To help save lives and improve human health, it is crucial for policy makers to ensure equity in access to drugs, drug efficacy, quality and safety, and rational use of drugs, which are indicators of the National Drug Policy (NDP) in evaluation of pharmaceutical system performance in every country. There are some retrospective evaluation methods that are currently used to appraise the success of NDP and adherence to its objectives in the relevant countries.
Nikfar et al. (2005) for the first time designed a weighted questionnaire system by a nominal group technique based on the answers to the questions for adding a single drug to the National Drug List (NDL). They found that although the system, structures, and mechanisms are in place, they do not function properly in some topics. Assessment of 59 dossiers of approved drugs for adding to the NDL during a 5-year period showed that decision makers pay more attention to efficacy, safety, and rationality in use rather than accessibility and affordability. Nikfar et al. (2005) concluded that every country must establish a survey system to check implementation of the processes to achieve the NDP's objectives of better protection of public health and equal access to pharmaceuticals [1]. Our belief is that the problem is still there and has not been resolved yet by the national drug authority (NDA) and the objectives of national drug policies (NDP) have not been met yet [2].
Adding or deleting medicines from the essential lists, establishing national formularies, registering the health professionals, facilities and goods, financial interventions, pricing and reimbursement, supervising the supply chain, pharmacovigilance, regulating advertising, and promoting rational drug use are some of the daily decisions that are made by NDAs. On the other hand, some factors in the market such as product specifications, prices, supply system and promotion affect the decisions. Furthermore, the cost of drugs is a hurdle for utilization as despite availability it affects accessibility [3–5].
These mixed variables complicate decisions that are needed to be made even in uncertain conditions. The NDAs usually cannot predict and evaluate consequences of their decisions. Although some national and international organizations offer some key indicators for evaluation of NDP on the basis of availability, affordability, quality, and rationality, these indicators are often retrospective and they need time-consuming data collecting procedures.
Lack of key indicators for evaluation of NDP in many countries causes failure in determining the relationship between health indices and the volume of the pharmaceutical market [2]. Although these countries have organizations and rules for regulating medicines, the system often does not function properly [6, 7]. Sometimes lack of tools for predicting the future leads to some policies which could not be evaluated at the time of decision making [5]. In the case of safety, efficacy, and quality, NDAs usually try to follow major organizations such as the FDA or EMEA; but to enhance availability and affordability, each country needs its own local solutions. Accurate national economic and health data, sufficient human resources, clarified rules and efficient structure are some prerequisites for calculation of the indicators that are the main weaknesses of most countries. Of course, the consequences of these defects would be extended to the life of all people and the community and health policy makers [8].
According to the mentioned explanations, pharmaceutical policy makers need a kind of decision support system which should be responsible for the following tasks:
– shortening the decision process,
– increasing the rationality of actions,
– evaluating the different alternatives,
– reducing the costs,
– decreasing the human-derived mistakes,
– increasing reliability and validity,
– providing potentials for sensitivity analysis and repeatability.
Recently, modelling and simulation techniques have created an opportunity to reduce the costs and risks of uncertain conditions. Searching bibliography databases shows the techniques that are used in artificial physician software and in the quantitative structuring of drugs. In managerial work and policy making, modelling and simulation have been applied in some fields [9, 10].
Although there are a few articles about the use of simulation models as a decision support system for hospitals or disease management, we think that developing a well-adjusted model is crucial to reduce the risk of decisions in pharmaceutical policy.
There are many models for simulation but only a few of them have been used in the health care system. They are as follows:
Mathematic models that try to put all relations and interactions between variables in a unique formula. Deferential equations, Fourier-type series and expansions are some methods in this modelling. Validation of the model strongly relies on the quantity and quality of variables which are used in the model [11].
Econometric models use statistical regression to make an equation between independent and dependent variables. Although there are many techniques to adjust the best model to variables, many unknown factors in pharmaceutical markets decrease the accuracy of the model. In spite of the accuracy of the models, regression models connect some input variables to one output variable. In this situation, we have many NDP indicators as the output variable and some output variables are inputs for other models. Thus we need at least one regression model for each output variable, which unfortunately would make the model too complicated [12].
Artificial neural networks simulate the neurons’ functions by connecting some input variables to some output variables. These models have to be trained to show an acceptable accuracy, but for training they need too many data. Usually it is impossible to find a good data source for model variables, especially in developing countries [12, 13].
System dynamic is another model to simulate many industrial, social and natural events. In these models, complicated events break down to simple parts, and then some stocks and flows are defined. Changes in flows and stocks levels can simulate the events. These models can adjust many social and managerial situations easily. Usually mathematical equations define relations between stocks [14, 15].
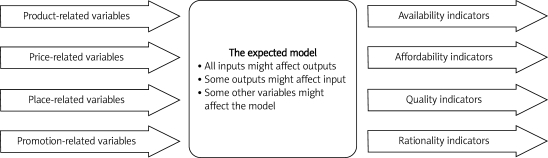
Here, to develop a decision support system to simulate NDP indicators, we propose 3 main groups of input variables and 4 main groups for output, as shown in Figure 1.
Figure 1.
The expected model of support system to simulate national drug policies indicators
The three main groups of input variables are:
Common variables such as population and its structure, economics and health situation.
Disease variables including prevalence, incidence, burden, duration and treatments.
Pharmaceutical market-related variables such as product specification, price, supply chain, promotion and people (4 Ps in marketing: Product, Price, Place, and Promotion).
Output variables are NDP indicators including availability, affordability, quality, and rationality indices. The model is supposed to simulate the interactions between input variables and predict changes in output variables that should have some specification:
It should be multi-variable to multi-variable, having the capability to connect many inputs to some output variables.
It doesn't need so many data for training.
The model has to be as simplest as possible.
Based on the specifications and limitations of mentioned models, a combination of the system dynamics models and econometrics can simulate the situations of the decision making process in NDAs in the environment of the pharmaceutical market and its variables. This simulation model creates an opportunity to evaluate the consequences of each decision in NDAs based on NDP indicators. The model can be implemented in software to help as a decision support system in reduction of the risk of decision making in uncertain situations.
References
- 1.Nikfar S, Kebriaeezadeh A, Majdzadeh R, Abdollahi M. Monitoring of national drug policy (NDP) and its standardized indicators; conformity to decisions of the national drug selecting committee in Iran. BMC Int Health Hum Rights. 2005;5:5. doi: 10.1186/1472-698X-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdollahiasl A, Nikfar S, Abdollahi M. Pharmaceutical market and health system in the Middle Eastern and Central Asian countries: time for innovations and changes in policies and actions. Arch Med Sci. 2011;7:365–7. doi: 10.5114/aoms.2011.23397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nikfar S, Khatibi M, Abdollahi-Asl A, Abdollahi M. Cost and utilization study of antidotes: an Iranian experience. Int J Pharmacol. 2011;7:46–9. [Google Scholar]
- 4.Abdollahiasl A, Kebriaeezadeh A, Nikfar S, Farshchi A, Ghiasi G, Abdollahi M. Patterns of antibiotic consumption in Iran during 2000-2009. Int J Antimicrob Agents. 2011;37:489–90. doi: 10.1016/j.ijantimicag.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 5.Kebriaeezadeh A, Abdollahi M. Iran seeks to boost domestic pharma production. Scrip. 2002;2695:16. [Google Scholar]
- 6.Using indicators to measure country pharmaceutical situations. World Health Organization; 2006. Fact Book on WHO Level I and Level II monitoring indicators. [Google Scholar]
- 7.The world medicines situation. World Health Organization; 2004. [Google Scholar]
- 8.White F, Nanan D. Community health case studies selected from developing and developed countries-common principles for moving from evidence to action. Arch Med Sci. 2008;4:358–63. [Google Scholar]
- 9.Weinstein MC, Toy EL, Sandberg EA, et al. Modeling for health care and other policy decisions: uses, roles, and validity. Value Health. 2001;4:348–61. doi: 10.1046/j.1524-4733.2001.45061.x. [DOI] [PubMed] [Google Scholar]
- 10.Davis P, Lay-Yee R, Pearson J. Using micro-simulation to create a synthesised data set and test policy options: the case of health service effects under demographic ageing. Health Policy. 2010;97:267–74. doi: 10.1016/j.healthpol.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Schlessinger L, Eddy DM. Archimedes: a new model for simulating health care systems – the mathematical formulation. Biomed Inform. 2002;35:37–50. doi: 10.1016/s1532-0464(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 12.Mullahy J. Econometric modeling of health care costs and expenditures a survey of analytical issues and related policy considerations. Med Care. 2009;47(7 Suppl 1):S104–8. doi: 10.1097/MLR.0b013e31819c9593. [DOI] [PubMed] [Google Scholar]
- 13.DiRusso SM, Sullivan T, Holly C, Cuff SN, Savino J. An artificial neural network as a model for prediction of survival in trauma patients: validation for a regional trauma area. J Trauma. 2000;49:212–20. doi: 10.1097/00005373-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Galea S, Hall C, Kaplan GA. Social epidemiology and complex system dynamic modelling as applied to health behaviour and drug use research. Int J Drug Policy. 2009;20:209–16. doi: 10.1016/j.drugpo.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonnell G, Heffernan M, Faulkner A. 2005. Using system dynamics to analyse health system performance within the WHO framework in international system dynamics Pty Ltd. [Google Scholar]