Abstract
Introduction
While some studies have indicated that alcohol intake is associated with a decreased risk of renal cell carcinoma, others have not. We conducted a meta-analysis of case-control studies to provide a quantitative assessment of the association between alcohol intake and the risk of renal cell carcinoma.
Material and methods
We identified studies by a literature search of PubMed and review of references of relevant articles. Both the fixed and random-effects models were used to obtain the summary risk estimates associated with the highest versus the lowest consumption categories depending on the heterogeneity of effects among studies. Dose-response meta-analysis was performed for studies reporting categorical risk estimates for a series of exposure levels.
Results
Fifteen studies were included in this meta-analysis. An inverse association between alcohol consumption and renal cell carcinoma was observed in both the overall alcohol intake group (OR 0.67, 95% CI 0.62-0.73) and subgroups stratified by sex, study design, geographical region, specific beverages and alcohol assessment. The dose-response meta-analysis showed that an increase in alcohol consumption of 12 g of ethanol per day was associated with a 5% statistically significant decreased risk of renal cell cancer.
Conclusions
High alcohol consumption exhibits a preventive effect for renal cell carcinoma in a dose-response manner. Further efforts should be made to clarify the underlying biological mechanisms.
Keywords: alcohol drinking, alcoholic beverages, ethanol, kidney neoplasms, meta-analysis
Introduction
Alcohol consumption is increasing in many countries and is an important cause of cancer worldwide [1]. A causal link has been established between alcohol consumption and cancers of the upper alimentary tract, liver, colorectum, and female breast [2]. Modifying alcohol consumption could be part of a prevention strategy of cancer through lifestyle changes.
Increasing incidence rates of renal cell carcinoma (RCC) have been reported worldwide [3]. Reasons for this phenomenon could be explained by both improvement in diagnostic workup and environmental factors. Smoking, obesity and hypertension are consistently associated with an increased risk of RCC [4-6], and the rising prevalences of obesity and hypertension likely have contributed to the upward cancer trends. The association between alcohol consumption and the risk of RCC has also been widely investigated, yielding inconclusive results. Most of the earlier case-control [7-3] and cohort studies [14-18] have shown no association, while recent prospective cohort studies [19-23] found a reduced incidence of RCC in alcohol drinkers.
Recently, a pooled analysis of 12 cohort studies showed that moderate alcohol consumption was associated with a decreased risk of RCC [24]. The purpose of the present study was to re-examine the epidemiological evidence regarding the association between alcohol consumption and the risk of RCC by summarizing the results of published case-control studies, including dose-response meta-analyses, to quantify the strength of this association.
Material and methods
Selection of studies
We identified publications in PubMed using alcohol, renal cell carcinoma, renal cell cancer, kidney cancer and case-control as keywords. Hand searches were also performed via cited references from the identified articles and reviews. The criteria for inclusion were (i) case-control studies evaluating the relationship between alcohol consumption and RCC; (ii) published in English between 1980 and March 2010; (iii) providing odds ratio (OR) estimates and corresponding 95% confidence intervals (95% CI), or information allowing us to compute them. In studies with overlapping patients or controls, the latest study with the largest sample size was included.
Data extraction
For each study, data were extracted for the first author, year of publication, the country in which the study was conducted, study design, number of cases, number and range of categories of exposure, adjusted effects estimates, types of alcohol exposure, adjusted covariates and exposure assessment. We extracted the maximally adjusted ORs and CIs. When sex-stratified ORs were provided in a study, the ORs were independently involved in the overall meta-analysis.
Statistical analysis
We first quantified the associations of alcohol with RCC risk using meta-analysis of OR estimates associated with the highest versus the lowest category of alcohol intake using fixed- and random-effects models that included a term for heterogeneity. Second, subgroup analyses were performed according to study design (hospital-based or population-based case-control studies), sex (men or women), geographical region (US/Canada or Europe), alcohol assessment (interview or questionnaire) and type of alcohol beverages (beer, wine or spirits).
For the dose-response meta-analysis, we estimated study-specific dose-response slopes from the correlated natural log of the ORs across categories of alcohol consumption using the method proposed by Greenland and Longnecker [25]. We converted all measures into grams of alcohol per day on the widely used estimation that a standard drink contains 12 g of alcohol regardless of alcohol type unless it was defined in the study population or the geographical area. The level of alcohol consumption was assigned from each study to these categories based on the calculated midpoint of alcohol consumption. When the highest category was open-ended, we assumed the width of the interval to be the same as in the preceding category.
We quantified the extent of heterogeneity using Q-test [26] and I 2 score [27] and statistical significance was considered when p < 0.05. Meta-regression analysis was used to explore the influence of study design, geographical region, alcohol assessment, and publication years in the heterogeneity. Publication bias was assessed using the tests of Egger [28] and Begg [29]. All statistical analyses were done with Stata Statistical Software, version 10.0.
Results
Characteristics of studies
We identified 24 articles [7-2, 30-47] that evaluated the association of RCC incidence and alcohol intake published between 1980 and March 2010. Four articles [10, 11, 42, 43] did not provide sufficient information to estimate a summary odds ratio and its 95% confidence intervals. One case-control study published results in two different articles [38, 44], while two studies published results in three different articles each [31, 37, 40, 46-48], and we extracted the latest and largest data sets from them [31, 37, 38, 40]. Of the fifteen selected studies, six were hospital-based case-control [7, 12, 30, 33, 36, 38], and nine were population-based case-control studies [8, 9, 31, 32, 34, 35, 37-40] (Table I), including a total of 9284 cases. Nine of these studies were conducted in the United States/Canada [7-9, 30, 32, 34, 37, 39-41], while 5 were in Europe [12, 33, 35, 36, 38] and 1 in multiple countries [31]. Nine articles reported the associations between consumption of specific alcoholic beverages (beer, wine or spirits) and the risk of RCC [9, 30, 31, 34-40]. Information on alcohol consumption was obtained by interview, self-administered questionnaire or both techniques.
Table I.
Study characteristics of published cohort and case-control studies on alcohol intake and renal cell carcinoma
| Authors, year | Study design | Country | Sex | Number of cases | Number of categories of exposure | Range of exposure | Specific alcohol beverages | Variables of adjustment | Alcohol assessment |
|---|---|---|---|---|---|---|---|---|---|
| Goodman et al., 1986 | Hospital-based case-control study | US | M/W | 267 | 2 | Non-drinker vs. drinkers | Beer, wine, spirits | Sex, race, age, and time of admission | Interview |
| Brownson et al., 1988 | Hospital-based case-control study | US | M/W | 326 | 2 | Non-drinker vs. drinkers | No | Age and smoking | Questionnaire |
| Maclure et al., 1990 | Population-based case-control study | US | M/W | 203 | 2 | ≤1 cup/week vs.≥2 cups/week | Beer, wine, spirits | Age, sex, smoking | Interview |
| Benhamou et al., 1993 | Hospital-based case-control study | France | M/W | 196 | 2 | Non-drinker vs. drinkers | No | Sex | Interview |
| Kreige et al., 1993 | Population-based case-control study | Canada | M/W | 518 | 3 | The first quartile vs. the fourth quartile | No | Age, active cigarette smoking status, and combined Quetelet ndex | Questionnaire |
| Wolk et al., 1996 | Population-based case-control study | Multiple countries | M/W | 1185 | 5 | <1 vs. ≥15 drinks/week | Beer, wine, spirits | Age, sex, study centre, body mass index, smoking and total calories | Interview |
| Yuan et al., 1998 | Population-based case-control study | US | M/W | 1204 | 7 | Non-drinker vs. ≥43 drinks/week | No | Level of education, body mass index, history of hypertension, number of cigarettes per day, current smoking status, total grams of analgesics consumed over lifetime and regular use of amphetamines | Interview |
| Parker et al., 2002 | Population-based case-control study | US | M/W | 406 | 3 | Non-drinkers vs. >35 g/week | Beer, wine, spirits | Age, pack-years of smoking, history of hypertension, history of bladder infection, family history of kidney cancer, exercise, consumption of red meat, and consumption of fruit | Interview and questionnaire |
| Mattioli et al., 2002 | Hospital-based case-control study | Italy | M/W | 324 | 6 (men) 3 (women) | Non-drinkers vs. >48 g/day | No | Sex, age, place of birth, residence environment | Questionnaire |
| Greving et al., 2007 | Population-based case-control study | Sweden | M/W | 855 | 6 | Non-drinkers vs. ≥620 g/month | Light beer, medium-strong beer, strong beer, white wine, red wine, strong wine, hard liquor | Age, sex, BMI, and cigarette smoking | Questionnaire |
| Hsu et al., 2007 | Hospital-based case-control study | Russia, Romania, Poland, and the Czech Republic | M/W | 1065 | 4 | Non-drinkers vs. ≥137.5 g/week | Beer, wine, spirits | Age, country, gender, tobacco pack-years of smoking, education, body mass index, hypertension medication use, and tertiles of total vegetable, total white meat, and total red meat consumption | Interview |
| Pelucchi et al., 2008 | Hospital-based case-control study | Italy | M/W | 1115 | 4 | Non-drinkers vs. >8 drinks/day | Beer, wine, spirits | Age, sex, study, study centre, education, smoking habits, body mass index and family history | Interview |
| Benedetti et al., 2009 | Population-based case-control study | Canada | M/W | 156 | 3 | Non-drinkers vs. ≥7 drinks/week | Beer, wine, spirits | Age, log of the SES index, years of schooling, ethnicity, respondent status, fruit and vegetable consumption, and smoking | Interview |
| Brock et al., 2009 | Population-based case-control study | US | M/W | 323 | 4 | Non-drinkers vs. >2 drinks/day | No | Age, sex, proxy status, smoking, BMI at age 40, blood pressure and vegetable consumption | Questionnaire |
| Fu et al., 2008 and 2009 | Population-based case-control study | Canada | M/W | 1141 | 4 | Non-drinkers vs. >13.6 g/day | Beer, wine, spirits | 10-year age group, province, education, body mass index, sex, pack-years of smoking, total consumption of vegetables and fruit, and total energy intake of meat, total consumption of vegetables and fruit, and total energy intake | Questionnaire |
Among 15 case-control studies, seven reported significantly decreased risks of RCC in drinkers as compared with non-drinkers [30, 31, 34, 35, 37, 38, 40, 41], whereas seven studies found no association [7, 8, 12, 32, 33, 36, 41]. A study conducted by Maclure et al. [9] provided odds ratios for three alcoholic beverages but no data on overall alcohol intake.
Highest versus lowest
In Figure 1 we present the overall ORs of RCC comparing the highest versus the lowest alcohol consumption categories. When all these case-control studies were analysed together, we observed a statistically significant 30% reduced risk of RCC. In analysis by study design, population-based case-control studies (OR 0.65, 95% CI 0.52-0.79) reported a lower risk of RCC in drinkers compared to hospital-based case-control studies (OR 0.78, 95% CI 0.65-0.91). The results were heterogeneous across case-control studies (P Het = 0.02, I 2 = 48.2%). There was no evidence of heterogeneity among hospital-based case-control studies, but some evidence in population-based studies.
Figure 1.
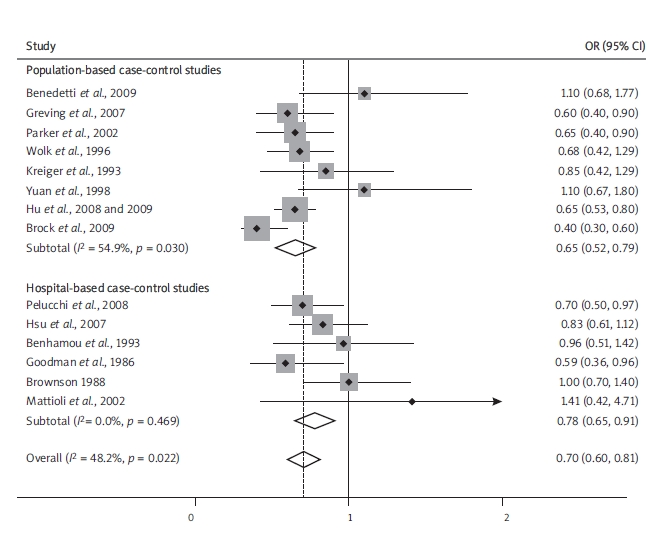
A forest plot showing risk estimates from case-control studies estimating the association between overall alcohol consumption and risk of renal cell carcinoma
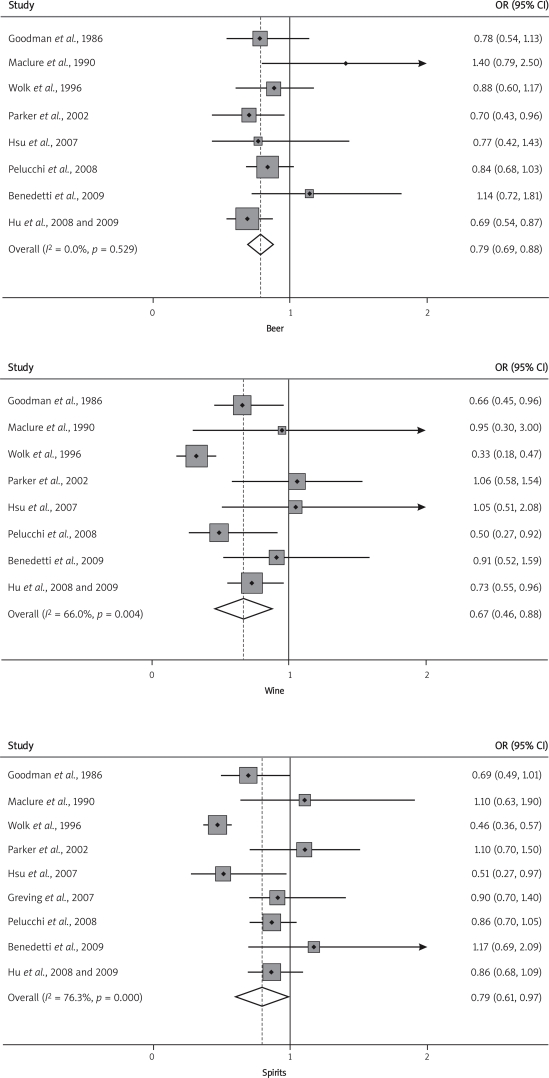
In Table II, we assessed associations separately for sex (men or women), geographical region (US/Canada or Europe) and alcohol assessment (interview or questionnaire). The OR estimates from subgroup analyses varied little, showing that alcohol consumption was consistently associated with a decreased risk of RCC. In analysis by specific beverages (Figure 2), we also found a significantly decreased risk of renal cancer for intake of beer, wine and spirits. There was no evidence of significant publication bias either with Egger’s or Begg’s test in any subgroup.
Table II.
Summary of pooled risk ratios of renal cell carcinoma by sex, geographical region, type of alcoholic beverages, body mass index, and smoking status
| Subgroup | Number of studies | Pooled OR (95% CI) | Pheterogeneity (I2 score) | Egger’s test Value of p | Begg’s test Value of p |
|---|---|---|---|---|---|
| All studies | 14[7, 8, 12, 13, 30-37, 39-41] | 0.70 (0.60, 0.81) | 0.02 (48.2%) | 0.17 | 0.32 |
| Sex | |||||
| Men | 9[7, 8, 12, 30, 31, 33, 34, 37, 38] | 0.80 (0.69, 0.91) | 0.47 (0) | 0.66 | 0.60 |
| Women | 9[7, 8, 12, 30, 31, 33, 34, 37, 38] | 0.61 (0.47, 0.76) | 0.56 (0) | 0.49 | 1 |
| Geographical region | |||||
| Europe | 5[12, 33, 35, 36, 38] | 0.73 (0.60, 0.87) | 0.55 (0) | 0.38 | 0.46 |
| US/Canada | 8[7, 8, 30, 32, 34, 37, 39-41] | 0.71 (0.54, 0.87) | 0.01 (64.8%) | 0.40 | 0.62 |
| Alcohol assessment | |||||
| Interview | 7[12, 30-32, 36, 38, 39] | 0.75 (0.64, 0.87) | 0.43 (0) | 0.30 | 0.76 |
| Questionnaire | 6[7, 8, 33, 35, 40, 41] | 0.62 (0.44, 0.79) | 0.02 (64.6%) | 0.57 | 0.57 |
Figure 2.
Forest plots showing the odds ratio of each study and the pooled odds ratios for specific alcoholic beverages
Dose-response analysis
Nine studies [32-41] were included in the dose-response analysis of the association between alcohol intake and risk of RCC. We excluded 4 studies with only 2 categories of alcohol consumption [7, 9, 12, 30] and one publication that did not provide the number of cases and controls, and one without exact dosage limits of alcohol for each stratum. Figure 3 shows the dose-response relationship between risk of RCC and alcohol consumption. There was a 5% (95% CI 3%-7%) decrease of risk of RCC for an increase of 12 g alcohol intake (approximately 1 drink) per day. The result was heterogeneous (p < 0.01).
Figure 3.
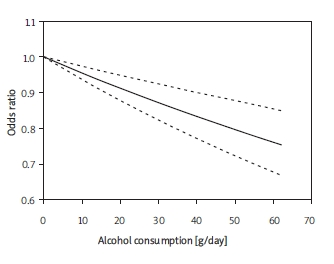
Odds ratio for renal cell carcinoma by doses of alcohol intake based on the results of the doseresponse meta-analyses. Solid line represents the estimated odds ratios and the dotted lines represent the 95% confidence intervals
Meta-regression analysis
We also performed meta-regression analysis to explore the influence of publication year, geographical region, study design, and method of alcohol assessment on the heterogeneity. However, none of the above was identified as a possible source of heterogeneity among all the included studies.
Discussion
In this meta-analysis we have observed an inverse association between alcohol intake and the risk of RCC. This finding is consistent with the previous pooled analysis by Lee et al. [24], which used the patient-level data and provided more convincing results and deeper analysis. However, in that study the case-control studies were not included. We included all the case-control studies so far for a total of 9,284 RCC cases. Our results showed that case-control studies, which may be subject to selection and recall bias, also provided support for a negative relationship between alcohol consumption and RCC. There was no evidence of heterogeneity among studies included in this analysis. Furthermore, we performed a meta-analysis of dose-response relationship between alcohol intake and the risk of RCC. An increase in alcohol consumption of 12 g of ethanol per day was statistically significantly associated with a 5% decrease in risk of RCC.
Our results from subgroup analyses suggested that an inverse relationship between alcohol intake and risk of RCC was seen in both men and women, and a stronger association was observed in women compared to men, although the difference in risk estimate was not statistically significant. This gender-specific association may suggest an underlying hormonal mechanism. There is some evidence that oestrogens increase risk of RCC [49, 50]. However, the data regarding effects of alcohol on oestrogen levels are inconsistent [51, 52], and recent studies suggest that alcohol’s favourable effect does not appear to involve hormonal mechanisms [53]. The summarized OR estimates were not statistically different across different alcoholic beverage types, indicating that the negative association is attributable to ethanol intake itself rather than to a specific beverage, though certain ingredients in beer and wine, such as xanthohumol and resveratrol, have been demonstrated to possess cancer chemopreventive properties [54, 55]. Our results also suggest that the association between alcohol consumption and RCC was not modified by different geographical regions or methods of alcohol assessment.
Several biological mechanisms for the negative relationship of alcohol consumption with the development of RCC have been proposed. One potential explanation is that the diuretic effect of alcoholic beverages may reduce the concentration of carcinogens and decrease the time that carcinogens stay in the kidney. This hypothesis could be verified by investigating the relationship of total fluid intake and RCC. However, a pooled analysis of 2 cohorts detected no association and a population-based case-control study found a positive relationship between total fluid intake and risk of RCC [22, 40]. Enhanced insulin sensitivity might be a mechanism by which alcohol intake exerts its protective effect against RCC. Obesity and diabetes are risk factors for RCC [56, 57], and light to moderate alcohol intake is associated with improved insulin sensitivity [58]; thus it is possible that insulin is a potential intermediate component in the association between alcohol consumption and RCC. It would be informative to determine whether the protective effect exists in patients with different insulin sensitivity levels, and well-designed cohort studies are needed to further clarify the consistency within diabetic and non-diabetic subjects.
Several potential limitations of our study need to be considered. First, as this meta-analysis is based on case-control studies, the possibility of bias and uncontrolled or residual confounding factors cannot be excluded, although we extracted the maximally adjusted ORs, which have been controlled for variables that might be related to RCC in most of the studies. However, different studies may have adjusted for different covariates, which could probably bias the results. Second, we did not attempt to uncover unpublished observations, which could bring a publication bias, even though no significant evidence of a publication bias was observed in Egger’s and Begg’s test. Third, both volume of alcohol consumption and patterns of drinking have been shown to influence the alcohol-related burden of disease, while most of the included studies did not provide data on alcohol intake over time or life drinking patterns. Consequently, we did not have sufficient data to evaluate the risk of RCC associated with these other dimensions of alcohol intake.
In conclusion, this study applied a detailed meta-analytic approach for combining OR estimates from case-control studies on the relationship between RCC incidence and alcohol consumption. We found that high alcohol consumption was consistently associated with a lower risk of renal cell cancer when stratified by sex, study design, geographical region and alcoholic types, and decreased risk for RCC in a dose-response manner. Future research to determine the likely biological mechanism is warranted.
References
- 1.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–56. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- 2.Poschl G, Seitz HK. Alcohol and cancer. Alcohol Alcohol. 2004;39:155–65. doi: 10.1093/alcalc/agh057. [DOI] [PubMed] [Google Scholar]
- 3.Mathew A, Devesa SS, Fraumeni JF, Jr, Chow WH. Global increases in kidney cancer incidence, 1973-1992. Eur J Cancer Prev. 2002;11:171–8. doi: 10.1097/00008469-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Tobacco smoke and involuntary smoking. IARC Monogr Eval Carcinog Risks Hum. 2004;83:1–1438. [PMC free article] [PubMed] [Google Scholar]
- 5.Pischon T, Lahmann PH, Boeing H, et al. Body size and risk of renal cell carcinoma in the European Prospective Investigation into Cancer and Nutrition (EPIC) Int J Cancer. 2006;118:728–38. doi: 10.1002/ijc.21398. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin JK, Lipworth L, Tarone RE. Epidemiologic aspects of renal cell carcinoma. Semin Oncol. 2006;33:527–33. doi: 10.1053/j.seminoncol.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Brownson RC. A case-control study of renal cell carcinoma in relation to occupation, smoking, and alcohol consumption. Arch Environ Health. 1988;43:238–41. doi: 10.1080/00039896.1988.9934940. [DOI] [PubMed] [Google Scholar]
- 8.Kreiger N, Marrett LD, Dodds L, Hilditch S, Darlington GA. Risk factors for renal cell carcinoma: results of a population-based case-control study. Cancer Causes Control. 1993;4:101–10. doi: 10.1007/BF00053150. [DOI] [PubMed] [Google Scholar]
- 9.Maclure M, Willett W. A case-control study of diet and risk of renal adenocarcinoma. Epidemiology. 1990;1:430–40. doi: 10.1097/00001648-199011000-00004. [DOI] [PubMed] [Google Scholar]
- 10.McLaughlin JK, Gao YT, Gao RN, et al. Risk factors for renal-cell cancer in Shanghai, China. Int J Cancer. 1992;52:562–5. doi: 10.1002/ijc.2910520411. [DOI] [PubMed] [Google Scholar]
- 11.Yu MC, Mack TM, Hanisch R, Cicioni C, Henderson BE. Cigarette smoking, obesity, diuretic use, and coffee consumption as risk factors for renal cell carcinoma. J Natl Cancer Inst. 1986;77:351–6. [PubMed] [Google Scholar]
- 12.Benhamou S, Lenfant MH, Ory-Paoletti C, Flamant R. Risk factors for renal-cell carcinoma in a French case-control study. Int J Cancer. 1993;55:32–6. doi: 10.1002/ijc.2910550107. [DOI] [PubMed] [Google Scholar]
- 13.Pelucchi C, La Vecchia C, Negri E, Talamini R, Franceschi S. Alcohol drinking and renal cell carcinoma in women and men. Eur J Cancer Prev. 2002;11:543–5. doi: 10.1097/00008469-200212000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt W, De Lint J. Causes of death of alcoholics. Q J Stud Alcohol. 1972;33:171–85. [PubMed] [Google Scholar]
- 15.Pell S, D'Alonzo CA. A five-year mortality study of alcoholics. J Occup Med. 1973;15:120–5. [PubMed] [Google Scholar]
- 16.Monson RR, Lyon JL. Proportional mortality among alcoholics. Cancer. 1975;36:1077–9. doi: 10.1002/1097-0142(197509)36:3<1077::aid-cncr2820360335>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Jensen OM. Cancer morbidity and causes of death among Danish brewery workers. Int J Cancer. 1979;23:454–63. doi: 10.1002/ijc.2910230404. [DOI] [PubMed] [Google Scholar]
- 18.Adami HO, McLaughlin JK, Hsing AW, et al. Alcoholism and cancer risk: a population-based cohort study. Cancer Causes Control. 1992;3:419–25. doi: 10.1007/BF00051354. [DOI] [PubMed] [Google Scholar]
- 19.Nicodemus KK, Sweeney C, Folsom AR. Evaluation of dietary, medical and lifestyle risk factors for incident kidney cancer in postmenopausal women. Int J Cancer. 2004;108:115–21. doi: 10.1002/ijc.11532. [DOI] [PubMed] [Google Scholar]
- 20.Mahabir S, Leitzmann MF, Virtanen MJ, et al. Prospective study of alcohol drinking and renal cell cancer risk in a cohort of finnish male smokers. Cancer Epidemiol Biomarkers Prev. 2005;14:170–5. [PubMed] [Google Scholar]
- 21.Rashidkhani B, Akesson A, Lindblad P, Wolk A. Alcohol consumption and risk of renal cell carcinoma: a prospective study of Swedish women. Int J Cancer. 2005;117:848–53. doi: 10.1002/ijc.21231. [DOI] [PubMed] [Google Scholar]
- 22.Lee JE, Giovannucci E, Smith-Warner SA, et al. Total fluid intake and use of individual beverages and risk of renal cell cancer in two large cohorts. Cancer Epidemiol Biomarkers Prev. 2006;15:1204–11. doi: 10.1158/1055-9965.EPI-05-0889. [DOI] [PubMed] [Google Scholar]
- 23.Setiawan VW, Stram DO, Nomura AM, Kolonel LN, Henderson BE. Risk factors for renal cell cancer: the multiethnic cohort. Am J Epidemiol. 2007;166:932–40. doi: 10.1093/aje/kwm170. [DOI] [PubMed] [Google Scholar]
- 24.Lee JE, Hunter DJ, Spiegelman D, et al. Alcohol intake and renal cell cancer in a pooled analysis of 12 prospective studies. J Natl Cancer Inst. 2007;99:801–10. doi: 10.1093/jnci/djk181. [DOI] [PubMed] [Google Scholar]
- 25.Greenland S. A meta-analysis of coffee, myocardial infarction, and coronary death. Epidemiology. 1993;4:366–74. doi: 10.1097/00001648-199307000-00013. [DOI] [PubMed] [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 30.Goodman MT, Morgenstern H, Wynder EL. A case-control study of factors affecting the development of renal cell cancer. Am J Epidemiol. 1986;124:926–41. doi: 10.1093/oxfordjournals.aje.a114482. [DOI] [PubMed] [Google Scholar]
- 31.Wolk A, Gridley G, Niwa S, et al. International renal cell cancer study. VII. Role of diet. Int J Cancer. 1996;65:67–73. doi: 10.1002/(SICI)1097-0215(19960103)65:1<67::AID-IJC12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 32.Yuan JM, Gago-Dominguez M, Castelao JE, et al. Cruciferous vegetables in relation to renal cell carcinoma. Int J Cancer. 1998;77:211–6. doi: 10.1002/(sici)1097-0215(19980717)77:2<211::aid-ijc7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 33.Mattioli S, Truffelli D, Baldasseroni A, et al. Occupational risk factors for renal cell cancer: a case – control study in northern Italy. J Occup Environ Med. 2002;44:1028–36. doi: 10.1097/00043764-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Parker AS, Cerhan JR, Lynch CF, Ershow AG, Cantor KP. Gender, alcohol consumption, and renal cell carcinoma. Am J Epidemiol. 2002;155:455–62. doi: 10.1093/aje/155.5.455. [DOI] [PubMed] [Google Scholar]
- 35.Greving JP, Lee JE, Wolk A, et al. Alcoholic beverages and risk of renal cell cancer. Br J Cancer. 2007;97:429–33. doi: 10.1038/sj.bjc.6603890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hsu CC, Chow WH, Boffetta P, et al. Dietary risk factors for kidney cancer in Eastern and Central Europe. Am J Epidemiol. 2007;166:62–70. doi: 10.1093/aje/kwm043. [DOI] [PubMed] [Google Scholar]
- 37.Hu J, Chen Y, Mao Y, Desmeules M, Mery L. Alcohol drinking and renal cell carcinoma in Canadian men and women. Cancer Detect Prev. 2008;32:7–14. doi: 10.1016/j.cdp.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Pelucchi C, Galeone C, Montella M, et al. Alcohol consumption and renal cell cancer risk in two Italian case-control studies. Ann Oncol. 2008;19:1003–8. doi: 10.1093/annonc/mdm590. [DOI] [PubMed] [Google Scholar]
- 39.Benedetti A, Parent ME, Siemiatycki J. Lifetime consumption of alcoholic beverages and risk of 13 types of cancer in men: results from a case-control study in Montreal. Cancer Detect Prevent. 2009;32:352–62. doi: 10.1016/j.canep.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Hu JF, Mao Y, DesMeules M, et al. Total fluid and specific beverage intake and risk of renal cell carcinoma in Canada. Cancer Epidemiol. 2009;33:355–62. doi: 10.1016/j.canep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Brock KE, Gridley G, Chiu BC, et al. Dietary fat and risk of renal cell carcinoma in the USA: a case-control study. Br J Nutr. 2009;101:1228–38. doi: 10.1017/S0007114508056043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Asal NR, Risser DR, Kadamani S, et al. Risk factors in renal cell carcinoma: I. Methodology, demographics, tobacco, beverage use, and obesity. Cancer Detect Prev. 1988;11:359–77. [PubMed] [Google Scholar]
- 43.McLaughlin JK, Mandel JS, Blot WJ, et al. A population-based case-control study of renal cell carcinoma. J Natl Cancer Inst. 1984;72:275–84. [PubMed] [Google Scholar]
- 44.Talamini R, Baron AE, Barra S, et al. A case-control study of risk factor for renal cell cancer in northern Italy. Cancer Causes Control. 1990;1:125–31. doi: 10.1007/BF00053163. [DOI] [PubMed] [Google Scholar]
- 45.Mellemgaard A, Engholm G, McLaughlin JK, Olsen JH. Occupational risk factors for renal-cell carcinoma in Denmark. Scand J Work Environ Health. 1994;20:160–5. doi: 10.5271/sjweh.1413. [DOI] [PubMed] [Google Scholar]
- 46.Lindblad P, Wolk A, Bergstrom R, Adami HO. Diet and risk of renal cell cancer: a population-based case-control study. Cancer Epidemiol Biomarkers Prev. 1997;6:215–23. [PubMed] [Google Scholar]
- 47.Hu J, Mao Y, White K. Diet and vitamin or mineral supplements and risk of renal cell carcinoma in Canada. Cancer Causes Control. 2003;14:705–14. doi: 10.1023/a:1026310323882. [DOI] [PubMed] [Google Scholar]
- 48.Mellemgaard A, Engholm G, McLaughlin JK, Olsen JH. Risk factors for renal cell carcinoma in Denmark. I. Role of socioeconomic satus tobacco use, beverages, and family history. Cancer Causes Control. 1994;5:105–13. doi: 10.1007/BF01830256. [DOI] [PubMed] [Google Scholar]
- 49.McLaughlin JK, Lipworth L. Epidemiologic aspects of renal cell cancer. Semin Oncol. 2000;27:115–23. [PubMed] [Google Scholar]
- 50.Li JJ, Hou X, Banerjee SK, et al. Overexpression and amplification of c-myc in the Syrian hamster kidney during estrogen carcinogenesis: a probable critical role in neoplastic transformation. Cancer Res. 1999;59:2340–6. [PubMed] [Google Scholar]
- 51.Ginsburg ES. Estrogen, alcohol and breast cancer risk. J Steroid Biochem Mol Biol. 1999;69:299–306. doi: 10.1016/s0960-0760(99)00047-3. [DOI] [PubMed] [Google Scholar]
- 52.Cauley JA, Gutai JP, Kuller LH, LeDonne D, Powell JG. The epidemiology of serum sex hormones in postmenopausal women. Am J Epidemiol. 1989;129:1120–31. doi: 10.1093/oxfordjournals.aje.a115234. [DOI] [PubMed] [Google Scholar]
- 53.Longnecker MP, Tseng M. Alcohol, hormones, and postmenopausal women. Alcohol Health Res World. 1998;22:185–9. [PMC free article] [PubMed] [Google Scholar]
- 54.Gerhauser C. Beer constituents as potential cancer chemopreventive agents. Eur J Cancer. 2005;41:1941–54. doi: 10.1016/j.ejca.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 55.Goswami SK, Das DK. Resveratrol and chemoprevention. Cancer Lett. 2009;284:1–6. doi: 10.1016/j.canlet.2009.01.041. [DOI] [PubMed] [Google Scholar]
- 56.Bergstrom A, Hsieh CC, Lindblad P, et al. Obesity and renal cell cancer - a quantitative review. Br J Cancer. 2001;85:984–90. doi: 10.1054/bjoc.2001.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lindblad P, Chow WH, Chan J, et al. The role of diabetes mellitus in the aetiology of renal cell cancer. Diabetologia. 1999;42:107–12. doi: 10.1007/s001250051122. [DOI] [PubMed] [Google Scholar]
- 58.Davies MJ, Baer DJ, Judd JT, et al. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA. 2002;287:2559–62. doi: 10.1001/jama.287.19.2559. [DOI] [PubMed] [Google Scholar]