Abstract
Introduction
Stroke is the second leading cause of death in the world. However, there is still no approved neuroprotective drug for acute ischaemic stroke. To clarify the neuroprotective efficacy and safety of dextromethorphan in stroke, the following study was carried out.
Material and methods
Forty patients with acute stroke causing moderate deficit were randomized to be treated with either dextromethorphan 300 mg per day or placebo for 5 days. Plasma level of dextromethorphan and its active metabolite was not evaluated in this study. The NIHSS score was calculated on day 5 and the Barthel activities of daily living index and Rankin score were checked after 3 months by a blinded investigator. Collected data were analysed using the t-test and χ2 test.
Results
In the dextromethorphan-treated group, the mean NIHSS score was 16.8 ±3.9 at baseline, and was 14.2 ±4.8 for the placebo-treated group (p = 0.069). At day 5, there was also no significant difference regarding NIHSS score (p = 0.167). At the 3-month follow-up, there was no significant difference regarding Barthel scale and Rankin score between the dextromethorphan and placebo groups.
Conclusions
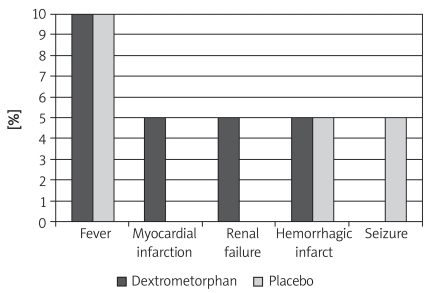
The results of our study suggest that although low-dose and short-term oral administration of dextromethorphan seems to be not neuroprotective, it does not worsen either patients’ condition or NIHSS score. Moreover, patients treated with dextromethorphan showed a significant reduction in seizures (complication after stroke), but had increased chance of MI and renal failure by almost 5% when compared to the placebo-treated groups. More prolonged studies with a higher number of cases are recommended.
Keywords: stroke, neuroprotection, dextromethorphan, treatment
Introduction
Stroke is the second leading cause of death in the world [1]. Stroke is the most common cause of serious, long-term disability in adults. The total cost of stroke in the USA is estimated to be more than US $56 billion in 2005 [2]. In addition, stroke patients have a high risk of myocardial infarction or vascular death [3]. During stroke, brain tissue does not obtain enough nutrients and oxygen and dies within a few hours [1]. Various therapies such as surgery, medications and post-stroke rehabilitation are not completely successful in controlling its consequences. Despite encouraging results with neuroprotective drugs in experimental models, none of them has been approved to date by the U.S. Food and Drug Administration for acute ischaemic stroke [1].
Dextromethorphan (an NMDA receptor antagonist) is a highly effective and widely used non-opioid antitussive drug [4]. It has been observed to provide neuroprotection in a variety of in vitro and in vivo experimental models of central nervous system (CNS) injury [5-7]. Adverse reactions with this drug are infrequent and usually not severe [5, 6].
Neuroprotective effects of dextromethorphan in animal models have been suggested in many previous studies [5, 8-11]. However, there is a lack of clinical data regarding use of this medication as a neuroprotective medication in humans. To better clarify the efficacy and safety of dextromethorphan in patients with ischaemic stroke, the following study was carried out.
Material and methods
This was a randomized placebo-controlled clinical trial that was performed in Al-Zahra Hospital, Isfahan, Iran. Ethical committee clearance was achieved before start of the study. Informed consent was also obtained from all of the participants. Forty patients who were in the acute phase of stroke (deficit having lasted more than 6 h and less than 24 h) and their stroke deficit was of moderate degree (NIHSS less than 17 and more than 7) were included in this study. Computed tomography (CT) scan and relevant laboratory tests were performed to exclude patients with intracerebral, subdural, epidural or subarachnoid haemorrhage. Patients with cerebral degenerative, demyelinating diseases, brain tumour, previous stroke, metabolic disorders and muscular or neuromuscular junction diseases were also excluded.
To have a homogeneous study group, only patients with middle cerebral artery territory infarction (based on Duplex sonography of the cervical artery and transcranial Doppler and CT scan on admission and the 2nd day after stroke and correlation with neurological examination) were included.
All of the patients were examined and scored using the National Institutes of Health Stroke Scale (NIHSS) by a blinded investigator on the admission day and the 5th day. The patients were randomized into 2 groups each including 20 patients. Group A was treated with a 60 mg loading dose of oral dextromethorphan and daily oral 300 mg of dextromethorphan divided into 5 doses for 5 days. Group B were treated with a placebo that was made of the basic materials of the tablet. Both the drug and placebo were provided by Poor Sina Company.
All of the patients were also treated with acetylsalicylic acid (enteric coated) 80 mg, daily. Both the active and placebo groups received the acetylsalicylic acid. It has already been shown that salicylic acid does not affect the plasma level of dextromethorphan and its metabolite [12]. No other medication was prescribed for the patients.
The Barthel activities of daily living index and Rankin score were checked after 3 months by a blinded investigator. The study end point was the completion of the 90-day follow-up period. Collected data were analysed using SPSS version 15.00 software and statistical tests including t-test and χ2 test.
Results
Forty patients (20 treated with dextromethorphan and 20 receiving placebo) entered the study. The mean age in the dextromethorphan group was 68.65 years and in the placebo group was 69.25 years and there was no significant difference regarding age between the 2 groups (p = 0.86). Out of 20 patients in the dextromethorphan group, 12 patients (60%) were male and 8 patients (40%) were female. Out of 20 patients in the placebo group, 7 patients (35%) were male and 13 patients (65%) were female (Table I).
Table I.
Demographic characteristics of the patients in the dextromethorphan and placebo groups (p > 0.05)
| Treatment group | Dextromethorphan | Placebo |
|---|---|---|
| Mean of age [years] | 68.65 | 69.25 |
| M/F (out of 20 patients) | 12/8 | 7/13 |
| Lag time to start treatment [h] | 11.9 | 10.4 |
There was no significant difference regarding risk factors between the dextromethorphan and placebo groups (Table II). Complications of stroke were seen in both groups (after 5 days of administration of drug or placebo) (Figure 1). However, no acute or chronic side effects attributable to dextromethorphan were observed.
Table II.
Comparison between risk factors in the dextromethorphan and placebo groups
| Treatment group | Dextromethorphan | Placebo | Value of p |
|---|---|---|---|
| Hypertension history (+/-) | 11/9 (55%/45%) | 16/4 (80%/20%) | 0.088 |
| Hyperlipidemia (+/-) | 2/18 (10%/90%) | 6/14 (30%/70%) | 0.118 |
| Ischemic Heart Disease (+/-) | 6/14 (30%/70%) | 5/15 (25%/75%) | 0.5 |
| Smoking (+/-) | 7/13 (35%/65%) | 4/16 (20%/80%) | 0.24 |
| Opium Addiction(+/-) | 2/18 (10%/90%) | 0/20 (0%/100%) | 0.244 |
Figure 1.
Complications of stroke in the dextro - methorphan and placebo groups (after 5 days’ administration of drug or placebo)
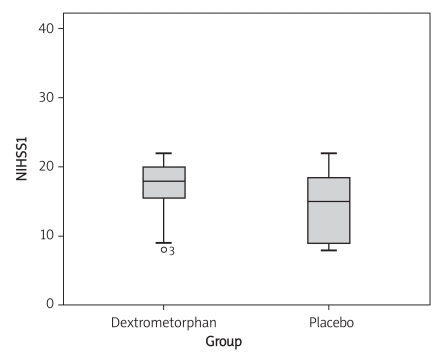
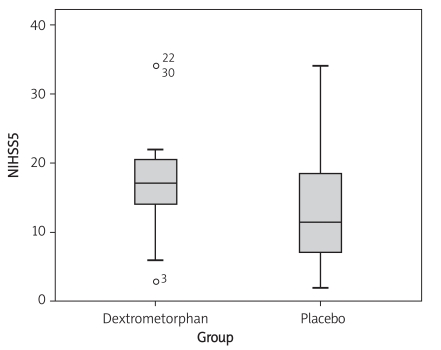
In the dextromethorphan-treated group, the mean NIHSS score was 16.8 ±3.9 at baseline, and was 14.2 ±4.8 for the placebo-treated group. There was no significant difference regarding NIHSS score at baseline (p = 0.069) (Figure 3). At day 5, there was also no significant difference regarding NIHSS score (p = 0.167) (Figure 4, Table III). At the 3-month follow-up, there was no significant difference regarding Barthel scale between the dextromethorphan and placebo groups (p = 0.268) (Table IV).
Figure 3.
Comparison of NIHSS score at day 1 in the dextromethorphan and placebo groups
Figure 4.
Comparison of NIHSS score at day 5 in the dextromethorphan and placebo groups
Table III.
Comparisson of the NIHSS between the dextrometorhphan and placebo groups at day 1 and day 5
| NIHSS | N | Mean | Standard deviation | Standard error, mean | |
|---|---|---|---|---|---|
| Day 1 | Dextromethorphan | 20 | 16.85 | 3.93 | 0.88 |
| Placebo | 20 | 14.25 | 4.81 | 1.07 | |
| Day 5 | Dextromethorphan | 20 | 17.25 | 7.62 | 1.70 |
| Placebo | 20 | 13.5 | 9.12 | 2.03 | |
Table IV.
Comparison of Barthel scale in the dexrtometorhpane and placebo group at day 90
| Treatment group | N | Mean | Standard deviation | Standard error |
|---|---|---|---|---|
| Dextromethorphan | 20 | 48.75 | 38.62 | 8.63 |
| Placebo | 20 | 62.50 | 38.71 | 8.65 |
In addition, at the 3-month follow-up, there was no significant difference regarding Rankin scale between the dextromethorphan and placebo groups (p = 0.268) (Table V).
Table V.
Comparison of Rankin scale in the dexrtromethorhpan and placebo group at day 90
| Treatment group | N | Mean | Standard deviation | Standard error |
|---|---|---|---|---|
| Dextromethorphan | 20 | 3.55 | 1.63 | 0.36 |
| Placebo | 20 | 2.8 | 1.90 | 0.42 |
Discussion
Many preclinical studies have shown the neuroprotective properties of dextromethorphan in various CNS injury models including focal and global ischaemia, seizure, and traumatic brain injury. Many of these protective actions seem functionally related to its inhibitory effects on glutamate-induced neurotoxicity via NMDA receptor antagonist, sigma-1 receptor agonist, and voltage-gated calcium channel antagonist actions. The capability of dextromethorphan to protect dopamine neurons in Parkinson models may be due to inhibition of neurodegenerative inflammatory responses [13].
Albers et al. treated 10 patients with a history of recent stroke with oral dextromethorphan (60 mg q.i.d.) for 3 weeks in a placebo-controlled, double-blind, and crossover tolerance study. They documented no clinical evidence of toxicity attributed to dextromethorphan in this preliminary study [14]. Also in another study by Albers et al. similar results were obtained; however, side effects including lightheadedness, drowsiness, nausea, decreased coordination, and unsteady gait were reported by several patients [15-17]. In the current study, we used shorter duration of treatment but with a higher number of patients. We also observed no evidence of toxicity attributable to dextromethorphan.
Steinberg et al. found that in rabbits with focal ischaemia the area of neocortical ischaemic neuronal damage was significantly reduced in the group treated with dextromethorphan compared with controls [18]. In addition other studies have shown a neuroprotective effect of dextromethorphan in animal models [8-11].
We chose dextromethorphan as a neuroprotective agent because of its assumed neuroprotective effect (as shown by animal studies), its safety and its effect on control of seizures and headaches [4-7, 13, 14, 19, 20].
Because the majority of stroke patients arrive at the hospital several hours after appearance of symptoms, the therapeutic window of 24 h was our proposed design. However, the usually accepted therapeutic window for neuroprotective medications is up to 6 h. Therefore, more studies proposing this fact are recommended. Nevertheless, animal model studies have shown that evaluation of inclusive changes to infarction is not limited to the first 24 h [7, 18]. In the current study, plasma levels of dextromethorphan and its active metabolite were not evaluated.
The results of our study suggest that although low-dose and short-term oral administration of dextromethorphan seems not to be neuroprotective, it does not worsen either patients’ condition or NIHSS score. In addition, patients treated with dextromethorphan showed significant reduction in seizures (complication after stroke), but increased chance of MI and renal failure by almost 5% when compared to the placebo-treated group.
The current study was the first clinical trial to evaluate the neuroprotective effect of dextromethorphan in stroke patients. Regarding the lack of clinical data about using dextromethorphan as a neuroprotective agent and the risk of possible side effects, we designed this study with a limited number of patients and short duration of the treatment. The results of the current study showed no significant improvement of the neurological deficit in the dextromethorphan-treated group as compared with the control group. Rankin and disability score and Barthel activities of daily living index also did not show any significant difference between groups. However, to better clarify the neuroprotective effect of this agent, more extensive studies with longer duration of the treatment and follow-up are recommended.
Figure 2.
Age distribution of patients in the 2 groups
References
- 1.Biller J, Love BB. Vascullar disease of nervouse system. In: Bradlly WG, editor. Neurology in clinical practice. Vol. 1197. Philadelphia: 2004. p. 1239. [Google Scholar]
- 2.Davis S, Lees K, Donnan G. Treating the acute stroke patient as an emergency: current practices and future opportunities. Int J Clin Prac. 2006;60:399–407. doi: 10.1111/j.1368-5031.2006.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakajima M, Doi H, Hirano T, Uchino M. Coronary flow velocity reserve in patients with ischemic stroke. Med Sci Monit. 2009;15:CR383–8. [PubMed] [Google Scholar]
- 4.Bern JL, Peck R. Dextromethorphan An overview of safety issues. Drug Saf. 1992;7:1909. doi: 10.2165/00002018-199207030-00004. [DOI] [PubMed] [Google Scholar]
- 5.Britton P, Lu XC, Laskosky MS, Tortella FC. Dextromethorphan protects against cerebral injury following transient, but not permantent focal ischemia in rats. Life Sci. 1997;60:1729–40. doi: 10.1016/s0024-3205(97)00132-x. [DOI] [PubMed] [Google Scholar]
- 6.Tortellan FC, et al. Dextromethrophan and neuromodulation: old drug coughs up new activities. Trends Phannacol Sci. 1989;10:5017. doi: 10.1016/0165-6147(89)90050-3. [DOI] [PubMed] [Google Scholar]
- 7.Swan JH, Evans MC, Meldrum BS. Long term development of selective neuronal loss and the mechanism of protection by 2amino7phosphonoheptanoatein rat model of incomplete forebrain ischemia. J Cereb Blood Flow Metab. 1988;8:64–78. doi: 10.1038/jcbfm.1988.9. [DOI] [PubMed] [Google Scholar]
- 8.Schmid Elsaesser R, Zausinger S, Hungerhuber E, Baethmann A, Reulen HJ. Monotherapy with dextromethorphan or tirilazed but not a combination of both improves out come after transient focal cerebral ischemia in rats. Exp Brain Res. 1998;122:12–7. doi: 10.1007/s002210050498. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg GK, Saleh J, Kunis D. Delayed treatment with dextromethorphan and dextrophan reduces cerebral damage after transient focal ischemia. Neurosci Lett. 1988;89:193–7. doi: 10.1016/0304-3940(88)90380-1. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg GK, Yoon EJ, Kunis DM, Sun GH, Maier CM, Gran GA. Neuroprotection by N-methyl D aspartate antagonist in focal cerebral ischemia dependent on continued maintenance dosing. Neuroscience. 1995;64:99–107. doi: 10.1016/0306-4522(94)00374-e. [DOI] [PubMed] [Google Scholar]
- 11.Steinberg GK, George CP, DelaPaz R, Shibate DK, Gross T. Dextromethorphan protects against cerebral injury following transient focal ischemia in rabbits. Stroke. 1988;19:111–28. doi: 10.1161/01.str.19.9.1112. [DOI] [PubMed] [Google Scholar]
- 12.Kristensen HT. Simultaneous determination of dextromethorphan and its metabolites in human plasma by capillary electrophoresis. J Pharm Biomed Anal. 1998;18:827–38. doi: 10.1016/s0731-7085(98)00273-8. [DOI] [PubMed] [Google Scholar]
- 13.Werling LL, Lauterbach EC, Calef U. Dextromethorphan as a potential neuroprotective agent with unique mechanisms of action. Neurologist. 2007;13:272–93. doi: 10.1097/NRL.0b013e3180f60bd8. [DOI] [PubMed] [Google Scholar]
- 14.Albers GW, Saens RE, Moses JA, Jr, Choi DW. Safety and tolerance of oral dextromethorphan in patients at risk for brain ischemia. Stroke. 1991;22:1075–7. doi: 10.1161/01.str.22.8.1075. [DOI] [PubMed] [Google Scholar]
- 15.Albers GW, Saens RE, Moses JA., Jr Tolerability of oral dextromethorphan in patients with a history of brain ischemia. Clin Neuropharmacol. 1992;15:509–14. doi: 10.1097/00002826-199212000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Albers GW, Atkinson RP, Kelley RE, Rosenbaum DM. Safety tolerability and pharmacokinetics of the N-methyl-D-aspartate antagonist dextrorphan in patients with acute stroke. Stroke. 1995;26:254–8. doi: 10.1161/01.str.26.2.254. [DOI] [PubMed] [Google Scholar]
- 17.Safety tolerability and pharmacokinetics of the N-methyl- D-aspartate antagonist Ro-01-6794 706 in patients with acute ischemic stroke. The Dextrorphan Study Group and HoffmannLa Roche. Ann N Y Acad Sci. 1995;765:249–61. [PubMed] [Google Scholar]
- 18.Steinberg GK, Saleh J, Kunis D, Delapaz R, Zarnegar SR. Protective effect of N-methyl D aspartate antagonists after focal cerebral ischemia in rahbits. Stroke. 1989;20:1247–52. doi: 10.1161/01.str.20.9.1247. [DOI] [PubMed] [Google Scholar]
- 19.Cheng YD, Al-Kjwury L, Zivin JA. Neuroprotection for ischemic stroke two decades of success and failure. NeuroRx. 2004;1:36–45. doi: 10.1602/neurorx.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albers GW, Goldberg MP, Choi DW. N-methyl D aspartate antagonists: ready for clinical trial in brain ischemia? Ann Neurol. 1989;25:398–403. doi: 10.1002/ana.410250412. [DOI] [PubMed] [Google Scholar]