were significant in breast cancer patients in Iran.
Material and methods
Patient data
Studies were conducted on 294 carcinoma breast cancer patients treated with chemotherapy and 306 controls comprising postmenopausal women genotyped for MTHFR, aged 45-55 years.
All patients were from the chemotherapy ward in the Special Medical Centre, Tehran, Iran.
This study was ethically approved by the local Ethical Committee of Islamic Azad University from the point of view of patients’ and controls’ rights.
A questionnaire and a consent form including questions on breast cancer risk factors were completed by each patient. The blood samples were collected from patients and controls prior to the start of treatment. Subjects were genotyped for MTHFR SNPS using genomic DNA extracted from peripheral blood lymphocytes. DNA was isolated from peripheral blood using a FlexiGene DNA extraction kit (Qiagen Germany).
Genotyping
The polymorphisms were detected using a modified PCR-RFLP method [12, 13, 16]. The PCR primers were synthesized by TAG Copenhagen A/S Primers which were:
-
A1298C polymorphism (256 bp), forward: 5’-CTTCTACCTGAAGAGCAAGTC-3’, reverse: 5’-CATGTCCACAGCATGGAG-3’.
The cycling conditions were 94°C, 30 min; 61°C, 30 min (35 cycles); 72°C, 60 min. The PCR products were digested with 1 unit of MboII (Figure 1).
-
C677T (183 bp), forward primer: GACCTGAAGCACTTGAAGGA, reverse primer: CGAGCTTATGGGCTCTCCTG.
The cycling conditions were 94°C, 30 min; 61°C, 30 min (35 cycles); 72°C, 60 min. The PCR products were digested with 1 unit of HinfI, and separated on a 4% agarose gel (Figure 2).
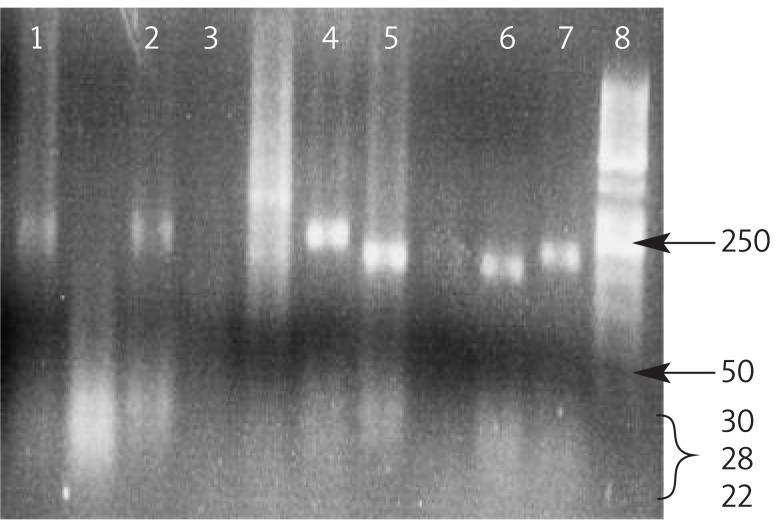
Figure 1.
Polymorphism analysis of MTHFR 1298. The PCR products were digested with restriction enzyme MboII in groups: 1,2; CA genotype (256 bp, 176 bp, 52 bp, 30, 28, 22 bp), 5,6 CC genotype (176, 52, 30, 28, 22 bp), 4,7; AA genotype (256 bp), 3; negative control, 8; (ladder 50 bp)
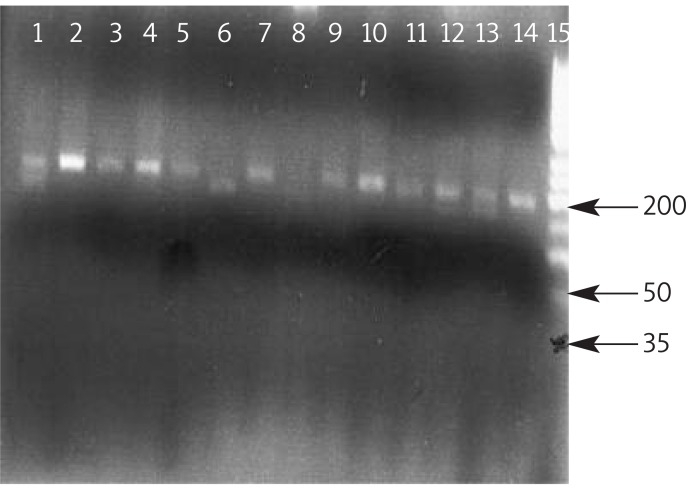
Figure 2.
Polymorphism analysis of MTHFR 677. The PCR products were digested with restriction enzyme Hinf I in groups: 1,12,13; CT genotype (183 bp, 153 bp, 35 bp), 2,3,4,5,7,9,10,11,14 CC genotype (183 bp) 6; TT genotype (153 bp, 35 bp), 8; negative control, 15; (ladder 50 bp)
This method is able to detect all three possible genotypes for the polymorphism: homozygous wild type, heterozygous variant type and homozygous variant type.
The genotypes and allelic frequencies of MTHFR polymorphisms in patient and control groups were analysed by χ2 and Fisher’s exact tests. P values < 0.05 were considered significant.
Results
There was a significant result for MTHFR 1298 and 677 polymorphism in relation to breast cancer risk. Analyses of affected and controls show that homozygote genotype MTHFR 677CC has the highest frequency in both groups (28.3% in patients and 25.3% in control group), p = 0.001.
On the other hand, the homozygous genotype MTHFR 1298 CC was more increased in the patient group (27.3%) compared with controls (17.7%), p = 0.001 (Tables I, II and Figure 3).
Table I.
MTHFR 677 and 1298 genotype frequencies [n (%)] for patients and controls: Analyses of 294 affected women and 306 controls show the highest frequency for C/C MTHFR 677 genotype (28.3 and 25.3 respectively) and C/C MTHFR 1298 genotype (27.3 and 17.7 respectively)
| Genotype | Patients n (%) | Controls n (%) | Total n (%) |
|---|---|---|---|
| n | 294 | 306 | 600 |
| MTHFR (677) | |||
| CC | 168 (28.3) | 150 (25.3) | 318 (53.5) |
| CT | 84 (14.1) | 90 (15.2) | 174 (29.3) |
| TT | 42 (7.1) | 60 (10.1) | 102 (17.2) |
| MTHFR (1298) | |||
| CC | 162 (27.3) | 105 (17.7) | 267 (44.9) |
| AC | 96 (16.2) | 135 (22.7) | 231 (38.9) |
| AA | 36 (6.1) | 60 (10.1) | 96 (16.2) |
Table II.
Comparison between genotypes, odds ratio and p value showed that p value of genotype MTHFR 677 CT was the most important risk factor in our population; TC odds ratio, 1.6 (95% confidence interval; CI, 1.019-2.513), p = 0.041, CC odds ratio, 1.2 (95% CI, 0.829-1.737), p = 0.334, TT odds ratio, 1.333 (95% CI, 0.814-2.185), p = 0.253. Genotype MTHFR 1298 AC was the most important risk factor in our population; AC odds ratio, 2.571 (95% confidence interval; CI, 1.590-4.158), p = 0.001, AA odds ratio, 1.185 (95% CI, 0.727-1.933), p = 0.496, CC odds ratio, 2.170 (95% CI, 1.515-3.106), p = 0.002
| Genotype MTHFR (1298) | Odds ratio | 95% confidenceinterval | P value |
|---|---|---|---|
| AC | 2.571 | 1.590-4.158 | 0.001*** |
| CC | 2.170 | 1.515-3.106 | 0.002*** |
| AA | 1.185 | 0.727-1.933 | 0.496 |
| Genotype MTHFR (677) | |||
| CC | 1.2 | 0.829-1.737 | 0.334 |
| CT | 1.6 | 1.019-2.513 | 0.041 |
| TT | 1.333 | 0.814-2.185 | 0.253 |
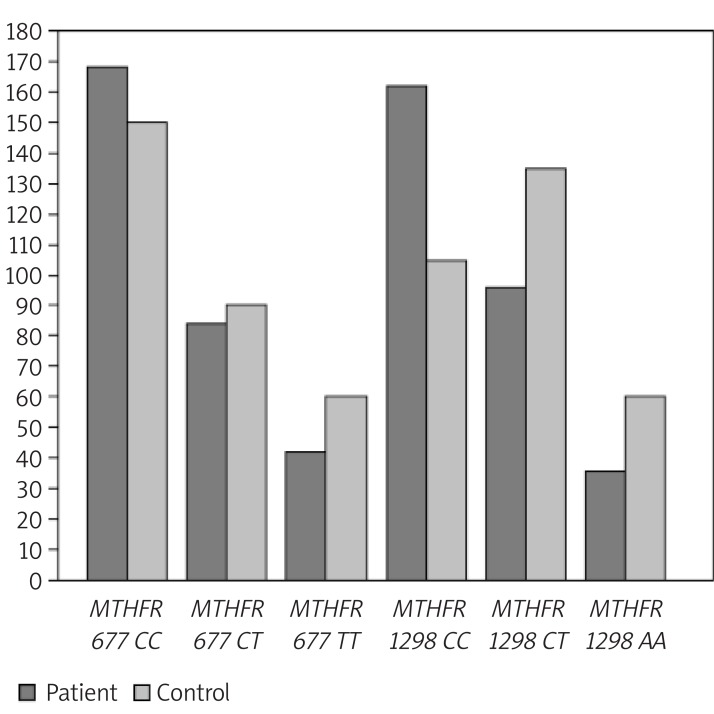
Figure 3.
MTHFR 677 and 1298 genotype (X) frequencies (Y) [n (%)] for cases and controls: Analyses of 294 affected women and 306 controls showed the highest frequency for C/C MTHFR 677 genotype (28.3 and 25.3 respectively) and C/C MTHFR 1298 genotype (27.3 and 17.7 respectively). On the other hand, a decreased frequency of MTHFR 677 CT, TT and 1298 CT, AA was found for patients compared to controls
The genotype MTHFR 677CT and genotype MTHFR 1298AC were found or appeared to be important risk factors in our population (odds ratio: 1.6, 95% CI: 1.019-2.513, p= 0.041; and odds ratio: 2.575, 95% CI: 1.590-4.158, p = 0.001 respectively), while MTHFR 677 CC did not show any statistical significance (odds ratio: 1.2, p = 0.334).
Of course, MTHFR 1298 CC (27.3%) and MTHFR 677 CC (28.3%) have the highest frequency compared with MTHFR 677 and MTHFR 1298 polymorphism.
In our study there was an association between C677T and A1298C polymorphism and breast cancer risk.
We conclude that not only was 1298 CC associated with increased risk for breast cancer but also there is a relation between the presence of 677 CC and increased breast cancer risk.
Discussion
Martin DN [14] found that the MTHFR SNPs C677T and A1298C were associated with breast cancer survival.
Jakubowska found that MTHFR_677_C > T was associated with an increased risk while 1298_A > C polymorphism was associated with a decreased risk for breast and ovarian cancer. It appears that functional polymorphisms in the MTHFR gene modify the risk of breast cancer and may potentially alter the risk of ovarian cancer in women with an inherited predisposition [15].
Shrubsole MJ did not observe any effect of A1298C genotypes on breast cancer risk. He suggests that the MTHFR C677T polymorphisms may modify the association between dietary folate intake and breast cancer risk [16, 17].
However, other studies on colorectal cancer [18], colorectal adenoma [19-21], gastric cancer [22], lung cancer [23], and acute myeloid leukaemia [24] did not find any association or an increased risk of cancer for individuals with the TT genotype.
The C677T polymorphism has been examined in relation to several cancers [6, 22]. Many studies have also examined the correlation between MTHFR 677TT and breast cancer risk [18–20, 25-28].
In the first study in Jewish women, there was no significant difference of MTHFR C677T genotype between sporadic cases and controls [26].
In Caucasian women it was reported that the MTHFR 677T allele was more prevalent in cases than controls [27], while in other studies the reported risk for breast cancer was associated with both the C677T and A1298C polymorphisms [28].
In our study, a statistically significant association between MTHFR genotype and breast cancer risk was found. Therefore, we can conclude that there might be a relation between the presence of MTHFR 1298AA and 677CC genotype and increasing risk of breast cancer, whereas there was a decreased frequency for MTHFR 677 CT, TT and 1298 CT, AA compared with controls.
Acknowledgments
We thank all the patients for their kind collaboration and also the Islamic Azad University for supporting this research. Finally, we thank the head and physicians of the Special Medical Centre, Tehran, Iran, for help during this research.
References
- 1.Ueland PM, Hustad S, Schneede J, et al. Biological and clinical implications of the MTHFR C677T polymorphism. Trends Pharmacol Sci. 2001;22:195–201. doi: 10.1016/s0165-6147(00)01675-8. [DOI] [PubMed] [Google Scholar]
- 2.Das PM, Singal R. DNA methylation and cancer. J Clin Oncol. 2004;22:4632–42. doi: 10.1200/JCO.2004.07.151. [DOI] [PubMed] [Google Scholar]
- 3.Frosst P, Blom HJ, Milos RA, et al. Candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–3. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 4.Weisberg I, Tran P, Christensen B, et al. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998;64:169–72. doi: 10.1006/mgme.1998.2714. [DOI] [PubMed] [Google Scholar]
- 5.Kim YI. Folate and carcinogenesis: evidence mechanisms and implications. J Nutr Biochem. 1999;10:66–88. doi: 10.1016/s0955-2863(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 6.Mason JB, Choi SW. Folate and carcinogenesis: developing a unifying hypothesis. Adv Enzyme Regul. 2000;40:127–41. doi: 10.1016/s0065-2571(99)00037-0. [DOI] [PubMed] [Google Scholar]
- 7.Matthews RG, Sheppard C, Goulding C, et al. Methylenetetrahydrofolate reductase and methionine synthase: biochemistry and molecular biology. Eur J Pediatr. 1998;157(Suppl. 2):S54–S59. doi: 10.1007/pl00014305. [DOI] [PubMed] [Google Scholar]
- 8.Fodinger M, Horl WH, Sunder-Plassmann G. Molecular biology of 5,10 methylenetetrahydrofolate reductase. J Nephrol. 2000;13:20–33. [PubMed] [Google Scholar]
- 9.Frosst P, Blom HJ, Milos P, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–3. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 10.Weisberg I, Tran P, Christensen B, et al. A second genetic polymorphism in methylenetetrahydrofolate reductase (MTHFR) associated with decreased enzyme activity. Mol Genet Metab. 1998;64:169–72. doi: 10.1006/mgme.1998.2714. [DOI] [PubMed] [Google Scholar]
- 11.Weisberg IS, Jacques PF, Selhub J, et al. The 129XA-C polymorphism in methylenetetrah drofolate reductase (MTHFR): in vitro expression and association with homocysteine. Artherosclerosis. 2001;156:409–15. doi: 10.1016/s0021-9150(00)00671-7. [DOI] [PubMed] [Google Scholar]
- 12.Hosseini M, Houshmand M, Ebrahimi A. Breast cancer risk not only was not associated with CYP17/A2allele but also was related to A1 allele. Arch Med Sci. 2009;1:102–6. [Google Scholar]
- 13.Hosseini M, Houshmand M, Ebrahimi A. The ERCC2 K751 polymorphism is associated with breast cancer risk. Arch Med Sci. 2009;3:455–9. [Google Scholar]
- 14.Martin DN, Boersma BJ, Howe TM, et al. Association of MTHFR gene polymorphisms with breast cancer survival. BMC Cancer. 2006;27:257–10. doi: 10.1186/1471-2407-6-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jakubowska A, Gronwald J, Menkiszak J, et al. Methylenetetrahydrofolate reductase polymorphisms modify BRCA1-associated breast and ovarian cancer risks. Breast Cancer Res Treat. 2007;104:299–308. doi: 10.1007/s10549-006-9417-3. [DOI] [PubMed] [Google Scholar]
- 16.Shrubsole MJ, Gao YT, Cai Q, et al. MTHFR polymorphisms, dietary folate intake, and breast cancer risk: results from the Shanghai Breast Cancer Study. Cancer Epidemiol Biomarkers Prev. 2004;13:190–6. doi: 10.1158/1055-9965.epi-03-0273. [DOI] [PubMed] [Google Scholar]
- 17.Ericson UC, Ivarsson MI, Sonestedt E, et al. Increased breast cancer risk at high plasma folate concentrations among women with the MTHFR 677T allele. Am J Clin Nutr. 2009;90:1380–9. doi: 10.3945/ajcn.2009.28064. [DOI] [PubMed] [Google Scholar]
- 18.Slattery ML, Potter JD, Samowitz W, et al. Methylenetetrahydrofolate reductase, diet, and risk of colon cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:513–18. [PubMed] [Google Scholar]
- 19.Chen J, Giovannucci E, Hankinson SE, et al. A prospective study of methylenetetrahydrofolate reductase and methionine synthase gene polymorphisms, and risk of colorectal adenoma. Carcinogenesis. 1998;19:2129–132. doi: 10.1093/carcin/19.12.2129. [DOI] [PubMed] [Google Scholar]
- 20.Ulrich CM, Kampman E, Bigler J, et al. Colorectal adenomas and the C677T MTHFR polymorphism: evidence for gene-environment interaction? Cancer Epidemiol Biomarkers Prev. 1999;8:659–68. [PubMed] [Google Scholar]
- 21.Nomura M, Watari J, Yokota K, et al. Morphogenesis of nonpolypoid colorectal adenomas and early carcinomas assessed by cell proliferation and apoptosis. Virchows Arch. 2000;437:17–24. doi: 10.1007/s004280000198. [DOI] [PubMed] [Google Scholar]
- 22.Shen H, Xu Y, Zheng Y, et al. Polymorphisms of 5, 10-methylenetetrahydrofolate reductase and risk of gastric cancer in a Chinese population: a case-control study. Int J Cancer. 2001;95:332–6. doi: 10.1002/1097-0215(20010920)95:5<332::aid-ijc1058>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 23.Shen H, Spitz MR, Wang LE, et al. Polymorphisms of methylene-tetrahydrofolate reductase and risk of lung cancer: a casecontrol study. Cancer Epidemiol Biomarkers Prev. 2001;10:397–401. [PubMed] [Google Scholar]
- 24.Burbee DG, Forgacs E, Zochbauer-Muller S, et al. Epigenetic inactivation of RASSF1A in lung and breast cancers and malignant phenotype suppression. J Natl Cancer Inst. 2001;93:691–9. doi: 10.1093/jnci/93.9.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J, Stampfer MJ, Giovannucci E, et al. Methylenetetrahydrofolate reductase polymorphism dietary interactions and risk of colorectal cancer. Cancer Res. 1997;57:1098–102. [PubMed] [Google Scholar]
- 26.Gershoni-Baruch R, Dagan E, Israeli D, et al. Association of the C677T polymorphism in the MTHFR gene with breast and/or ovarian cancer risk in Jewish women. Eur J Cancer. 2000;36:2313–16. doi: 10.1016/s0959-8049(00)00306-3. [DOI] [PubMed] [Google Scholar]
- 27.McGlynn KA, Wang L, Patrick-Acevedo NY, et al. Methylene tetrahydrofolate reductase, methionine synthase, folate, alcohol and breast cancer; The 91st Annual AACR Meeting; 2000 April 1–5; San Francisco. [Google Scholar]
- 28.Sharp L, Little J, Schofield AC, et al. Folate and breast cancer: the role of polymorphisms in methylenetetrahydrofolate reductase (MTHFR) Cancer Lett. 2002;181:65–71. doi: 10.1016/s0304-3835(02)00030-7. [DOI] [PubMed] [Google Scholar]