Abstract
Introduction
Cigarette smoking is related to many pathological conditions; however, chemical substances affect the oral cavity first, so it is important to consider its influence on oral mucosa and oral potentially pre-malignant lesions. The aim of this study was to investigate the effect of smoking on microvessel density in oral lichen planus. Special emphasis was placed on examining the relationship between the expression of c-Met receptor in blood vessels and smoking habits.
Material and methods
This study included 34 patients with oral lichen planus diagnosed clinically and verified by histopathological examination and 12 healthy individuals as controls. Biopsy of oral mucosa was performed and specimens were examined for immunohistochemical CD34 and c-Met receptor expression. The microvessel density was established by evaluation of the five most vascular areas within a section.
Results
Compared to normal oral mucosa, in lichen planus patients, significantly higher blood vessel density and c-Met expression were noted. Irregular distribution of microvessels was typical for oral lichen planus. Also, microvessel density was higher in cigarette smoking patients’ tissues than in non-smoker specimens. Furthermore, the association of c-Met expression with smoking habit was statistically significant.
Conclusions
Cigarette smoking habit has a direct impact on the oral lichen planus course; therefore, close follow-up of these patients is mandatory.
Keywords: oral lichen planus, cigarette smoking, c-Met, microvessel density
Introduction
Cigarette smoking is the most established risk factor for lung carcinogenesis, and lung cancer is the leading cause of cancer mortality worldwide [1, 2]. An association with other malignancies (i.e., cancer of head and neck, pancreas, liver, uterine cervix, oesophagus, stomach and kidney) has also been shown [3, 4]. Cigarette smokers are at 2 to 5 times higher risk of oral cancer than non-smokers, and the risk increases with the number of cigarettes and years smoked [5]. Oral cancer cases and deaths are due to both individual predisposition, linked to specific genetic characteristics, and exposure to carcinogens, among which 20-30% of overall cases are attributable to tobacco smoking [6-8]. Nevertheless, smokeless tobacco (betel-quid) may also lead to malignant transformation – 50% of men and almost 90% of women presenting this habit [8, 9].
Smoking is also associated with potentially malignant disorders of the oral and oropharyngeal mucosa, especially with leukoplakia and erythroplakia [10, 11], with risk increasing in a dose-dependent manner and declining with the duration of smoking cessation [12]. However, little is known about the influence of cigarette smoke content on the course of oral lichen planus (OLP) and its potential of malignant transformation [13]. Some researchers speculate that the development of cancer in OLP could result from an interaction of the atrophic mucosa with tobacco carcinogens [14]. Betel-quid lichenoid lesion has been described as a reaction to chewing tobacco, but a causal role for betel has not been clearly established [15]. Moreover, tobacco could support tissue destruction in OLP through irritation and other well-established mechanisms, such as functional alterations in chemotaxis and phagocytosis of neutrophils, elevating levels of TNF, PGE2, neutrophil elastase and MMPs, as well as vasoconstrictory effect with lower oxygen concentration in tissue [16-19].
In this study, we investigated cigarette smoking effects on microvessels of OLP. Special concern was given to the expression of c-Met receptor, which has been shown to be overexpressed in human malignancies and to promote cancer cell growth [20, 21].
Material and methods
Patients
This study included 34 patients (24 women and 10 men) referred for the first time to the Department of Periodontology and Oral Mucosal Diseases of the Medical University of Lodz during the period of 2007-2009, with clinically diagnosed oral lichen planus. Clinical diagnosis of lichen planus was confirmed by iodide test. The mean age of examined patients was 61.5 ±8.2 years for women (range: 52 to 68 years), and 64 ±5.8 years for men (range: 58 to 69 years). The duration of the disease prior to presentation ranged from 3 weeks to 3 years. Healthy individuals [12] undergoing periodontal procedures were enrolled as controls. All the patients considered as cigarette smokers smoked about 10-20 cigarettes a day, and have been smoking for longer than 20 years. Non-smokers have never smoked at all; therefore in our group there were no ex-smokers.
The research has been conducted in full accordance with ethical principles including the World Medical Association Declaration of Helsinki (ver. 2002). The study has been reviewed and approved by the ethical board of the Medical University of Lodz.
Biopsy
The biopsy from the lichen planus lesions was fixed in 10% formalin and then routinely processed for paraffin embedding and haematoxylin and eosin (H&E) staining to confirm the clinical diagnosis of OLP.
Immunohistochemistry
Tissue samples were fixed in 10% buffered formalin and embedded in paraffin. Tissue sections were cut (4 µm), deparaffinized with xylene, and rehydrated through graduated ethanol solutions. The slides were treated with target retrieval solution (Dako, Carpenteria, CA) at 97°C for 45 min. Further, the sections were treated with 3% hydrogen peroxide in methanol for 30 min to quench endogenous peroxidase activity. Non-specific reactions were then blocked with 5% dried milk in phosphate-buffered saline (PBS) for 1 h at room temperature and the sections incubated overnight at 4°C with rabbit anti-human c-Met polyclonal antibody (1 : 50; Santa Cruz Biotechnology, Santa Cruz, CA), and mouse anti-human CD34 monoclonal antibody (1 : 50; DakoCytomation Denmark A/S). Multi-use secondary antibody (EnVision System; Dako) was applied to the tissues for 1 h at room temperature. Peroxidase activity was visualized with a 3,3'-diaminobenzidine tetrahydrochloride solution containing 0.05% hydrogen peroxidase (DAB Liquid System; Dako) and the sections were counterstained with haematoxylin for 3 min, dehydrated through graduated ethanol solutions, cleared with xylene, and mounted. Staining intensity was evaluated by systematically screening all slices and evaluating them according to an established 0-3 scale. The immunohistochemical results for c-Met protein were classified as follows: 0 – no staining, 1 – weak, 2 – moderate, 3 – strong. The membrane staining and cytoplasmic staining were evaluated. Samples graded as 0 and 1 were considered negative, 2 and 3 as positive.
Microvessel density
Immunohistochemical staining for CD34 to highlight the endothelial cells was performed. For the determination of microvessel density (MVD), the total microvessels number per chosen area was evaluated. The five most vascular areas within a section were selected and counted under a light microscope with 200-fold magnification (i.e., 20× objective lens and 10× ocular lens; 0.7386 mm2/field) as described by Weindner et al. [22]. Each area was counted twice and the arithmetical mean of values was used to calculate the mean for a section.
Statistical analysis
The correlation between c-Met receptor expression and cigarette smoking, as well as the expression of the protein and clinicopathological parameters were evaluated using Fisher's exact test.
Pearson’s correlation coefficient analysis was performed to determine the overall correlation between MVD and smoking habit and c-Met expression. All p-values < 0.05 were considered statistically significant.
Results
Clinical examination of all patients revealed papules and plaques with some irregular reticular aspects and the erythematous background. Intraorally, the most affected anatomical site was the buccal mucosa (67%), followed by tongue (20%), alveolar mucosa (10%), and palate (3%). Painful and burning symptoms were reported in all cases.
Histopathological analysis confirmed the clinical diagnosis of OLP in all cases. All specimens showed infiltration of inflammatory cells with a predominance of lymphocytes forming a dense band under the epithelium. Features of hydropic degeneration of the basal cell layer with local loss of distinct epithelial-connective tissue interface were observed. Hyperkeratosis was noted. Further, irregular acanthotic hyperplasia in 50% of the cases was found. No epithelial dysplasia was present.
In this study, we concentrated on the microvessel aspect of OLP. In normal tissues and OLP specimens, microvessels were mainly located immediately underneath the epithelium. In control tissues of non-smokers, microvessels were regularly distributed and had rather regular cross-sectional shapes. In OLP tissues of non-smokers, the vessels were irregularly distributed and had usually elongated cross-sections, and vessels with very small diameter were numerous. However, in cigarette smoking patients of both groups, the diameter of vessel cross-sections was smaller in comparison to non-smoking patients (Figure 1). The accumulation of small-diameter microvessels in OLP specimens of smokers was markedly higher than in OLP tissues of non-smokers. Within each of the investigated groups, differences in microvessels morphology or density between specimens derived from men or women were not observed.
Figure 1.
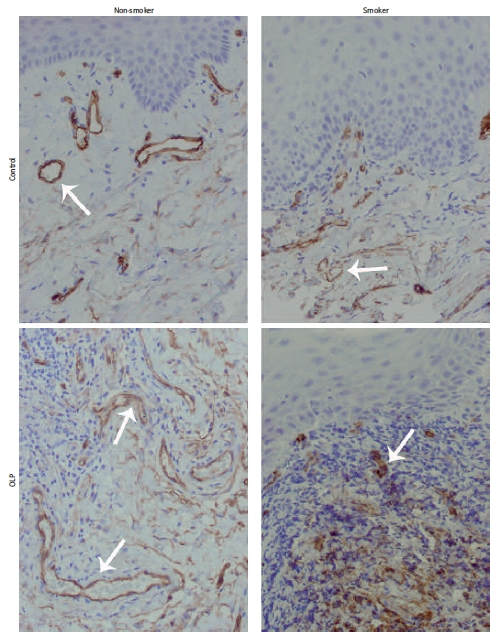
Blood vessels pattern by CD34 staining in control and OLP tissues. Blood vessels distributed regularly in control samples of non-smokers, with regular cross-sections, but with smaller diameter in smoking control samples (upper pictures). Elongated vessels irregularly distributed in OLP non-smokers; in OLP smokers numerous smalldiameter cross-sections of vessels (lower pictures). All presented samples derived from women. Arrows mark blood vessels (mag. 200×)
Generally, in non-smoking groups, the microvessel density (MVD; mean ± SD) was 24.8 ±4.4 in control, and 37.6 ±6.1 in OLP tissues. Nevertheless, MVD was higher in cigarette smoking patients’ tissues than in non-smoker specimens (Figure 2), and in both examined groups statistically correlated with cigarette smoking habit (p < 0.05). Cigarette smokers with OLP showed MVD at the level of 52.8 ±8.6, but non-smokers with OLP presented MVD at the level of 37.6 ±6.1, which indicated the statistical correlation between smoking habit and MVD increase in the OLP group (p < 0.05).
Figure 2.
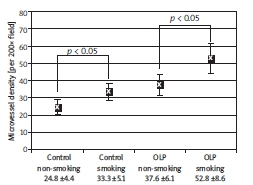
Comparison of MVD between control and OLP patients with regard to smoking habit. The MVD was markedly higher in cigarette smoking groups of control and OLP. Mean values and standard deviation are presented
Immunohistochemical staining for c-Met is presented in Figure 3. Of all OLP samples, 70.6% were positive for membrane and cytoplasmic c-Met staining in endothelial cells; among them, 6 samples of non-smokers and 18 samples of cigarette smokers (Table I). The association of c-Met expression with smoking habit was statistically significant within the OLP group (p < 10–6). In the group of healthy individuals, 50% of samples were positive for c-Met endothelial staining, and among them, 41.64% (5 samples) were obtained from cigarette smokers. Fisher’s exact test correlation for c-Met expression and smoking habit was statistically significant (p = 0.039, Table II).
Figure 3.
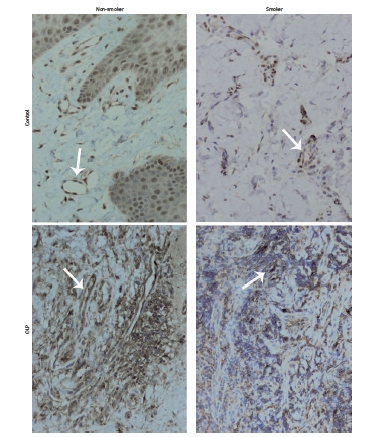
c-Met staining in control and OLP tissues. Increased staining of c-Met receptor in endothelial cells of OLP groups was noted. Smaller cross-sections of vessels in OLP smokers were characteristic. All presented samples derived from women. Arrows mark blood vessels (mag. 200×)
Table I.
Correlation between the expression of c-Met and smoking habit in OLP patients
| Habit | c-Met | |
|---|---|---|
| Negative | Positive | |
| Non-smokers | 10 (29.4%) | 6 (17.6%) |
| Smokers | 0 | 18 (53%) |
Correlation, p < 10–6
Table II.
Correlation between the expression of c-Met and smoking habit in healthy patients
| Habit | c-Met | |
|---|---|---|
| Negative | Positive | |
| Non-smokers | 5 (41.66%) | 1 (8.34%) |
| Smokers | 1 (8.34%) | 5 (41.66%) |
Correlation, p = 0.039
Furthermore, mean MVD was 54.0 ±9.3 in c-Met positive OLP samples and 40.2 ±6.2 in c-Met negative OLP samples. Therefore, it was higher in samples expressing c-Met. Pearson’s correlation was significant in OLP patients (p < 0.05). Seventy-five percent of c-Met positive patients smoked tobacco. Within the c-Met positive group, OLP smokers showed MVD at the level of 55.1 ±2.6 and OLP non-smokers at the level of 49.3 ±5.3, and the difference was statistically significant (p < 0.05).
Discussion
To evaluate the role of angiogenesis in OLP lesions with special concern for the influence of tobacco smoke on vascularization, we analysed MVD in healthy and diseased individuals with or without smoking habits, and compared the MVD between patients with OLP diagnosed with or without such addiction. First, we considered MVD in normal and OLP specimens, and we found a significant difference between these two groups. The MVD in the OLP group was higher in comparison to the control group; however, some previous studies have shown no significant difference between normal mucosa and pre-malignant lesions [23]. Li et al. have also shown no significant correlation; however, in their study, MVD increase was parallel to tumour volume during its growth [24]. In the present study, the higher MVD in OLP specimens might be the result of inflammation and the increase in blood vessels due to pro-inflammatory cytokine action since all patients showed an erythematous background of OLP lesions, which indicates that local microvasculature may undergo an intense process of inflammation-dependent angiogenesis [17]. Similarly, dermal MVD was also increased in cutaneous lichen planus as checked by CD34 staining [25]. Further, by videocapillaroscopy, an increase in capillary diameter and density in lichen planus of gingiva compared to the control patients has been reported [26]. Moreover, Lopez de Blanc et al. showed that lesions of lichen planus are more congestive than leukoplakia [27].
Next, we observed increased MVD in patients of OLP with smoking habit in comparison to those of OLP non-smokers. The MVD was by almost one-third higher when OLP patients smoked. The increased MVD in samples obtained from smoking patients might be the result of compensatory angiogenesis due to lower oxygen concentration in tissue, and the smaller diameter of blood vessels was the effect of vasoconstriction evoked by tobacco smoke. Interestingly, there are no other reports showing such correlation.
Further, we analysed the expression of c-Met in endothelial cells. c-Met protein is a receptor for hepatocyte growth factor (HGF), which was originally identified as a potent mitogen that stimulates the growth of hepatocytes, as well as the proliferation, migration, survival, angiogenesis and invasion in human malignancies [28]. Moreover, c-Met is known as an oncogene and its overexpression has been reported in many human tumours [29]. Previously, we have shown that c-Met together with CD151 tetraspanin controls the signal passage for proliferation in human salivary gland cells [21], and that the mentioned protein complex is important in human breast cancer cells, MDA-MB-231, for cord-like structure formation, which imitate the morphogenesis or angiogenesis in vivo[30]. Interestingly, since c-Met and its ligand play a role in blood vessel formation, inhibition of the HGF/c-Met signalling pathway has been shown to stop angiogenesis in multiple in vitroand in vivomodels [31]. Therefore c-Met is recognized as a promising target in cancer therapy.
In our study, all control and OLP samples showed expression of c-Met in endothelial cells. However, in some cases the expression was weak, which could not be considered positive staining. Nevertheless, 70.6% of OLP were positive for c-Met staining and the data directly correlated with smoking habit. Moreover, we showed increased values of MVD in OLP smokers in comparison to non-smokers. Of all c-Met positive samples, 75% were derived from smokers. Previously, the expression of c-Met on endothelial cells has been reported, and omnipresence of this receptor was frequently accompanied by increased HGF concentration in many pathological conditions [32]. Furthermore, it has been reported that nicotine upregulated HGF expression in lung cancer tissues, as well as in type II normal pneumocytes with overexpression of c-Met frequently detected in adenocarcinoma cells [33]. Thus smoking might play a key role in promoting disease progression via activation of the HGF/c-Met pathway.
This all is convergent with our study, where smoking habit correlated with both MVD and c-Met positive staining. The results support the role of cigarette smoking in the progression of microvascular changes in OLP tissues. Therefore, in the light of previous reports and our conclusions, it is of high importance to advise patients with OLP cigarette smoking cessation, and follow them closely in order not to overlook any signs of malignant transformation.
Acknowledgments
This study was supported by the grant for scientific research No. 502-12-613, from the Medical University of Lodz, as well as by a grant for scientific research in 2007-2010, from the Ministry of Science and Higher Education of Poland (NN401097833 to SKK).
References
- 1.Winn DM. Tobacco use and oral disease. J Dent Educ. 2001;65:306–12. [PubMed] [Google Scholar]
- 2.Van der Vaart H, Postma DS, Timens W, Hacken NH. Acute effects of cigarette smoke on inflammation and oxidative stress: a review. Thorax. 2004;59:713–21. doi: 10.1136/thx.2003.012468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mena S, Ortega A, Estrela JM. Oxidative stress in environmental-induced carcinogenesis. Mutat Res. 2009;674:36–44. doi: 10.1016/j.mrgentox.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 4.Dautzenberg B. Tobacco-related diseases. Rev Prat. 2004;54:1877–82. [PubMed] [Google Scholar]
- 5.Wald NJ, Hackshaw AK. Cigarette smoking: an epidemiological overview. Br Med Bull. 1996;52:3–11. doi: 10.1093/oxfordjournals.bmb.a011530. [DOI] [PubMed] [Google Scholar]
- 6.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Hecht SS. Tobacco carcinogens, their biomarkers and tobacco-induced cancer. Nat Rev Cancer. 2003;3:733–44. doi: 10.1038/nrc1190. [DOI] [PubMed] [Google Scholar]
- 8.Hashibe M, Brennan P, Benhamous S, et al. Alcohol drinking in never users of tobacco, cigarette smoking in never drinkers, and the risk of head and neck cancer: pooled analysis in the international head and neck cancer epidemiology consortium. J Natl Cancer Inst. 2007;99:777–89. doi: 10.1093/jnci/djk179. [DOI] [PubMed] [Google Scholar]
- 9.Nair U, Bartsch H, Nair J. Alert for an epidemic of oral cancer due to use of the betel quid substitutes gutkha and pan masala: a review of agents and causative mechanisms. Mutagenesis. 2004;19:251–62. doi: 10.1093/mutage/geh036. [DOI] [PubMed] [Google Scholar]
- 10.Dietrich T, Reichart PA, Scheifele C. Clinical risk factors of oral leukoplakia in a representative sample of the US population. Oral Oncol. 2004;40:158–63. doi: 10.1016/s1368-8375(03)00145-3. [DOI] [PubMed] [Google Scholar]
- 11.Banoczy J, Gintner Z, Dombi C. Tobacco use and oral leukoplakia. J Dent Educ. 2001;65:322–7. [PubMed] [Google Scholar]
- 12.Morse DE, Psoter WJ, Cleveland D, et al. Smoking and drinking in relation to oral cancer and oral epithelial dysplasia. Cancer Causes Contr. 2007;18:919–29. doi: 10.1007/s10552-007-9026-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandolfo S, Richiardi L, Carrozo M, et al. Risk of squamous cell carcinoma in 402 patients with oral lichen planus: a follow-up in an Italian population. Oral Oncol. 2004;40:77–83. doi: 10.1016/s1368-8375(03)00139-8. [DOI] [PubMed] [Google Scholar]
- 14.Gonzales-Moles MA, Scully C, Gil-Montoya JA. Oral lichen planus: controversies surrounding malignant transformation. Oral Dis. 2008;14:229–43. doi: 10.1111/j.1601-0825.2008.01441.x. [DOI] [PubMed] [Google Scholar]
- 15.Ismail SB, Kumar SK, Zain RB. Oral lichen planus and lichenoid reactions: etiopathogenesis, diagnosis, management and malignant transformation. J Oral Sci. 2007;49:89–106. doi: 10.2334/josnusd.49.89. [DOI] [PubMed] [Google Scholar]
- 16.Newman MG, Takei HH, Klokkevold PR, Carranza FA. 10th. Missouri: Saunders Elsevier; 2007. Carranza’s clinical periodontology; pp. 254–5. [Google Scholar]
- 17.Regezi JA, Sciubba JJ, Jordan RCK. Clinical Pathologic Correlations. 5th. Missouri: Saunders Elsevier; 2008. Oral pathology; pp. 90–5. [Google Scholar]
- 18.La Rocca G, Anzalone R, Magno F, et al. Cigarette smoke exposure inhibits extra cellular MMP-2 (gelatinases) activity in human lung fibroblasts. Respir Res. 2007;8:1186–465. doi: 10.1186/1465-9921-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaddah S, Rashed L, Obaia E, Sabry D. A preliminary study: matrix metalloproteinase expression as an indicator of the hazards of shisha (nargila) smoking. Arch Med Sci. 2009;5:570–6. [Google Scholar]
- 20.Klosek SK, Nakashiro KI, Hara S, Li C, Shintani S, Hamakawa H. Constitutive activation of Stat3 correlates with increased expression of the c-Met/HGF receptor in oral squamous cell carcinoma. Oncol Rep. 2004;12:293–6. [PubMed] [Google Scholar]
- 21.Klosek SK, Nakashiro K, Hara S, Shintani S, Hasegawa H, Hamakawa H. CD151 forms a functional complex with c-Met in human salivary gland cancer cells. Biochem Biophys Res Commun. 2005;336:408–16. doi: 10.1016/j.bbrc.2005.08.106. [DOI] [PubMed] [Google Scholar]
- 22.Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–9. [PMC free article] [PubMed] [Google Scholar]
- 23.Tae K, El-Naggar AK, Yoo E, et al. Expression of vascular endothelial growth factor and microvessel density in head and neck tumorigenesis. Clin Cancer Res. 2000;6:2821–8. [PubMed] [Google Scholar]
- 24.Li C, Shintani S, Terakado N, et al. Microvessel density and expression of vascular endothelial growth factor, basic fibroblast growth factor, and platelet-derived endothelial growth factor in oral squamous cell carcinomas. Int J Oral Maxillofac Surg. 2005;34:559–65. doi: 10.1016/j.ijom.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 25.Hussein MR. Evaluation of angiogenesis in normal and lichen planus skin by CD34 protein immunohistochemistry: preliminary findings. Cell Biol Int. 2007;31:1292–5. doi: 10.1016/j.cellbi.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 26.Scardina GA, Cacioppo A, Carini F, Ruggieri A, Valenza V, Messina P. Periodontal morphological microcirculation in oral lichen planus. Ital J Anat Embriol. 2007;112:281–91. [PubMed] [Google Scholar]
- 27.Lopez de Blanc S, Gendelman H, Itoiz ME, Lanfranchi H. Study of the vascular pattern in oral lichen planus. Acta Odontol Latinoam. 1996;9:27–36. [PubMed] [Google Scholar]
- 28.Nakashiro K, Hara S, Shinohara Y, et al. Phenotypic switch from paracrine to autocrine role of hepatocyte growth factor in an androgen-independent human prostatic carcinoma cell line, CWR22R. Am J Pathol. 2004;165:533–40. doi: 10.1016/s0002-9440(10)63318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma PC, Tretiakova MS, Mackinnon AC, et al. Expression and mutational analysis of MET in human solid cancers. Genes Chrom Cancer. 2008;47:1025–37. doi: 10.1002/gcc.20604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klosek SK, Nakashiro K, Hara S, Goda H, Hasegawa H, Hamakawa H. CD151 regulates HGF-stimulated morphogenesis of human breast cancer cells. Biochem Biophys Res Commun. 2009;379:1097–100. doi: 10.1016/j.bbrc.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 31.You WK, McDonald DM. The hepatocyte growth factor/c-Met signaling pathway as a therapeutic target to inhibit angiogenesis. BMB Rep. 2008;41:833–9. doi: 10.5483/bmbrep.2008.41.12.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori T, Shimizu A, Masuda Y, Fukuda Y, Yamanaka N. Hepatocyte growth factor – stimulating endothelial cell growth and accelerating glomerular capillary repair in experimental progressive glomerulonephritis. Nephron Exp Nephrol. 2003;94:44–54. doi: 10.1159/000071283. [DOI] [PubMed] [Google Scholar]
- 33.Chen JT, Lin TS, Chow KC, et al. Cigarette smoking induces overexpression of hepatocyte growth factor in type II pneumocytes and lung cancer cells. Am J Respir Cell Mol Biol. 2006;34:264–73. doi: 10.1165/rcmb.2005-0117OC. [DOI] [PubMed] [Google Scholar]


