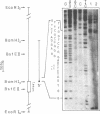
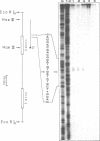
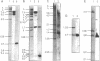Abstract
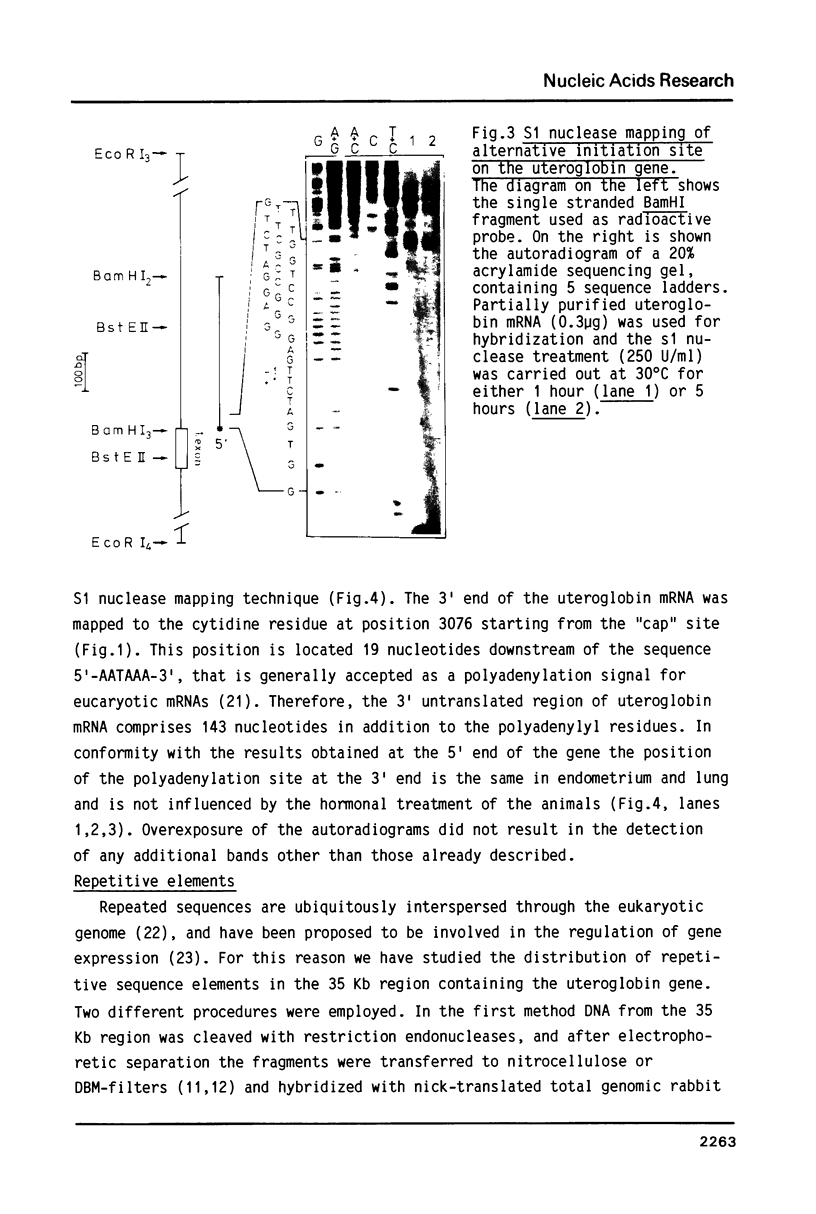
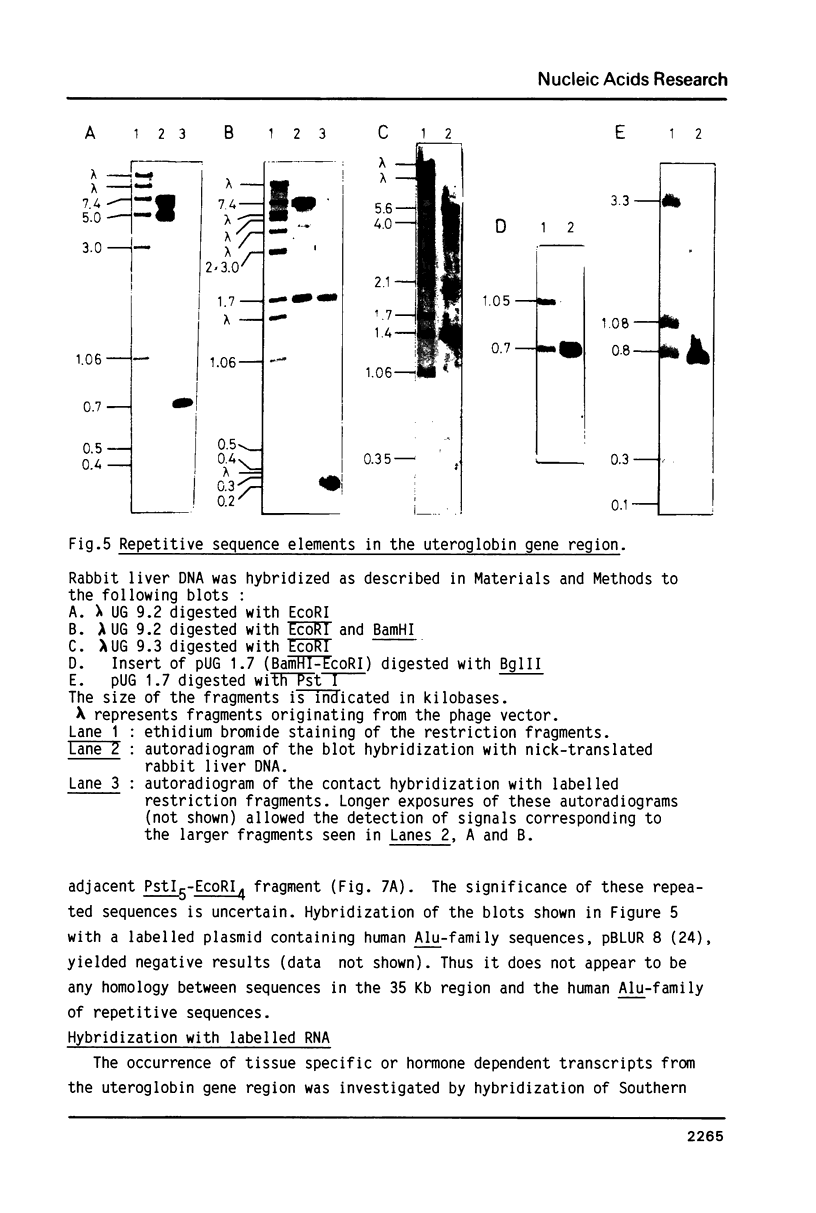
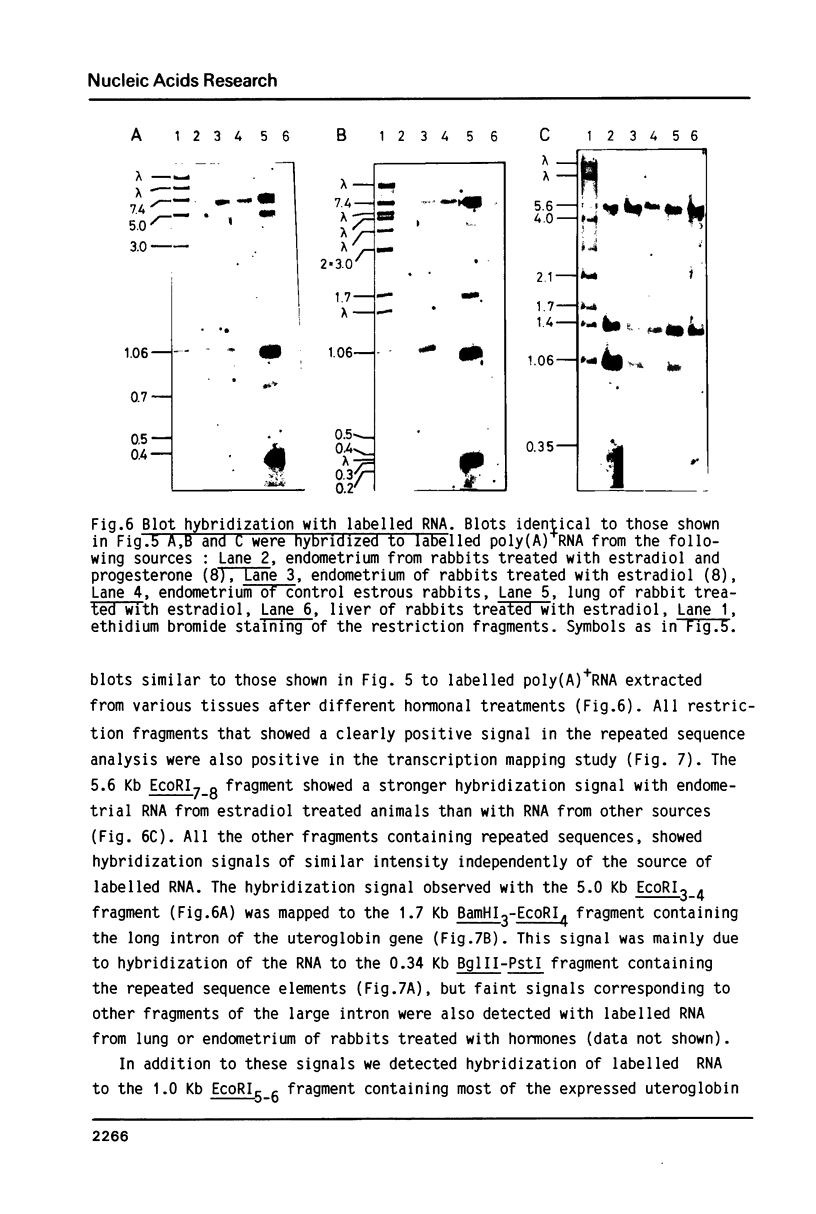
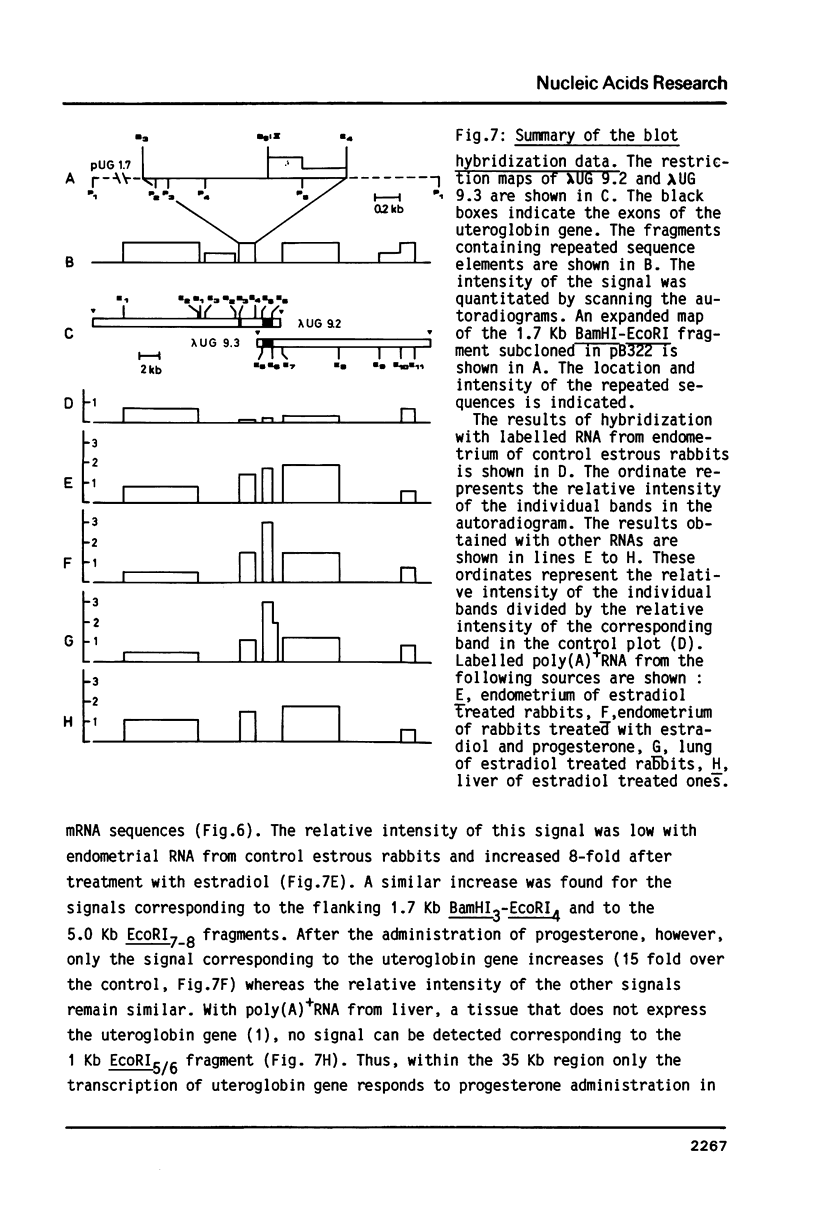
Differential uteroglobin induction represents an appropriate model for the molecular analysis of the mechanism by which steroid hormones control gene expression in mammals. We have analyzed the structure and hormonal regulation of a 35 Kb region of genomic DNA in which the uteroglobin gene is located. The complete sequence of 3,700 nucleotides including the uteroglobin gene and its flanking regions has been determined, and the limits of the gene established by S1 nuclease mapping. Several regions containing repeated sequences were mapped by blot hybridization, one of which is located within the large intron in the uteroglobin gene. Analysis of the RNAs extracted from endometrium, lung and liver, after treatment with estrogen and/or progesterone shows that within the 35 Kb region, the uteroglobin gene is the only DNA segment whose transcription into stable RNA is induced by progesterone.
Full text
PDF




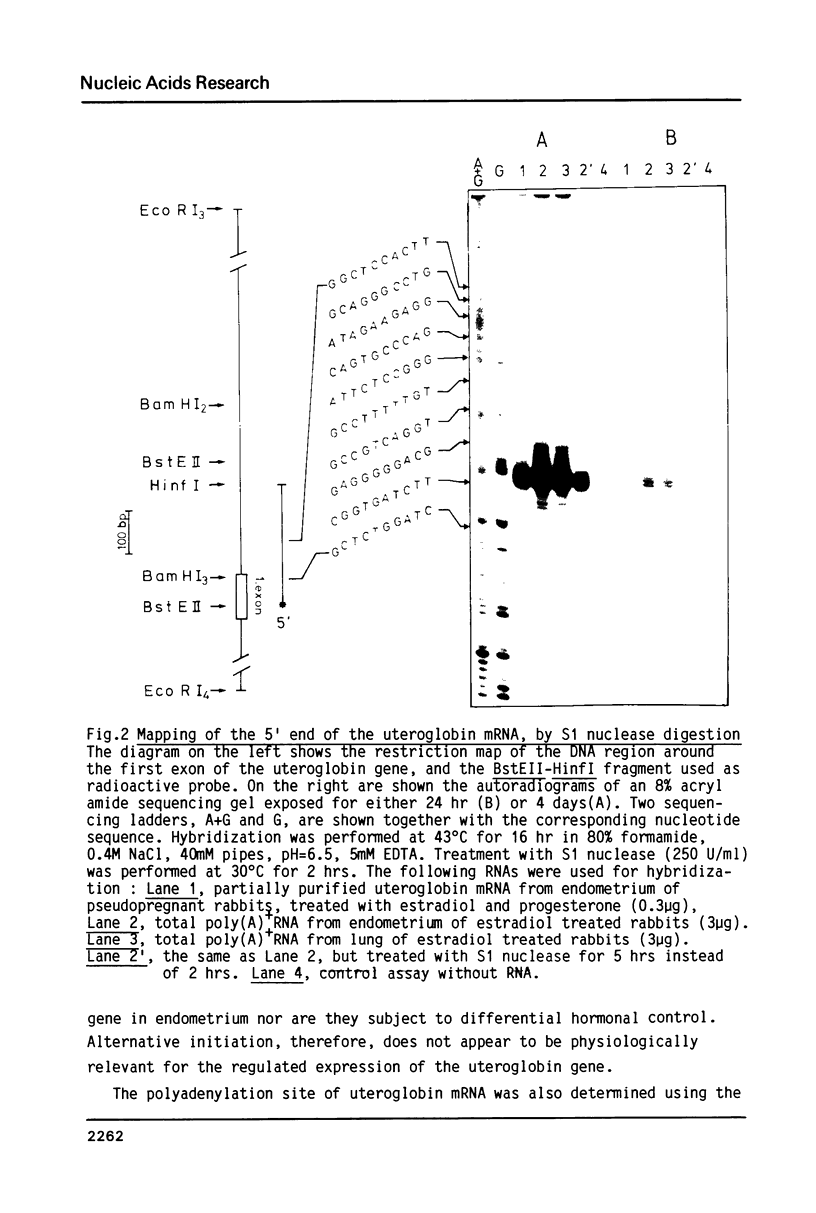





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnemann J., Heins B., Beato M. Purification and properties of rabbit uterus preuteroglobin mRNA. Nucleic Acids Res. 1977 Nov;4(11):4023–4036. doi: 10.1093/nar/4.11.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Blin N., Stafford D. W. A general method for isolation of high molecular weight DNA from eukaryotes. Nucleic Acids Res. 1976 Sep;3(9):2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten R. J., Davidson E. H. Gene regulation for higher cells: a theory. Science. 1969 Jul 25;165(3891):349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Cochet M., Chang A. C., Cohen S. N. Characterization of the structural gene and putative 5'-regulatory sequences for human proopiomelanocortin. Nature. 1982 May 27;297(5864):335–339. doi: 10.1038/297335a0. [DOI] [PubMed] [Google Scholar]
- Dierks P., van Ooyen A., Mantei N., Weissmann C. DNA sequences preceding the rabbit beta-globin gene are required for formation in mouse L cells of beta-globin RNA with the correct 5' terminus. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1411–1415. doi: 10.1073/pnas.78.3.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasel N., Pearson K., Buetti E., Diggelmann H. The region of mouse mammary tumor virus DNA containing the long terminal repeat includes a long coding sequence and signals for hormonally regulated transcription. EMBO J. 1982;1(1):3–7. doi: 10.1002/j.1460-2075.1982.tb01115.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisse S., Scheidereit C., Westphal H. M., Hynes N. E., Groner B., Beato M. Glucocorticoid receptors recognize DNA sequences in and around murine mammary tumour virus DNA. EMBO J. 1982;1(12):1613–1619. doi: 10.1002/j.1460-2075.1982.tb01363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kröger M., Kröger-Block A. A flexible new computer program for handling DNA sequence data. Nucleic Acids Res. 1982 Jan 11;10(1):229–236. doi: 10.1093/nar/10.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N. M., Chandra T., Woo S. L., Bullock D. W. Transcriptional activity of the uteroglobin gene in rabbit endometrial nuclei during early pregnancy. Endocrinology. 1982 Oct;111(4):1115–1120. doi: 10.1210/endo-111-4-1115. [DOI] [PubMed] [Google Scholar]
- LeMeur M., Glanville N., Mandel J. L., Gerlinger P., Palmiter R., Chambon P. The ovalbumin gene family: hormonal control of X and Y gene transcription and mRNA accumulation. Cell. 1981 Feb;23(2):561–571. doi: 10.1016/0092-8674(81)90152-5. [DOI] [PubMed] [Google Scholar]
- Maizels N. Dictyostelium 17S, 25S, and 5S rDNAs lie within a 38,000 base pair repeated unit. Cell. 1976 Nov;9(3):431–438. doi: 10.1016/0092-8674(76)90088-x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Menne C., Suske G., Arnemann J., Wenz M., Cato A. C., Beato M. Isolation and structure of the gene for the progesterone-inducible protein uteroglobin. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4853–4857. doi: 10.1073/pnas.79.16.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulvihill E. R., LePennec J. P., Chambon P. Chicken oviduct progesterone receptor: location of specific regions of high-affinity binding in cloned DNA fragments of hormone-responsive genes. Cell. 1982 Mar;28(3):621–632. doi: 10.1016/0092-8674(82)90217-3. [DOI] [PubMed] [Google Scholar]
- Müller H., Beato M. RNA synthesis in rabbit endometrial nuclei. Hormonal regulation of transcription of the uteroglobin gene. Eur J Biochem. 1980 Nov;112(2):235–241. doi: 10.1111/j.1432-1033.1980.tb07199.x. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Savouret J. F., Loosfelt H., Atger M., Milgrom E. Differential hormonal control of a messenger RNA in two tissues Uteroglobin mRNA in the lung and the endometrium. J Biol Chem. 1980 May 10;255(9):4131–4136. [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Schmid W., Scherer G., Danesch U., Zentgraf H., Matthias P., Strange C. M., Röwekamp W., Schütz G. Isolation and characterization of the rat tryptophan oxygenase gene. EMBO J. 1982;1(10):1287–1293. doi: 10.1002/j.1460-2075.1982.tb00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Reeder R. H. The nucleotide sequence of the initiation and termination sites for ribosomal RNA transcription in X. laevis. Cell. 1979 Oct;18(2):485–499. doi: 10.1016/0092-8674(79)90066-7. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. I., Nickol J., Felsenfeld G. Repeated sequence organization and RNA transcription map of the chicken adult beta-globin gene region. J Biol Chem. 1981 Feb 25;256(4):1502–1506. [PubMed] [Google Scholar]
- Zasloff M., Santos T. Reiteration frequency mapping: analysis of repetitive sequence organization within cloned DNA fragments containing the human initiator methionine tRNA gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5668–5672. doi: 10.1073/pnas.77.10.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooyen A., van den Berg J., Mantei N., Weissmann C. Comparison of total sequence of a cloned rabbit beta-globin gene and its flanking regions with a homologous mouse sequence. Science. 1979 Oct 19;206(4416):337–344. doi: 10.1126/science.482942. [DOI] [PubMed] [Google Scholar]