Abstract
Introduction
Recent evidence suggests that the implantation of bone marrow-derived mesenchymal stem cells improves peripheral nerve regeneration. In this study we aimed to investigate whether adipose-derived stem cells (ADSCs) can be used for peripheral nerve repair.
Material and methods
In a rat model, nerve regeneration was evaluated across a 15 mm lesion in the sciatic nerve by using an acellular nerve injected with allogenic ADSCs. The walking behaviour of rats was measured by footprint analysis, and electrophysiological analysis and histological examination were performed to evaluate the efficacy of nerve regeneration.
Results
Cultured ADSCs became morphologically homogeneous with a bipolar, spindle-like shape after ex vivo expansion. Implantation of ADSCs into the rat models led to (i) improved walking behaviour as measured by footprint analysis, (ii) increased conservation of muscle-mass ratio of gastrocnemius and soleus muscles, (iii) increased nerve conduction velocity, and (iv) increased number of myelinated fibres within the graft.
Conclusions
Adipose-derived stem cells could promote peripheral nerve repair in a rat model. Although the detailed mechanism by which ADSCs promote peripheral nerve regeneration is being investigated in our lab, our results suggest that ADSCs transplantation represents a powerful therapeutic approach for peripheral nerve injury.
Keywords: adipose-derived stem cells, peripheral nerve repair, cell transplantation, sciatic nerve
Introduction
Mesenchymal stem cells (MSCs) are an attractive cell source for the regeneration of nerve tissue due to their self-renewal ability, high growth rate and multi-potent differentiation properties [1]. In particular, the implantation of bone marrow-derived mesenchymal stem cells (BMMSCs) has been shown to exert a beneficial effect on peripheral nerve regeneration [2-5]. However, it is a highly invasive and painful procedure to isolate BMMSCs and the frequency of MSCs in bone marrow is relatively low [6]. Therefore, an alternative cell source is in urgent demand [7, 8].
Adipose-derived stem cells (ADSCs) have similar phenotypic and gene expression profiles to BMMSCs [9, 10], but have unique advantages: they can be harvested easily by a safe and conventional liposuction procedure from subcutaneous fat tissue; the frequency of ADSCs in adipose tissue is much higher than that of MSCs in bone marrow [11]; and ADSCs proliferate significantly faster than BMMSCs [12, 13]. The apparent advantages of ADSCs led us to investigate whether they may be ideal transplantable cells for peripheral nerve repair. In this study we employed a rat model of peripheral nerve injury and demonstrated that implantation of ADSCs promotes peripheral nerve repair.
Material and methods
Preparation and characterization of adipose-derived stem cells
Rat ADSCs were isolated as described previously [14]. Briefly, the inguinal fat pad was harvested from a 4-week old male Wistar rat and the adipose tissue was carefully dissected. The adipose tissue was digested using collagenase type I (Gibco, USA) and then dissociated mechanically. The suspension was centrifuged to separate the floating adipocytes from the stromal vascular fraction. The cells in the stromal vascular fraction were cultured in DMEM (Gibco) supplemented with 10% fetal bovine serum (FBS, Gibco) and the culture medium was changed 24 h later to eliminate the non-adherent cells. The cells were passaged 3-5 times before being used for subsequent experiments. To characterize the multi-potential differentiation capacity of ADSCs, the cells grown to at least 80% confluence were treated for 3 weeks with either osteogenic induction medium or adipogenic induction medium. Osteogenic induction medium was DMEM supplemented with 10% FBS, 0.1 µM dexamethasone, 50 µM ascorbate-2-phosphate, and 10 mM β-glycerophosphate. Adipogenic induction medium was DMEM supplemented with 10% FBS, 0.5 mM isobutyl-methylxanthine (IBMX), 1 µM dexamethasone, 10 µM insulin, and 200 µM indomethacin. Rat ADSCs were then stained with Alizarin Red S or Oil-Red O to confirm the osteogenic and adipogenic differentiation, respectively.
In vitro construction of nerve graft
A total of 2 × 106 ADSCs in 100 µl DMEM were injected into a 1.5-cm long acellular nerve allograft using a microinjector under an SXP-10 microscope at 10× magnification. To perform the injection, the microinjector was inserted through the full length of the nerve section, and cells were injected in equal volumes at four evenly spaced points as the injector was withdrawn. Another set of acellular nerve was used as the control group with no ADSC-laden grafts, in which 100 µl DMEM was injected into the nerve grafts. The nerve grafts were then incubated in DMEM supplemented with 10% FBS in a humidified atmosphere with 5% CO2 at 37°C for 48 h after which they were quickly used for in vivo experiments.
Grafting procedure
Twenty adult female Wistar rats (200-250 g), divided into ADSC-treated (n = 10) and control (n = 10) groups, were anesthetized with chloral hydrate (350 mg/kg) during all surgical procedures. After skin incision, the sciatic nerve was exposed using a muscle splitting incision. With the aid of an operation microscope, the right sciatic nerve (15-mm) was severed and removed near the obturator tendon in the mid-thigh. A 15-mm nerve graft was interposed into this nerve gap. The graft was coapted to the host nerve stumps by epineurial neurorrhaphy using one 9-0 Ethilon suture at each end. The ADSC-treated group received ADSC-laden grafts and the control group received non-ADSC-laden grafts. Both proximal and distal nerve stumps were anchored into the graft with 9-0 nylon microsutures, and the skin was closed with wound clips.
Walking-track analysis
Functional recovery was assessed by calculating the sciatic functional index (SFI) value [15]. SFI = –38.3[(EPL-NPL)/NPL] + 109.5[(ETS-NTS)/NTS] + + 13.3[(EIT-NIT)/NIT] – 8.8 based on analysis of walking tracks [16, 17]. Postoperatively, animals were assessed every 4 weeks to week 12. The investigators were blinded to the animal groups during walking-track analysis.
Electrophysiological analysis
The rats were anesthetized with chloral hydrate (350 mg/kg) and the right sciatic nerve was exposed thoroughly. A crook-shaped silver needle electrode was placed on the proximal end and distal end of grafts. The proximal end was stimulated, and was record at the distal end. The distance between the two electrodes was measured with a sliding caliper of 0.2 mm precision. Nerve conduction velocity, latent period, and peak amplitude were recorded.
Weight of the gastrocnemius and soleus muscles
The rats were anaesthetized with chloral hydrate (350 mg/kg) 12 weeks after operation. The gastrocnemius and soleus muscles were harvested from the experimental and control sides, tendons were trimmed and muscles were weighed. A conservation muscle-mass ratio was recorded for each animal by dividing the experimental-side muscle mass by the control-side muscle mass [18, 19].
Histological examination
The segments of the nerves were immersed in 2.5% Na-cacodylate-buffered glutaraldehyde solution for 2 h, and fixed for 2 h in 2% Na-cacodylate-buffered osmium tetroxide, then serially dehydrated in increasing concentrations of ethanol, infiltrated and embedded in Epon 812 (Ted Pella, CA, USA). A 1-µm-thick cross-section was obtained and then stained with toluidine blue to evaluate the efficacy of nerve regeneration.
Statistical analysis
The data were expressed as mean ± SD and analysed by SPSS 13.0 software. Analysis of variance was used for significant difference test. Student’s t-test was used for inter-group comparison. Value of p < 0.05 was considered statistically significant.
Results
Characterization of cultured adipose-derived stem cells
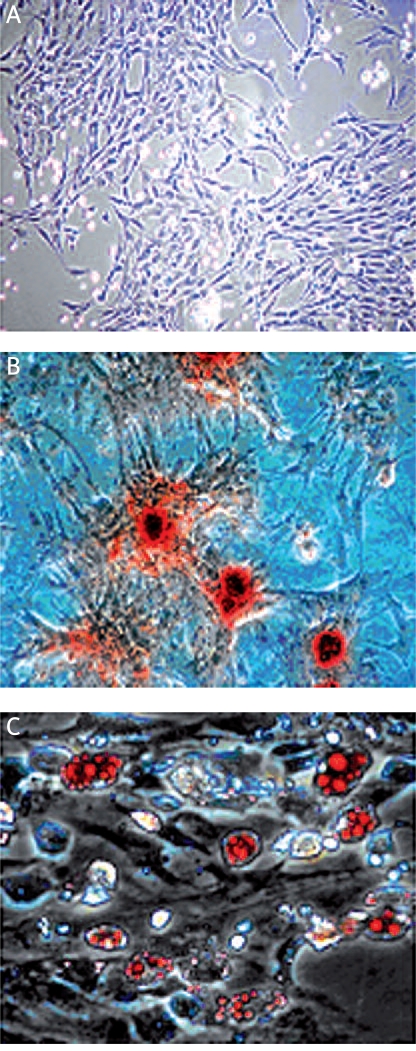
Rat ADSCs within 3-5 passages appeared as a monolayer of large and flat cells (Figure 1 A). When induced with appropriate lineage-specific induction medium, ADSCs underwent adipogenesis (Figure 1 B) and osteogenesis (Figure 1 C), demonstrating that we successfully isolated ADSCs with stem cell characteristics.
Figure 1.
Rat ADSCs exhibit properties of stem cells. Rat ADSCs were passaged 3-5 times after initial plating of the primary culture. A – under phase contrast microscope, cultured ADSCs appeared as spindle-shaped. Rat ADSCs could undergo multilineage differentiation, including osteogenesis (B) and adipogenesis (C), as visualized by staining with Alizarin Red S or Oil-Red O, respectively (light microscope, ×200)
Adipose-derived stem cells implantation promotes nerve regeneration
None of the 20 rats developed any serious post-surgical complications. Wounded tissues healed spontaneously, and there were no trophic ulcerations on the operated legs. About 3 or 4 weeks later, muscular atrophy of operated legs of control groups was obvious. The adherence was very slight between the grafts and around tissues with no formation of neuroma.
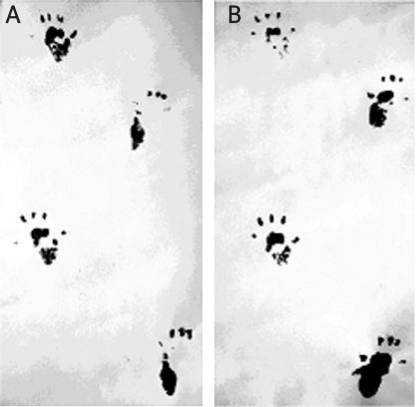
To evaluate the efficacy of ADSCs in functional improvement in peripheral nerve injured rats, we first compared the SFI between the ADSC-treated group and the control group. Typical walking tracts obtained from the rats 12 weeks after surgery are shown in Figure 2. The SFI analysis showed that functional recovery of the ADSC-treated group was significant better than that of the control group (p < 0.05) (Table I).
Figure 2.
Walking tract analysis. A – control group. B – ADSC-treated group
Table I.
Functional nerve regeneration (SFI)
| Postoperative week | Control group | ADSC-treated group |
|---|---|---|
| 4 | –97.50 ±0.12 | –96.18 ±0.57 |
| 8 | –90.70 ±0.09 | –82.82 ±0.49* |
| 12 | –86.79 ±0.4 | –80.51 ±0.09* |
Values were mean ± SD
p < 0.05 compared to control group
In addition, electrophysiological analysis revealed higher nerve conduction velocities and peak amplitudes, and shorter incubation period in the ADSC-treated group compared to the control group and the differences were statistically significant (p < 0.05) (Table II). After 12 weeks of nerve injury, the right gastrocnemius and soleus muscles degenerated and lost weight. The muscle-mass conservation ratio showed that ADSC transplantation could reduce sciatic nerve injury-induced weight loss (Table II).
Table II.
Electrophysiological index and conservation muscle-mass ratio of gastrocnemius and soleus muscles (n = 10)
| Group conservation | Nerve conduction velocity [m/s] | Incubation period [ms] | Peak amplitude[mm] | Muscle-mass ratio [%] |
|---|---|---|---|---|
| Control | 8.16 ±0.42 | 1.85 ±0.05 | 3.04 ±0.31 | 38.13 ±3.76 |
| ADSC-treated | 10.72 ±0.58* | 1.39 ±0.07* | 6.29 ±0.52* | 44.50 ±4.63* |
Values were mean ± SD
p < 0.05 compared to control group
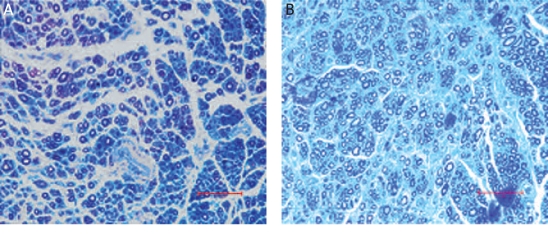
To evaluate the regeneration efficacy of myelinated nerves after grafting with nerve grafts, we examined semi-thin sections of the distal ends of the grafts. Light microscopic examination of toluidine blue-stained cross-sections of these distal ends revealed an obvious difference between the experimental and control group. In particular, the experimental ADSC grafts had a comparatively higher density of well-myelinated fibres in the distal portion of the graft (Figure 3 B). In contrast, the control grafts showed a relatively low density of well-myelinated fibres (Figure 3 A). The percentages of nerve fibre density and nerve fibre diameter were significantly higher in the experimental group compared to the control group (Table III). These data indicate greater axonal regeneration in ADSC-laden acellular nerve grafts than in non-ADSC-laden grafts.
Figure 3.
Improved nerve regeneration after ADSC graft. Semi-thin cross-sections of the distal portion of nerve graft were subjected to Toluidine blue stain 12 weeks post-surgery. A – the control, non-ADSC-laden graft group showed impaired regeneration with thin remyelinization. B – the ADSC-laden graft showed a higher density of well-myelinated nerve fibres. Scale bar = 50 µm
Table III.
Regeneration of myelinated fibre (n = 6)
| Group | Myelinated fibre number [root/mm2] | Myelin sheath thickness [µm] | Myelinated fibres/total nerves [%] |
|---|---|---|---|
| Control | 1378 ±91 | 0.47 ±0.07 | 39.63 ±3.50 |
| ADSC-treated | 1671 ±122* | 0.92 ±0.09* | 50.88 ±6.08* |
Values were mean ± SD,
p < 0.05 compared to control group
Discussion
Cell transplantation has emerged as a novel therapeutic approach for peripheral nerve defects. The ideal transplantable cells should be easily accessible, proliferate in culture and successfully integrate into host tissue with immunological tolerance [20]. Adipose-derived stem cells can be isolated simply by conventional liposuction procedures from abundant subcutaneous fat deposits. Furthermore, these cells can be expanded in culture for extended periods and proliferate rapidly [13, 21].
In the present study, we provided several lines of evidence that ADSC implantation could promote the repair of 10-mm nerve defects in acellular nerve: (i) improved walking behaviour as measured by footprint analysis, (ii) increased conservation of muscle-mass ratio of gastrocnemius and soleus muscles, (iii) increased nerve conduction velocity, and (iv) increased the number of myelinated fibres within the graft. These data indicate that ADSCs have great potential to promote the regeneration of peripheral nerve.
However, it is important to note that we only examined the effects of ADSC implantation on the regeneration of peripheral nerve 12 weeks after the grafting, which is one limitation of the present study. Future multiple-time-point and long-term investigations are necessary to find out how long implanted ADSCs can promote the peripheral nerve regeneration and at what time point the ADSCs exhibit the best effects.
In conclusion, our study demonstrated that ADSCs promote peripheral nerve repair in a rat model. Although the detailed mechanism by which ADSCs promote peripheral nerve regeneration is being investigated in our lab, our results reported here suggest that ADSC transplantation represents a powerful therapeutic approach for peripheral nerve injury.
Acknowledgments
This study was supported by grants from Shenyang Science and Technology Development Fund (No. F10-205-1-69) and Liaoning Science and Technology Development Fund (No. 2010225029), and Heilongjiang Youth Science Fund (No. QC2010040).
References
- 1.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 2.Cuevas P, Carceller F, Dujovny M. Peripheral nerve regeneration by bone marrow stromal cells. Neurol Res. 2002;24:634–8. doi: 10.1179/016164102101200564. [DOI] [PubMed] [Google Scholar]
- 3.Cuevas P, Carceller F, Garcia-Gomez I. Bone marrow stromal cell implantation for peripheral nerve repair. Neurol Res. 2004;26:230–2. doi: 10.1179/016164104225013897. [DOI] [PubMed] [Google Scholar]
- 4.Chen CJ, Ou YC, Liao SL. Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp Neurol. 2007;204:443–53. doi: 10.1016/j.expneurol.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 5.Dong Wang, Xiao-Lin Liu, Jia-Kai Zhu. Bridging small-gap peripheral nerve defects using acellular nerve allograft implanted with autologous bone marrow stromal cells in primates. Brain Res. 2008;1188:44–53. doi: 10.1016/j.brainres.2007.09.098. [DOI] [PubMed] [Google Scholar]
- 6.Zuk PA, Zhu M, Mizuno H. Multilineage cells from human adiposetissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 7.Lee TH, Yoon JG. Intracerebral transplantation of human adipose tissue stromal cells after middle cerebral artery occlusion in rats. J Clin Neurosci. 2008;15:907–12. doi: 10.1016/j.jocn.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Li X, Gong P, Liao D. In vitro neural/glial differentiation potential of periodontal ligament stem cells. Arch Med Sci. 2010;6:678–85. doi: 10.5114/aoms.2010.17080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Ugarte DA, Morizono K, Elbarbary A. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–9. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 10.Strem BM, Hicok KC, Zhu M. Multipotential differentiation of adipose tissue-derived stem cells. Keio J Med. 2005;54:132–41. doi: 10.2302/kjm.54.132. [DOI] [PubMed] [Google Scholar]
- 11.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100:1249–60. doi: 10.1161/01.RES.0000265074.83288.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshimura H, Muneta T, Nimura A. Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 2007;327:449–62. doi: 10.1007/s00441-006-0308-z. [DOI] [PubMed] [Google Scholar]
- 13.Liao D, Gong P, Li X, Tan Z, Yuan Q. Co-culture with Schwann cells is an effective way for adipose-derived stem cells neural transdifferentiation. Arch Med Sci. 2010;6:145–51. doi: 10.5114/aoms.2010.13885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu YF, Liu L, Li Y. Myelin-forming ability of Schwann cell-like cells induced from rat adipose derived stem cells in vitro. Brain Res. 2008;1239:49–55. doi: 10.1016/j.brainres.2008.08.088. [DOI] [PubMed] [Google Scholar]
- 15.Bain JR, MacKinnon SE, Hunter DA. Functional evalution of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989;83:129–36. doi: 10.1097/00006534-198901000-00024. [DOI] [PubMed] [Google Scholar]
- 16.de Medinaceli L, Freed WJ, Wyatt RJ. An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks. Exp Neurol. 1982;77:634–43. doi: 10.1016/0014-4886(82)90234-5. [DOI] [PubMed] [Google Scholar]
- 17.Carlton JM, Goldberg NH. Quantitating intergrated muscle function following reinnervation. Surg Forum. 1986;37:611–2. [Google Scholar]
- 18.Newman JP, Verity AN, Hawatmeh S. Ciliary neurotrophic factor enhances peripheral nerve regeneration. Arch Otolaryngol Head Neck Surg. 1996;122:399–403. doi: 10.1001/archotol.1996.01890160041008. [DOI] [PubMed] [Google Scholar]
- 19.Zhi Li. Effects of local release of hepatocyte growth factor on peripheral nerve regeneration in acellular nerve grafts. Exp Neurol. 2008;214:47–54. doi: 10.1016/j.expneurol.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 20.Tohill M, Terenghi G. Stem-cell plasticity and therapy for injuries of the peripheral nervous system. Biotechnol Appl Biochem. 2004;40:17–24. doi: 10.1042/BA20030173. [DOI] [PubMed] [Google Scholar]
- 21.Zuk PA, Zhu M, Ashjian P. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]