Abstract
Low-molecular-weight heparin/protamine microparticles (LH/P MPs) serve as carriers for controlled release of heparin-binding cytokines. LH/P MPs were stably coated onto plastic surfaces by drying. The purpose of this study is to evaluate a culture method for selective expansion of CD34+ cells using LH/P MPs as cytokine-binding matrix. Ficoll-purified mouse bone marrow cells (mouse FP-BMCs) containing CD34+ cells were cultured on LH/P MP-coated plates in the presence of stem cell factor (SCF), thrombopoietin (Tpo), and Flt-3 ligand (Flt-3) in hematopoietic progenitor growth medium (HPGM) supplemented with 4% heat-inactivated fetal bovine serum (FBS). After 8 days of culture, the total cell count increased 4.6-fold, and flow cytometry analyses revealed that 23.8% of the initial cells and 57.4% of the expanded cells were CD34 positive. Therefore, CD34+ cells were estimated to have increased 11.0-fold. In contrast, cultured CD34+ cells on uncoated tissue culture plates increased 5.8-fold in an identical medium.
Keywords: CD34-positive hematopoietic cells (CD34 cells), cell proliferation, cytokines
Introduction
Cellular expression of the CD34 antigen identifies a morphologically and immunologically heterogeneous cell population that is functionally characterized by its in vitro capability to generate clonal aggregates derived from early and late progenitors in hematopoiesis and its in vivo capacity to reconstitute the myeloid hematopoietic system in a supralethally irradiated host.1 On the other hand, there are several recent reports that a certain CD34+ cells form cooperative vascular networks as endothelial progenitor cells,2 and human CD34+ cells have been used in clinical trials for treatment of myocardial infarction.3 Furthermore, synergistic actions of CD34+ hematopoietic cells and mesenchymal stem/progenitor cells in vascularizing bioengineered tissues have been reported.4 Thus, CD34+ cells are important cells for vascularizing bioengineered tissues as well as transplantation of bone marrow. Bone marrow and peripheral blood are the sources of immature hematopoietic precursors identified as CD34+ cells. Large numbers of CD34+ cells can be obtained from massive marrow harvests, such as for transplantation purposes.1,5,6 A diagnostic marrow sample contains only a small number of CD34+ cells, while even fewer are present in the peripheral samples taken under steady-state conditions. Recently developed cell separation procedures such as panning, combination of galactose-bound vinyl polymer and soybean agglutinin,7 and immunomagnetic beads allow for the recovery of a highly enriched CD34+ cell population.8 However, this is generally achieved through a specific and often unacceptable cell loss. In contrast, in vitro expansion of CD34+ cells may be of particular relevance for cell transplantation.
CD34+ cells undergo regulated proliferation, conservation, and differentiation in the bone marrow microenvironment. Therefore, the cell pool can be preserved while allowing controlled cell proliferation and differentiation.9,10 CD34+ cells are localized in stem cell niches and local area networks in the microenvironment, where they interact with the components of their niche, including stromal cells, extracellular matrix proteins, heparan sulfate proteoglycans, and cytokines. Regional variation in these components within the hematopoietic microenvironment may create niches that are specific to cells at various stages of differentiation.11–13 However, the identity and structural characteristics of macromolecules that mediate the formation of these niches are not well known. In addition, a method for selecting ex vivo amplification of CD34+ cells from bone marrow has not yet been established.14
Human CD34+ cells proliferate and mature in semisolid media when stimulated with exogenous hematopoietic cytokines such as stem cell factor (SCF), thrombopoietin (Tpo), and Flt-3 ligand (Flt-3).15–18 They also proliferate in association with bone marrow–derived stromal cells,19,20 although biologically active amounts of hematopoietic cytokines cannot be detected in stromal culture supernatants.18 It is possible that hematopoietic cytokines are synthesized by stromal cells but remain bound to them and/or their extracellular matrix. In fact, it has been demonstrated that natural and recombinant hematopoietic cytokines, such as IL-3 and granulocyte macrophage colony–stimulating factor (GM-CSF), can be absorbed by heparan sulfate, the major sulfated glycosaminoglycan in bone marrow stroma.17,18 Furthermore, SCF, Tpo, and Flt-3 bind to LH/P MP-coated plates. These cytokines, once bound, can be presented in their biologically active form to the CD34+ cells.19,20 Other studies have demonstrated that heparinoids (heparin, heparan sulfate, and other heparin-like molecules) serve as cofactors to promote binding of SCF to high-affinity receptors, thus enhancing its activity.19,20 These findings may have important implications for the use of heparinoids as biomaterials for selective ex vivo expansion of CD34+ cells.
Because heparin and low-molecular-weight heparin (LH) are known to interact with a variety of functional proteins, such as growth factors, cytokines, extracellular matrix components, and adhesion molecules,21,22 heparin could be used as a therapeutic agent for various pathological conditions in which these functional proteins are involved.23 However, high-dose heparin cannot be used because of the excessive risk of bleeding.24 In contrast, LH has pharmacological and practical advantages as well as low protein binding, which produces a low, stable, and predictable anticoagulant response, thereby obviating the need for laboratory monitoring for dose adjustment.25. In addition, one or two subcutaneous injections per day are sufficient to maintain therapeutic concentrations because of the long plasma half-life of LH.25
On the other hand, protamine, a purified mixture of proteins obtained from fish sperms, neutralizes heparin and LH by forming a stable complex, which lacks anticoagulant activity.26 Protamine is also used clinically as an antidote to reverse the anticoagulant activity of heparin following cardiopulmonary bypass and in cases of heparin-induced bleeding.27 In this study, we used LH (for comparison with heparin) as a heparinoid, and protamine to prepare LH/P MPs.28 The purpose of this study is to evaluate LH/P MPs as a stem cell niche for the controlled release of hematopoietic cytokines, which stimulate the selective growth of CD34+ cells. We report here that CD34+ cells derived from mouse bone marrow exhibited a significantly higher proliferation on LH/P MP-coated plates in hematopoietic progenitor growth medium (HPGM) supplemented with adequate cytokines and 4% fetal bovine serum (FBS) than those on uncoated plates
Materials and methods
Preparation of LH/P MPs
LH/P MPs were synthesized as described previously.28 Briefly, 0.35 ml of protamine solution (10 mg/ml; Mochida Pharmaceutical Co., Tokyo, Japan) was added drop by drop to 0.7 ml of LH (fragmin solution: 6.4 mg/ml; Kissei Pharmaceutical Co., Tokyo, Japan) with vigorous vortexing for approximately 2 min. To maximize the production of microparticles, protamine and LH were mixed in a ratio of 1:2 (vol:vol) in this study. The LH/P MPs solution (1.05 ml) was then washed twice with phosphate-buffered saline (PBS) to remove nonreactants, and finally adjusted to 1 ml with PBS. More than 6 mg of dry LH/P MPs was obtained from 1.05 ml of the LH/P MPs solution.
Binding of LH/P MPs onto plastic plates and preparation of LH/P MP-coated plates
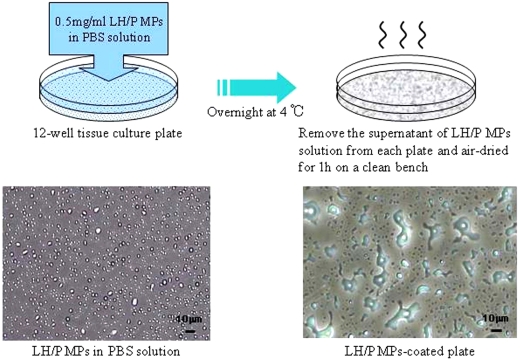
Twelve-well culture plates (well area: 360 mm2; Sumitomo Bakelite Corp., Tokyo, Japan) were coated overnight at 4°C with 1 ml of 0.5 mg/ml LH/P MPs in the PBS solution. LH/P MPs solutions were removed from the wells by pipetting. LH/P MPs were loosely bound to the plastic surface, and the unbound particles were easily washed away with PBS. The LH/P MPs were stably coated onto the plastic surface by air-drying the 12-well plate-bound LH/P MPs for 1 h on a clean bench (Figure 1).
Figure 1.
Preparation of LH/P MP-coated plates. LH aqueous (6.4 mg/ml) and protamine solutions (10 mg/ml) were mixed in a ratio of 1:2 (vol:vol) to produce LH/P MPs. The LH/P MPs consist of insoluble round complexes of approximately 1.0–0.5 μm in diameter (average: approximately 0.7 μm in diameter) and can be stably coated onto plastic surfaces by drying.
Isolation of mouse FP-BMCs from femurs of mice
Bone marrow cells were isolated by flushing both femurs of a C57BL/6J mouse (Japan SLC, Inc., Tokyo, Japan), and debris were eliminated by filtration through a 100 μm cell strainer (BD Biosciences Discovery Labware, Bedford, MA, USA). Furthermore, red blood cells and platelets were eliminated by density gradient centrifugation with Ficoll-Paque (1.077 g/ml; Amersham Biosciences, Uppsala, Sweden) at 400 g for 30 min. Mouse FP-BMCs were suspended in Dulbecco’s modified Eagle’s medium (DMEM) without FBS before culturing. These animal experiments were approved and performed in accordance with the guidelines for animal experimentation of the National Defense Medical College (Saitama, Japan).
Culture of FP-BMCs from mouse bone marrow
Prepared mouse FP-BMCs were plated at an initial density of about 100,000 cells/well on either 12-well tissue culture plates (Sumitomo Bakelite Corp., Tokyo, Japan) or LH/P MP-coated plates in 2.5 ml of HPGM (Lonza Japan Corp., Tokyo, Japan) supplemented with the indicated concentrations of inactivated FBS (JRH Biosciences, Inc., KS, USA) containing antibiotics (100 U/ml penicillin G and 100 μg/ml streptomycin), and the indicated concentrations of recombinant human SCF (Acris Corp., Hiddenhausen, Germany), Tpo (Acris Corp.), and Flt-3 (Acris Corp.). The cells were cultured at 37°C in an atmosphere of 5% CO2 and 100% relative humidity for the indicated periods. Almost all mouse FP-BMCs were easily suspended in the medium by pipetting the cell cultures, and the number of cells was counted using a hemocytometer (Sigma-Aldrich, Tokyo, Japan). The culture medium was not changed during the 8-day culture.
Selection of culture medium
Mouse FP-BMCs were plated on LH/P MP-coated plates using either one of the four types of basal media (Asahi Techno Glass Co., Tokyo, Japan) including DMEM, (RPMI-1640), DMEM/Ham’s nutrient mixture F12 (DMEM/F12), and HPGM. Each medium was supplemented with 4% FBS, antibiotics (100 U/ml penicillin G and 100 μg/ml streptomycin), and 1/2× cytokines (SCF (10 ng/ml), Tpo (20 ng/ml), and Flt-3 (20 ng/ml)). The concentrations of SCF (20 ng/ml), Tpo (40 ng/ml) and Flt-3 (40 ng/ml) in HPGM are represented as 1×.
Flow cytometry analyses
Cultured mouse FP-BMCs (approximately 1.0 × 105 cells) were suspended in 1 ml of stain buffer (FBS (10 ml) + PBS (40 ml) + NaN3 (0.5 g)) and centrifuged twice at 3000 rpm for 2 min to wash the cells. A 5-µl aliquot of Phycoerythrin PE-labeled anti-mouse CD34 (BD Biosciences Pharmingen, San Diego, CA, USA) was added to the pellet and incubated in an ice bath at 4°C for 15 min. The cells were washed twice with 1 ml of stain buffer, and then 500 µl of IsoFlow (Beckman Coulter, Fullerton, CA, USA) was added to the pellet. The samples were analyzed using EPICS®XL (Beckman Coulter).
Statistical analyses
Statistical analyses were performed by Student’s t-test. Probability values were calculated using the software, Stat Mate III for Windows (ATMS Co., Tokyo, Japan). The values of p < 0.05 were considered to be statistically significant.
Results
LH/P MP-coating and determination of culture conditions for expansion of CD34+ cells
Although added LH/P MPs were loosely bound to the plastic surface, particles were easily washed away from the surface with PBS. Alternatively, LH/P MPs were stably coated onto the plastic surface by air-drying bound LH/P MPs for 1 h on a clean bench (Figure 1). While most FP-BMCs did not bind to both the LH/P MP-coated and uncoated tissue culture plates, only a small number of cells (<20%) loosely bound to both the LH/P MP-coated and uncoated tissue culture plates. Almost all of these bound cells could be easily released by washing with the culture medium.
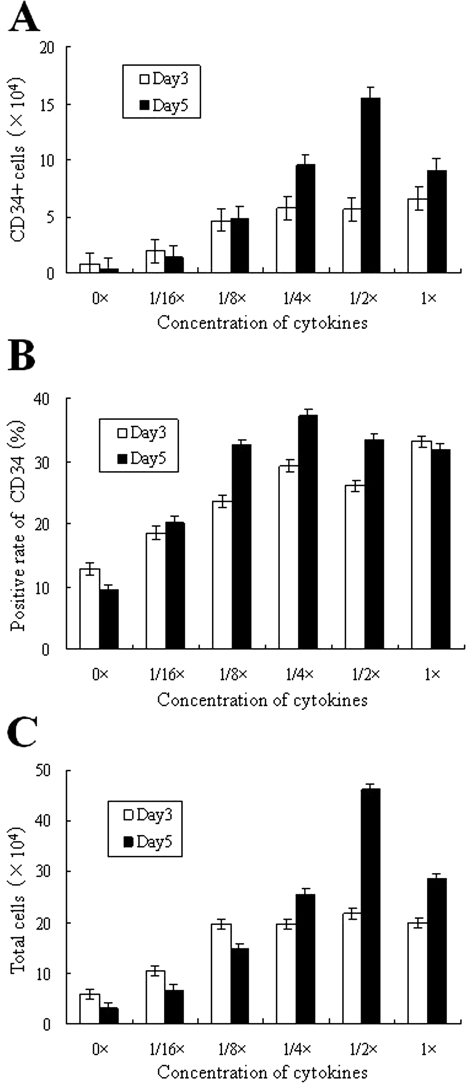
The optimal concentrations of SCF, Tpo, Flt-3, and FBS in HPGM were tested for maximum expansion of mouse FP-BMCs and CD34+ cells on LH/P MP-coated plates (Figures 2 and 3). In this study, we used 12-well culture plates using 2.5 ml of the medium for culturing mouse FP-BMCs because over 50,000 cells were required to evaluate the marker CD34 by flow cytometry as well as for estimating the cell numbers. When mouse FP-BMCs (approximately 100,000 cells) were plated on 12-well tissue culture plates, few cells adhered to the plates, and no cell colonies were obtained in these cultures. However, the optimal expansion of CD34+ cells as well as total cells (mouse FP-BMCs) was obtained on LH/P MP-coated tissue culture plates containing concentrations (1/2×) of SCF (10 ng/ml), Tpo (20 ng/ml), and Flt-3 (20 ng/ml) in HPGM supplemented with 4% FBS (Figure 2). The total cell number increased 4.6-fold under these conditions after 5 days of culture (Figure 2C). Because flow cytometry analysis (Figure 2B) revealed that 23.8% of original mouse FP-BMCs and 33.4% of the expanded cells after 5 days of culture were CD34+, CD34+ cells were estimated to have increased 6.5-fold after 5 days of culture (Figure 2A).
Figure 2.
Optimal concentrations of cytokines. Optimal concentrations of SCF/Tpo/Flt-3 were determined on LH/P MP-coated plates incubated for 3 and 5 days in the presence of the indicated cytokines in HPGM supplemented with 4% FBS. The concentrations of SCF (20 ng/ml), Tpo (40 ng/ml), and Flt-3 (40 ng/ml) in HPGM are represented as 1×. White bars (Day 3) and black bars (Day 5) represent total cells (mouse FP-BMCs) (C) and CD34+ cells (A), which were calculated using data from flow cytometry analyses shown in (B). The ratio of CD34+ to total cells is represented in (B). Data represent mean ± SD of six determinations.
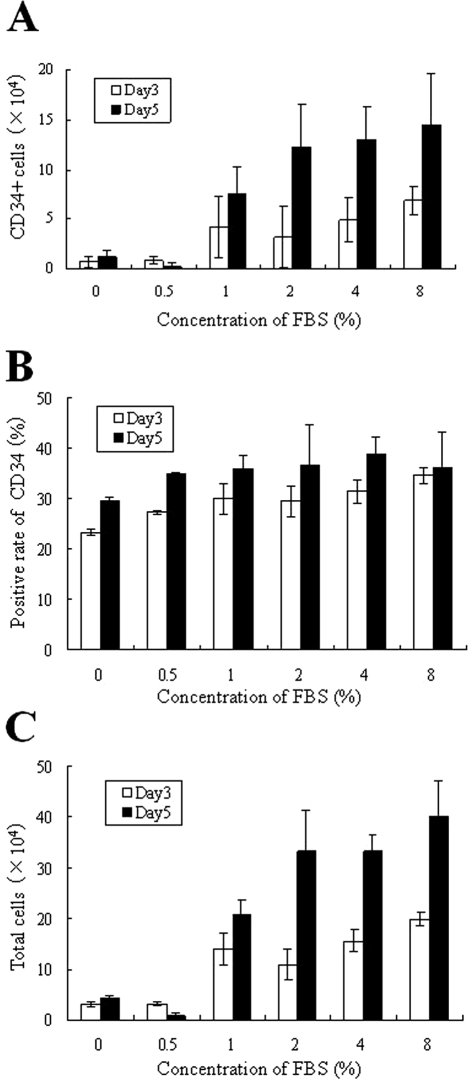
Figure 3.
Optimal concentrations of serum. Optimal concentrations of FBS were determined on LH/P MP-coated plates incubated for 3 and 5 days in the presence of the indicated concentrations of FBS with 1/2× cytokines in HPGM. White bars (Day 3) and black bars (Day 5) represent total cells (mouse FP-BMCs) (C) and CD34+ cells (A), which were calculated using data from flow cytometry analyses shown in (B). The ratio of CD34+ to total cells is represented in (B). Data represent mean ± SD of six determinations.
Since FBS was required for expansion of cells, the optimal concentration of FBS was also determined in the presence of the cytokines (1/2×), SCF (10 ng/ml), Tpo (20 ng/ml), and Flt-3 (20 ng/ml) in HPGM (Figure 3). The total cell (mouse FP-BMCs) number increased 3.3-fold by 4% or more FBS after 5 days of culture (Figure 3C). Since flow cytometry analysis (Figure 3B) revealed that 23.8% of original mouse FP-BMCs and 39.0% of the expanded cells after 5 days of culture were CD34+, CD34+ cells were estimated to have expanded 5.5-fold after 5 days of culture (Figure 3A). Considering mouse FP-BMC growth and the ratio of CD34+ cells to the total cells, we chose concentrations (1/2×) of SCF (10 ng/ml), Tpo (20 ng/ml), and Flt-3 (20 ng/ml) in HPGM supplemented with 4% FBS for optimal culture conditions for CD34+ cells on LH/P MP-coated plates.
Evaluation of various culture media for growth of CD34+ cells
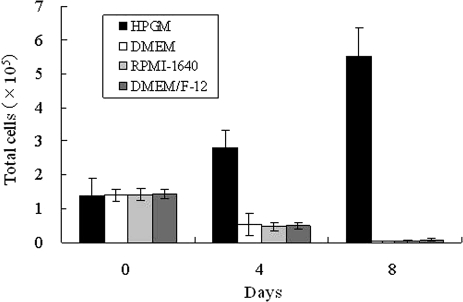
To define the optimal media for expansion of CD34+ cells, mouse FP-BMCs were plated on LH/P MP-coated tissue culture plates containing various basal culture media, including DMEM, RPMI-1640, DMEM/F12, and HPGM supplemented with 4% FBS, antibiotics, and 1/2× cytokines (SCF (10 ng/ml), Tpo (20 ng/ml), and Flt-3 (20 ng/ml)). The CD34+ cells as well as mouse FP-BMCs were able to grow only in HPGM (Figure 4).
Figure 4.
Evaluation of various culture media for growth of CD34+ cells. To define the optimal media for expansion of CD34+ cells, mouse FP-BMCs were plated on LH/P MP-coated tissue culture plates containing various basal culture media supplemented with 4% FBS, antibiotics, and 1/2× cytokines. Data represent mean ± SD of six determinations.
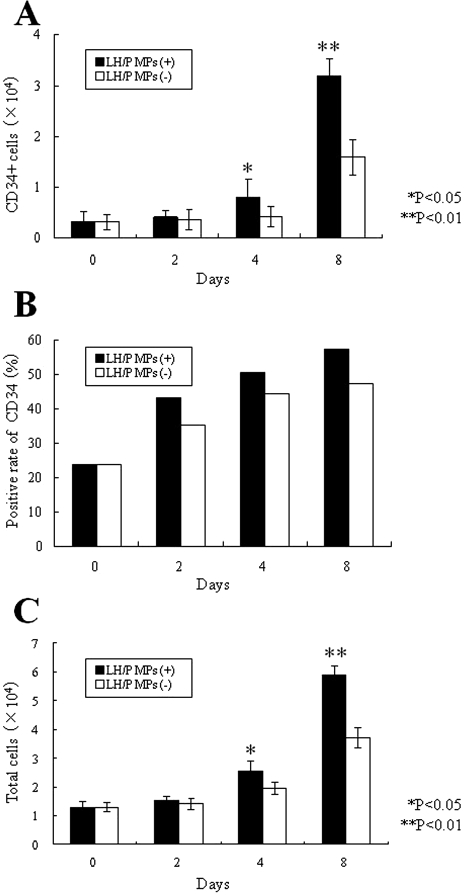
Assessment of expansion of CD34+ cells
When mouse FP-BMCs were plated on both LH/P MP-coated and uncoated tissue culture plates, the cells did not adhere to either of the plates. However, these cells were able to grow in suspension culture with concentrations (1/2×) of SCF (10 ng/ml), Tpo (20 ng/ml), and Flt-3 (20 ng/ml) in HPGM supplemented with 4% FBS. The total cell number increased by 4.6- and 2.9-fold after 8 days of culture on LH/P MP-coated and uncoated tissue culture plates, respectively (Figure 5C). Since flow cytometry analysis revealed that 23.8% of initial mouse FP-BMCs, and 57.4% and 47.5% of the expanded cells on LH/P MP-coated and uncoated tissue culture plates, respectively, were CD34+ (Figure 5B), CD34+ cells were estimated to have expanded 11.0-fold after 8 days of culture on LH/P MP-coated plates (Figure 5A). The ratios of CD34+ cells to total cells on LH/P MP-coated plates were always higher than those on uncoated plates as determined on Days 2, 4, and 8.
Figure 5.
Expansion of CD34+ cells on LH/P MP-coated plates. Expansion of total cells (mouse FP-BMCs) and CD34+ cells on both LH/P MP-coated and uncoated tissue culture plates was examined in HPGM supplemented with 1/2× cytokines and 4% FBS for 8 days. Total cells (C) were counted using a hemocytometer, and CD34+ cells (A) were calculated using flow cytometry data (B) to determine the ratio of CD34+ cells to total cells (mouse FP-BMCs). Data represent mean ± SD of six determinations.
Discussion
We previously reported that LH/P MPs, which are approximately 1 μm in diameter, facilitate the fibroblast growth factor-2 (FGF-2) injection.28 FGF-2-containing LH/P MPs induce substantial vascularization and fibrous tissue formation because of the gradual release of FGF-2 molecules from the LH/P MPs. In this study, LH/P MP-coating as a stem cell niche stimulated the growth of CD34+ cells in HPGM with adequate cytokines (SCF, Tpo, and Flt-3). Mouse FP-BMCs in the presence of concentrations (1/2×) of cytokines (SCF (10 ng/ml), Tpo (20 ng/ml), and Flt-3 (20 ng/ml)) in HPGM supplemented with 4% FBS exhibited 4.6- and 2.4-fold expansion after 8 days of culture on LH/P MP-coated and uncoated tissue culture plates, respectively. Furthermore, since flow cytometry analysis (Figure 5B) revealed that the percentage of CD34+ in the expanding cells on LH/P MP-coated plates increased from 23.8% to 57.4% during the 8 days of culture (Figure 5B), the CD34+ cells expanded 11.0- and 5.8-fold after 8 days of culture on LH/P MP-coated and uncoated tissue culture plates, respectively. Thus, the LH/P MP-coated plate in the presence of appropriate cytokines is a useful culture method for selective expansion of CD34+ cells in mouse FP-BMCs. However, we still need to characterize the expanded cell population with respect to various surface antigens in addition to CD34 and different morphologies/functions in the expanded cells.
CD34+ cells are localized in stem cell niches and local area networks in the microenvironment of bone marrow, where they interact with components of their niche including stromal cell surfaces, extracellular matrix proteins, heparan sulfate proteoglycans, and cytokines.17,18 Heparan sulfate proteoglycans are found in the extracellular matrix produced by stromal cells. They are prime candidates for selectively retaining CD34+ cells stimulatory factors in the niche (stromal layers), and they may regulate hematopoiesis.19 In addition, the immobilization of cytokines (SCF, Tpo, and Flt-3) onto LH/P MP-coated plates may provide a matrix-like stem cell niche that is bioactive for the proliferation of CD34+ cells.
It is recognized in polymer chemistry that positively and negatively charged polymers interact ionically.29 Basic protamine molecules complexed with acidic molecules such as LH form microparticles through ionic interactions. The amounts of LH and protamine on the LH/P MP-coated plates used for culturing CD34+ cells did not alter for at least 8 days (data not shown). However, it probably appears that polypeptides such as SCF, Tpo, and Flt-3, once bound to the LH/P MPs are gradually released from the LH/P MP-coated plates and decreased by half within 5 days of culture.19 Although LH/P MPs are not biodegradable in vitro, the inclusion of various cytokines, such as SCF, Tpo, and Flt-3, into the coating will provide an excellent cytokine-containing matrix for CD34+ cells.15–18 We have previously demonstrated that SCF, Tpo, and Flt-3 are heparin-binding proteins, that all three interact with LH/P MPs, and that the immobilized cytokines are gradually released into the culture medium over 6 days.19,20 Furthermore, it has been shown that heparin addition enhances the expansion of cord blood hematopoietic progenitor cells in three-dimensional coculture with stromal cells.30
The induction of hematopoietic cell differentiations stimulated by several late-acting cytokines may be considered as consequence of proliferation of earlier hematopoietic population such as CD34+ cells stimulated by several cytokines such as SCF and leukemia inhibitory factor (LIF).31 The majority of late-acting cytokines such as CSFs, interleukins (ILs), Tpo, and erythropoietin (EPO) support the proliferation and maturation of lineage-committed progenitors and the functional properties of differentiated cells. Intermediate-acting, lineage-nonspecific cytokines including IL-3, IL-4, and GM-CSF are mainly directed toward progenitor cells in the intermediate stages of hematopoietic development.32 Therefore, it may be possible that a specific hematopoietic population in BMCs can be selectively expanded by various combinations of appropriate cytokines using the LH/P MP-coated plates.
Dalteparin is an LH that has an anticoagulant activity much lower than that of the native heparin and can therefore be administered subcutaneously.25,26 Therefore, dalteparin as LH was used to prepare LH/P MPs in these studies. No bleeding complications were observed in animals injected with LH/P MPs in a previous study.28 In addition, since the two components for LH/P MPs, LH, and protamine, are already in clinical use, their clinical safeties are ensured.
Acknowledgments
We acknowledge expertise and advice of Associate Professor Koichi Fukuda and also thank the personnel of Institute of Laboratory Animals, Graduate School of Medicine, and National Defense Medical College for expert care of animals.
Conflict of Interest
None Declared
References
- 1. Stella CC, Cazzola M, Fabritiis PD, et al. CD34-positive cells: biology and clinical relevance. Haematologica 1995; 80(4): 367–387 [PubMed] [Google Scholar]
- 2. Guo S, Cheng Y, Ma Y, et al. Endothelial progenitor cells derived from CD34+ cells from cooperative vascular networks. Cell Physiol Biochem 2010; 26(4–5): 679–688 [DOI] [PubMed] [Google Scholar]
- 3. Wang J, Zhang S, Rabinovich B, et al. Human CD34+ cells in experimental myocardial infarction: long-term survival, sustained functional improvement, and mechanism of action. Circ Res 2010; 106(12): 1904–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moioli EK, Clark PA, Chen M, et al. Synergistic actions of hematopoietic and mesenchymal stem/progenitor cells in vascularizing bioengineered tissues. PLoS One 2008; 3(12): e3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sutherland RD, Keating A. The CD34 antigen: structure, biology and potential clinical application. J Hematother 1992; 1(2): 115–129 [DOI] [PubMed] [Google Scholar]
- 6. Andrews RG, Singer JW, Bernstein ID. Monoclonal antibody 12.8 recognizes a 115 kD molecule present on both unipotent and multipotent hematopoietic colony-forming cells and their precursors. Blood 1986; 67(3): 842–852 [PubMed] [Google Scholar]
- 7. Yura H, Kanatani Y, Ishihara M, et al. Selection of hematopoietic stem cells with combination of galactose-bound vinyl polymer and soybean agglutinin, a galactose-specific lectin. Transfusion 2008; 48(3): 561–566 [DOI] [PubMed] [Google Scholar]
- 8. Traycott CM, Abboud MR, Laver J, et al. Rapid exit from G0/G1 phases of cell cycle in response to stem cell factor confers on umbilical cord blood CD34+ cells an enhanced ex vivo expansion potential. Exp Hematol 1994; 22(13): 1264–1272 [PubMed] [Google Scholar]
- 9. Parmar K, Mauch P, Vergilio JA, et al. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A 2007; 104(13): 5431–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hung SC, Chen NJ, Hsieh SL, et al. Isolation and characterization of size-sieved stem cells from human bone marrow. Stem Cells 2002; 20(3): 249–258 [DOI] [PubMed] [Google Scholar]
- 11. Hofmeister CC, Zhang J, Knight KL, et al. Ex vivo expansion of umbilical cord blood stem cells for transplantation: growing knowledge from the hematopoietic niche. Bone Marrow Transplant 2007; 39(1): 11–23 [DOI] [PubMed] [Google Scholar]
- 12. Wilson A, Trumpp A. Bone-marrow haemetopoietic-stem-cell niches. Nat Rev Immunol 2006; 6(2): 93–106 [DOI] [PubMed] [Google Scholar]
- 13. Moore KA, Lemischka IR. Stem cells and their niches. Science 2006; 311(5769): 1880–1885 [DOI] [PubMed] [Google Scholar]
- 14. Zubler RH. Ex vivo expansion of haematopoietic stem cells and gene therapy development. Swiss Med Wkly 2006; 136(49–50): 795–799 [DOI] [PubMed] [Google Scholar]
- 15. Gupta P, Oegema TR, Jr, Brazil JJ, et al. Structurally specific heparan sulfates support primitive human hematopoiesis by formation of a multimolecular stem cell niche. Blood 1998; 92(13): 4641–4651 [PubMed] [Google Scholar]
- 16. Alvarez-Silva M, Borojevic R. GM-CSF and IL-3 activities in schistosomal liver granulomas are controlled by stroma-associated heparan sulfate proteoglycans. J Leukoc Biol 1996; 59(3): 435–441 [DOI] [PubMed] [Google Scholar]
- 17. Roberts R, Gallagher J, Spooncer E, et al. Heparan sulfate bound growth factors: a mechanism for stromal cell mediated haemopoiesis. Nature 1988; 332(6162): 376–378 [DOI] [PubMed] [Google Scholar]
- 18. Gordon MY, Riley GP, Watt SM, Greaves MF. Compartmentalization of a haematopoietic growth factor (GM-CSF) by glycosaminoglycans in the bone marrow microenvironment. Nature 1987; 326(6111): 403–405 [DOI] [PubMed] [Google Scholar]
- 19. Kishimoto S, Nakamura S, Nakamura SI, et al. Cytokine-immobilized microparticle-coated plates for culturing hematopoietic progenitor cells. J Control Release 2009; 133(3): 185–190 [DOI] [PubMed] [Google Scholar]
- 20. Kishimoto S, Nakamura S, Hattori H, et al. Human stem cell factor (SCF) is a heparin-binding cytokine. J Biochem 2009; 145(3): 275–278 [DOI] [PubMed] [Google Scholar]
- 21. Ishihara M, Ono K. Structure and function of heparin and heparan sulfate: heparinoid library and modification of FGF-activities. Trends Glycosci Glycotechnol 1998; 10(52): 223–233 [Google Scholar]
- 22. Ishihara M. Biosynthesis, structure, and biological activity of basic FGF binding domains of heparan sulfate. Trends Glycosci Glycotechnol 1993; 5(25): 343–354 [Google Scholar]
- 23. Salmivirta M, Lidholt K, Lindahl U. Heparan sulfate: a piece of information. FASEB J 1996; 10(11): 1270–1279 [DOI] [PubMed] [Google Scholar]
- 24. Lindahl U, Lidholt K, Spillmann D, Kjellen L. More to ‘heparin’ than anticoagulation. Thromb Res 1994; 75(1): 1–32 [DOI] [PubMed] [Google Scholar]
- 25. Hirsh J, Warkentin TE, Shaughnessy SG, et al. Heparin and low-molecular-weight heparin: mechanisms of action, pharmacokinetics, dosing, monitoring, efficacy, and safety. Chest 2001; 119(1): 64S–94S [DOI] [PubMed] [Google Scholar]
- 26. Wolzt M, Wetermann A, Nieszpaur-Los M, et al. Studies on the neutralizing effects of protamine on unfractionated and low molecular weight heparin (FragminR) at the site of activation of the coagulation system in man. Thromb Haemost 1995; 73(3): 439–443 [PubMed] [Google Scholar]
- 27. Pan M, Lezo JS, Medina A, et al. In-laboratory removal of femoral sheath following protamine administration in patients having intracoronary stent implantation. Am J Cardiol 1997; 80(10): 1336–1338 [DOI] [PubMed] [Google Scholar]
- 28. Nakamura S, Kanatani Y, Kishimoto S, et al. Controlled release of FGF-2 using fragmin/protamine microparticles and effect on neovascularization. J Biomed Mater Res A 2009; 91(3): 814–823 [DOI] [PubMed] [Google Scholar]
- 29. Nakamura S, Nambu M, Ishizuka T, et al. Effect of controlled release of fibroblast growth factor-2 from chitosan/fucoidan micro complex hydrogel on in vitro and in vivo vascularization. J Biomed Mater Res A 2008; 85(3): 619–627 [DOI] [PubMed] [Google Scholar]
- 30. Okamoto T, Takagi M, Soma T, et al. Effect of heparin addition on expansion of cord blood hematopoietic progenitor cells in three-dimensional coculture with stromal cells in nonwoven fabrics. J Artif Organs 2004; 7(4): 194–202 [DOI] [PubMed] [Google Scholar]
- 31. Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood 1993; 81(11): 2844–2853 [PubMed] [Google Scholar]
- 32. Sonoda Y, Yang YC, Wong GG, et al. Analysis in serum-free culture of the targets of recombinant human hematopoietic factors: interleukin-3 and granulocyte/macrophage colony–stimulating factor are specific for early developmental stages. Proc Natl Acad Sci U S A, 1988; 85(12): 4360–4364 [DOI] [PMC free article] [PubMed] [Google Scholar]