Abstract
Introduction
The purpose of this study was to compare the clinical, microbiological and immunological effects of subgingival irrigation with Dentol™ and 0.2% chlorhexidine in human advanced periodontitis.
Material and methods
Twenty cases of advanced periodontitis patients were randomly treated either with Dentol™ (as test) or 0.2% chlorhexidine (as control) every other day for 29 days after primary scaling, root planing and oral hygiene instruction. Pocket depth (PD), bleeding on probing (BOP) and plaque index (PI) were considered as clinical parameters. Total counts of aerobic and anaerobic bacteria were measured in subgingival plaque. At the end, concentrations of TNF-α and IL-1β in gingival crevicular fluid were measured as inflammatory markers.
Results
PD and BOP decreased in both groups on the 29th day and two weeks afterward. PI did not show any statistical difference either within or among groups (p > 0.05). Results indicate that both chlorhexidine and Dentol™ diminished total count of subgingival anaerobic bacteria significantly (p < 0.05). Total count of subgingival aerobic bacteria increased in both groups, but the differences between the two groups were statistically significant in favour of Dentol™ (p < 0.05). The level of IL-1β in the crevicular fluid was reduced in both groups but it was more significant in the test group (p < 0.05). The TNF-a level decreased in both groups but the statistical difference between the two groups was negligible (p > 0.05).
Conclusions
Subgingivally irrigation with Dentol™, containing 0.02% carvacrol, every other day for 29 days is equally or more effective than 0.2% chlorhexidine in reducing clinical and immunological inflammatory indices.
Keywords: periodontitis, Dentol™, chlorhexidine, irrigation, oxidative stress, Satureja khuzestanica
Introduction
Progressive periodontal disease is characterized by inflammatory lesions in periodontal connective tissues, derangement of periodontal fibres, alveolar bone loss, proliferation, ulceration and apical migration of junctional epithelium. Although host modulation of the inflammatory response is effective in controlling periodontal diseases, microbial plaque elimination is the only practical way to control them [1]. In order to achieve this goal, oral hygiene instruction following professional bacterial plaque elimination is a critical part of the periodontal treatment. Systemic antibiotic administration in adult periodontitis does not help in scaling and root planing, which are the initial steps in periodontal treatment [2]. Moreover, long-term prescriptions of antibiotics may lead to a change in body natural microflora and adverse reactions.
Local antimicrobial drug prescription is a site-specific method for periodontal treatment which has several benefits such as limitation of the drug in a specific region and prevention of adverse effects in other sites. Among various different antimicrobials which have been used for subgingival irrigation, chlorhexidine is widely accepted by most practitioners due to its proven antimicrobial effects and also availability and low cost. Since the majority of freshly isolated subgingival bacteria are inhibited in vitro by extremely low concentrations of chlorhexidine, it might be speculated that subgingival irrigation with this antiseptic agent could arrest periodontitis [3]. Oosterwaal et al. used a syringe with a blunted needle to apply 2% chlorhexidine gel three times within 10 min into the bottom of periodontal pockets of single rooted teeth and reported 99.4% reduction in total colony forming units and black-pigmented Bacteroides, a reduction comparable to the effects of scaling and root planing [4]. The local side effects of chlorhexidine and other cationic antiseptics limit their long-term use for prevention of periodontal disease. The most important side effect of chlorhexidine, external tooth staining, is still the greatest side effect [5]. Other local side effects include taste perturbation, oral mucosal erosion, unilateral or bilateral parotid swelling and enhanced supragingival calculus formation [5]. A growing number of people worldwide are using herbal products for preventive and therapeutic purposes. Twenty percent of the 20,000 herbal plants are used for medical and 10% for commercial purposes. The genus Satureja belongs to the family Lamiaceae (Labiatae), subfamily Nepetoideae and tribe Mentheae. The major constituents of the essential oils in most species are found to be phenols, carvacrol, thymol, p-cymene, β-caryophyllene, linalool, monoterpenes, sesquiterpenes, alcohols, phenolic acids, labiatic acids, and flavonoids [6]. Dentol™, which is commercially available as oral drops for dental pain, is an essence obtained from Satureja khuzestanica growing in southern Iran. The main component of essential oil of S. khuzestanicais carvacrol [7]. According to an in vitro study by Wagner et al.[8] carvacrol could prevent prostaglandin synthesis. Helander et al. [9] found that essential oil components including carvacrol and thymol can cause disruption in the cell wall of gram negative bacteria. Gill et al. [10] found that plant oil aromatics cause cellular membrane disruption in Escherichia coli, Listeria monocytogenesand Lactobacillus sakei.In vitro anti-inflammatory activity of carvacrol has been proven by Landa et al. [11]. They found that carvacrol has a strong inhibitory activity against cyclooxygenase-2 biosynthesis in vitro and suggested its possible contribution to anti-inflammatory activity of traditionally used carvacrol-rich plant drugs. Recently, Botelho et al. [12] demonstrated that the local application of a dental gel based on carvacrol prevents alveolar bone resorption and has an anti-inflammatory and antimicrobial effect in experimental periodontitis. As reviewed by Momtaz & Abdollahi [13], the most common uses which have been listed for S. khuzestanicaare as antioxidant, antidiabetic, anti-hyperlipidaemic, antibacterial, anti-inflammatory, antinociceptive as well as reproduction stimulatory properties. The aim of this study was to compare the clinical, microbiological and immunological effects of subgingival irrigation with Dentol™ (diluted Satureja khuzestanicaessential oil containing 0.02% carvacrol) and 0.2% chlorhexidine in advanced periodontitis patients.
Materials and methods
Material
Dentol™ (10%) was provided from Khorraman Pharmaceutical Co. (Lorestan). ELISA kits for determination of TNF-α and IL-1β were purchased from Bender MedSystems (Austria). Other materials were purchased from Merck chemical company (Tehran).
Clinical protocol and patients
Dentol™ is the essential oil obtained from Satureja khuzestanicagrowing in southern parts of Iran. 10% Dentol™ has been registered and is available in pharmacy stores as local teeth analgesic drops. According to related articles, the effective antimicrobial concentration of carvacrol was considered two times the minimum inhibitory concentration in vitro [14]. Consequently, the effective antimicrobial dose was 0.02% after calculation for Porphyromonas gingivalisand Prevotella intermedia, which are two major periodontal pathogens [15, 16].
Twenty advanced periodontitis patients aged 35 to 45 years were chosen from patients referred to the periodontics department of TUMS at the school of dentistry. All teeth were probed in six sites (mesiobuccal, mid-buccal, distobuccal, mesiolingual, mid-lingual and distolingual). According to the latest classification for periodontal diseases, generalized severe chronic periodontitis was defined as 5 mm or more attachment loss in more than 30% of the sites [17]. In this paper, generalized severe chronic periodontitis was briefly called advanced periodontitis. Exclusion criteria included pregnancy, nursing, history of allergy to drugs and history of antibiotic consumption three months prior to the study; also any systemic disease was considered as an exclusion criterion. After selection, the patients were informed about the process of the study and individually signed a consent form to participate in the study. Then phase one of the periodontal treatment (including scaling and root planing and oral hygiene instruction) was done for all patients. Following plaque index reduction to the score of 30% or less, the patients were randomly assigned to test and control groups. Subgingival irrigation was performed in 15 sessions every other day during a period of 29 days. Dentol was irrigated in the test group and chlorhexidine in the control group. The irrigation was performed with a blunt ended needle syringe and by a practitioner. The frequency of appointments was selected on the basis of patient compliance and presence of an expert team to examine the patients carefully.
Clinical examination
Pocket depth (PD) was measured from the depth of the pocket to the gingival margin. Bleeding on probing (BOP) to the bottom of the clinical pocket was assessed during measurement of PD. The percentage of bleeding sites out of the total number of experimental sites was calculated for each treatment group [18]. The oral hygiene status was evaluated by modified O’Leary Plaque Control Record. After application of the disclosing solution, presence or absence of supragingival plaque was recorded for six aspects of each tooth. The complete mouth index was defined as the percentage of plaque-infected surfaces [19]. These clinical parameters were measured before the first subgingival irrigation, at the last irrigation session and two weeks later at the follow-up session. Data were collected by a practitioner who was unaware of the study assignment and then compared between the two groups.
Microbiological examination
Subgingival plaque samples were removed after supragingival plaque removal on two teeth in posterior and anterior regions with a 1-2 Gracey curette. The samples were transferred in sterile normal saline as the transport medium to the microbiology laboratory. Cultivation was performed within 60 minutes after sampling. Each sample was shaken for 30 seconds in a whirling mixer. One ml of the transport medium was diluted to 10–2 and 10–4 times. 0.1 ml from each dilution was evenly distributed on the surface of Brain Heart Infusion (BHI) and another 0.1 ml on the surface of Soybean Casein Digest Agar (SCDA). BHI plates were incubated anaerobically and SCDA plates were incubated aerobically for 36 hours. After formation of colony units, plates containing 30 to 300 grown colonies were chosen for total count analysis and the others were discarded. Plaque samplings were performed immediately before the irrigation at the first, 5th, 10th and 15th session in both groups.
Immunological examination
Two teeth in each patient were selected and isolated with cotton roll from saliva. A Dura pore strip was inserted 1 mm into the sulcus for 15 s, and 3 min later a second Dura pore strip was inserted in the same site for another 15 s. This process was repeated for the other site. The two mentioned strips were then placed into a microcentrifuge tube and were immediately frozen at –70°C until the day of analysis. In case of visible contamination with blood, the strips were discarded. On the day of analysis, 350 µl of phosphate buffered saline was added to the microtubes and shaken for 1 min and then centrifuged at 2000 g for 5 min. After strip removal, the supernatant was divided into two parts: one part for IL-1β and the other part for TNF-a analysis. The amount of these cytokines was determined in gingival crevicular fluid (GCF) with ELISA kits obtained from Bender MedSystems GmbH (Austria). Samplings of GCF were performed immediately before irrigation at the first session and the last session of subgingival irrigation in both groups.
Ethics
The study protocol was approved by the Ethics Committee of Tehran University of Medical Sciences (TUMS). There is no conflict of interest for the authors and there was no connection with the company producing Dentol.
Statistical analysis
The means of cytokine changes were compared between the two groups by analysis of covariance. For other variables, repeated measurement analysis was used considering the baseline measurements as covariates. For all of the variables except for cytokines, the test group was considered as between subject comparison. Statistical tests were done using SPSS ver. 11.5.
Results
Clinical results
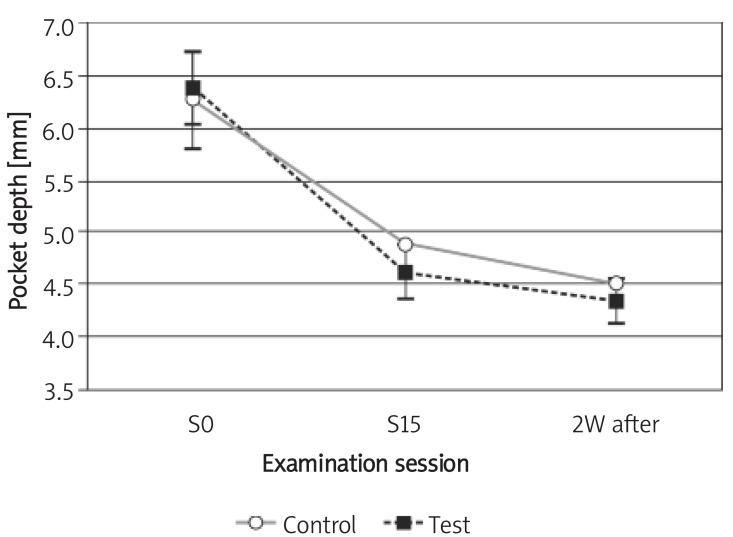
The mean PD in both test and control groups decreased from the mean (SD) of 6.4 (0.87) mm and 6.28 (0.99) mm to 4.62 (0.72) mm and 4.90 (0.77) mm after 29 days, respectively. Two weeks after the last irrigation session, the mean (SD) for PD decreased to 4.35 (0.57) mm and 4.50 (0.52) mm in test and control groups, respectively. No statistically significant difference between groups was found at the 1st and 15th sessions (the 29th day) or 2 weeks later, at the follow-up session (p > 0.05); however, there was a significant reduction in PD in each group (p< 0.05; Figure 1).
Figure 1.
Changes of pocket depth during the study Control – chlorhexidine group, Test – Dentol™ group, S0 – session before irrigation, S15 – 15th session of irrigation, 2W after – 2 weeks after the last session of irrigation. Data are mean ± SE
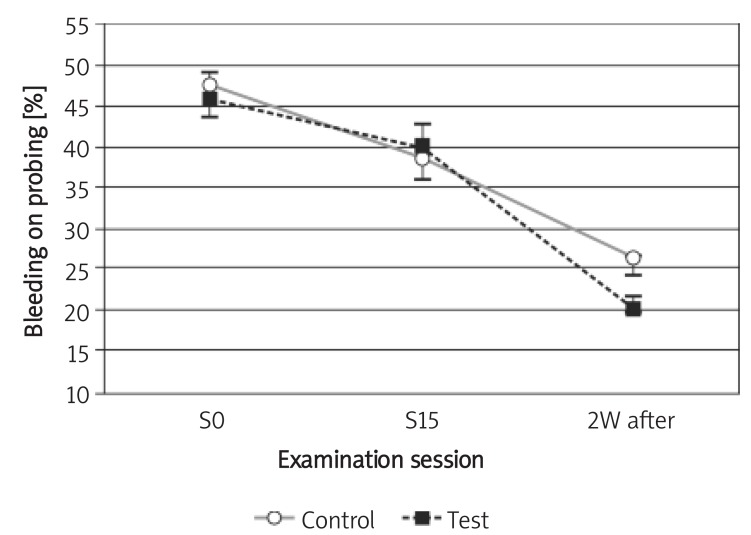
The mean BOP in both test and control groups decreased from the mean (SD) of 45.80 (13.61)% and 47.50 (12.96)% to 40.00 (10.54)% and 39.00 (12.20)% after 29 days, respectively. Two weeks after the last irrigation session the mean (SD) for BOP decreased to 20.20 (8.57)% and 26.30 (6.32)% in the test and control groups, respectively. According to the statistical analysis, no statistically significant difference between the two groups was detected at the 1st and 15th sessions (the 29th day) or 2 weeks later (p > 0.05); however, the reduction was statistically significant in both groups (p< 0.05; Figure 2).
Figure 2.
Changes of bleeding on probing during the study
Control – chlorhexidine group, Test – Dentol™ group, S0 – session before irrigation, S15 – 15th session of irrigation, 2W after – 2 weeks after the last session of irrigation. Data are mean ± SE
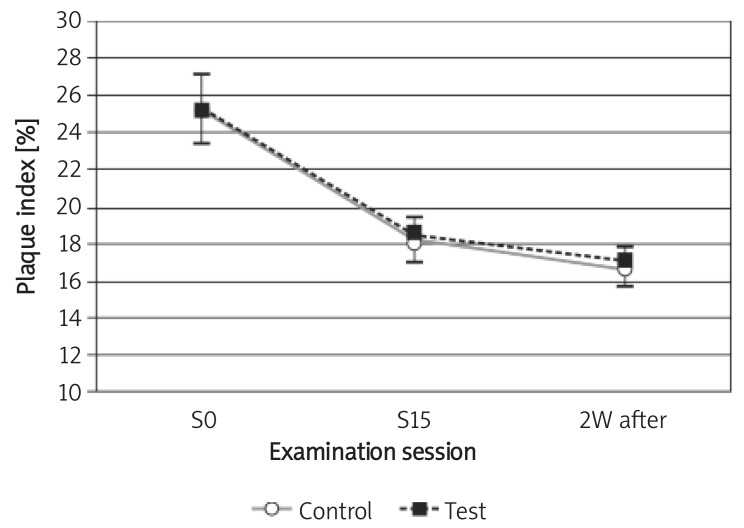
The mean PI in the test and control group decreased from a mean (SD) of 25.40 (3.65)% and 25.30 (4.83)% to 18.50 (2.99)% and 18.20 (3.29)% after 29 days, respectively. Two weeks after the last irrigation session, the mean (SD) for PI decreased to 17.10 (3.60)% and 16.70 (2.75)% in the test and control groups, respectively. No statistically significant difference was found between the two groups either at the 1st session or at the 15th session (the 29th day) or 2 weeks later (p > 0.05); furthermore, the reduction was not significant in each group (p> 0.05; Figure 3).
Figure 3.
Changes of plaque index during the study Control – chlorhexidine group, Test – Dentol™ group, S0– session before irrigation, S15 – 15th session of irrigation, 2W after – 2 weeks after the last session of irrigation. Data are mean ± SE
Microbiological results
The mean total colony forming units of aerobic bacteria increased significantly in both groups at the 5th, 10th, and 15th sessions compared to the baseline (p < 0.05). The difference between the test and control groups was statistically significant (p < 0.05). Details are shown in Table I.
Table I.
Changes in colony forming units (CFU) of aerobic bacteria during the study
| S0 | S5 | S10 | S15 | p value* | |
|---|---|---|---|---|---|
| Control | 4.5 × 104 (5.84 × 104) | 1.75 × 105 (2.22 × 105) | 3.93 × 105 (4.56 × 105) | 1.15 × 106 (1.56 × 106) | < 0.05 |
| Test | 1.26 × 105 (1.07 × 105) | 9.33 × 106 (1.06 × 107) | 8.87 × 107 (1.29 × 108) | 1.08 × 108 (1.24 × 108) | < 0.05 |
S0 – session before irrigation, S5 – 5th session of irrigation, S10 – 10th session of irrigation, S15 – 15th session of irrigation, Control – chlorhexidine group, Test – Dentol™ group.
p value of changes in CFU from S0 to S15. Data are mean (SD)
The mean total colony forming units of anaerobic bacteria decreased significantly in both groups at the 5th, 10th, and 15th session as compared to the baseline (p < 0.05). The difference between test and control groups was not statistically significant (p > 0.05). Details are shown in Table II.
Table II.
Changes in colony forming units (CFU) of anaerobic bacteria during the study
| S0 | S5 | S10 | S15 | p value* | |
|---|---|---|---|---|---|
| Control | 6.68 × 107 (6.41 × 107) | 1.60 × 106 (3.33 × 106) | 4.37 × 104 (7.62 × 104) | 1.08 × 103 (4.05 × 102) | < 0.05 |
| Test | 8.99 × 107 (8.92 × 107) | 9.85 × 105 (1.01 × 106) | 1.07 × 105 (3.06 × 105) | 1.38 × 104 (3.36 × 104) | < 0.05 |
S0 – session before irrigation, S5 – 5th session of irrigation, S10 – 10th session of irrigation, S15 – 15th session of irrigation, Control – chlorhexidine group, Test – Dentol™ group.
p value of changes in CFU from S0 to S15. Data are mean (SD)
Immunological results
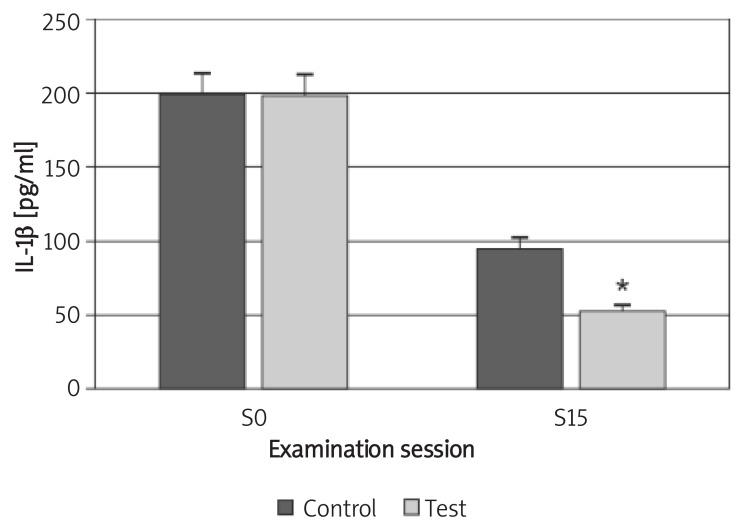
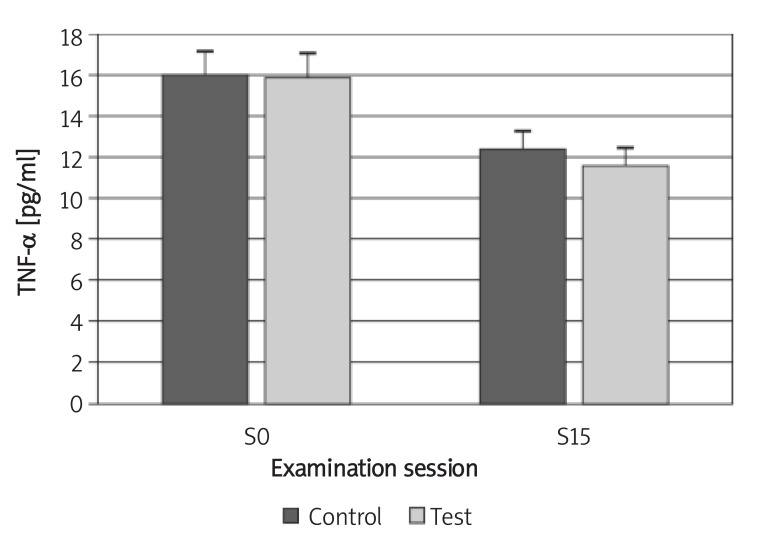
The mean concentration of IL-1β in the test and control groups decreased from a mean (SD) of 197.31 (41.11) pg/ml and 198.39 (27.94) pg/ml to 51.39 (28.05) and 93.97 (31.69) pg/ml, respectively. Although the difference between the two groups was not statistically significant (p > 0.05) at the baseline, the reduction was statistically significant for the test group at the 15th session (p < 0.05; Figure 4). The mean concentration of TNF-α in both groups decreased from 15.89 (4.55) pg/ml and 15.94 (3.46) pg/ml to 11.58 (1.92) pg/ml and 12.34 (1.11) pg/ml, respectively. The difference between the two groups was not statistically significant either at the baseline or at the 15th session (p > 0.05; Figures 4, 5).
Figure 4.
Changes of IL-1β concentration in GCF during the study
Control – chlorhexidine group, Test – Dentol™ group, S0– session before irrigation, S15 – 15th session of irrigation. Data are mean ± SE. * Difference between test and control groups is significant at p < 0.05
Figure 5.
Changes of TNF-α concentration in GCF during the study
Control – chlorhexidine group, Test – Dentol™ group, S0 – session before irrigation, S15 – 15th session of irrigation. Data are mean ± SE
Discussion
The results of the present study demonstrated that subgingival irrigation with Dentol™ or chlorhexidine is effective in reduction of pocket depth and bleeding on probing; however, it does not affect plaque index. This indicates that this intervention has affected the inflammatory indicators by influencing the subgingival plaque. This effect might be related to the anti-inflammatory or antibacterial effects of the irrigators and intervention in plaque maturation. According to the guidelines, absence of BOP is an excellent predictor of periodontal stability [20]. This achievement was observed in the Dentol™ group, which is in agreement with the study of Wagner et al. and Landa et al. demonstrating anti-inflammatory action for carvacrol [8, 11]. It was found in an animal study by Botelho et al. [12] that a carvacrol and chalcones combination gel showed a positive effect in experimental periodontitis. The present study was the first trial to investigate the effect of carvacrol in human periodontal disease and demonstrated its efficiency in reducing PD and BOP.
The results of this study indicated that subgingivally irrigated Dentol™ and also 0.2% chlorhexidine enhanced the growth of aerobic bacteria, although Dentol™ was more effective. On the other hand, Dentol™ and 0.2% chlorhexidine were equally effective in reducing the total count of anaerobic bacteria. In other words, Dentol™ subgingival irrigation resulted in a morphotype shift of periodontal microbiota to a healthy type. According to the study of Stabholz et al., subgingival irrigation with 0.12% chlorhexidine shifts the bacterial morphotype to periodontal health [21]. Based on the previous in vitro studies, essential oil components could disrupt the cell wall of gram negative bacteria [9]. Moreover, treatment with combined carvacrol and chalcones gel reduced tissue lesions in the histopathology, decreased myeloperoxidase activity in gingival tissue and inhibited the growth of oral microorganisms [12]. This evidence provided sufficient support for our study, which was the first study investigating the antibacterial effects of Dentol™ on humans. In the present clinical trial, Dentol™ was more effective than chlorhexidine in reducing the concentration of IL-1β, although it showed similar effects on reduction of TNF-α in GCF. Sharmas et al. investigated the effects of various mouthwashes on the levels of interleukin 2 and interferon-ã in chronic gingivitis [22]. They reported that the application of chlorhexidine, essential oils, povidone iodine and Azadirachta extract as mouthwash reduces inflammatory cytokines including IL-1β and TNF-α in GCF. Although there was no comparing evaluation in Sharma’s study between different mouthwashes, the present investigation confirms the superior effects for Dentol™ compared to 0.2% chlorhexidine in GCF IL-1β reduction. In support of the present findings, evidence about the safety of Satureja essential oil is complete and recent reports indicate its potential benefit in various inflammatory conditions such as inflammatory bowel disease and glucose metabolism or prevention of toxicity [23-30]. As a strong relationship between periodontitis and several systemic diseases such as cardiovascular diseases has been demonstrated in previous studies [31], the clinical implications of this study suggest that it could be considered as an alternative way to control such systemic diseases.
There were some limitations in the present study, some of which were inevitable; for instance, the relatively short term of treatment was because the patients had to come to the periodontics department several times according to the method of the study; thus it was not practical to consider a longer time in order to obtain more accurate results. Moreover, due to time constraints, the sample size was limited to 20. The results of the study would be more reliable if the sample size had been greater.
Dentol™ containing 0.02% carvacrol when irrigated subgingivally every other day for 29 days is as effective as 0.2% chlorhexidine or even superior to it in reducing clinical and immunological inflammatory indices. Additionally, it could result in subgingival colonization of healthy microbiota.
Acknowledgments
This study was supported by a grant from the Dentistry Research Center, TUMS and carried out with the collaboration of the Pharmaceutical Sciences Research Center, TUMS. The authors wish to thank Mohamad Javad Kharazifard for his assistance in statistical analysis of data.
References
- 1.Axelsson P, Lindhem J, Nyström B. On the prevention of caries and periodontal disease. Results of a 15-year longitudinal study in adults. J Clin Periodontol. 1991;18:182–9. doi: 10.1111/j.1600-051x.1991.tb01131.x. [DOI] [PubMed] [Google Scholar]
- 2.Listgarten MA, Lindhe J, Hellen L. Effect of tetracycline and/or scaling on human periodontal disease. J Clin Periodontol. 1978;5:246–71. doi: 10.1111/j.1600-051x.1978.tb01918.x. [DOI] [PubMed] [Google Scholar]
- 3.Shiloah J, Hovious LA. The role of subgingival irrigations in the treatment of periodontitis. J Periodontol. 1993;64:835–43. doi: 10.1902/jop.1993.64.9.835. [DOI] [PubMed] [Google Scholar]
- 4.Oosterwaal PJM, Mikx FHM, Van't Hof MA, Renggli HH. Shortterm bactericidal activity of chlorhexidine gel, stannous fluoride gel and amine fluoride gel tested in periodontal pockets. J Clin Periodontol. 1991;18:97–100. doi: 10.1111/j.1600-051x.1991.tb01696.x. [DOI] [PubMed] [Google Scholar]
- 5.Flötra L, Gjermo P, Rölla G, Waerhaug J. Side effects of chlorhexidine mouthwashes. Scand J Dent Res. 1971;79:119–25. doi: 10.1111/j.1600-0722.1971.tb02001.x. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez de Rojas VR, Somoza B, Ortega T, Villar AM. Isolation of vasodilatory active flavonoids from the traditional remedy Satureja obovata. Planta Med. 1996;62:272–4. doi: 10.1055/s-2006-957876. [DOI] [PubMed] [Google Scholar]
- 7.Farsam H, Amanlou M, Radpour MR, Salehinia AN, Shafiee A. Composition of the essential oils of wild and cultivated Satureuja khuzistanica Jamzad from Iran. Flavour Frag J. 2004;19:308–10. [Google Scholar]
- 8.Wagner H, Wierer M, Bauer R. Invitro inhibition of prostaglandin biosynthesis by essential oils and phenolic compounds. Planta Med. 1986;52:184–7. [PubMed] [Google Scholar]
- 9.Helander IM, Alakomi HL, Latuakala K, Sandholm M. Characterization of the action of essential oil components on gram negative bacteria. J Agric Food Chem. 1998;46:3590–5. [Google Scholar]
- 10.Gill AO, Holley RA. Disruption of Escherichia coli, Listeria monocytogenes and lactobactillus sakei cellular membrane by plant oil aromatics. Int J Food Microbial. 2006;108:1–9. doi: 10.1016/j.ijfoodmicro.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Landa P, Kokoska L, Pribylova M, Vanek T, Marsik P. In vitro anti-inflammatory activity of Carvacrol: Inhibitory effect on COX-2 catalyzed prostaglandin E(2) biosynthesis. Arch Pharm Res. 2009;32:75–8. doi: 10.1007/s12272-009-1120-6. [DOI] [PubMed] [Google Scholar]
- 12.Botelho MA, Martins JG, Ruela RS, Santos JA. Protective effect of locally applied Carvacrol gel on ligature-induced periodontitis in rats: a tapping mode AFM study. Phytother Res. 2009;22:442–9. doi: 10.1002/ptr.2798. [DOI] [PubMed] [Google Scholar]
- 13.Momtaz S, Abdollahi M. An update on pharmacology of Satureja species; from antioxidant, antimicrobial, antidiabetes and anti-hyperlipidemic to reproductive stimulation. Int J Pharmacol. 2010;6:454–61. [Google Scholar]
- 14.Chami N, Bennis S, Chami F, Aboussekhra A, Remmal A. Study of anticandidal activity of Carvacrol and eugenol in vitro and in vivo. Oral Microbiol Immunol. 2005;20:106–11. doi: 10.1111/j.1399-302X.2004.00202.x. [DOI] [PubMed] [Google Scholar]
- 15.Maxwell J, Greenberg M, Stawski B, Broderick K. Breath Freshening and Oral Cleansing Product Using Carvacrol. United States Patent 20040253278. Available from: http//www.freepatentsonline.com/y2004/0253278.html . 2008. [Accessed November 10].
- 16.Carranza FA, Klokkevold PR, Takei HH, Newman MG. Carranza’s clinical periodontology. 10th. Vol. 160. China: Elsevier; 2007. [Google Scholar]
- 17.Armitage G. Development of a Classification System for Periodontal diseases and Conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Trombelli L, Scabbia A, Carotta V, Scapoli C, Calura G. Clinical effect of subgingival tetracycline irrigation and tetracycline loaded fiber application in the treatment of adult periodontitis. Quintessence Int. 1996;27:19–25. [PubMed] [Google Scholar]
- 19.O’Leary TJ, Drake RB, Naylor JE. The plaque control record. J Periodontol. 1972;43:38–40. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 20.Carranza FA, Klokkevold PR, Takei HH, Newman MG. Carranza’s clinical periodontology. 10th. Vol. 553. China: Elsevier; 2007. [Google Scholar]
- 21.Stabholz A, Nicolas AA, Zimmerman GJ, Wikesjö UM. Clinical and antimicrobial effects of a single episode of subgingival irrigation with tetracycline HCl or chlorhexidine in deep periodontal pockets. J Clin Periodontol. 1998;25:794–800. doi: 10.1111/j.1600-051x.1998.tb02372.x. [DOI] [PubMed] [Google Scholar]
- 22.Sharma S, Saimbi CS, Koirala B, Shukla R. Effect of various mouthwashes on the levels of interleukin-2 and interferon gamma in chronic gingivitis. J Clin Pediatr Dent. 2008;32:111–4. doi: 10.17796/jcpd.32.2.u01p135561161476. [DOI] [PubMed] [Google Scholar]
- 23.Vosough-Ghanbari S, Rahimi R, Kharabaf S, et al. Effects of Satureja khuzestanica on serum glucose, lipids and markers of oxidative stress in patients with type 2 diabetes mellitus: a double-blind randomized controlled trial. Evid Based Complement Alternat Med. 2010;7:465–70. doi: 10.1093/ecam/nen018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basiri S, Esmaily H, Vosough-Ghanbari S, Mohammadirad A, Yasa N, Abdollahi M. Improvement by Satureja khuzestanica essential oil of malathion-induced red blood cells acetylcholinesterase inhibition and altered hepatic mitochondrial glycogen phosphorylase and phosphoenolpyruvate carboxykinase activities. Pestic Biochem Physiol. 2007;89:124–9. [Google Scholar]
- 25.Ghazanfari G, Minaie B, Yasa N, et al. Biochemical and histopathological evidences for beneficial effects of Satureja khuzestanica jamzad essential oil on the mouse model of inflammatory bowel diseases. Toxicol Mech Methods. 2006;16:365–72. doi: 10.1080/15376520600620125. [DOI] [PubMed] [Google Scholar]
- 26.Haeri S, Minaie B, Amin G, et al. Effect of Satureja khuzestanica essential oil on male rat fertility. Fitoterapia. 2006;77:495–9. doi: 10.1016/j.fitote.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 27.Saadat M, Pournourmohammadi S, Donyavi M, et al. Alteration of rat hepatic glycogen phosphorylase and phosphoenolpyruvate carboxykinase activities by Satureja khuzestanica Jamzad essential oil. J Pharm Sci. 2004;7:310–4. [PubMed] [Google Scholar]
- 28.Abdollahi M, Salehnia A, Mortazavi SH, et al. Antioxidant, anti diabetic, antihyperlipidemic, reproduction stimulatory properties and safety of essential oil of Satureja Khuzestanica in rat in vivo: a oxicopharmacological study. Med Sci Monit. 2003;9:331–5. [PubMed] [Google Scholar]
- 29.Rezvanfar M, Sadrkhanlou R, Ahmadi A, et al. Protection of cyclophosphamide-induced toxicity in reproductive tract histology, sperm characteristics, and DNA damage by an herbal source; evidence for role of free-radical toxic stress. Hum Exp Toxicol. 2008;27:901–10. doi: 10.1177/0960327108102046. [DOI] [PubMed] [Google Scholar]
- 30.Rezvanfar MA, Farshid AA, Sadrkhanlou RA, et al. Benefit of Satureja khuzestanica in subchronically rat model of cyclophosphamide-induced hemorrhagic cystitis. Exp Toxicol Pathol. 2010;62:323–30. doi: 10.1016/j.etp.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Shanker J, Kakkar V. Role of periodontal infection in cardiovascular disease: a current perspective. Arch Med Sci. 2009;5:125–34. [Google Scholar]