Abstract
The pathways leading to autoimmunity remain enigmatic despite numerous lines of experimental inquiry and epidemiological evidence. The mechanisms leading to the initiation and perpetuation of specific diseases such as primary biliary cirrhosis (PBC) or multiple sclerosis (MS) remain largely enigmatic, although it is established that a combination of genetic predisposition and environmental stimulation is required. The growing number of genome-wide association studies and the largely incomplete concordance for autoimmune diseases in monozygotic twins concur to support the role of the environment (including infectious agents and chemicals) in the breakdown of tolerance leading to autoimmunity through different mechanisms. In the present article we illustrate the current hypotheses related to an environmental impact on the onset of PBC and MS as two representative conditions investigated with complementary approaches. Indeed, while a role of post-translational antigen modifications has been proposed for MS, this field remain unexplored in PBC where, conversely, most evidence is gathered from geoepidemiology and experimental data on xenobiotics or infectious agents.
Keywords: tolerance breakdown, multiple sclerosis, autoantibodies, antigen modification, glycosilation
Introduction
The field of autoimmunity currently recognizes a wide variety of conditions ranging from pure autoimmune to chronic inflammatory diseases and approximately 100 different entities [1] affecting up to 5% of the general population in Westernized countries [2]. These prevalence rates lead to significant economic costs for healthcare systems worldwide also based on the possible degrees of disability resulting from the multi-organ involvement and the high comorbidity rate [3-5].
Several common factors are shared by the pathogenetic mechanisms of different autoimmune disease and manifest as the coined “mosaic of autoimmunity” to support the multifactorial origin and clinical diversity [6-8]. Four groups include the major factors contributing to the mosaic: i.e. genetics, environment, immune defects, and hormones [9]. An ideal example to represent the former two groups is given by the disease concordance rates in monozygotic compared to dizygotic twins or other family members [10], as illustrated in Table I. Somehow surprisingly, autoimmune disease concordance rates in monozygotic twins are largely incomplete and are often based on small series, as in the case of primary biliary cirrhosis (PBC). Nevertheless, data support the hypothesis that genetics per se are insufficient to determine disease onset and that additional factors, likely related to the environment, may play a significant role [11]. The present article will discuss the current views in terms of gene-environment interplay based on two paradigmatic examples, i.e. PBC and multiple sclerosis (MS).
Table I.
Pairwise concordance rates of autoimmune disease in monozygotic and dizygotic twins (n of concordant sets/n of studied sets)
| Disease | MZ concordance rate | DZ concordance rate |
|---|---|---|
| Primary biliary cirrhosis | 0.63 | 0.00 |
| Primary sclerosing cholangitis | Concordant pair reported | – |
| Systemic lupus erithematosus | 0.24 | 0.02 |
| Sjögren’s syndrome | Concordant pair reported | – |
| Type I diabetes mellitus | 0.21-0.70* | 0.00-0.13 |
| Rheumatoid arthritis | 12.3-15.4 | 3.50-3.60 |
| Graves’ disease | 0.17-0.29 | 0.00-0.02 |
| Multiple sclerosis | 0.25-0.31* | 0.03-4.7 |
| Celiac disease | 0.75-0.83 | 0.11 |
Following a minimum of 7.5 years of observation
Primary biliary cirrhosis is a chronic cholestatic liver disease characterized by the immune-mediated destruction of intrahepatic bile ducts resulting in chronic liver injury and ultimately fibrosis, cirrhosis and liver failure [12]. The disease recognizes an autoimmune pathogenesis as well represented by the specific presence of serum antimitochondrial antibodies (AMA) and autoreactive T cells [13]. On the other hand, MS is a chronic inflammatory neurodegenerative autoimmune disease affecting the central nervous system [14, 15]. Both conditions manifest a largely incomplete concordance among monozygotic twins, 63% in PBC [16] and 30% in MS [17] despite a significant genetic component [18-20]. One interesting feature characterizing both diseases, similar to autoimmunity per se [8], is related to the geographical pattern in prevalence and incidence which also support the environmental influence [4, 7, 21, 22]. These observations are now unified into the novel field of clinical geoepidemiology [6, 23].
Epidemiology and geoepidemiology
Thanks to the globalization and the consequently easy comparison of data between different geographical areas the discipline of geoepidemiology was developed in recent years [6] to represent the approach by which epidemiological data across different geographical regions and populations are compared [4, 8, 24]. It is then possible to identify genetic, environmental, and socioeconomical factors involved in the autoimmune disease development. On one side it is possible to determine the contribution of ethnogenetic factors in development of autoimmune diseases, analyzing prevalence among different racial groups sharing the same geographical locations. On the other hand, it allows to study the impact of nutrition, light exposure, infections, xenobiotics and other environmental factors. An example is given by the evidence that different diseases share similar geoepidemiological distributions, as in the cases of type 1 diabetes mellitus, multiple sclerosis, and inflammatory bowel disease. These three chronic inflammatory diseases share the highest prevalence rates in European Caucasian populations, particularly in Northern Europe and North America, originally settled by northern-European people [8] and this observation suggests common determinants and offers a cue for future studies, although we cannot overlook the possible role of disease awareness in different areas. Of note, a latitudinal gradient was observed for most autoimmune conditions [4], with highest prevalence in industrialized countries, especially Northern Europe and North America, suggesting a central role played by Westernized lifestyle and focusing the attention also on other factors such as sunlight (UV) exposure affecting vitamin D levels, nutritional habits, and xenobiotics (i.e. chemicals).
The environmental factors and their mechanisms in autoimmunity
The search for environmental factors triggering autoimmunity has led to numerous candidates via four possible mechanisms [25]. The first mechanism, molecular mimicry, is perhaps the most widely investigated mechanism by which infections may induce autoimmunity. It is characterized by the cross-reactivity between epitopes (i.e. proteins, carbohydrates, or DNA sequences) shared by the pathogen and the host, as well illustrated by rheumatic fever, a disease often associated with heart involvement and acute polyarthritis in a large proportion of cases following streptococcal infections. In this case streptococcal epitopes (i.e. N-acetyl-glucosamine and M protein) mimic cardiac myosin and α-helical proteins present in cardiac valves, resulting in cardiac damage [26]. Another classic example is Guillain-Barre` syndrome (GBS), an acute polyradiculoneuritis following various microbial infections or vaccinations. A particular attention was given to the association between GBS and Campylobacter jejuni. Antibodies cross-reacting with both C. jejuni and peripheral nerve gangliosides are detected in the sera of GBS patients [27] and recently a significant homology was found between lipo-oligosaccharides present in C. jejuni and in ganglioside GM1 [28]. Furthermore, antibodies directed against the CMV-derived protein UL94 have been found to cross-react with the cell surface tetraspanin transmembrane 4 superfamily member 7 (TM4SF7 or NAG-2) molecule inducing apoptosis of endothelial cells and activation of fibroblasts in patients with systemic sclerosis [29]. Second, a polyclonal activation of B-cell occurs when a lymphotrophic virus infects B cells leading to B-cell proliferation causing enhanced antibody production and the accumulation of circulating immune complexes that may cause damage to self tissues [30]. One evocative example is mixed cryoglobulinemia (MC) induced by hepatitis C virus (HCV). Mixed cryoglobulinemia is considered the result of such persistent polyclonal activation [31] eventually resulting in the appearance of a mixture of monoclonal and polyclonal autoantibodies that may result in autoimmunity [31]. The third mechanism is provided by epitope spreading occuring when, in an inflammatory state, there is strong local activation of antigen-presenting cells (APC). This may result in overprocessing and overpresentation of antigens, thus priming large numbers of T cells with broad specificities, possibly against self antigens [32], as recently described in translational settings [33, 34]. Fourth and last, the bystander activation occurs when pro-inflammatory cytokine production is enhanced after an infection. An inflammatory microenvironment is created, with cytokines such as tumor necrosis factor α (TNF-α), lymphotoxin, nitric oxide (NO), and others, leading to bystander killing of uninfected cells in the proximity. Ultimately, this manifests as bystander activation of autoreactive T cells that were already present within the self tissue but, due to their prior low number, were unable to cause an overt autoimmune disease [35, 36].
Microbial infections
The most widely studied environmental factors are represented by infectious agents through two pathways: (i) directly causing the breakdown of tolerance irrespective of the individual susceptibility; or (ii) triggering autoimmunity in genetically susceptible individuals. The issue is largely enigmatic as most recently supported by solid data on a hygiene hypothesis [37]. The former pattern is exemplified by rheumatic fever, in which antibodies against streptococcal antigens cross-react with a myocardial antigen causing heart injury [38] while the latter appears more likely in autoimmune diseases [39, 40] as exemplified by Epstein-Barr virus (EBV) infection in systemic lupus erythematosus (SLE) [41] but also in rheumatoid arthritis [42], multiple sclerosis [43], Sjögren’s syndrome [44], autoimmune thyroiditis [45], autoimmune hepatitis [46], and Kawasaki disease [47]. Besides EBV, also hepatitis B (HBV) and cytomegalovirus (CMV) infections have been associated with autoimmune diseases, as represented by the prevalence of serum autoantibodies in patients with chronic or acute infections. In particular, relevant titers of antimitochondrial antibody (AMA), liver kidney microsome (LKM), antinuclear antibodies (ANA), and smooth muscle antibodies (SMA) were observed in chronic HBV infected subjects [48-50]. A potential role for CMV in the development of SLE has been suggested [51], similar to inflammatory bowel disease [52], antiphospholipid syndrome [53] and diabetes mellitus [54, 55]. Whether these are stochastic associations or significant pathogenetic links remains to be elucidated.
Vaccines
One of the hottest debates in modern medicine stemmed from the reports of an association between vaccination and autoimmune diseases, even though vaccinations protect from infections and thus may have an indirectly protective role towards autoimmune diseases. This is well illustrated by the proposed association between autism and vaccines [56]. The most studied associations are GBS after 1976 swine influenza vaccine, immune thrombocytopenic purpura after measles, mumps and rubella vaccine, and myopericarditis after smallpox vaccination [57]. It has been reported an increased relative risk of developing autoimmune manifestations in adults receiving HBV vaccine. The autoimmune manifestations include SLE (OR = 9.1, p < 0.0001, 95% CI = 2.3-76), rheumatoid arthritis (OR = 18, p < 0.0001, 95% CI = 3.1-740), multiple sclerosis (OR = 5.2, p < 0.0003, 95% CI = 1.9-20), and thrombocytopenia (OR = 2.3, p < 0.04, 95% CI = 1.02-6.2) [58]. The mechanism underlying the proposed relationship between vaccination and autoimmune diseases is not clearly understood, although it is thought to be caused by both vaccine immunogenic content and adjuvants, which are used to increase the immune reaction [59]. Nevertheless, we submit that the data supporting a causative link remain circumstantial [60] and do not justify a blind refusal of vaccination.
Tobacco
Tobacco smoking has been demonstrated to alter cytokines release and to exacerbate autoimmunity modulating pro-inflammatory cytokines levels (TNF-α, IL-1, IL-6, IL-8, and GM-CSF) and to decrease anti-inflammatory cytokines levels (IL-10) [61-63]. The autoimmune diseases associated with smoking include rheumatoid arthritis, SLE, Graves’ hyperthyroidism, Crohn’s disease, Goodpasture syndrome, thromboangioitis obliterans, PBC, systemic sclerosis, MS, and fibromyalgia. Indeed, higher risk and worse prognosis have been reported in past and current smokers for these conditions while we should also note that in some cases tobacco was associated with a reduced risk of disease, i.e. ulcerative colitis and Behçet’s disease [9, 64, 65]. One significant link has been proposed for rheumatoid arthritis. Citrullination, also termed deimination, is a post-translational modification of arginine side chains catalyzed by peptidyl arginine deiminase enzymes with the potential to alter the structure, antigenicity, and function of proteins. In rheumatoid arthritis, antibodies to cyclic citrullinated peptides are used for diagnosis, though we argue that the identification of specific citrullinated antigens, as whole proteins, is awaited. Four citrullinated antigens, fibrinogen, vimentin, collagen type II, and α-enolase, have been identified among others. All four proteins are expressed in the joint connective tissue and there is evidence that antibodies to citrullinated fibrinogen and collagen type II mediate inflammation by the formation of immune complexes, both in humans and animal models. Antibodies to citrullinated proteins are associated with HLA ‘shared epitope’ alleles, and autoimmunity to at least one antigenic sequence, the CEP-1 peptide from citrullinated α-enolase (KIHACitEIFDSCitGNPTVE), demonstrates a specific association with HLA-DRB1*0401, HLA-DRB1*0404, 620W PTPN22, and smoking [66]. In a complementary fashion, a higher expression of peptidyl arginine deiminase and higher levels of citrullinated proteins have been observed in the bronchoalveolar lavage cells of smokers [67].
Nutrition
Numerous systematic studies have confirmed that nutritional deficiencies can alter the immune response [68]. In recent years we have witnessed important advances in “nutritional immunology” [69], as well represented by the relationship of vitamins and minerals with autoimmune conditions. Among these, vitamin D has been demonstrated to be a modulator of the innate and adaptive immunity [70] and low levels of vitamin D are associated with numerous autoimmune diseases such as multiple sclerosis, SLE, and psoriasis [71, 72]. Accordingly, there is evidence of the beneficial effects of vitamin D supplementation [73-75]. Other examples include the lower risk of developing rheumatoid arthritis with a higher weekly consumption of fish oil, rich in omega-3 polyunsaturated fatty acids [76], the increased iodine consumption as a trigger for autoimmune thyroiditis in genetically susceptible individuals [21], and effects of low doses of probiotics on disease activity in inflammatory bowel disease [77]. Most recently, an association between anti-gluten or anti-gliadin serum antibodies and autoimmune disease different from celiac disease, such as MS, was reported [78]. We foresee that clinical and epidemiological studies focused on the impact of dietary factors on the geographical gradient of autoimmunity may help focus on this promising field of research with potential improvement in patient management [68].
Ultraviolet light
Ultraviolet (UV) light represents an additional proposed environmental factor capable of producing direct negative effects in predisposed individuals. The skin is a barrier against external agents while hosting both innate immune and adaptive immune cells and specialized antigen presenting cells, i.e. Langerhans cell [79]. Of note, photosensitivity is the most common skin manifestation in SLE [80]. Cutaneous SLE lesions often arise in sun-exposed areas and it is well recognized that sun exposure may also exacerbate or induce systemic manifestations of this disease [81] via proposed mechanisms [79]. It has been demonstrated that human keratinocytes can bind autoantibodies on their surface following UV exposure and antibodies against La, Ro, Sm, RNP, lupus-specific antigens, are specifically associated to the membrane blebs of cells undergoing apoptosis after UV damage. The ability of UV light to induce keratinocyte apoptosis and autoantigen exposure suggests the role of UV in the lupus pathogenesis [82]. Conversely, low-dose UV light can also have immunosuppressive effects, as well represented by the beneficial effects in psoriasis. Finally, pre-vitamin D3 is produced from 7-dehydrocholesterol by effect of UV light [79].
Xenobiotics
Xenobiotics represent additional etiologic factors proposed for autoimmune diseases. Xenobiotics include substances of synthetic, natural, or biologic origin that may exertan immunosuppressive effect due to direct toxicity on the immune system cells, or an immunostimulant effect resulting in autoimmune manifestations, as well illustrated by the pseudo-autoimmune conditions triggered by exposure to chemicals. In 1981 the ingestion of contaminated rape oil seed resulted in the toxic oil syndrome with cases of SLE-like disease, scleroderma-like disease and a number of general clinical manifestations that persisted in 10% of the subjects [83]. In the late 1980s in the United States, the eosinophilic myalgia syndrome was reported following exposure to L-tryptophan [84]. Since these earlier reports our knowledge of the links between chemicals and autoimmune conditions has considerably grown. As of now silica exposure is considered a trigger for SLE [85] and scleroderma [86] while organic solvents such as vinyl chloride and epoxy resins may also be associated with scleroderma-like manifestations [87]. Several other associations have been proposed through three mechanisms [88-90]. First, a potential direct toxic effect of xenobiotics may cause cell death by apoptosis or oncosis, inducing the generation of immunogenic autoepitopes. Second, chemical modifications of native cellular proteins by removal and/or exchange of a hapten has been shown to change processing in antigen-presenting cells and may lead to the presentation of cryptic, potentially immunogenic peptides. Third, xenobiotics may have the potential to modify host proteins to form neoantigens. Neoantigen specific T cells and B cells, once primed, may cross-react with the formerly inert native autoantigens.
The case of primary biliary cirrhosis
As previously mentioned PBC is a slowly progressive autoimmune disease in which the loss of bile ducts leads to decreased bile secretion and the retention of toxic substances within the liver, resulting in further hepatic damage, fibrosis, cirrhosis, and eventually, liver failure. It primarily affects women. Commonly it develops mainly in the fifth decade of life and it is uncommon in persons under 25 years of age [91].
Geoepidemiology
Although PBC is considered a rare disease, recent data suggest an increasing trend in its incidence and prevalence, but several factors that make difficult to study PBC epidemiology have to be considered. First of all, thus far there are no solid population-based studies on PBC, due to the rarity of this condition, and most of the data we have are descriptive data. Then the accuracy of diagnosis is difficult to obtain, since it does not exist a perfect diagnostic marker. Finally, disease awareness in healthcare providers is raising and may reflect the increase in prevalence and incidence of PBC cases, reported in several studies [92]. Based on the epidemiological data on PBC, it was observed that there are areas presenting a higher prevalence of PBC cases. Reported prevalence and incidence rates of PBC cases are highest in the United Kingdom [93, 94], the scandinavian region [95], and the northern United States (Table II) [96]. In this case it is difficult to determine whether this data are due to a true higher prevalence of cases, possibly influenced by environmental factors, or to a higher number of epidemiological studies lead in these areas. Instead, a lower prevalence and incidence have been reported in other countries, such as Canada, Australia, and the Mediterranean countries [97-104]. An interesting population-based study lead in Australia showed a significant higher PBC prevalence in Italian, British, and Greek immigrants, compared to native population [105], an evidence that may underline the genetic component on the etiology of PBC or specific environmental priming occurring early in life. Finally data on PBC epidemiology are not available in large parts of the world such as Africa or Asia.
Table II.
Distribution of prevalence, incidence and sex ratio of primary biliary chirrosis in different countries [7]
| Geographical area | Year | Number of cases | Incidence (per million) | Prevalence (per million) | Gender (M : F) |
|---|---|---|---|---|---|
| Europe | 1984 | 569 | 4 | 23 | 1 : 10 |
| Sweden | 1985 | 111 | 13,3 | 151 | 1 : 6 |
| Newcastle, UK | 1989 | 347 | 19 | 154 | 1 : 9 |
| Canada | 1990 | 225 | 3.26 | 22 | 1 : 13 |
| Victoria, Australia | 1995 | 84 | – | 19 | 1 : 11 |
| Estonia | 1995 | 69 | 2.27 | 26.9 | 1 : 22 |
| Norway | 1998 | 21 | 16 | 146 | 1 : 9 |
| USA | 2000 | 46 | 27 | 402 | 1 : 8 |
| Newcastle, UK | 2001 | 770 | 31 | 251 | 1 : 10 |
| Victoria, Australia | 2004 | 249 | – | 51 | 1 : 10 |
| Japan | 2005 | 9761 | – | 78 | 1 : 9 |
| Canada | 2009 | 227 | 30 | 227 | 1 : 9 |
Environmental factors
Based on earlier data on PBC autoantibody reactivity with xenobiotic-modified autoantigens [106], Amano et al. [107] studied a large number of xenobiotics with a structure similar to the lipoic acid residue on the epitope of PDC-E2, the E2 subunit of the mitochondrial pyruvate dehydrogenase complex, that is the main autoreactive antigen identified so far in PBC [108]. It was observed that replacement of lipoic acid by certain xenobiotics enhanced PBC sera reactivity against the PDC-E2 epitope. Particularly, one of the xenobiotics, 2-nonynoic acid, induced reactivity of PBC sera stronger than that of the native lipoic acid residue. Interestingly, the methyl ester of 2-nonynoic acid has a viol-/peach-like scent and is used as an ingredient in perfumes. But this is only one of the several example, the number of exogenous compounds potentially leading to modifications is enormous, from food preservatives to home detergents to water pollutants [106] and new mechanisms for haptenization have been recently described [109]. In addition one such halogenated compound has been shown to induce AMA production in rabbits without requiring the peptide backbone of PDC-E2 [110] but failed to produce liver lesions. It could reflect the evidence that in humans AMA titer is detectable several years before the appearance of liver injury. Finally, xenobiotics are able to induce epigenetic modifications, as DNA methylation and histone modifications, that can lead to gene expression alteration and then phenotypic changes. These processes may play a fundamental role in the onset of PBC [111].
Several lines of evidence support the hypothesis that also some infectious agents are able to be a trigger in PBC development. In particular it was suggested by the observation of a significantly higher rate of urinary tract infections sustained by Escherichia coli among PBC patients and the presence of bacterial products in mononuclear cells surrounding damaged bile ducts [112]. In addition it has been demonstrated that a history of urinary tract infections increases the risk of having PBC [113]. The proposed mechanism to explain this hypothesis is molecular mimicry, discussed above. This theory is mostly based on experiments that demonstrated a cross reactivity between AMA and/or autoreactive T cells with prokaryotic antigens of several microbes, including E. coli [114] which share an ExDK amino acid motif within their mimicry epitopes.
More recently, Novosphingobium aromaticivorans, an aerobic, free-living Gram-negative bacterium has been investigated in PBC [115] as supported by several observations. First, the bacterium is known to be ubiquitous in the environment (soil, water), non-pathogenic to humans and demonstrates the ability to modulate activation of estrogens [116], which could be linked to the well-documented female predominance of PBC. Then it was observed in 100% of anti-PDC-E2 positive and in a fraction of AMA-negative sera a serum reactivity against two lipoylated proteins of 47 and 50 KDa from N. aromaticivorans [117]. Titers of antibodies detected against lipoylated bacterial proteins of N. aromaticivoranswere 1000-fold higher compared to those against E. coli in PBC patients[117]. Lactobacilli and Chlamydia, which show some structural homology with the autoantigen (although reactivity against them is considerably less than that against either E. colior N. aromaticivorans), have also been implicated as putative pathogens, as well as Helicobacter pylori and Mycobacterium gordonae [118, 119]. However, no compelling data have been provided to show that one individual infectious agent can reproducibly be detected in patients with PBC. In conclusion our current knowledge of the environmental triggers of PBC includes numerous suspected agents involved in PBC development, but no specific agents have been identified as able to be causative of disease.
The case of multiple sclerosis
Multiple sclerosis is an inflammatory, demyelinating disease of the central nervous system (CNS). The disease is highly heterogeneous particularly in its clinical subforms, with an extremely variable evolution over time. Temporal profile and combination of neurological findings, appearing as relapses and remissions, or gradually progressive dysfunctions, led to classifying MS in clinical subforms coined relapsing-remitting MS (RRMS), secondary progressive MS (SPMS), primary progressive MS (PPMS), and progressive relapsing MS.
Geoepidemiology
The total number of people affected by MS worldwide is estimated to be 2-2.5 million, but the distribution of the disease changes significantly in different part of the world. Its prevalence varies between less than 5 cases per 100,000 people in tropical areas or Asia and more than 100-200 cases per 100,000 in temperate areas such as the United States, Canada, New Zealand and parts of Australia [120, 121]. Furthermore it exists a well-accepted latitudinal gradient for MS [122]. Highest rates are recorded in Northern Europe, the British Isles and Scandinavia, and the areas originally settled from these locations, which are North America, Australia and New Zealand [8, 120]. Interestingly it was observed a lower risk to develop MS in adult migrants from tropical countries to the UK than local population, although these differences were less evident in following generations [123, 124] thus confirming also the familial risk [19].
There are also important differences in MS prevalence in different races. Caucasians seems to be more affected by MS than blacks and Asian populations, whereas Australian Aboriginals, New Zealand Maori and North and South-American Indians have a very low prevalence [121, 125]. After a large study conducted by WHO it was recently proposed a socioeconomic gradient, since it has been observed a higher MS prevalence in Western Countries, even though it may reflect the availability of more precise but expensive diagnostic instruments [126].
Disease pathogenesis and the role of post-translational modifications in creating neo-antigens
Multiple sclerosis pathogenesis remains unclear, but growing evidence suggests that the disease could be due to a consequence of multiple genetic, immunological, and environmental factors [18, 20, 127].
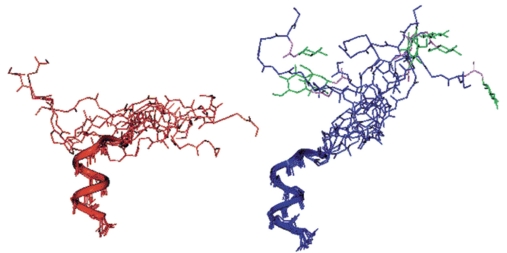
Different hypotheses have been formulated to explain MS pathology putting into evidence the heterogeneous and homogeneous features of MS [128-130]. However, data support the hypothesis proposed by Lucchinetti et al. [128] of a heterogeneous mechanism of demyelination in which the following four MS pathological patterns can be identified: (I) T-cell/macrophage-mediated demyelination, (II) antibody/complement-associated demyelination, (III) distal oligodendrogliopathy, and (IV) oligodendrocyte degeneration in the periplaque white matter. Nevertheless, the difficulty in the comprehension of MS pathology can be connected to the presence of post-translational modifications (PTM) that increase the complexity of myelin proteins. It is of note that if there are 22 proteinogenic amino acids (20 of which directly encoded by the universal genetic code), once considering PTMs the possible combinations will become over 400. Moreover, growing evidence indicates that PTMs (i.e., acetylation, lipidation, citrullination, glycosylation, etc.), either native or aberrant, may play a fundamental role for specific autoantibody recognition in autoimmune diseases [131].The role of PTMs in MS has not been fully understood; in fact, it is currently a matter of debate whether PTMs can have an effective role in MS pathology and if they can be related to an autoimmune response [131-133] or a neurodegenerative process [134, 135].These observations account, at least in part, for the limited results in the discovery of biomarkers using proteomic analysis and/or protein microarrays. A number of autoimmune diseases have been associated with PTMs, demonstrating that some modifications can generate new self-antigens or even mask antigens normally recognized by the immune system in physiological conditions [131, 136]. It is well accepted that a number of these chemical modifications introduced in proteins, during or after their synthesis, could influence the central and peripheral tolerance, and subsequent inducing autoimmune responses to otherwise ignored self-protein [131]. On the other hand, the neurodegenerative hypothesis is based on metabolic changes in myelin constituents that altering the PTMs destabilize the membrane structure leading to myelin degradation [135]. Once myelin is modified by PTMs some antigenic fragments could be exposed to the autoimmune response. Numerous studies indicate that PTMs, either native or aberrant, may play a fundamental role in generating neo-epitopes and thus triggering an autoimmune response, particularly in MS. For example, rheumatoid arthritis recognizes anti-citrullinated peptide/protein autoantibodies in sera of patients because of an aberrant deimination [137]. In MS, the role of different PTMs in triggering an autoimmune response have been investigated considering the possible mechanisms that could pathologically alter the PTM profile (e.g. environmental factors and oxidative stress, pathogens and molecular mimicry). It was previously reported that Pb metal can enhance proteins immunogenicity [138] while lead exposure (PbOAc2) can induce autoantibodies to nervous system proteins, including MBP [139, 140]. MBP represents 20-30% of total proteins of myelin sheath and is important for myelin structure [141]. The putative antigen MBP is widely studied in MS because it acts as an inter-membrane adhesion protein enabling myelin structure stability thanks also to its dynamic pattern of PTMs. MBP binds to the negatively charged lipids in myelin sheath and is characterized by different PTMs that differ in their extent in physiological vs. pathological conditions [142]. Mice immunized with Pb-altered MBP developed elevated anti-MBP antibody titer [143] but a possible correlation with MS disease activity is far from convincing. Nitric oxide (NO) is involved in axon and oligodendrocyte degeneration in MS lesions [144, 145] and increased levels of nitrite have been detected in cerebrospinal fluid of MS patients and correlate with clinical activity [146, 147]. Boullerne et al. reported an antibody response against the uncommon nitrosylated epitope: SNO-cysteine-BSA, in which BSA was used as a carrier to expose the modified cysteine [148]. Anti-SNO-cysteine-BSA IgM antibodies when compared with healthy controls were detected in: 8/8 (100%) of RRMS patients in relapse, 6/18 (33%) of clinically stable RRMS patients, and 16/23 (70%) SPSM patients. Further, the anti-SNO-cysteine-BSA antibody titer correlates with disease activity and increases during clinical activity in MS and was detected also in sera from Lewis rats with actively induced MS-like murine disease and it was shown that anti-SNO-cysteine-BSA antibodies precede anti-MBP antibodies. The specificity of IgM binding to SNO-cysteine-BSA was demonstrated by inhibition experiments at different concentrations. Another PTM widely studied in MS is glycosylation as it can affect the immune response and immune tolerance [131]. Recently, IgM autoantibodies to recombinant MOG, a putative self-antigen component of normal myelin of the CNS, have been reported to be predictors of clinically definite MS after a first demyelinating event [149, 150]. However, these results were not confirmed by other studies probably because differently folded and modified forms of MOG have been used (Table III) [151, 152]. In fact a recently report demonstrated for the first time that a solid-phase ELISA based on a conformationally controlled rMOGED(His)6 on MS sera and controls failed to detect IgM or IgG antibodies. Interestingly the rMOGED(His)6 used was expressed in E. coli after subcloning the cDNA of the extracellular domain of rat MOG, performing a refolding procedure on column and affinity purification. The far-UV Circular Dichroism (CD) spectra of rMOGED(His)6 manifested a β-sheet, a characteristic feature of the Ig-fold [153]. MOG is a myelin protein presenting a unique N-glycosylation site at position 31 (Asn31) and it was previously reported that N-glucosylation of a MOG peptide epitope improved the detection of specific antibodies in MS sera [154]. Moreover, structure-activity relationship studies demonstrated that the two MOG derived peptides hMOG(30-50) and the glycosylated analog [Asn31(N-β-Glc)]hMOG(30-50) adopt similar conformations in the environment used for NMR investigations (water/HFA solution). Thus, the specific antibody-recognition of [Asn31(N-β-Glc)]hMOG(30-50) is driven by direct interactions of the antibody binding site with the N-linked glucose (Figure 1) [155]. Therefore, specifically glucosylated synthetic peptides, as MS antigenic probes, have been demonstrated to be powerful tools for increasing autoantibodies recognition in MS patients sera, with higher affinity and specificity compared to recombinant MOG.
Table III.
Different types of MOG used as antigens for the detection of autoantibodies in multiple sclerosis
| Authors | MOG type |
|---|---|
| Berger et al. [149] | hMOGED – extracellular, unfolded, and unglycosylated hMOG(28-130) |
| Gaertner et al. [150] | mMOG – full length native, folded, and randomly glycosylated mMOG(1-247) |
| Mantegazza et al. [151] | hMOGED – extracellular, unfolded, and unglycosylated hMOG(29-149) |
| Lampasona et al. [152] | hMOG – full length and unglycosylated hMOG(1-241) |
| Gori et al. [153] | rMOGED – extracellular, folded, and unglycosylated rMOG(1-125) |
Figure 1.
Superposition of the 10 optimized structures of hMOG(30-50) (left) and [Asn31(N-β-Glc)]hMOG(30-50) (right) with the C-terminal part at the bottom showing that the two peptides adopt similar conformations in water/HFA solution. Autoantibodies detected in a relevant number of MS sera by the MOG glycopeptide and not by the unglycosylated sequence are directed against the N-linked glucose [155]. Copyright © 2001, American Chemical Society
Final thoughts on the cases of primary biliary cirrhosis and multiple sclerosis
The etiology of autoimmune diseases remains a complex issue that encompasses epidemiology, clinics, genetics, and experimental data. The genomic predisposition remains preponderant but insufficient to explain the different geographic distribution of these diseases or the incomplete concordance in MZ twins. Environmental factors are thus ideal candidates to fill this gap but the mechanisms remain to be elucidated. Our current knowledge of the environmental triggers of PBC is limited to numerous suspected agents involved in its development, such as xenobiotics or infectious agents (e.g. E. coli, N. aromaticivorans), but no specific agents have been identified as able to be causative of disease and data obtained in animal models await confirmation. The case of MS, on the other hand, illustrates a different approach, mostly based on the PTMs in creating neo-antigens, but due to the heterogeneity of MS, a clear and rational association of PTMs with the activity of MS has not been yet fully elucidated. Geoepidemiology may provide an important contribution in researching (looking for) potential environmental factors involved in autoimmune diseases. Nevertheless, it remains uncertain whether the latitudinal gradient observed for most autoimmune conditions reflects a real different distribution of the disease, possibly influenced by environmental factors, or it is due to a greater number of epidemiological studies lead in certain regions or to the availability of more precise but expensive diagnostic instruments.
References
- 1.Shoenfeld Y, Selmi C, Zimlichman E, Gershwin ME. The autoimmunologist: geoepidemiology, a new center of gravity, and prime time for autoimmunity. J Autoimmun. 2008;31:325–30. doi: 10.1016/j.jaut.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Cooper GS, Bynum ML, Somer EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. 2009;33:197–207. doi: 10.1016/j.jaut.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eaton WW, Rose NR, Kalaydjian A, Pedersen MG, Mortensen PB. Epidemiology of autoimmune diseases in denmark. J Autoimmun. 2007;29:1–9. doi: 10.1016/j.jaut.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandran V, Raychaudhuri SP. Geoepidemiology and environmental factors of psoriasis and psoriatic arthritis. J Autoimmun. 2010;34:J314–21. doi: 10.1016/j.jaut.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Zeki AA, Schivo M, Chan AL, et al. Geoepidemiology of copd and idiopathic pulmonary fibrosis. J Autoimmun. 2010;34:J327–38. doi: 10.1016/j.jaut.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Youinou P, Pers JO, Gershwin ME, Shoenfeld Y. Geo-epidemiology and autoimmunity. J Autoimmun. 2010;34:J163–7. doi: 10.1016/j.jaut.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Invernizzi P. Geoepidemiology of autoimmune liver diseases. J Autoimmun. 2010;34:J300–6. doi: 10.1016/j.jaut.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 8.Shapira Y, Agmon-Levin N, Shoenfeld Y. Defining and analyzing geoepidemiology and human autoimmunity. J Autoimmun. 2010;34:J168–77. doi: 10.1016/j.jaut.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 9.Shoenfeld Y, Zandman-Goddard G, Stojanovich L, et al. The mosaic of autoimmunity: hormonal and environmental factors involved in autoimmune diseases - 2008. Isr Med Assoc J. 2008;10:8–12. [PubMed] [Google Scholar]
- 10.Wandstrat A, Wakeland E. The genetics of complex autoimmune diseases: non-mhc susceptibility genes. Nat Immunol. 2001;2:802–9. doi: 10.1038/ni0901-802. [DOI] [PubMed] [Google Scholar]
- 11.Alarcon-Riquelme ME. Recent advances in the genetics of autoimmune diseases. Ann N Y Acad Sci. 2007;1110:1–9. doi: 10.1196/annals.1423.001. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261–73. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 13.Hohenester S, Oude-Elferink RP, Beuers U. Primary biliary cirrhosis. Semin Immunopathol. 2009;31:283–307. doi: 10.1007/s00281-009-0164-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapira E, Brodsky B, Proscura E, Nyska A, Erlanger-Rosengarten A, Wormser U. Amelioration of experimental autoimmune encephalitis by novel peptides: involvement of t regulatory cells. J Autoimmun. 2010;35:98–106. doi: 10.1016/j.jaut.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 15.Wu GF, Shindler KS, Allenspach EJ, et al. Limited sufficiency of antigen presentation by dendritic cells in models of central nervous system autoimmunity. J Autoimmun. 2011;36:56–64. doi: 10.1016/j.jaut.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selmi C, Mayo MJ, Bach N, et al. Primary biliary cirrhosis in monozygotic and dizygotic twins: genetics, epigenetics, and environment. Gastroenterology. 2004;127:485–92. doi: 10.1053/j.gastro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Handunnetthi L, Handel AE, Ramagopalan SV. Contribution of genetic, epigenetic and transcriptomic differences to twin discordance in multiple sclerosis. Expert Rev Neurother. 2010;10:1379–81. doi: 10.1586/ern.10.116. [DOI] [PubMed] [Google Scholar]
- 18.Akkad DA, Hoffjan S, Petrasch-Parwez E, Beygo J, Gold R, Epplen JT. Variation in the il7ra and il2ra genes in german multiple sclerosis patients. J Autoimmun. 2009;32:110–5. doi: 10.1016/j.jaut.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Arora-Singh RK, Assassi S, del Junco DJ, et al. Autoimmune diseases and autoantibodies in the first degree relatives of patients with systemic sclerosis. J Autoimmun. 2010;35:52–7. doi: 10.1016/j.jaut.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gourh P, Agarwal SK, Martin E, et al. Association of the c8orf13-blk region with systemic sclerosis in north-american and european populations. J Autoimmun. 2010;34:155–62. doi: 10.1016/j.jaut.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burek CL, Talor MV. Environmental triggers of autoimmune thyroiditis. J Autoimmun. 2009;33:183–9. doi: 10.1016/j.jaut.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tobon GJ, Youinou P, Saraux A. The environment, geoepidemiology, and autoimmune disease: rheumatoid arthritis. J Autoimmun. 2010;35:10–4. doi: 10.1016/j.jaut.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Cooper GS, Bynum ML, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. 2009;33:197–207. doi: 10.1016/j.jaut.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hemminki K, Li X, Sundquist J, Sundquist K. The epidemiology of graves' disease: evidence of a genetic and an environmental contribution. J Autoimmun. 2010;34:J307–13. doi: 10.1016/j.jaut.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 25.Christen , Hintermann E, Holdener M, von Herrath MG. Viral triggers for autoimmunity: is the 'glass of molecular mimicry' half full or half empty? J Autoimmun. 2010;34:38–44. doi: 10.1016/j.jaut.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guilherme L, Kalil J, Cunningham M. Molecular mimicry in the autoimmune pathogenesis of rheumatic heart disease. Autoimmunity. 2006;39:31–9. doi: 10.1080/08916930500484674. [DOI] [PubMed] [Google Scholar]
- 27.Neisser A, Schwerer B, Bernheimer H, Moran AP. Ganglioside-induced antiganglioside antibodies from a neuropathy patient cross-react with lipopolysaccharides of campylobacter jejuni associated with guillain-barre syndrome. J Neuroimmunol. 2000;102:85–8. doi: 10.1016/s0165-5728(99)00159-9. [DOI] [PubMed] [Google Scholar]
- 28.Yuki N, Susuki K, Koga M, et al. Carbohydrate mimicry between human ganglioside gm1 and campylobacter jejuni lipooligosaccharide causes guillain-barre syndrome. Proc Natl Acad Sci U S A. 2004;101:11404–9. doi: 10.1073/pnas.0402391101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lunardi C, Bason C, Navone R, et al. Systemic sclerosis immunoglobulin g autoantibodies bind the human cytomegalovirus late protein ul94 and induce apoptosis in human endothelial cells. Nat Med. 2000;6:1183–6. doi: 10.1038/80533. [DOI] [PubMed] [Google Scholar]
- 30.Ferri C, Zignego AL. Relation between infection and autoimmunity in mixed cryoglobulinemia. Curr Opin Rheumatol. 2000;12:53–60. doi: 10.1097/00002281-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Agmon-Levin N, Ram M, Barzilai O, et al. Prevalence of hepatitis c serum antibody in autoimmune diseases. J Autoimmun. 2009;32:261–6. doi: 10.1016/j.jaut.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Lehmann PV, Forsthuber T, Miller A, Sercarz EE. Spreading of t-cell autoimmunity to cryptic determinants of an autoantigen. Nature. 1992;358:155–7. doi: 10.1038/358155a0. [DOI] [PubMed] [Google Scholar]
- 33.Chamberlain JL, Pittock SJ, Oprescu AM, et al. Peripherinigg association with neurologic and endocrine autoimmunity. J Autoimmun. 2010;34:469–77. doi: 10.1016/j.jaut.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Routsias JG, Tzioufas AG. B-cell epitopes of the intracellular autoantigens ro/ssa and la/ssb: tools to study the regulation of the autoimmune response. J Autoimmun. 2010;35:256–64. doi: 10.1016/j.jaut.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Murali-Krishna K, Altman JD, Suresh M, et al. Counting antigen-specific cd8 t cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–87. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 36.Sellner J, Hemmer B, Muhlau M. The clinical spectrum and immunobiology of parainfectious neuromyelitis optica (devic) syndromes. J Autoimmun. 2010;34:371–9. doi: 10.1016/j.jaut.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 37.Ege MJ, Mayer M, Normand AC, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364:701–9. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 38.Malkiel S, Liao L, Cunningham MW, Diamond B. T-cell-dependent antibody response to the dominant epitope of streptococcal polysaccharide, n-acetyl-glucosamine, is cross-reactive with cardiac myosin. Infect Immun. 2000;68:5803–8. doi: 10.1128/iai.68.10.5803-5808.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tomer Y. Hepatitis c and interferon induced thyroiditis. J Autoimmun. 2010;34:J322–6. doi: 10.1016/j.jaut.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pratesi F, Tommasi C, Anzilotti C, et al. Antibodies to a new viral citrullinated peptide, vcp2: fine specificity and correlation with anti-cyclic citrullinated peptide (ccp) and anti-vcp1 antibodies. Clin Exp Immunol. 2011;164:337–45. doi: 10.1111/j.1365-2249.2011.04378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barzilai O, Ram M, Shoenfeld Y. Viral infection can induce the production of autoantibodies. Curr Opin Rheumatol. 2007;19:636–43. doi: 10.1097/BOR.0b013e3282f0ad25. [DOI] [PubMed] [Google Scholar]
- 42.Balandraud N, Roudier J, Roudier C. Epstein-barr virus and rheumatoid arthritis. Autoimmun Rev. 2004;3:362–7. doi: 10.1016/j.autrev.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen TR, Pedersen M, Rostgaard K, Frisch M, Hjalgrim H. Correlations between epstein-barr virus antibody levels and risk factors for multiple sclerosis in healthy individuals. Mult Scler. 2007;13:420–3. doi: 10.1177/1352458506071470. [DOI] [PubMed] [Google Scholar]
- 44.Padalko EY, Bossuyt X. Anti-dsdna antibodies associated with acute ebv infection in sjogren's syndrome. Ann Rheum Dis. 2001;60:992. doi: 10.1136/ard.60.10.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vrbikova J, Janatkova I, Zamrazil V, Tomiska F, Fucikova T. Epstein-barr virus serology in patients with autoimmune thyroiditis. Exp Clin Endocrinol Diabetes. 1996;104:89–92. doi: 10.1055/s-0029-1211428. [DOI] [PubMed] [Google Scholar]
- 46.Vento S, Guella L, Mirandola F, et al. Epstein-barr virus as a trigger for autoimmune hepatitis in susceptible individuals. Lancet. 1995;346:608–9. doi: 10.1016/s0140-6736(95)91438-2. [DOI] [PubMed] [Google Scholar]
- 47.Lee SJ, Lee KY, Han JW, Lee JS, Whang KT. Epstein-barr virus antibodies in kawasaki disease. Yonsei Med J. 2006;47:475–9. doi: 10.3349/ymj.2006.47.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bogdanos DP, Mieli-Vergani G, Vergani D. Virus, liver and autoimmunity. Dig Liver Dis. 2000;32:440–6. doi: 10.1016/s1590-8658(00)80266-2. [DOI] [PubMed] [Google Scholar]
- 49.Hansen KE, Arnason J, Bridges AJ. Autoantibodies and common viral illnesses. Semin Arthritis Rheum. 1998;27:263–71. doi: 10.1016/s0049-0172(98)80047-4. [DOI] [PubMed] [Google Scholar]
- 50.Kansu A, Kuloglu Z, Demirceken F, Girgin N. Autoantibodies in children with chronic hepatitis b infection and the influence of interferon alpha. Turk J Gastroenterol. 2004;15:213–8. [PubMed] [Google Scholar]
- 51.Su BY, Su CY, Yu SF, Chen CJ. Incidental discovery of high systemic lupus erythematosus disease activity associated with cytomegalovirus viral activity. Med Microbiol Immunol. 2007;196:165–70. doi: 10.1007/s00430-007-0040-7. [DOI] [PubMed] [Google Scholar]
- 52.Criscuoli V, Rizzuto MR, Cottone M. Cytomegalovirus and inflammatory bowel disease: is there a link? World J Gastroenterol. 2006;12:4813–8. doi: 10.3748/wjg.v12.i30.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blank M, Asherson RA, Cervera R, Shoenfeld Y. Antiphospholipid syndrome infectious origin. J Clin Immunol. 2004;24:12–23. doi: 10.1023/B:JOCI.0000018058.28764.ce. [DOI] [PubMed] [Google Scholar]
- 54.Filippi C, von Herrath M. How viral infections affect the autoimmune process leading to type 1 diabetes. Cell Immunol. 2005;233:125–32. doi: 10.1016/j.cellimm.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 55.Roberts BW, Cech I. Association of type 2 diabetes mellitus and seroprevalence for cytomegalovirus. South Med J. 2005;98:686–92. doi: 10.1097/01.SMJ.0000163310.12516.2D. [DOI] [PubMed] [Google Scholar]
- 56.Altmann D. Autism and measles, mumps, and rubella vaccine. Lancet. 2000;355:409–10. doi: 10.1016/S0140-6736(05)74033-2. [DOI] [PubMed] [Google Scholar]
- 57.Salemi S, D'Amelio R. Could autoimmunity be induced by vaccination? Int Rev Immunol. 2010;29:247–69. doi: 10.3109/08830181003746304. [DOI] [PubMed] [Google Scholar]
- 58.Geier DA, Geier MR. A case-control study of serious autoimmune adverse events following hepatitis b immunization. Autoimmunity. 2005;38:295–301. doi: 10.1080/08916930500144484. [DOI] [PubMed] [Google Scholar]
- 59.Cohen AD, Shoenfeld Y. Vaccine-induced autoimmunity. J Autoimmun. 1996;9:699–703. doi: 10.1006/jaut.1996.0091. [DOI] [PubMed] [Google Scholar]
- 60.Belloni C, Avanzini MA, De Silvestri A, et al. No evidence of autoimmunity in 6-year-old children immunized at birth with recombinant hepatitis b vaccine. Pediatrics. 2002;110:e4. doi: 10.1542/peds.110.1.e4. [DOI] [PubMed] [Google Scholar]
- 61.Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM. Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol. 2002;89:1117–9. doi: 10.1016/s0002-9149(02)02284-1. [DOI] [PubMed] [Google Scholar]
- 62.Glossop JR, Dawes PT, Mattey DL. Association between cigarette smoking and release of tumour necrosis factor alpha and its soluble receptors by peripheral blood mononuclear cells in patients with rheumatoid arthritis. Rheumatology (Oxford) 2006;45:1223–9. doi: 10.1093/rheumatology/kel094. [DOI] [PubMed] [Google Scholar]
- 63.Hagiwara E, Takahashi KI, Okubo T, et al. Cigarette smoking depletes cells spontaneously secreting th(1) cytokines in the human airway. Cytokine. 2001;14:121–6. doi: 10.1006/cyto.2001.0860. [DOI] [PubMed] [Google Scholar]
- 64.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34:J258–65. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 65.Harel-Meir M, Sherer Y, Shoenfeld Y. Tobacco smoking and autoimmune rheumatic diseases. Nat Clin Pract Rheumatol. 2007;3:707–15. doi: 10.1038/ncprheum0655. [DOI] [PubMed] [Google Scholar]
- 66.Wegner N, Lundberg K, Kinloch A, et al. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol Rev. 2010;233:34–54. doi: 10.1111/j.0105-2896.2009.00850.x. [DOI] [PubMed] [Google Scholar]
- 67.Makrygiannakis D, Hermansson M, Ulfgren AK, et al. Smoking increases peptidylarginine deiminase 2 enzyme expression in human lungs and increases citrullination in bal cells. Ann Rheum Dis. 2008;67:1488–92. doi: 10.1136/ard.2007.075192. [DOI] [PubMed] [Google Scholar]
- 68.Selmi C, Tsuneyama K. Nutrition, geoepidemiology, and autoimmunity. Autoimmun Rev. 2010;9:A267–70. doi: 10.1016/j.autrev.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 69.Fernandes G. Progress in nutritional immunology. Immunol Res. 2008;40:244–61. doi: 10.1007/s12026-007-0021-3. [DOI] [PubMed] [Google Scholar]
- 70.Shapira Y, Agmon-Levin N, Shoenfeld Y. Mycobacterium tuberculosis, autoimmunity, and vitamin d. Clin Rev Allergy Immunol. 2010;38:169–77. doi: 10.1007/s12016-009-8150-1. [DOI] [PubMed] [Google Scholar]
- 71.Carvalho JF, Blank M, Kiss E, Tarr T, Amital H, Shoenfeld Y. Anti-vitamin d, vitamin d in sle: preliminary results. Ann N Y Acad Sci. 2007;1109:550–7. doi: 10.1196/annals.1398.061. [DOI] [PubMed] [Google Scholar]
- 72.Orbach H, Zandman-Goddard G, Amital H, et al. Novel biomarkers in autoimmune diseases: prolactin, ferritin, vitamin d, and tpa levels in autoimmune diseases. Ann N Y Acad Sci. 2007;1109:385–400. doi: 10.1196/annals.1398.044. [DOI] [PubMed] [Google Scholar]
- 73.Munger KL, Zhang SM, O'Reilly E, et al. Vitamin d intake and incidence of multiple sclerosis. Neurology. 2004;62:60–5. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 74.Cutolo M, Otsa K, Uprus M, Paolino S, Seriolo B. Vitamin d in rheumatoid arthritis. Autoimmun Rev. 2007;7:59–64. doi: 10.1016/j.autrev.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 75.Birlea SA, Costin GE, Norris DA. Cellular and molecular mechanisms involved in the action of vitamin d analogs targeting vitiligo depigmentation. Curr Drug Targets. 2008;9:345–59. doi: 10.2174/138945008783954970. [DOI] [PubMed] [Google Scholar]
- 76.Rosell M, Wesley AM, Rydin K, Klareskog L, Alfredsson L. Dietary fish and fish oil and the risk of rheumatoid arthritis. Epidemiology. 2009;20:896–901. doi: 10.1097/EDE.0b013e3181b5f0ce. [DOI] [PubMed] [Google Scholar]
- 77.Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic bifidobacterium lactis hn019. Am J Clin Nutr. 2001;74:833–9. doi: 10.1093/ajcn/74.6.833. [DOI] [PubMed] [Google Scholar]
- 78.Ben-Ami Shor D, Zandman-Goddard G. The 6th autoimmunity congress: meeting highlights 6th international congress on autoimmunity, 9-13 september 2008, Porto, Portuga. Immunotherapy. 2009;1:171–176. doi: 10.2217/1750743X.1.2.171. [DOI] [PubMed] [Google Scholar]
- 79.Maverakis E, Miyamura Y, Bowen MP, Correa G, Ono Y, Goodarzi H. Light, including ultraviolet. J Autoimmun. 2010;34:J247–57. doi: 10.1016/j.jaut.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lehmann P, Homey B. Clinic and pathophysiology of photosensitivity in lupus erythematosus. Autoimmun Rev. 2009;8:456–61. doi: 10.1016/j.autrev.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 81.Nived O, Johansen PB, Sturfelt G. Standardized ultraviolet-a exposure provokes skin reaction in systemic lupus erythematosus. Lupus. 1993;2:247–50. doi: 10.1177/096120339300200407. [DOI] [PubMed] [Google Scholar]
- 82.Utz PJ, Anderson P. Posttranslational protein modifications, apoptosis, and the bypass of tolerance to autoantigens. Arthritis Rheum. 1998;41:1152–60. doi: 10.1002/1529-0131(199807)41:7<1152::AID-ART3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 83.Alonso-Ruiz A, Zea-Mendoza AC, Salazar-Vallinas JM, Rocamora-Ripoll A, Beltran-Gutierrez J. Toxic oil syndrome: a syndrome with features overlapping those of various forms of scleroderma. Semin Arthritis Rheum. 1986;15:200–12. doi: 10.1016/0049-0172(86)90017-x. [DOI] [PubMed] [Google Scholar]
- 84.Hertzman PA, Blevins WL, Mayer J, Greenfield B, Ting M, Gleich GJ. Association of the eosinophilia-myalgia syndrome with the ingestion of tryptophan. N Engl J Med. 1990;322:869–73. doi: 10.1056/NEJM199003293221301. [DOI] [PubMed] [Google Scholar]
- 85.Parks CG, Cooper GS. Occupational exposures and risk of systemic lupus erythematosus. Autoimmunity. 2005;38:497–506. doi: 10.1080/08916930500285493. [DOI] [PubMed] [Google Scholar]
- 86.Ranque B, Mouthon L. Geoepidemiology of systemic sclerosis. Autoimmun Rev. 2010;9:A311–8. doi: 10.1016/j.autrev.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 87.D'Cruz D. Autoimmune diseases associated with drugs, chemicals and environmental factors. Toxicol Lett. 2000;112-113:421–32. doi: 10.1016/s0378-4274(99)00220-9. [DOI] [PubMed] [Google Scholar]
- 88.Yoshida S, Gershwin ME. Autoimmunity and selected environmental factors of disease induction. Semin Arthritis Rheum. 1993;22:399–419. doi: 10.1016/s0049-0172(05)80032-0. [DOI] [PubMed] [Google Scholar]
- 89.Rieger R, Gershwin ME. The x and why of xenobiotics in primary biliary cirrhosis. J Autoimmun. 2007;28:76–84. doi: 10.1016/j.jaut.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Griem P, Wulferink M, Sachs B, Gonzalez JB, Gleichmann E. Allergic and autoimmune reactions to xenobiotics: how do they arise? Immunol Today. 1998;19:133–41. doi: 10.1016/s0167-5699(97)01219-x. [DOI] [PubMed] [Google Scholar]
- 91.Talwalkar JA, Lindor KD. Primary biliary cirrhosis. Lancet. 2003;362:53–61. doi: 10.1016/S0140-6736(03)13808-1. [DOI] [PubMed] [Google Scholar]
- 92.Selmi C, Invernizzi P, Zuin M, Podda M, Gershwin ME. Genetics and geoepidemiology of primary biliary cirrhosis: following the footprints to disease etiology. Semin Liver Dis. 2005;25:265–80. doi: 10.1055/s-2005-916319. [DOI] [PubMed] [Google Scholar]
- 93.Metcalf JV, Bhopal RS, Gray J, Howel D, James OF. Incidence and prevalence of primary biliary cirrhosis in the city of newcastle upon tyne, england. Int J Epidemiol. 1997;26:830–6. doi: 10.1093/ije/26.4.830. [DOI] [PubMed] [Google Scholar]
- 94.Triger DR. Primary biliary cirrhosis: an epidemiological study. Br Med J. 1980;281:772–5. doi: 10.1136/bmj.281.6243.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Danielsson A, Boqvist L, Uddenfeldt P. Epidemiology of primary biliary cirrhosis in a defined rural population in the northern part of sweden. Hepatology. 1990;11:58–64. doi: 10.1002/hep.1840110317. [DOI] [PubMed] [Google Scholar]
- 96.Kim WR, Lindor KD, Locke GR, 3rd, et al. Epidemiology and natural history of primary biliary cirrhosis in a us community. Gastroenterology. 2000;119:1631–6. doi: 10.1053/gast.2000.20197. [DOI] [PubMed] [Google Scholar]
- 97.Triger DR, Berg PA, Rodes J. Epidemiology of primary biliary cirrhosis. Liver. 1984;4:195–200. doi: 10.1111/j.1600-0676.1984.tb00927.x. [DOI] [PubMed] [Google Scholar]
- 98.Lofgren J, Jarnerot G, Danielsson D, Hemdal I. Incidence and prevalence of primary biliary cirrhosis in a defined population in sweden. Scand J Gastroenterol. 1985;20:647–50. doi: 10.3109/00365528509089711. [DOI] [PubMed] [Google Scholar]
- 99.Myszor M, James OF. The epidemiology of primary biliary cirrhosis in north-east england: an increasingly common disease? Q J Med. 1990;75:377–85. [PubMed] [Google Scholar]
- 100.Witt-Sullivan H, Heathcote J, Cauch K, et al. The demography of primary biliary cirrhosis in ontario, canada. Hepatology. 1990;12:98–105. doi: 10.1002/hep.1840120116. [DOI] [PubMed] [Google Scholar]
- 101.Watson RG, Angus PW, Dewar M, Goss B, Sewell RB, Smallwood RA. Low prevalence of primary biliary cirrhosis in victoria, australia. Melbourne liver group. Gut. 1995;36:927–30. doi: 10.1136/gut.36.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Remmel T, Remmel H, Uibo R, Salupere V. Primary biliary cirrhosis in estonia With special reference to incidence, prevalence, clinical features, and outcome. Scand J Gastroenterol. 1995;30:367–71. doi: 10.3109/00365529509093292. [DOI] [PubMed] [Google Scholar]
- 103.Prince MI, Chetwynd A, Diggle P, Jarner M, Metcalf JV, James OF. The geographical distribution of primary biliary cirrhosis in a well-defined cohort. Hepatology. 2001;34:1083–8. doi: 10.1053/jhep.2001.29760. [DOI] [PubMed] [Google Scholar]
- 104.Sakauchi F, Mori M, Zeniya M, Toda G. A cross-sectional study of primary biliary cirrhosis in Japan: utilization of clinical data when patients applied to receive public financial aid. J Epidemiol. 2005;15:24–8. doi: 10.2188/jea.15.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sood S, Gow PJ, Christie JM, Angus PW. Epidemiology of primary biliary cirrhosis in victoria, australia: high prevalence in migrant populations. Gastroenterology. 2004;127:470–5. doi: 10.1053/j.gastro.2004.04.064. [DOI] [PubMed] [Google Scholar]
- 106.Long SA, Quan C, Van de Water J, et al. Immunoreactivity of organic mimeotopes of the e2 component of pyruvate dehydrogenase: connecting xenobiotics with primary biliary cirrhosis. J Immunol. 2001;167:2956–63. doi: 10.4049/jimmunol.167.5.2956. [DOI] [PubMed] [Google Scholar]
- 107.Amano K, Leung PS, Rieger R, et al. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. J Immunol. 2005;174:5874–83. doi: 10.4049/jimmunol.174.9.5874. [DOI] [PubMed] [Google Scholar]
- 108.Lleo A, Battezzati PM, Selmi C, Gershwin ME, Podda M. Is autoimmunity a matter of sex? Autoimmun Rev. 2008;7:626–30. doi: 10.1016/j.autrev.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 109.McFadden JP, White JM, Basketter DA, Kimber I. Does hapten exposure predispose to atopic disease? The haptenatopy hypothesis. Trends Immunol. 2009;30:67–74. doi: 10.1016/j.it.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 110.Leung PS, Quan C, Park O, et al. Immunization with a xenobiotic 6-bromohexanoate bovine serum albumin conjugate induces antimitochondrial antibodies. J Immunol. 2003;170:5326–32. doi: 10.4049/jimmunol.170.10.5326. [DOI] [PubMed] [Google Scholar]
- 111.Selmi C, Gershwin ME. The role of environmental factors in primary biliary cirrhosis. Trends Immunol. 2009;30:415–20. doi: 10.1016/j.it.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 112.Tsuneyama K, Harada K, Kono N, et al. Scavenger cells with gram-positive bacterial lipoteichoic acid infiltrate around the damaged interlobular bile ducts of primary biliary cirrhosis. J Hepatol. 2001;35:156–63. doi: 10.1016/s0168-8278(01)00084-8. [DOI] [PubMed] [Google Scholar]
- 113.Gershwin ME, Selmi C, Worman HJ, et al. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42:1194–202. doi: 10.1002/hep.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shimoda S, Nakamura M, Shigematsu H, et al. Mimicry peptides of human pdc-e2 163-176 peptide, the immunodominant t-cell epitope of primary biliary cirrhosis. Hepatology. 2000;31:1212–6. doi: 10.1053/jhep.2000.8090. [DOI] [PubMed] [Google Scholar]
- 115.Kaplan MM. Novosphingobium aromaticivorans: a potential initiator of primary biliary cirrhosis. Am J Gastroenterol. 2004;99:2147–9. doi: 10.1111/j.1572-0241.2004.41121.x. [DOI] [PubMed] [Google Scholar]
- 116.Fujii K, Kikuchi S, Satomi M, Ushio-Sata N, Morita N. Degradation of 17beta-estradiol by a gram-negative bacterium isolated from activated sludge in a sewage treatment plant in Tokyo, Japan. Appl Environ Microbiol. 2002;68:2057–60. doi: 10.1128/AEM.68.4.2057-2060.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Padgett KA, Selmi C, Kenny TP, et al. Phylogenetic and immunological definition of four lipoylated proteins from novosphingobium aromaticivorans, implications for primary biliary cirrhosis. J Autoimmun. 2005;24:209–19. doi: 10.1016/j.jaut.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 118.Leung PS, Park O, Matsumura S, Ansari AA, Coppel RL, Gershwin ME. Is there a relation between chlamydia infection and primary biliary cirrhosis? Clin Dev Immunol. 2003;10:227–33. doi: 10.1080/10446670310001642429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Abdulkarim AS, Petrovic LM, Kim WR, Angulo P, Lloyd RV, Lindor KD. Primary biliary cirrhosis: an infectious disease caused by chlamydia pneumoniae? J Hepatol. 2004;40:380–4. doi: 10.1016/j.jhep.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 120.Milo R, Kahana E. Multiple sclerosis: geoepidemiology, genetics and the environment. Autoimmun Rev. 2010;9:A387–94. doi: 10.1016/j.autrev.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 121.Rosati G. The prevalence of multiple sclerosis in the world: an update. Neurol Sci. 2001;22:117–39. doi: 10.1007/s100720170011. [DOI] [PubMed] [Google Scholar]
- 122.Ebers GC, Sadovnick AD. The geographic distribution of multiple sclerosis: a review. Neuroepidemiology. 1993;12:1–5. doi: 10.1159/000110293. [DOI] [PubMed] [Google Scholar]
- 123.Dean G, McLoughlin H, Brady R, Adelstein AM, Tallett-Williams J. Multiple sclerosis among immigrants in greater london. Br Med J. 1976;1:861–4. doi: 10.1136/bmj.1.6014.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Elian M, Nightingale S, Dean G. Multiple sclerosis among united kingdom-born children of immigrants from the indian subcontinent, africa and the west indies. J Neurol Neurosurg Psychiatry. 1990;53:906–11. doi: 10.1136/jnnp.53.10.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Flachenecker P. Epidemiology of neuroimmunological diseases. J Neurol. 2006;253(Suppl 5):V2–8. doi: 10.1007/s00415-006-5001-3. [DOI] [PubMed] [Google Scholar]
- 126.Atlas multiple sclerosis resources in the world. WHO report. Geneva [Google Scholar]
- 127.Lutton JD, Winston R, Rodman TC. Multiple sclerosis: etiological mechanisms and future directions. Exp Biol Med (Maywood) 2004;229:12–20. doi: 10.1177/153537020422900102. [DOI] [PubMed] [Google Scholar]
- 128.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–17. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 129.Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55:458–68. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- 130.Breij EC, Brink BP, Veerhuis R, et al. Homogeneity of active demyelinating lesions in established multiple sclerosis. Ann Neurol. 2008;63:16–25. doi: 10.1002/ana.21311. [DOI] [PubMed] [Google Scholar]
- 131.Doyle HA, Mamula MJ. Post-translational protein modifications in antigen recognition and autoimmunity. Trends Immunol. 2001;22:443–9. doi: 10.1016/s1471-4906(01)01976-7. [DOI] [PubMed] [Google Scholar]
- 132.Doyle HA, Mamula MJ. Posttranslational protein modifications: new flavors in the menu of autoantigens. Curr Opin Rheumatol. 2002;14:244–9. doi: 10.1097/00002281-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 133.Doyle HA, Mamula MJ. Posttranslational modifications of self-antigens. Ann N Y Acad Sci. 2005;1050:1–9. doi: 10.1196/annals.1313.001. [DOI] [PubMed] [Google Scholar]
- 134.Harauz G, Musse AA. A tale of two citrullines - structural and functional aspects of myelin basic protein deimination in health and disease. Neurochem Res. 2007;32:137–58. doi: 10.1007/s11064-006-9108-9. [DOI] [PubMed] [Google Scholar]
- 135.Moscarello MA, Mastronardi FG, Wood DD. The role of citrullinated proteins suggests a novel mechanism in the pathogenesis of multiple sclerosis. Neurochem Res. 2007;32:251–6. doi: 10.1007/s11064-006-9144-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Willison HJ, Yuki N. Peripheral neuropathies and anti-glycolipid antibodies. Brain. 2002;125:2591–625. doi: 10.1093/brain/awf272. [DOI] [PubMed] [Google Scholar]
- 137.Vossenaar ER, van Venrooij WJ. Citrullinated proteins: sparks that may ignite the fire in rheumatoid arthritis. Arthritis Res Ther. 2004;6:107–11. doi: 10.1186/ar1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lawrence DA. In vivo and in vitro effects of lead on humoral and cell-mediated immunity. Infect Immun. 1981;31:136–43. doi: 10.1128/iai.31.1.136-143.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Waterman SJ, El-Fawal HAN, Snyder CA. Lead alters the immunogenicity of two neural proteins: a potential mechanism for the progression of lead-induced neurotoxicity. Environ Health Perspect. 1994;102:1052–6. doi: 10.1289/ehp.941021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Evans HL. Markers of neurotoxicity: from behavior to autoantibodies against brain proteins. Clin Chem. 1995;41:1874–81. [PubMed] [Google Scholar]
- 141.Min Y, Kristiansen K, Boggs JM, Husted C, Zasadzinski JA, Israelachvili J. Interaction forces and adhesion of supported myelin lipid bilayers modulated by myelin basic protein. Proc Natl Acad Sci U S A. 2009;106:3154–9. doi: 10.1073/pnas.0813110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.uurlink BHJ DR, Doucette JR, Nazarali AJ, Schreyer DJ, Verge VMK, editors. Cell biology and pathology of myelin: evolving biological concepts and therapeutic approaches. New York: Plenum Press; 1997. pp. 13–25. [Google Scholar]
- 143.Waterman SJ, el-Fawal HA, Snyder CA. Lead alters the immunogenicity of two neural proteins: a potential mechanism for the progression of lead-induced neurotoxicity. Environ Health Perspect. 1994;102:1052–6. doi: 10.1289/ehp.941021052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Boullerne AI, Nedelkoska L, Benjamins JA. Role of calcium in nitric oxide-induced cytotoxicity: egta protects mouse oligodendrocytes. J Neurosci Res. 2001;63:124–35. doi: 10.1002/1097-4547(20010115)63:2<124::AID-JNR1004>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 145.Trojano M, Avolio C, Ruggieri M, et al. Serum soluble intercellular adhesion molecule-i in ms: relation to clinical and gd-mri activity and to rifn beta-ib treatment. Mult Scler. 1998;4:183–7. doi: 10.1177/135245859800400318. [DOI] [PubMed] [Google Scholar]
- 146.Brundin L, Morcos E, Olsson T, Wiklund NP, Andersson M. Increased intrathecal nitric oxide formation in multiple sclerosis, cerebrospinal fluid nitrite as activity marker. Eur J Neurol. 1999;6:585–90. doi: 10.1046/j.1468-1331.1999.650585.x. [DOI] [PubMed] [Google Scholar]
- 147.Svenningsson A, Petersson AS, Andersen O, Hansson GK. Nitric oxide metabolites in csf of patients with ms are related to clinical disease course. Neurology. 1999;53:1880–2. doi: 10.1212/wnl.53.8.1880. [DOI] [PubMed] [Google Scholar]
- 148.Boullerne AI, Rodriguez JJ, Touil T, et al. Anti-s-nitrosocysteine antibodies are a predictive marker for demyelination in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J Neurosci. 2002;22:123–32. doi: 10.1523/JNEUROSCI.22-01-00123.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Berger T, Rubner P, Schautzer F, et al. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med. 2003;349:139–45. doi: 10.1056/NEJMoa022328. [DOI] [PubMed] [Google Scholar]
- 150.Gaertner S, de Graaf KL, Greve B, Weissert R. Antibodies against glycosylated native mog are elevated in patients with multiple sclerosis. Neurology. 2004;63:2381–3. doi: 10.1212/01.wnl.0000147259.34163.33. [DOI] [PubMed] [Google Scholar]
- 151.Mantegazza R, Cristaldini P, Bernasconi P, et al. Anti-mog autoantibodies in italian multiple sclerosis patients: specificity, sensitivity and clinical association. Int Immunol. 2004;16:559–65. doi: 10.1093/intimm/dxh056. [DOI] [PubMed] [Google Scholar]
- 152.Lampasona V, Franciotta D, Furlan R, et al. Similar low frequency of anti-mog igg and igm in ms patients and healthy subjects. Neurology. 2004;62:2092–4. doi: 10.1212/01.wnl.0000127615.15768.ae. [DOI] [PubMed] [Google Scholar]
- 153.Gori F, Mulinacci B, Massai L, et al. Igg and igm antibodies to the refolded mog(1-125) extracellular domain in humans. J Neuroimmunol. 2011;233:216–20. doi: 10.1016/j.jneuroim.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 154.Mazzucco S, Mata S, Vergelli M, et al. A synthetic glycopeptide of human myelin oligodendrocyte glycoprotein to detect antibody responses in multiple sclerosis and other neurological diseases. Bioorg Med Chem Lett. 1999;9:167–72. doi: 10.1016/s0960-894x(98)00698-2. [DOI] [PubMed] [Google Scholar]
- 155.Carotenuto A, D'Ursi AM, Nardi E, Papini AM, Rovero P. Conformational analysis of a glycosylated human myelin oligodendrocyte glycoprotein peptide epitope able to detect antibody response in multiple sclerosis. J Med Chem. 2001;44:2378–81. doi: 10.1021/jm010811t. [DOI] [PubMed] [Google Scholar]
- 156.Selmi C, Invernizzi P, Miozzo M, Podda M, Gershwin ME. Primary biliary cirrhosis: does x mark the spot? Autoimmun Rev. 2004;3:493–9. doi: 10.1016/j.autrev.2004.05.003. [DOI] [PubMed] [Google Scholar]