Abstract
Introduction
The clinical value of double contrast-enhanced ultrasonography (DCUS) in determining the Lauren classification of advanced gastric carcinoma needed investigation.
Material and methods
Fifty-eight patients with gastric cancer proved by endoscopic biopsy underwent preoperative DCUS examination in which an oral contrast agent was combined with an intravenous agent, and the findings were compared with the postoperative pathological findings using haematoxylin-eosin and Alcian Blue-Periodic Acid Schiff (AB-PAS) staining.
Results
Of 58 patients, 34 (59%) were the intestinal type and 24 (41%) the diffuse type on pathological examination of resected specimens. Among intestinal type patients, 30 (88%) showed homogeneous vascular enhancement and 4 (12%) heterogeneous enhancement with the “sandwich” pattern in 2 patients (50%) and “barrier” pattern in 2 patients (50%). In the diffuse type, 22 of 24 patients (92%) enhanced heterogeneously, with stippled and peripheral enhancement in 9 (41%), the “sandwich” pattern in 8 (36%) and “barrier” pattern in 5 (23%). Two of 24 patients (8%) with the diffuse type enhanced homogeneously. The proportion of heterogeneous enhancement was significantly different between the 2 subtypes of tumour (p = 0.0001). The sensitivity and specificity of heterogeneous enhancement in diagnosing the diffuse type of advanced gastric cancer were 92% and 88%, respectively. Youden’s index was 0.8.
Conclusions
Double contrast-enhanced ultrasonography is a new and useful method to determine Lauren classification in patients with gastric carcinoma.
Keywords: ultrasonography, microbubbles, contrast, gastric carcinoma, Lauren classification
Introduction
Gastric cancer is one of the most frequent causes of cancer-related death in East Asia [1, 2]. Despite the recent advances in diagnostic techniques for early detection and the improvement in surgical treatment, gastric carcinoma remains the second most common cause of death from cancer [3]. It is essential to predict precisely the risk of recurrence in order to minimize adverse effects and to maximize the therapeutic effect in the treatment of cancer patients. At least 4 different histological classification systems for gastric adenocarcinoma are in common use (Goseki, Lauren, Ming, and the World Health Organization (WHO) [4-7].
From an epidemiological standpoint, one helpful method is the Lauren classification [8], which divides gastric cancers into 4 histological types: intestinal and diffuse, mixed, and unclassified. We investigated the intestinal and diffuse types in our study. Patients with the mixed and unclassified types were not investigated because we did not have these patients.
There are pronounced differences in pathology, epidemiology, biological behaviour, and prognosis between the intestinal and diffuse types. The diffuse type has a worse prognosis [9]. Compared with the WHO classification and Lauren classification, Roukos reported that, in general, well and moderately differentiated cancers according to the WHO classification correspond to the intestinal type of Lauren, whereas poorly or undifferentiated and signet ring cell carcinomas correspond to the diffuse type [10]. Different types of Lauren gastric carcinoma have different biological behaviours [11-13]. For the intestinal type, the macroscopic margins correspond approximately to the microscopic extent of the tumour. The diffuse type of a poorly differentiated cancer may extend submucosally far beyond its macroscopic borders. This difference in tumour spread is important for selecting the appropriate treatment [14]. The Lauren classification has proven useful in evaluating the natural history of gastric carcinoma, especially with regard to incidence trends, clinicopathological correlations, and aetiological precursors [14]. Different types of Lauren classification may have different biological behaviours and prognosis. The choice of surgical procedure in resectable gastric carcinoma is dictated by size, location, and ability to achieve adequate margins clear of disease. Other methods used to diagnose and stage gastric carcinoma include endoscopy, upper gastrointestinal barium computed tomography, magnetic resonance imaging, abdominal ultrasound, positron emission tomography scans, and staging laparoscopy.
The use of pathological features in addition to the standard Lauren classification better separates chronic cancers (survivors beyond 5 years after diagnosis) from cured cancers and shows differences in behaviour between histological types [15]. The purpose of this study was to evaluate the Lauren classification of patients with advanced gastric cancer preoperatively using double contrast-enhanced ultrasonography (DCUS) in which an oral ultrasonic contrast agent is combined with an intravenous contrast agent.
Material and methods
Seventy-nine patients with gastric cancer proven by endoscopic biopsy were examined with DCUS preoperatively. Of the 79 patients, 6 were excluded because their tumours were unresectable and another 15 because of the early stage of their carcinomas. The remaining 58 patients were enrolled in this study. The 58 patients included 41 men and 17 women, mean age 54 ±18 years. All patients were studied in the second affiliated hospital of Wenzhou Medical College in China.
Surgical resections were performed within 5 days after the DCUS examination. None of the patients received non-steroidal anti-inflammatory drugs, chemotherapy, radiotherapy, or immunotherapy before surgery.
Informed consent was obtained from all patients prior to their examination. The local ethics committee approved this study.
Double contrast-enhanced ultrasonography was performed after fasting for at least 6 h [16]. Atropine sulfate (0.05 mg/kg) was administered by intramuscular injection half an hour before the examination to inhibit gastric peristalsis [16]. An Acuson Sequoia 512 system (Siemens, Mountain View, CA), equipped with a 4V1 vector™ transducer (frequency: 1.0-4.0 MHz) and the microbubble-specific contrast pulse sequencing (CPS) technology, was used [17].
The oral contrast agent Xinzhang® (Huqingyutang, HangZhou, China) was supplied as a powder which is derived from rice and soya (48 g per package). It was reconstituted by adding 500 ml of boiling water and gently agitating by hand to form a palatable homogeneous suspension [16].
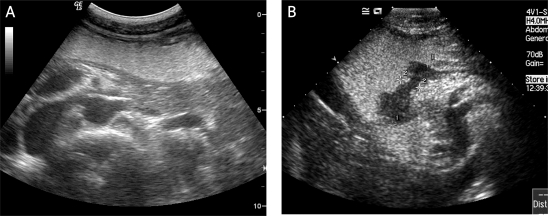
The distal oesophagus and the cardia of all patients were studied in real time B-mode using conventional tissue settings while the patients ingested the oral agent to expand the stomach and displace the air within it [16]. The lumen appears as a mid-grey, homogeneous region that acts as an acoustic window and improves visualization of the inner wall (Figures 1A-B). Then the rest of the stomach and the duodenal bulb were examined in turn with the patient in the supine and both decubitus positions to facilitate complete filling and visualization of the stomach wall. When a suspected lesion was identified, it was measured routinely. For lesions less than 1.5 cm thick, write-zoom was used to improve spatial resolution.
Figure 1.
The effect of the oral contrast agent. A) Following swallowing of the Xinzhang® oral contrast agent, the gastric lumen appears as a mid-grey, homogeneous region that acts as an acoustic window and improves visualization of the inner wall (arrows). This was a normal patient. B) In another patient, a gastric carcinoma is clearly displayed as a hypoechogenic region (between two cursors) after taking the Xinzhang® oral contrast agent
Afterwards, dynamic real-time contrast-enhanced sonography was performed with the CPS mode at a transmit frequency of 1.5 MHz and a low mechanical index (0.20), selected to minimize microbubble disruption. Vascular contrast-enhanced sonographic studies were performed after the administration of 2.4 ml of SonoVue (Bracco SpA, Milan, Italy) as a bolus via a 19-gauge peripheral intravenous cannula followed by a 10 ml saline flush. A timer on the sonography unit was started at the time of injection, and the entire movie sequence (at least 5 min) was stored on magnetic optical disks for analysis. The contrast study could be repeated a second time if necessary.
The cine loops of the 58 lesions were retrospectively reviewed by 3 independent radiologists who are experts in sonography and microbubble contrast agents. These 3 reviewers were unaware of the final diagnosis and other imaging information at the time of the analysis.
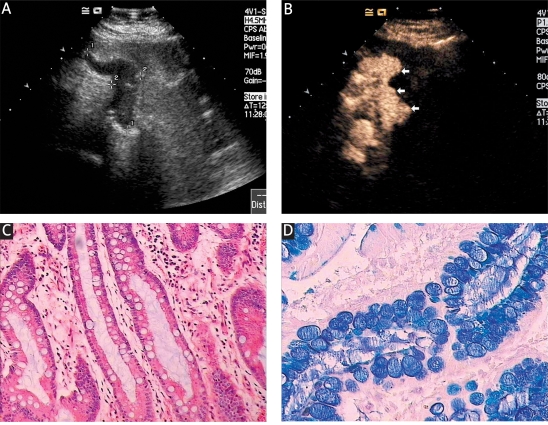
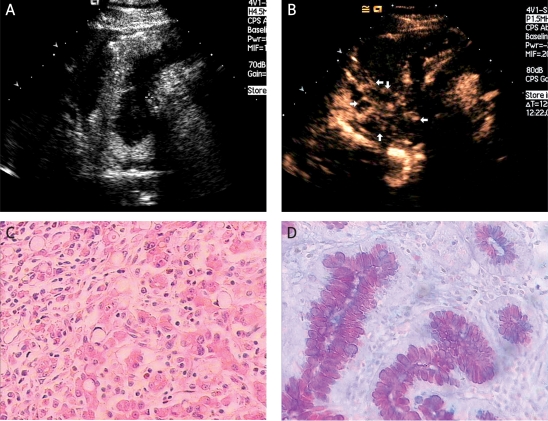
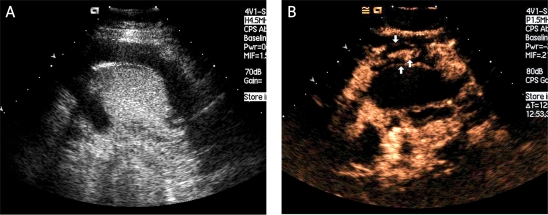
Vascular enhancement of gastric carcinomas was classified into 2 patterns: homogeneous enhancement and heterogeneous enhancement. Homogeneous enhancement was defined as an even signal intensity over the whole tumour with no signal defects during the early arterial phase (lasting 25 seconds after injection) (Figures 2A-D). Heterogeneous enhancement was defined as an uneven signal intensity over the lesion during the early arterial phase with stippled and peripheral enhancement (Figures 3A-D). In the “sandwich” pattern, the enhancement took the form of layers in the tumour (Figures 4A-B). In the “barrier” pattern, the enhancement began at the serosa and progressed to the mucosa (Figures 5A-B). Agreement regarding the classification of all lesions was reached by consensus.
Figure 2.
Intestinal type of gastric carcinoma. A) The tumour is displayed as a hypoechoic mass (between two cursors) after taking the Xinzhang® oral contrast agent. B) This tumour enhanced homogeneously with no signal defects during the early arterial phase (white arrow). C) The tumour proved to be a well differentiated intestinal type of carcinoma (×200 HE). D) On AB-PAS staining, the acid mucus took up the blue dye (×200)
STO - stomach
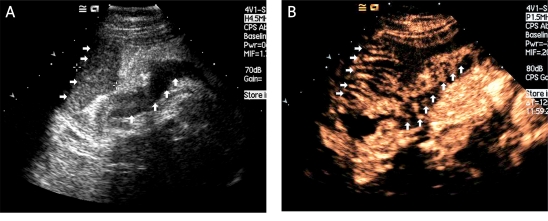
Figure 3.
Diffuse type of gastric carcinoma. A) In another patient, a gastric carcinoma was again clearly displayed (between two cursors) as an echo-poor region after taking the Xinzhang® oral contrast agent. B) The tumour enhanced heterogeneously during the early arterial phase with stippled and peripheral enhancement (white arrows). C) This carcinoma was poorly differentiated; the blue arrows show the signet-ring cells (×200 HE). D) AB-PAS staining shows the neutral mucus (×200)
Figure 4.
Diffuse type of gastric carcinoma showing the sandwich pattern. A) The oral contrast agent highlights the hypoechogenic tumour (between the cursors). B) During the early arterial phase of i.v. enhancement the tumour enhanced hetero geneously with the sandwich pattern (white arrows)
Figure 5.
Diffuse type of gastric carcinoma showing the barrier pattern. A) The echopoor gastric carcinoma is clearly displayed (white arrows) using oral contrast ultrasonography. B) This lesion of the diffuse type enhanced heterogeneously during the early arterial phase with the barrier pattern (white arrows)
STO - stomach
The surgically resected specimens were immediately fixed in 85% ethanol and embedded in paraffin. Ten to 15 pieces of tumour tissue were obtained from each sample, dehydrated routinely with graded ethanol, embedded in paraffin, and cut into 4-m thick sections. For each block, 2 slides were prepared, one stained with haematoxylin and eosin (H&E) for pathological diagnosis and the other one stained for histochemical analysis. Haematoxylin and eosin and alcian blue (pH 2.5) - periodic acid Schiff (AB-PAS) staining were performed [18].
Briefly, for AB-PAS staining, the paraffin slides were deparaffinized with xylene. One hundred microlitres of AB staining solution (Fluka Company) was added to the slides for staining in wet boxes for 20-30 min and washed with distilled water for 3-5 min. One hundred ml of 0.5% periodic acid solution was applied for oxidation for 10 min and washed with distilled water for 3-5 min. Staining was performed with Schiff solution for 10 min. The slides were then washed with distilled water for 5-10 min. Finally, the slides were sealed with neutral gum. The tumours were classified according to Lauren’s description [8] as intestinal (Figure 2A-D) or diffuse (Figure 3A-D).
SPSS version 12.0 (SPSS Inc. Chicago, USA) was used for the statistical analysis. Numerical data were compared using χ2 test between the 2 subtypes of tumour. Sensitivity, specificity, and Youden’s index were calculated. Youden’s index is the sum of the sensitivity and specificity (expressed as fractions) minus one. For all analyses, a p value of less than 0.05 was considered statistically significant.
Results
Among the 58 patients included in the study, 34 (59%) were histologically diagnosed as the intestinal type and 24 (41%) as the diffuse type. Thirty of the 34 patients (88%) with the intestinal type showed global and homogeneous enhancement. Four of the 34 patients (12%) showed heterogeneous enhancement with the “sandwich” pattern in 2 patients (50%) and the “barrier” pattern in 2 patients (50%). Among the 24 patients with diffuse lesions, 22 (92%) enhanced heterogeneously, with stippled and peripheral enhancement in 9 patients (41%), the “sandwich” pattern in 8 patients (36%), and the “barrier” pattern in 5 patients (23%). The remaining 2 diffuse cases enhanced homogeneously. Final tumour staging according to the gold standard was 0 patients at stage T1, 22 patients at stage T2, 30 patients at stage T3, and 6 patients at stage T4.
Chi-square analysis showed that the proportion of heterogeneous enhancement was significantly different between the 2 subtypes of tumour (p = 0.0001). Taking heterogeneous enhancement as a criterion for the diffuse type of advanced gastric cancer, the sensitivity and specificity were 92% and 88% respectively (Table I). Youden’s index was 0.8. The κ value of this method was 0.75.
Table I.
Evaluation of Lauren classification for gastric carcinoma using double contrast-enhanced ultra-sonography (DCUS)
| DCUS | Lauren classification | |
|---|---|---|
| Diffuse type | Intestinal type | |
| Heterogeneous enhancement | 22 | 4 |
| Homogeneous enhancement | 2 | 30 |
The proportion of heterogeneous enhancement was significantly different between the diffuse and intestinal types of gastric carcinoma (p = 0.0001). The sensitivity and specificity of heterogeneous enhancement in diagnosing the diffuse type of gastric carcinoma were 92% and 88%, respectively. Youden's index was 0.8
Discussion
In 1965, Lauren reported a histological classification in which gastric carcinomas were divided into intestinal and diffuse types. The intestinal type of gastric carcinoma is histologically characterised by the presence of cohesive cells forming glandular and papillary structures and acidic mucous as judged by Alcian blue pH 2.5)/periodic acid Schiff staining of mucous. The diffuse type of gastric carcinoma is characterized histologically by non-cohesive cells, the common presence of signet ring cells, and neutral mucous as judged by high iron diamine/Alcian blue pH 2.5 staining. The intestinal type is more common in men, typically arises in the antrum, and has a better prognosis. The diffuse type is only slightly more common in men and also occurs in younger patients. It generally arises in the fundus and is associated with blood group A.
Regional and ethnic group variations in gastric carcinoma incidence appear to be related to the prevalent histological type [19, 20]. The intestinal type predominates in epidemic zones such as the Far East [21]. When gastric carcinoma incidence declines, as it has in Europe and the United States, it has been predominantly the intestinal type that has diminished in frequency. The incidence of the diffuse type has remained unchanged or has decreased to a much lesser degree [19, 22-24]. Because the incidence of intestinal type cancer can change dramatically over a relatively short period, it has been hypothesized that its pathogenesis may be linked to environmental and socioeconomic factors. Additional support for the environmental hypothesis comes from studies of migrants, which demonstrate retention of parent-country risk in first generation migrants but not in later generations [25].
In contrast, diffuse type disease is thought to be largely genetic in aetiology. The intestinal type is characterized by cohesive neoplastic cells forming gland-like tubular structures, whereas in the diffuse type cell cohesion is absent so that individual cells infiltrate and thicken the stomach wall without forming a discrete mass. This difference in microscopic growth pattern is also reflected in the different macroscopic appearance of the 2 histological subtypes. [5]. The development of the intestinal type of gastric cancer is a progression of mucosal changes from superficial gastritis to chronic atrophic gastritis and metaplasia before malignancy appears [26, 27].
While there are many reports on the preoperative staging of gastric carcinoma using multidetector computed tomography with high accuracy [28-31], few reports have been reported on the preoperative Lauren classification of gastric carcinoma using imaging modalities [32, 33]. It is very difficult for conventional ultrasound to visualize gastric cancers because stomach gas interferes with imaging. The use of an oral contrast agent improves their detection [34].
Intravenous contrast-enhanced ultrasonography has been widely used to assess tumours in vivo.Double contrast-enhanced ultrasonography has been reported as a valid method to evaluate the microcirculatory perfusion of gastric carcinomas [34]. In this study, we sought to identify whether DCUS could be used to evaluate Lauren’s histological classification of gastric carcinomas.
The intestinal type of gastric carcinoma accounts for approximately 50% of gastric carcinomas and the diffuse type for 35%. The remaining 15% of gastric carcinomas are unclassified or mixed type carcinomas [5, 24, 35, 36]. Among our 58 patients, 34 (59%) were diagnosed as the intestinal type and 24 (41%) as the diffuse type. None of our patients had an unclassified or mixed type gastric carcinoma on pathological examination of the resected specimens.
The characteristics of gastric carcinoma DCUS examination correlated closely with the Lauren classification. Among 34 patients with the intestinal type, 30 lesions (88%) showed homogeneous enhancement after intravenous contrast injection. In 24 patients with the diffuse type, 22 lesions (92%) had heterogeneous enhancement. Therefore, homogeneous vascular enhancement could form a useful diagnostic criterion for the intestinal type of gastric cancer and heterogeneous enhancement for the diffuse type.
The sensitivity and specificity of the pattern of heterogeneous vascular enhancement on DCUS to detect the diffuse type of gastric cancer were 92% and 88%, respectively. Youden’s index of DCUS to predict the Lauren classification was 0.8. However, there were 4 patients (12%) with the intestinal type that enhanced in heterogeneous patterns, and 2 patients (8%) with the diffuse type that enhanced homogeneously.
In conclusion, DCUS shows promise as a new noninvasive, convenient method to distinguish the histological classification of gastric carcinoma and may have a role in evaluating the prognosis in patients with gastric carcinoma in vivo.
Acknowledgments
This study was supported by Zhejiang Wenzhou Science & Technology Bureau (No. H20060039).
References
- 1.Lawrence W., Jr Gastric cancer. CA Cancer J Clin. 1986;36:216–36. doi: 10.3322/canjclin.36.4.216. [DOI] [PubMed] [Google Scholar]
- 2.Boring CC, Squires TS, Tong T, et al. Cancer statistics. CA Cancer J Clin. 1991;41:19–36. doi: 10.3322/canjclin.41.1.19. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Goseki N, Takizawa T, Koike M. Differences in the mode of extension of gastric cancer classified by histological types: new histological classification of gastric carcinoma. Gut. 1992;33:606–12. doi: 10.1136/gut.33.5.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 6.Ming SC. Gastric carcinoma: a pathological classification. Cancer. 1977;39:2475–85. doi: 10.1002/1097-0142(197706)39:6<2475::aid-cncr2820390626>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 7.Oota K, Sobin LH. International histological classification of tumours. No 18: histological typing of gastric and oesophageal tumours. Geneva: WHO. 1977.
- 8.Lauren P. The two histological main types of gastric cancer: diffuse and so-called intestinal type carcinoma. Acta Pathol Microbiol Scand. 1965;64:31–9. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- 9.Polkowski W, van Sandick JW, Offerhaus GJ, et al. Prognostic value of Lauren classification and c-erbB-2 oncogene overexpression in adenocarcinoma of the esophagus and gastroesophageal junction. Ann Surg Oncol. 1999;6:290–7. doi: 10.1007/s10434-999-0290-2. [DOI] [PubMed] [Google Scholar]
- 10.Roukos DH. Current status and future perspectives in gastric cancer management. Cancer Treat Rev. 2000;26:243–55. doi: 10.1053/ctrv.2000.0164. [DOI] [PubMed] [Google Scholar]
- 11.Cho YE, Kim JY, Kim YW, et al. Expression and prognostic significance of human growth and transformation-dependent protein in gastric carcinoma and gastric adenoma. Hum Pathol. 2009;40:975–81. doi: 10.1016/j.humpath.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Jung EJ, Lee HS, et al. Comparative analysis of DNA methylation between primary and metastatic gastric carcinoma. Oncol Rep. 2009;21:1251–9. doi: 10.3892/or_00000348. [DOI] [PubMed] [Google Scholar]
- 13.Sung JY, Lee S, Kim YW, et al. Vascular endothelial growth factor receptor-3 is a favorable prognostic factor in advanced gastric carcinoma. Oncol Rep. 2008;19:939–44. [PubMed] [Google Scholar]
- 14.Fuchs CS, Mayer RJ. Gastric carcinoma. New Engl J Med. 1995;333:32–41. doi: 10.1056/NEJM199507063330107. [DOI] [PubMed] [Google Scholar]
- 15.MacDonald WC, Owen DA, Le N. Chronic advanced gastric cancer: clinicopathologic analysis of survival data. Hum Pathol. 2008;39:641–9. doi: 10.1016/j.humpath.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 16.Shiyan L, Pintong H, Zongmin W, et al. The relationship between enhanced intensity and microvessel density of gastric carcionoma using double contrast-enhanced ultrasonography. Ultrasound Med Biol. 2009;35:1086–1091. doi: 10.1016/j.ultrasmedbio.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 17.Phillips P, Gardner E. Contrast-agent detection and quantification. Eur Radiol. 2004;14(Suppl 8):P4–10. [PubMed] [Google Scholar]
- 18.Spicer SS, Horn RG, Leppi TJ. Histochemistry of connective tissue mucopolysaccharides. In: Wagner BM, Smith DE, editors. The Connective Tissue. Baltimore: Williams and Wilkins; 1967. pp. 251–303. [Google Scholar]
- 19.Correa P, Haenszel W, editors. Epidemiology of gastric cancer. In: Epidemiology of cancer of the digestive tract. The Hague: Martinus-Nijhoff; 1982. pp. 58–84. [Google Scholar]
- 20.Meyers WC, Damiano RJ, Postlethwait RW, et al. Adenocarcinoma of the stomach.Changing patterns over the last 4 decades. Ann Surg. 1987;205:1–8. doi: 10.1097/00000658-198701000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munoz N, Correa P, Cuello C, et al. Histologic types of gastric cancer in high- and low-risk areas. Int J Cancer. 1968;3:809–18. doi: 10.1002/ijc.2910030614. [DOI] [PubMed] [Google Scholar]
- 22.Sipponen P, Jarvi O, Kekki M, et al. Decreased incidences of intestinal and diffuse types of gastric cancer in Finland during a 20-year period. Scand J Gastroenterol. 1987;22:865–71. doi: 10.3109/00365528708991927. [DOI] [PubMed] [Google Scholar]
- 23.Howson CP, Hiyama T, Wynder EL. The decline in gastric cancer: epidemiology of an unplanned triumph. Epidemiol Rev. 1986;8:1–27. doi: 10.1093/oxfordjournals.epirev.a036288. [DOI] [PubMed] [Google Scholar]
- 24.Munoz N, Connelly R. Time trends of intestinal and diffuse types of gastric cancer in the United States. Int J Cancer. 1971;8:158–64. doi: 10.1002/ijc.2910080119. [DOI] [PubMed] [Google Scholar]
- 25.Haenszel W, Kurihara M, Segi M, et al. Stomach cancer among Japanese in Hawaii. J Natl Cancer Inst. 1972;49:969–88. [PubMed] [Google Scholar]
- 26.Correa P, Cuello C, Duque E, et al. Gastric cancer in Colombia. III. Natural history of precursor lesions. J Natl Cancer Inst. 1976;57:1027–35. doi: 10.1093/jnci/57.5.1027. [DOI] [PubMed] [Google Scholar]
- 27.Correa P, Haenszel W, Cuello C, et al. Gastric precancerous process in a high risk population: cohort follow-up. Cancer Res. 1990;50:4737–40. [PubMed] [Google Scholar]
- 28.Chamadol N, Wongwiwatchai J, Bhudhisawasd V, et al. Accuracy of spiral CT in preoperative staging of gastric carcinoma: correlation with surgical and pathological findings. J Med Assoc Thai. 2008;91:356–63. [PubMed] [Google Scholar]
- 29.Lee IJ, Lee JM, Kim SH, et al. Helical CT evaluation of the preoperative staging of gastric cancer in the remnant stomach. Am J Roentgenol. 2009;192:902–8. doi: 10.2214/AJR.07.3520. [DOI] [PubMed] [Google Scholar]
- 30.Heye T, Kuntz C, Dux M, et al. CT and endoscopic ultrasound in comparison to endoluminal MRI: preliminary results in staging gastric carcinoma. Eur J Radiol. 2009;70:336–41. doi: 10.1016/j.ejrad.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 31.Davies J, Chalmers AG, Sue-Ling HM, et al. Spiral computed tomography and operative staging of gastric carcinoma: a comparison with histopathological staging. Gut. 1997;41:314–9. doi: 10.1136/gut.41.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chin SY, Lee BH, Kim KH, et al. Radiological prediction of the depth of invasion and histologic type in early gastric cancer. Abdom Imaging. 1994;19:532–6. doi: 10.1007/BF00198254. [DOI] [PubMed] [Google Scholar]
- 33.Rossi M, Broglia L, Graziano P, et al. Local invasion of gastric cancer: CT findings and pathologic correlation using 5-mm incremental scanning, hypotonia, and water filling. Am J Roentgenol. 1999;172:383–8. doi: 10.2214/ajr.172.2.9930788. [DOI] [PubMed] [Google Scholar]
- 34.Li SY, Huang PT, Wang ZM, et al. The relationship between enhanced intensity and microvessel density of gastric carcinoma using double contrast-enhanced ultrasonography. Ultrasound Med Biol. 2009;35:1086–91. doi: 10.1016/j.ultrasmedbio.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Lauren PA, Nevalainen JT. Epidemiology of intestinal and diffuse types of gastric carcinoma: a new-trend study in Finland with comparison between studies from high and low-risk areas. Cancer. 1993;71:2926–33. doi: 10.1002/1097-0142(19930515)71:10<2926::aid-cncr2820711007>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 36.Roukos D, Lorenz M, Hottenrott C. Prognostic significance of the Lauren classification of patients with stomach carcinoma. A statistical analysis of long-term results following gastrectomy. Schweiz Med Wochenschr. 1989;119:755–9. [PubMed] [Google Scholar]