Abstract
The risk of avian influenza outbreaks in poultry is partially dependent on the probability of contact between domestic poultry and wild birds shedding avian influenza (AI) virus. The major objective of this study was to document wild bird activity on poultry farms to determine which wild bird species should be targeted for AI surveillance in Canada. We collected data in 2 major poultry producing regions of Canada, southwestern Ontario and the Fraser Valley of British Columbia, on the relative abundance of various wild bird species found on poultry farms and on how these species utilized habitat around poultry farms. We reviewed the published literature to determine what was known about AI pathobiology in the species we observed. From these results we created a list of 10 wild bird species that are a priority for further study. These species are the European starling, barn swallow, rock dove, American crow, northwestern crow, American robin, dark-eyed junco, song sparrow, horned lark, and common grackle. Abundance of these and other species varied between provinces and seasons.
Résumé
Utilisation de l’observation de l’activité des oiseaux sauvages dans les fermes avicoles et examen de la documentation afin de cibler les espèces prioritaires pour le dépistage de l’influenza aviaire dans 2 régions du Canada. Le risque d’éclosions d’influenza aviaire chez la volaille dépend en partie de la probabilité du contact entre la volaille domestique et les oiseaux sauvages sécrétant le virus de l’influenza aviaire (IA). L’objectif principal de cette étude consistait à documenter l’activité de la sauvagine dans les fermes avicoles afin de déterminer les espèces d’oiseaux sauvages qui devraient être ciblées pour la surveillance de l’IA au Canada. Nous avons recueilli des données dans 2 grandes régions avicoles du Canada, le Sud-Ouest de l’Ontario et la vallée du Fraser de la Colombie-Britannique, sur l’abondance relative des diverses espèces d’oiseaux sauvages trouvées dans les fermes avicoles et sur la façon dont ces espèces utilisaient l’habitat autour des fermes avicoles. Nous avons examiné la documentation publiée afin de déterminer ce qui est connu à propos de la pathobiologie de l’IA chez les espèces que nous avons observées. À partir de ces résultats, nous avons créé une liste de 10 espèces d’oiseaux sauvages qui représentent une priorité pour des études approfondies. Ces espèces sont l’étourneau sansonnet, l’hirondelle rustique, le pigeon biset, la corneille d’Amérique, la corneille d’Alaska, le merle d’Amérique, le junco ardoisé, le bruant chanteur, l’alouette hausse-col et le quiscale bronzé. L’abondance de ces espèces et d’autres espèces variait selon la province et la saison.
(Traduit par Isabelle Vallières)
Introduction
Avian influenza virus (AIV) has been detected in many species of wild birds throughout the world (1). The virus is most frequently detected in apparently healthy individuals during surveillance programs, but it has occasionally been detected after mortality incidents involving groups of wild birds (2–5). Surveillance of wild birds for AIV can provide information about the prevalence and strains of AIV circulating locally. It may also provide early warning of incursion of circulating highly pathogenic strains into a new geographic area (6).
Wild bird species are included in surveillance programs for reasons that include previously reported high AIV prevalence and accessibility for sampling (7). In Canada and throughout the world, many species of Anseriformes (waterfowl) and Charadriiformes (gulls and shorebirds) have been tested in large numbers and shown to be competent viral hosts (8,9). Prevalence of infection in Anseriformes and Charadriiformes varies greatly among closely related species, as well as with age and season (5,10). Wild bird species other than Anseriformes and Charadriiformes appear to be less susceptible to infection with AIV, and if infected, appear to excrete less virus (1,11,12); however, recent research has detected AIV in 2.3% (13) and 18% (14) of passerine species sampled near wetland habitat. Surveillance and inoculation studies have not been designed specifically to investigate which species might play a role in AIV transmission to poultry in Canada.
Anseriformes and Charadriiformes infected with AIV are generally asymptomatic and excrete large amounts of virus in their feces (11,15). Non-Anseriforme and Charadriiforme species might transmit infectious material either by active shedding, or by mechanical transfer of water droplets or waterbird feces containing AIV. Wild bird species that enter poultry barns could carry AIV particles directly into the poultry flock, while wild bird species spending time near poultry barns could contaminate surfaces near barns from which AIV could be carried into poultry barns by farm workers, equipment, pets, or rodent or insect pests. Depending on environmental conditions, AIV in water droplets, fecal material, or organic debris could remain infective for days to weeks (15,16)
Because detection of AIV, particularly H5 and H7 strains, in wild birds on or near poultry farms could result in implementation of disease control actions on poultry farms (17), surveillance of wild birds in Canada is functionally restricted to areas that are distant from commercial poultry farms. These areas have different habitat and human activity patterns than commercial poultry farms; therefore, sampling of wild birds off-farm may not reflect the species distribution on poultry farms and species that are most likely to have contact with poultry might be insufficiently represented to estimate their potential to transmit AIV (7). Determining which wild bird species are common on poultry farms would facilitate targeted sampling of these species in areas distant from poultry farms. In addition, laboratory studies of these species could be used to assess their ability to amplify and transmit AIV.
The major objective of this study was to determine which wild bird species in 2 major poultry producing regions of Canada should be targeted for further investigation of AIV–host interactions. We used bird counts to document which wild bird species occur on poultry farms and a literature review to gather published information on AI virus-host interactions in the observed species. Finally, we integrated the 2 sources of information to create a “high priority” species list that included Anseriforme and Charadriiformes we observed in proximity to poultry barns, and other species whose habitat use might create contact bridges between wild-bird source AIV and poultry. The study areas were i) southwestern Ontario (ON), which produces the largest amount of poultry products in Canada, and ii) the Fraser Valley of British Columbia (BC), which produces the third largest amount of poultry products in Canada and has had 3 isolations of AI in commercial poultry farms since 2004.
Materials and methods
Wild birds were counted on commercial poultry farms in southwestern British Columbia (BC) and Ontario (ON) between autumn 2007 and summer 2008. In BC, the study area was located between 49°03′ and 49°08′ N and 121°52′ and 122°33′ W, a 15 km × 80 km area in the Fraser Valley bounded to the north by the Fraser River, to the south by the US border, and to the east and west by the borders of the municipalities of Chilliwack and Langley. In ON, all farms were within 80 km of the city of Kitchener (43°26′ N, 80°28′ W).
Twenty poultry farms in BC and 21 farms in ON were recruited to participate. Because of privacy issues, a sampling frame was not available for farm recruitment. Instead, the provincial marketing boards were asked to provide contact information for members who would be willing to participate. A meeting was held with each participant to fully explain the project and obtain written consent. Participating farms used fully enclosed housing, and were members of the marketing board. Production types included broiler (20 farms), layer (12 farms), broiler breeder (10 farms), and turkey producers (5 farms). Six farms had different production types in adjacent barns.
A schematic map of each farm was created using data from the farm visit and Google Earth images (Google Earth 2009 version 5.1.3533, Google, Mountain View, California, USA). One skilled biologist in BC and another in ON were employed to perform all bird counts. Standardized methods for determining bird numbers and species abundance were used as described in the US Department of Agriculture Manual entitled Monitoring Bird Populations by Point Counts (18). Birds were counted 1 h after dawn using 10-minute unlimited distance circular point counts. Birds were counted at 3 points approximately 125 to 300 m apart that provided views of all areas of the poultry barns, so that in total, 30 min of data were collected. The species and the estimated bird numbers were noted, and an effort was made to count individual birds only once per 30-minute session. Observed bird locations within the farm or the surrounding habitat were recorded on the farm maps; each sighting of 1 or more birds of a single species at a particular location was treated as a single data point. Counts took place on each study farm in both regions in autumn (Oct–Nov) 2007, and in winter (Jan–Feb), spring (April–May), and summer (July–Aug) 2008. Dates for autumn and spring bird counts were chosen to coincide with autumn and spring migration (19) and spring breeding seasons (20,21), while dates for summer and winter counts were chosen to be equidistant in time from autumn and spring counts.
Maps were analyzed to classify bird location into 8 categories relative to the poultry barns and surrounding habitat. These categories were as follows: went into barn, perched on barn, in manure storage area, near feed silos (within 5 m), on ground near barn (within 5 m of barn perimeter), on ground in farmyard, flew over barn, flew over farmyard, in wetlands or ponds surrounding barns (within 300 m), and in croplands surrounding barns (within 300 m). The first 6 categories were collapsed to create a list of wild bird species that were observed in the immediate area of the poultry barns. Tabulation and descriptive analyses were performed using a statistical software package (Minitab 15 2007; Minitab, State College, Pennsylvania, USA). The distribution of bird species observed in the immediate barn area and in wetlands/croplands was evaluated for each province using a scattergram.
A literature review was undertaken to identify existing information about AI infection in species we observed on farms. Methods used to determine AIV status in research papers included polymerase chain reaction (PCR) detection of viral particles, viral culture, and detection of serum antibodies. The search methodology is presented in detail in supplementary online material available at http://centreforcoastalhealth.ca/index.php?option=com_content&view=article&id=146
In brief, the databases CAB Abstracts (OVID), Medline (OVID), Wildlife and Ecology Studies Worldwide (EBSCO), and Global Health (OVID) were searched using broad categories of bird type, individual common and scientific names of bird species, influenza, and testing as search concepts. The search was limited to papers published between 1960 and 2010 and to bird species found in North America. No publication type, study design, or language limits were applied to the search results. The most recent search was performed on January 21, 2010. Relevant papers from a comprehensive review (1) were also included. Information on recent surveillance in Canada was obtained from the Canadian Cooperative Wildlife Health Center Web site (http://www.ccwhc.ca).
After the data were summarized and reviewed, we created a list of bird species that met the 3 “high priority” criteria; i) Anseriforme and Charadriiforme species observed in the immediate barn area at least twice in either province, ii) any species we observed entering poultry barns, and iii) any species observed at least 10 times in both the immediate barn area and wetland/cropland areas in either province. Published information on prevalence of AIV in free-living individuals and laboratory studies of viral amplification and shedding were reviewed for each species on the “high-priority” list. Through this process, 2 risk groups were created: 1) a “high priority for continued surveillance” group for species that met 1 of the criteria listed and had been shown in at least 1 study of more than 100 individuals to have a virus prevalence of > 1%, and 2) a “high priority for initial data collection” group for species that met 1 of the criteria listed but had been tested at insufficient numbers (< 100) to gain an understanding of AI prevalence or host competence for AIV.
Results
Detailed bird count results are available in supplementary online material (http://centreforcoastalhealth.ca/index.php?option=com_content&view=article&id=146). In total, 37 005 birds of 121 species were observed on the 41 poultry farms; 88 in BC, and 68 in ON. Thirty-five species were observed in both provinces, while 53 were observed only in BC and 33 were observed only in ON.
In BC, 12 species were classified as non-migratory, 48 as migratory, and 28 as containing migratory and non-migratory populations. Twenty-six species were classified as summer visitors, 10 as winter visitors, and 50 as year-round residents, while 2 were classified as unusual visitors. In ON, 10 species were classified as non-migratory, 42 as migratory, and 16 as containing migratory and non-migratory populations. Thirty-seven species were classified as summer visitors, 3 as winter visitors and 25 as year round residents, while 3 were classified as passage migrants, and 1 was an unusual visitor.
European starlings were observed in the highest median number per farm in all seasons in both provinces, except in summer in BC, where they were second to barn swallows. Ranking of all other species was different between provinces and seasons (Tables S1 and S2), available at the following Web site: http://centreforcoastalhealth.ca/index.php?option=com_content&view=article&id=146
European starlings were observed on more than 50% of farms in both provinces during all seasons. No other species were seen on more than half of farms in all seasons and provinces; however, 19 species were observed on more than half of farms in at least 1 season in ON, while 28 species were observed on more than half of farms in at least 1 season in BC.
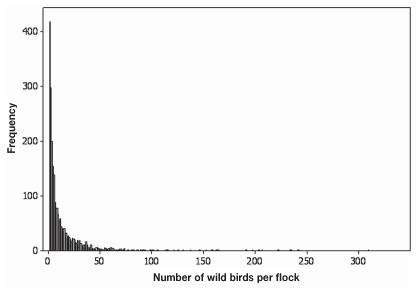
Flock size showed a right skewed distribution with a range of 1 to 3342 birds and a median flock size of 5 birds (Figure 1). Three species, European starlings, Canada geese, and mallards, were observed in flocks of more than 1000 individuals. All were observed in BC during autumn or winter. Seven species (European starling, northwestern crow, house sparrow, mew gull, northern pintail, ring-billed gull, trumpeter swan) were observed in flocks of 100 to 1000 individuals.
Figure 1.
Histogram of the number of wild birds of a single species per flock on 20 poultry farms in the Fraser Valley, British Columbia and 21 poultry farms in southwestern Ontario in autumn 2007, and winter, spring, and summer 2008. Three very large flocks of approximately 1253, 3000, and 3048 birds are not shown.
At least 1 Anseriforme or Charadriiforme species was seen in the immediate barn area on 27 farm visits (16%), 19 of 40 farm visits (48%) in BC and 8 of 44 farm visits (18%) in ON (Tables 1 and 2). Three Anseriforme and 2 Charadriiforme species were seen in the immediate barn area on 2 or more occasions in BC; these were the mallard, trumpeter swan, Canada goose, glaucous-winged gull, and mew gull. One Anseriforme and 2 Charadriiforme species were seen in the immediate barn area on 2 or more occasions in ON; these were the Canada goose, killdeer, and ring-billed gull. Waterfowl and killdeer were most commonly seen near feed silos, while gulls were most often seen near feed silos or perched on barn roofs.
Table 1.
Map location of wild birds counted by sight in 80 counts on 20 poultry farms in British Columbia. Only species observed in the immediate barn area or in wetlands on two or more counts are listed
| Included in immediate barn area
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total number of birds observed | In immediate barn area (n) | In immediate barn area (% of farms) | Went in barn | In manure storage area | Perched on barn | Near feed silos | On ground near barn | In farmyard | Flew over barn | Flew over farmyard | In cropland | In wetland/pond | |
| All | 1584 | 479 | 100 | 7 | 21 | 146 | 148 | 1 | 156 | 19 | 116 | 596 | 227 |
| European starling | 354 | 125 | 88 | 2 | 10 | 68 | 17 | 0 | 28 | 4 | 24 | 128 | 53 |
| Northwestern crow | 192 | 56 | 45 | 0 | 2 | 25 | 16 | 0 | 13 | 2 | 15 | 68 | 24 |
| American robin | 83 | 28 | 21 | 0 | 0 | 1 | 13 | 0 | 14 | 1 | 16 | 13 | 16 |
| House finch | 80 | 27 | 26 | 0 | 0 | 6 | 10 | 0 | 11 | 1 | 4 | 35 | 6 |
| Rock dove | 70 | 24 | 15 | 3 | 1 | 18 | 1 | 0 | 1 | 0 | 3 | 32 | 8 |
| House sparrow | 60 | 23 | 20 | 0 | 0 | 13 | 2 | 0 | 8 | 4 | 9 | 9 | 14 |
| White-crowned sparrow | 35 | 21 | 23 | 0 | 1 | 0 | 13 | 0 | 7 | 1 | 4 | 3 | 6 |
| Dark-eyed junco | 36 | 15 | 14 | 0 | 2 | 0 | 4 | 0 | 9 | 2 | 4 | 2 | 12 |
| Song sparrow | 41 | 15 | 14 | 0 | 1 | 0 | 3 | 0 | 11 | 0 | 4 | 3 | 19 |
| Glaucous-winged gull | 97 | 12 | 14 | 0 | 1 | 2 | 9 | 0 | 0 | 0 | 1 | 63 | 5 |
| Savannah sparrow | 27 | 11 | 9 | 0 | 0 | 2 | 8 | 0 | 1 | 1 | 4 | 5 | 5 |
| Red-tailed hawk | 13 | 9 | 10 | 0 | 0 | 0 | 2 | 1 | 6 | 0 | 0 | 1 | 1 |
| Golden-crowned sparrow | 17 | 8 | 8 | 0 | 1 | 0 | 2 | 0 | 5 | 0 | 5 | 1 | 3 |
| Spotted towhee | 15 | 8 | 8 | 0 | 1 | 0 | 2 | 0 | 5 | 0 | 2 | 0 | 5 |
| American goldfinch | 45 | 7 | 6 | 0 | 0 | 0 | 2 | 0 | 5 | 0 | 1 | 33 | 2 |
| Bald eagle | 20 | 7 | 9 | 0 | 0 | 0 | 4 | 0 | 3 | 0 | 0 | 6 | 1 |
| Black-capped chickadee | 11 | 7 | 9 | 0 | 0 | 0 | 2 | 0 | 5 | 0 | 1 | 1 | 2 |
| Brewers blackbird | 33 | 6 | 4 | 0 | 1 | 4 | 1 | 0 | 0 | 1 | 2 | 12 | 5 |
| Mallard | 44 | 6 | 6 | 0 | 0 | 0 | 5 | 0 | 1 | 0 | 1 | 24 | 3 |
| Trumpeter swan | 17 | 5 | 4 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 11 | 0 |
| Yellow-rumped warbler | 16 | 5 | 5 | 0 | 0 | 1 | 2 | 0 | 2 | 0 | 2 | 6 | 2 |
| American pipit | 12 | 4 | 5 | 0 | 0 | 0 | 3 | 0 | 1 | 1 | 0 | 5 | 1 |
| Canada goose | 29 | 4 | 4 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 1 | 17 | 1 |
| Great blue heron | 6 | 4 | 4 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 1 | 0 |
| Northern flicker | 8 | 4 | 5 | 0 | 0 | 1 | 0 | 0 | 3 | 1 | 1 | 2 | 0 |
| Red-winged blackbird | 20 | 4 | 4 | 0 | 0 | 2 | 1 | 0 | 1 | 0 | 3 | 9 | 2 |
| Barn swallow | 61 | 3 | 4 | 1 | 0 | 1 | 1 | 0 | 0 | 0 | 3 | 44 | 4 |
| Chestnut-backed chickadee | 5 | 3 | 4 | 0 | 0 | 0 | 2 | 0 | 1 | 0 | 0 | 0 | 2 |
| Bewick’s wren | 2 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Brown-headed cowbird | 6 | 2 | 3 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 1 |
| Mew gull | 11 | 2 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 6 | 0 |
| Peregrine falcon | 3 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 1 | 0 |
| Pine siskin | 8 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 2 | 1 |
| Townsend’s solitaire | 4 | 2 | 3 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | 1 |
| Stellar’s jay | 3 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 |
| Lincoln’s sparrow | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| Ring-billed gull | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Variegated thrush | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| Violet-green swallow | 19 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 15 | 2 |
| Western meadowlark | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 |
Table 2.
Map location of wild birds counted by sight in 84 counts on 21 poultry farms in Ontario. Only species observed in the immediate barn area or in wetlands on two or more counts are listed
| Included in immediate barn area
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total number of birds observed | In immediate barn area (n) | In immediate barn area (% of farms) | Went in barn | Perched on barn | In manure storage area | Near feed silos | On ground near barn | In farmyard | Flew over barn | Flew over farmyard | In cropland | In wetland/pond | |
| All | 799 | 407 | 100 | 0 | 51 | 25 | 244 | 8 | 79 | 13 | 54 | 1 | 324 |
| European starling | 140 | 67 | 56 | 0 | 19 | 6 | 27 | 0 | 15 | 3 | 8 | 1 | 61 |
| House sparrow | 141 | 47 | 38 | 0 | 22 | 5 | 11 | 0 | 9 | 6 | 13 | 0 | 75 |
| Horned lark | 57 | 46 | 48 | 0 | 0 | 1 | 44 | 0 | 1 | 0 | 1 | 0 | 10 |
| Red-winged blackbird | 36 | 30 | 24 | 0 | 0 | 0 | 24 | 2 | 4 | 0 | 0 | 0 | 6 |
| Savannah sparrow | 44 | 21 | 19 | 0 | 0 | 0 | 19 | 0 | 2 | 3 | 5 | 0 | 15 |
| Rock dove | 30 | 18 | 20 | 0 | 3 | 11 | 4 | 0 | 0 | 0 | 0 | 0 | 12 |
| Common grackle | 35 | 17 | 13 | 0 | 1 | 0 | 11 | 0 | 5 | 0 | 2 | 0 | 16 |
| Killdeer | 35 | 17 | 18 | 0 | 0 | 0 | 15 | 2 | 0 | 0 | 9 | 0 | 9 |
| American crow | 16 | 15 | 14 | 0 | 0 | 0 | 12 | 0 | 3 | 0 | 0 | 0 | 1 |
| American robin | 44 | 14 | 12 | 0 | 1 | 0 | 10 | 0 | 3 | 0 | 3 | 0 | 27 |
| Dark-eyed junco | 21 | 11 | 13 | 0 | 1 | 1 | 5 | 0 | 4 | 1 | 1 | 0 | 5 |
| American goldfinch | 15 | 10 | 11 | 0 | 0 | 0 | 7 | 0 | 3 | 0 | 0 | 0 | 5 |
| Mourning dove | 17 | 9 | 10 | 0 | 0 | 0 | 7 | 0 | 2 | 0 | 0 | 0 | 8 |
| Brown-headed cowbird | 12 | 7 | 7 | 0 | 1 | 1 | 4 | 0 | 1 | 0 | 0 | 0 | 5 |
| Blue jay | 10 | 7 | 7 | 0 | 0 | 0 | 2 | 0 | 5 | 0 | 0 | 0 | 3 |
| Chipping sparrow | 29 | 7 | 8 | 0 | 0 | 0 | 5 | 0 | 2 | 0 | 7 | 0 | 15 |
| Northern cardinal | 7 | 7 | 7 | 0 | 0 | 0 | 2 | 0 | 5 | 0 | 0 | 0 | 0 |
| Barn swallow | 23 | 6 | 6 | 0 | 2 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 17 |
| American kestrel | 5 | 5 | 5 | 0 | 1 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 |
| Canada goose | 5 | 5 | 7 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 0 | 0 |
| Black-capped chickadee | 7 | 4 | 5 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 0 | 3 |
| White-crowned sparrow | 6 | 4 | 2 | 0 | 0 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 2 |
| Ring-billed gull | 3 | 3 | 2 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 |
| Song sparrow | 13 | 3 | 4 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 10 |
| Great blue heron | 2 | 2 | 2 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gray catbird | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Yellow warbler | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 |
| Eastern kingbird | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Tundra swan | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
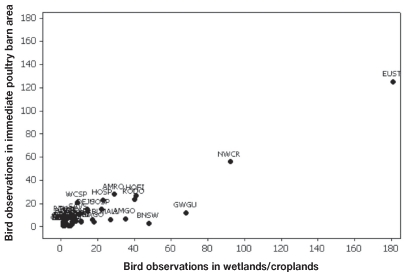
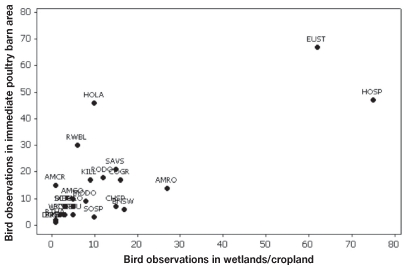
Birds were seen entering poultry barns either through roof vents or holes near the barn eaves 6 times, all in BC. The 3 species that entered barns were European starlings, rock doves, and barn swallows. Seventy-seven species (886 birds) were noted in the immediate area of poultry barns. Of these, 392 (44%) were observed near feed silos, 197 (22%) were perched on the barn roof and 46 (5%) were in the manure storage area. Eighty species (1295 birds) were recorded in wetlands and croplands around poultry farms, and 50 species were observed both in the immediate area of the barns and in wetlands/croplands. In BC, the 10 species that were observed in both wetlands/croplands and the immediate barn area on more than 10 occasions were the European starling, northwestern crow, glaucous-winged gull, house finch, rock dove, American robin, house sparrow, song sparrow, dark-eyed junco, and Savannah sparrow (Figure 2). In ON, the 7 species observed in both wetlands/croplands and the immediate barn area on at least 10 occasions were the house sparrow, European starling, American robin, common grackle, Savannah sparrow, rock dove, and horned lark (Figure 3).
Figure 2.
Scatterplot of the number of times wild bird species were observed in the immediate barn area and wetlands/croplands on 20 poultry farms in British Columbia. Standard four-letter ornithological codes are used for species labels.
Figure 3.
Scatterplot of the number of times wild bird species were observed in the immediate barn area and wetlands/croplands on 21 poultry farms in Ontario. Standard four-letter ornithological codes are used for species labels.
The literature review returned 249 unique references. Of these, 32 presented North American data on AI status of free-living individuals of species we observed. Nine references presented data for individuals tested outside North America, and five presented results from laboratory challenge studies. Information was available for 66 of the 121 species we observed. In general, low numbers of individual birds of any species have been tested (Table 3). Published information on 1000 individual birds or more was found for only 6 species, all waterfowl and gulls. Results of the literature review for each species observed are available in supplemental online material http://centreforcoastalhealth.ca/index.php?option=com_content&view=article&id=146#s6
Table 3.
Range of individual free-living birds tested for avian influenza virus reported in published information returned by the literature review for 121 wild bird species observed on 41 poultry farms in British Columbia and Ontario
| Number of individuals tested | Number of species |
|---|---|
| > 1000 | 6 |
| 100 to 1000 | 9 |
| 25 to 100 | 12 |
| < 25 | 37 |
| Tested outside North America only | 2 |
| No results found | 55 |
Three species met our criteria for classification as high priority for continued surveillance (Canada goose, mallard, and ring-billed gull) and 12 species met our criteria for high priority for initial data collection (killdeer, mew gull, trumpeter swan, American robin, barn swallow, common grackle, dark-eyed junco, European starling, horned lark, northwestern crow, rock dove, and song sparrow). A summary of AIV prevalence reported in previous studies, along with calculated confidence intervals and disease detection probabilities, are presented for these species in Table 4. In BC, Canada geese, mallards, American robins, European starlings, killdeer, northwestern crows, rock doves, and song sparrows were observed on 2 or more poultry farms in all 4 seasons. Dark-eyed juncos, mew gulls, and trumpeter swans were observed in autumn, winter, and spring, while barn swallows were observed in summer. In ON, Canada geese, European starlings, horned larks, and rock doves were seen on 2 or more farms in all 4 seasons. Ring-billed gulls were observed in autumn, spring, and summer while American robins, barn swallows, common grackles, and song sparrows were observed in spring and summer. Dark-eyed juncos were observed in autumn and winter.
Table 4.
Summary of AIV risk criteria and literature review results with calculated confidence intervals in 15 high-priority wild bird species
| Species | Province in which species met high-priority criteria
|
High priority criteriaa | Test positive/number tested in all studies returned by literature review | 90% confidence interval for reported prevalenceb | Probability of 0 test positives with an assumed true prevalence of 2%c | |
|---|---|---|---|---|---|---|
| BC | ON | |||||
| American robin | X | X | 2 | 0/33 | 0.51 | |
| Barn swallow | X | 2,3 | 3/6 (50%) | 15.3%–84.6% | ||
| Canada goose | X | X | 1 | 32/1393 (2.3%) | 1.7%–3.1% | |
| Common grackle | X | 2 | 0/0 | |||
| Dark-eyed junco | X | 2 | 1/31 (3.2%) | 0%–14.4% | ||
| European starling | X | X | 2,3 | 0/862 | 0.0 | |
| Horned lark | X | 2 | 0/2 | 0.96 | ||
| Killdeer | X | 1 | 0/1 | 0.98 | ||
| Mallard | X | 1 | 2691/12393 (21.7%) | 21.1%–22.3% | ||
| Mew gull | X | 1 | 0/10 | 0.82 | ||
| Northwestern crow | X | 2 | 0/0 | |||
| Ring-billed gull | X | 1 | 68/3456 (2.0%) | 1.6%–2.4% | ||
| Rock dove | X | X | 2,3 | 1/? | ||
| Song sparrow | X | 2 | 1/72 (1.4%) | 0%–6.4% | ||
| Trumpeter swan | X | 1 | 0/14 | 0.75 | ||
1 — Anseriforme or Charadriiforme present on at least 2 farms per province, 2 — Observed at least 10 times in immediate barn area and wetland/cropland, 3 — Entered poultry barn.
Confidence intervals calculated using the Exact method (54).
Calculated using Freecalc Version 2 (available online at: www.ausvet.com.au/content.php?page=software#freecalc).
Discussion
This study provides the first data on the distribution and abundance of wild bird species near commercial poultry farms, and provides novel information about which wild bird species might be most likely to create contact links to commercial poultry in 2 poultry producing regions of Canada. Within BC and ON, 362 and 318 wild bird species occur regularly (22). Our study observed approximately 20% of these around commercial poultry farms. For most seasons, published data on the relative abundance of species in the study areas was not available, so it was not possible to determine if the species we observed are the most abundant in general, or are specifically attracted to habitat near poultry farms. In our BC study area, a pilot project counting birds on berry crops found European starlings to be the most common bird during summer (23). In southwestern ON, a study using point counts in cropland detected 68 species during spring compared with the 45 species we observed (21). In general, the relative abundance of species was similar in both studies; however, European starlings and common grackles were found on 100% of poultry farms and only 55% and 36% of crop farms surveyed. In winter, Audubon Society Christmas Bird Counts (ASCBCs) showed European starlings to be the most common species in both provinces (24). Other species we observed regularly were also in the top 15 species reported in ASCBC data, except for horned larks, which were common on ON poultry farms, but less common in ASCBC data. These bird counts are performed by volunteer observers in points purposefully selected for species richness. One significant risk of the point count method used in this study and in ASCBCs is undercounting species that are well-camouflaged and non-vocal, and species that visit at different times of day or year (25,26). For example, we observed owl pellets containing white feathers that appeared to be domestic chicken feathers on fence posts near poultry barns; however, owls were not observed.
There is limited published information on the prevalence of AIV in most wild bird species that we observed near poultry farms. Species of Anseriformes and Charadriiformes have been tested in different regions of North America and have been demonstrated to shed high amounts of infectious virus in their feces during certain life stages and seasons (1,9,27,28). Avian influenza virus test results for other species have been reported in fewer studies, in lower numbers, and in limited geographical regions of North America (11,29–32). This might might be because active surveillance designed to monitor circulating AIV strains has focussed on species known to have high AIV prevalence, or might represent a bias in the literature towards reporting only positive results. Passive surveillance by veterinary pathology laboratories probably occurs, particularly in species such as American and northwestern crows, which are surveillance targets for West Nile virus, and in large or charismatic species such as bald eagles and red-tailed hawks (33); however, results are not published (34). In inconspicuous and common species, such as rock doves, song sparrows, and European starlings, individual mortalities are unlikely to be submitted to pathology laboratories, so unpublished passive AI surveillance data might be limited.
In order to fully understand the role of wild birds in transmission of AIV to poultry, information on the prevalence and transmission dynamics of AI should be available for all wild bird species that use habitat on or near poultry farms. However, because capturing and testing of wild bird species requires intensive effort and is expensive, we attempted to focus on species that have the greatest likelihood of playing a role in AI transmission to poultry. As a result, we focused on 2 groups of wild birds: i) species that, based on previous research (5), had a high likelihood of being infectious hosts for AIV (Canada goose, mallard, trumpeter swan, ring-billed gull, mew gull, killdeer), and ii) species that, based on our observations, had a high likelihood of creating contact “bridges” between Anseriformes and Charadriiformes and domestic poultry, and about which minimal information on AIV prevalence was returned by the literature review (American robin, barn swallow, common grackle, dark-eyed junco, European starling, horned lark, northwestern crow, rock dove, song sparrow). Because infection with AIV varies with bird age, migration, and season (1,35,36), it will be important to target further testing of each species to the season(s) when they are most abundant near poultry farms.
Anseriformes and Charadriiformes activities near poultry farms are of particular interest because of their role in the ecology of AIV. Our finding that Anseriformes and Charadriiformes occur in large flocks in farmland surrounding poultry barns, particularly in partially flooded pasture and harvested corn fields during winter in BC, is supported by previous studies showing that the Fraser Valley is an important wintering ground for waterfowl (19,37). Flooded farmland provides winter food for ducks, in particular mallards, American wigeons, and northern pintails (38). Three recent isolations of notifiable (H5 and H7) AIV from poultry in BC occurred in the winter months when waterfowl are at highest numbers near poultry farms (39–41). Of the 7 Anseriforme and Charadriiforme species we observed in the immediate barn area, limited information is available for mew gulls and trumpeter swans (32,42), while Canada geese, mallard and ring-billed gulls have been tested in large numbers and are frequently infected with AI viruses (5). Published information on kildeer is negligible, with only 1 negative result from a single individual. One previous study of glaucous-winged gulls in Alaska detected AIV at a prevalence of 0.13% (1/770) (43), which is lower than prevalence reported in studies including other gull species (9,42,44,45). From these data, we conclude that first priority should be given to continuing ongoing surveillance in mallards (BC all seasons), Canada geese (BC autumn, winter, spring; ON all seasons), and ring-billed gulls (ON autumn, spring, summer). Baseline information should also be collected on AIV prevalence and host competence in mew gulls (BC Winter), trumpeter swans (BC autumn, winter, spring), and killdeer (BC all seasons; ON autumn, spring, summer).
In our study, 3 species were observed entering poultry barns: European starlings, rock doves, and barn swallows. European starlings were also present in high numbers on nearly all farms in all seasons in both provinces. They were observed more often than any other species in both the immediate barn area and wet-land and cropland habitat in both provinces. Data on whether European starlings are competent vectors for AIV is equivocal. Of 868 European starlings tested for antibodies in Ohio by agar gel immunodiffusion, none were found to have antibodies to AIV (31); however, the method has since been shown to have a low sensitivity in wild birds (46). Free-living European starlings infected with AIV have been found in other parts of the world (47–49). Because populations of European starlings in BC and ON contain both migratory and non-migratory individuals, there is opportunity for mixing and long distance viral transmission. Further investigation of AIV prevalence and host competence in European starlings in all seasons in Canada is required.
Rock doves were the species we observed entering poultry barns the most times and they were present on farms in all seasons and provinces. Avian influenza virus has been isolated from 1 free-living rock dove in North America (50), and several in Asia (4) and Russia (47), but not from 140 free-living birds sampled in Europe (51,52). Rock doves can allow AIV to multiply and be shed after experimental inoculation; however, experimental studies have shown that they are less susceptible to H5 and H7 influenza subtypes than are many species (53). Because they are non-migratory, they are unlikely to be important in long distance transmission of novel viruses. Further investigation of AIV prevalence and host competence in rock doves in all seasons in Canada is required to eliminate them as potential AIV hosts. Investigation of rock doves as mechanical vectors for AIV should also be conducted.
Barn swallows were the third species we observed entering poultry barns, and they were common in both provinces during summer. In Slovakia, AIV was detected in 3/6 barn swallows tested near wetlands (14). Individuals migrate and gather in large flocks, possibly providing opportunity for mixing and long distance viral transmission of infection. Further investigation of AIV prevalence and host competence is needed for barn swallow populations that use farmyard habitat in Canada during summer.
Of the other species that were common on poultry farms in our study, we believe priority should be given to dark-eyed juncos and northwestern crows in winter in BC, as this is the season AIV was recently isolated from poultry (39–41). Avian influenza virus was isolated from 1 of 15 free-living dark-eyed juncos in eastern Canada (29). Prevalence estimates of AI have not been published for North American crow species; however, highly pathogenic H5N1 virus has been isolated from dead large-billed crows (Corvus macrorhynchos) in Japan (2). Laboratories testing for West Nile virus may be a source of existing data on AIV isolations from crows.
Next priority should be given to collecting information on American robins (BC and ON; spring, summer), song sparrows (BC; all seasons, ON; spring, summer), horned larks (ON; autumn, winter, spring), and common grackles (ON; spring, summer). Previously, 1 of 72 (1.4%) song sparrows tested in North America was positive for AIV (29,31), while 7 American robins (29,31,32) and 2 horned larks have tested negative (32). No information was available for common grackles.
Our bird counts were performed on a limited number of purposefully chosen poultry farms in specific areas of BC and ON. Participants in our study were highly involved in poultry industry management, so biosecurity, including practices aimed at decreasing bird activity near farm may have been more common than on other poultry farms. Despite this, the study provided data on which wild bird species occur on poultry farms in BC and ON and a review of existing information on AIV in these species. Species that we recommend as first priority for data collection include the 5 Anseriforme and Charadriiforme species we observed in the immediate barn areas: mallards, Canada geese, trumpeter swans, mew gulls and ring-billed gulls, and the 3 species observed entering poultry barns: European starlings, barn swallows, and rock doves. Dark-eyed juncos and northwestern crows should also be high priority because both were common on BC poultry farms during the winter months when most Anseriformes were observed near poultry farms, and when recent AI outbreaks in poultry have occurred (39–41). Other species that we believe are of priority for data collection include American robins (BC and ON; spring, summer), song sparrows (BC; all seasons, ON; spring, summer), horned larks (ON; autumn, winter, spring), and common grackles (ON; spring, summer).
Acknowledgments
We thank the poultry farmers who allowed us to count wild birds on their farms and the 2 reviewers for valuable feedback on an earlier version of the manuscript. CVJ
Footnotes
This project was completed through the Department of Population Medicine, University of Guelph as part of the PhD thesis of Dr. Burns.
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Stallknecht DE, Shane SM. Host range of avian influenza virus in free-living birds. Vet Res Commun. 1988;12:125–141. doi: 10.1007/BF00362792. [DOI] [PubMed] [Google Scholar]
- 2.Tanimura N, Tsukamoto K, Okamatsu M, et al. Pathology of fatal highly pathogenic H5N1 avian influenza virus infection in large-billed crows (Corvus macrorhynchos) during the 2004 outbreak in Japan. Vet Pathol. 2006;43:500–509. doi: 10.1354/vp.43-4-500. [DOI] [PubMed] [Google Scholar]
- 3.Becker WB. The isolation and classification of Tern virus: Influenza A-Tern South Africa — 1961. J Hyg (Lond) 1966;64:309–320. doi: 10.1017/s0022172400040596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis TM, Bousfield RB, Bissett LA, et al. Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathol. 2004;33:492–505. doi: 10.1080/03079450400003601. [DOI] [PubMed] [Google Scholar]
- 5.Olsen B, Munster VJ, Wallensten A, Waldenstrom J, Osterhaus AD, Fouchier RA. Global patterns of influenza a virus in wild birds. Science. 2006;312:384–388. doi: 10.1126/science.1122438. [DOI] [PubMed] [Google Scholar]
- 6.Rutz C, Dalessi S, Baumer A, Kestenholz M, Engels M, Hoop R. Avian influenza: Wild bird monitoring in Switzerland between 2003–2006. Schweiz Arch Tierheilkd. 2007;149:501–509. doi: 10.1024/0036-7281.149.11.501. [DOI] [PubMed] [Google Scholar]
- 7.Hoye BJ, Munster VJ, Nishiura H, Klaassen M, Fouchier RA. Surveillance of wild birds for avian influenza virus. Emerg Infect Dis. 2010;16:1827–1834. doi: 10.3201/eid1612.100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fouchier RA, Munster V, Wallensten A, et al. Characterization of a novel influenza A virus hemagglutinin subtype (H16) obtained from black-headed gulls. J Virol. 2005;79:2814–2822. doi: 10.1128/JVI.79.5.2814-2822.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parmley EJ, Bastien N, Booth TF, et al. Wild bird influenza survey, Canada, 2005. Emerg Infect Dis. 2008;14:84–87. doi: 10.3201/eid1401.061562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krauss S, Walker D, Pryor SP, et al. Influenza A viruses of migrating wild aquatic birds in North America. Vector Borne Zoonotic Dis. 2004;4:177–189. doi: 10.1089/vbz.2004.4.177. [DOI] [PubMed] [Google Scholar]
- 11.Roy G, Burton J, Lecomte J, Boudreault A. Role of passerine birds in the ecology of influenza viruses. Rev Can Biol Exp. 1983;42:73–81. [PubMed] [Google Scholar]
- 12.Fouchier RA, Olsen B, Bestebroer TM, et al. Influenza a virus surveillance in wild birds in Northern Europe in 1999 and 2000. Avian Dis. 2003;47:857–860. doi: 10.1637/0005-2086-47.s3.857. [DOI] [PubMed] [Google Scholar]
- 13.Peterson AT, Bush SE, Spackman E, Swayne DE, Ip HS. Influenza A virus infections in land birds, People’s Republic of China. Emerg Infect Dis. 2008;14:1644–1646. doi: 10.3201/eid1410.080169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gronesova P, Kabat P, Trnka A, Betakova T. Using nested RT-PCR analyses to determine the prevalence of avian influenza viruses in passerines in western Slovakia, during summer 2007. Scand J Infect Dis. 2008;40:954–957. doi: 10.1080/00365540802400576. [DOI] [PubMed] [Google Scholar]
- 15.Webster RG, Yakhno M, Hinshaw VS, Bean WJ, Murti KG. Intestinal influenza: Replication and characterization of influenza viruses in ducks. Virology. 1978;84:268–278. doi: 10.1016/0042-6822(78)90247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiwari A, Patnayak DP, Chander Y, Parsad M, Goyal SM. Survival of two avian respiratory viruses on porous and nonporous surfaces. Avian Dis. 2006;50:284–287. doi: 10.1637/7453-101205R.1. [DOI] [PubMed] [Google Scholar]
- 17.Canadian Food Inspection Agency. Notifiable avian influenza hazard specific plan [monograph on the Internet] Ottawa: Canadian Food Inspection Agency; c2007. [Last accessed December 14, 2011]. Available from http://www.inspection.gc.ca/english/anima/heasan/disemala/avflu/bacdoc/prepsume.shtml. [Google Scholar]
- 18.Ralph CJ, Droege S, Sauer JR. Monitoring Bird Populations by Point Counts. Pacific Southwest Research Station, United States Department of Agriculture; 1995. [Google Scholar]
- 19.Leach BA. The waterfowl of the Fraser Delta, British Columbia. Wildfowl. 1972;23:45–55. [Google Scholar]
- 20.Cyr A, Lepage D, Freemark K. In: Evaluation point count efficiency relative to territory mapping in cropland birds. Ralph CJ, Droege S, Sauer JR, editors. Pacific Southwest Research Station, United States Department of Agriculture; 1995. pp. 63–68. [Google Scholar]
- 21.Freemark KE, Kirk DA. Birds on organic and conventional farms in Ontario: Partitioning effects of habitat and practices on species composition and abundance. Biol Conserv. 2001;101:337–350. [Google Scholar]
- 22.Hinterland who’s who: Bird fact sheets. [homepage on the Internet] Kanata, Ontario: Canadian Wildlife Federation; c2009. [Last accessed December 14, 2011]. Available from http://www.hww.ca/hww.asp?id=7. [Google Scholar]
- 23.Steensma KM. Efficacy of bird deterrent devices in agricultural areas of the Fraser Valley of British Columbia: A pilot study. Langley, British Columbia: Trinity Western University; 2009. [Google Scholar]
- 24.The Christmas Bird Count Historical Results [database on the Internet] New York: Audubon; c2009. [Last accessed December 14, 2011]. Available from http://www.audubon.org/bird/cbc. [Google Scholar]
- 25.Ralph CJ, Droege S, Sauer JR. In: Managing and monitoring birds using point counts: Standards and applications. Ralph CJ, Droege S, Sauer JR, editors. Pacific Southwest Research Station, United States Department of Agriculture; 1995. pp. 161–168. [Google Scholar]
- 26.Pendleton GW. In: Effects of sampling strategy, detection probability, and independance of counts on the use of point counts. Pacific Southwest Research Station, United States Department of Agriculture. Ralph CJ, Droege S, Sauer JR, editors. 1995. pp. 131–133. [Google Scholar]
- 27.Parmley EJ, Lair S, Leighton FA. Canada’s inter-agency wild bird influenza survey. Integr Zoo. 2009;4:409–417. doi: 10.1111/j.1749-4877.2009.00177.x. [DOI] [PubMed] [Google Scholar]
- 28.Deliberto TJ, Swafford SR, Nolte DL, et al. Surveillance for highly pathogenic avian influenza in wild birds in the USA. Integr Zoo. 2009;4:426–439. doi: 10.1111/j.1749-4877.2009.00180.x. [DOI] [PubMed] [Google Scholar]
- 29.Boudreault A, Lecomte J, Hinshaw VS. Characterisation antige-nique des virus influenza A isoles des oiseaux catures dans Ontario, Le Quebec et les provinces Maritimes durant la saison 1977. Rev Can Biol. 1980;39:107–114. [PubMed] [Google Scholar]
- 30.Boudreault A, Lecomte J. Isolation of influenzavirus from different avian species in Canada in 1978. Rev Can Biol. 1981;40:139–145. [PubMed] [Google Scholar]
- 31.Morishita TY, Aye PP, Ley EC, Harr BS. Survey of pathogens and blood parasites in free-living passerines. Avian Dis. 1999;43:549–552. [PubMed] [Google Scholar]
- 32.Ip HS, Flint PL, Franson JC, et al. Prevalence of influenza A viruses in wild migratory birds in Alaska: Patterns of variation in detection at a crossroads of intercontinental flyways. Virol J. 2008;5:4. doi: 10.1186/1743-422X-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Snelling J, Greiner EC, Kocan AA. Some infectious and parasitic diseases of Oklahoma raptors. J Wildl Dis. 1977;13:304–312. doi: 10.7589/0090-3558-13.3.304. [DOI] [PubMed] [Google Scholar]
- 34.Nemeth NM, Beckett S, Edwards E, Klenk K, Komar N. Avian mortality surveillance for West Nile virus in Colorado. Am J Trop Med Hyg. 2007;76:431–437. [PubMed] [Google Scholar]
- 35.Graves IL. Influenza viruses in birds of the Atlantic flyway. Avian Dis. 1992;36:1–10. [PubMed] [Google Scholar]
- 36.Hinshaw V, Webster RG, Turner B. The perpetuation of orthomyxoviros and paramyxoviruses in Canadian waterfowl. Can J Microbiol. 1980;26:622–629. doi: 10.1139/m80-108. [DOI] [PubMed] [Google Scholar]
- 37.Butler RW. Abundance, distribution and conservation of birds in the vicinity of Boundary Bay, British Columbia. In: Butler RW, editor. Technical Report Series No. 155. Canadian Wildlife Service, Pacific and Yukon Region; British Columbia: 1992. [Google Scholar]
- 38.Hirst SM, Easthope CA. Use of agricultural lands by waterfowl in southwestern British Columbia. J Wildl Dis. 1981;45:454–462. [Google Scholar]
- 39.Precautionary quarantine in place at Fraser Valley Farm. [homepage on the Internet] Ottawa: Canadian Food Inspection Agency; c2005. [Last accessed December 14, 2011]. Available from http://www2.news.gov.bc.ca/news_releases_2005-2009/2005AL0023-001069.htm. [Google Scholar]
- 40.Bowes VA, Ritchie SJ, Byrne S, Sojonky K, Bidulka JJ, Robinson JH. Virus characterization, clinical presentation, and pathology associated with H7N3 avian influenza in British Columbia broiler breeder chickens in 2004. Avian Dis. 2004;48:928–934. doi: 10.1637/7218-060304R. [DOI] [PubMed] [Google Scholar]
- 41.Avian influenza detected in British Columbia [homepage on the Internet] Ottawa: Canadian Food Inspection Agency; c2009. [Last accessed December 14, 2011]. Available from http://www.inspection.gc.ca/english/corpaffr/newcom/2009/20090124e.shtml. [Google Scholar]
- 42.Nettles VF, Wood JM, Webster RG. Wildlife surveillance associated with an outbreak of lethal H5N2 avian influenza in domestic poultry. Avian Dis. 1985;29:733–741. [PubMed] [Google Scholar]
- 43.Winker K, Spackman E, Swayne DE. Rarity of influenza A virus in spring shorebirds, southern Alaska. Emerg Infect Dis. 2008;14:1314–1316. doi: 10.3201/eid1408.080083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanson BA, Luttrell MP, Goekjian VH, et al. Is the occurrence of avian influenza virus in Charadriiformes species and location dependent? J Wildl Dis. 2008;44:351–361. doi: 10.7589/0090-3558-44.2.351. [DOI] [PubMed] [Google Scholar]
- 45.Dugan VG, Chen R, Spiro DJ, et al. The evolutionary genetics and emergence of avian influenza viruses in wild birds. PLoS Pathog. 2008;4:e1000076. doi: 10.1371/journal.ppat.1000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown JD, Luttrell MP, Berghaus RD, et al. Prevalence of antibodies to type a influenza virus in wild avian species using two serologic assays. J Wildl Dis. 2010;46:896–911. doi: 10.7589/0090-3558-46.3.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.L’vov DK, Shchelkanov MI, Prilipov AG, et al. Interpretation of the epizootic outbreak among wild and domestic birds in the south of the European part of Russia in December 2007. Vopr Virusol. 2008;53:18–23. [PubMed] [Google Scholar]
- 48.Lipkind M, Shihmanter E, Shoham D. Further characterization of H7N7 avian influenza virus isolated from migrating starlings wintering in Israel. Zentralbl Veterinarmed B. 1982;29:566–572. doi: 10.1111/j.1439-0450.1982.tb01256.x. [DOI] [PubMed] [Google Scholar]
- 49.Nestorowicz A, Kawaoka Y, Bean WJ, Webster RG. Molecular analysis of the hemagglutinin genes of Australian H7N7 influenza viruses: Role of passerine birds in maintenance or transmission? Virology. 1987;160:411–418. doi: 10.1016/0042-6822(87)90012-2. [DOI] [PubMed] [Google Scholar]
- 50.Chen R, Holmes EC. Frequent inter-species transmission and geographic subdivision in avian influenza viruses from wild birds. Virology. 2009;383:156–161. doi: 10.1016/j.virol.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dovc A, Zorman-Rojs O, Vergles RA, Bole-Hribovsek V, Krapez U, Dobeic M. Health status of free-living pigeons (Columba livia domestica) in the city of Ljubljana. Acta Vet Hung. 2004;52:219–226. doi: 10.1556/AVet.52.2004.2.10. [DOI] [PubMed] [Google Scholar]
- 52.Lillehaug A, Monceyron JC, Bergsjo B, et al. Screening of feral pigeon (Colomba livia), mallard (Anas platyrhynchos) and graylag goose (Anser anser) populations for Campylobacter spp., Salmonella spp., avian influenza virus, and avian paramyxovirus. Acta Vet Scand. 2005;46:193–202. doi: 10.1186/1751-0147-46-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaleta EF, Honicke A. Review of the literature on avian influenza A viruses in pigeons and experimental studies on the susceptibility of domestic pigeons to influenza A viruses of the haemagglutinin subtype H7. Dtsch Tierarztl Wochenschr. 2004;111:467–472. [PubMed] [Google Scholar]
- 54.Dohoo I, Martin W, Strynn H. Veterinary Epidemiological Research. 2nd ed. Charlottetown, PEI: VER Inc; 2009. p. 84. [Google Scholar]