Abstract
CD9 and CD81 are closely related tetraspanins that regulate cell motility and signaling by facilitating the organization of multimolecular membrane complexes, including integrins. We show that CD9 and CD81 are down-regulated in smoking-related inflammatory response of a macrophage line, RAW264.7. When functions of CD9 and CD81 were ablated with monoclonal antibody treatment, small interfering RNA transfection, or gene knock-out, macrophages were less motile and produced larger amounts of matrix metalloproteinase (MMP)-2 and MMP-9 than control cells in vitro. In line with this, CD9/CD81 double-knock-out mice spontaneously developed pulmonary emphysema, a major pathological component of chronic obstructive pulmonary disease (COPD). The mutant lung contained an increased number of alveolar macrophages with elevated activities of MMP-2 and MMP-9 and progressively displayed enlarged airspace and disruption of elastic fibers in the alveoli. Secretory cell metaplasia, a finding similar to goblet cell metaplasia in cigarette smokers, was also observed in the epithelium of terminal bronchioles. With aging, the double-knockout mice showed extrapulmonary phenotypes, including weight loss, kyphosis, and osteopenia. These results suggest that the tetraspanins CD9 and CD81 regulate cell motility and protease production of macrophages and that their dysfunction may underlie the progression of COPD.
Chronic obstructive pulmonary disease (COPD),5 a disease defined by incompletely reversible airflow limitation, results from abnormal inflammatory response to chronic cigarette smoking. Pulmonary emphysema is a major component of COPD, and a dominant hypothesis in its pathophysiology is that persistent infiltration of inflammatory cells and production of proteases, including matrix metalloproteinases (MMPs) in the lung, lead to tissue destruction and airspace enlargement (1, 2). In patients with emphysema, there was an increase in bronchoalveolar lavage fluid (BALF) concentrations and macrophage expression of MMP-9 (3). Studies of human samples have shown increases of MMP-2 and MMP-9 in smoking-related emphysema (4). Alveolar macrophages secrete elastolytic enzymes, including MMP-2, MMP-9, and MMP-12, and play a pivotal role in the pathophysiology of COPD. There was a marked increase in the numbers of macrophages in airways, lung parenchyma, BALF, and sputum in patients with emphysema (2). Macrophages are activated by cigarette smoke to release inflammatory mediators such as TNF-α, chemokines, and reactive oxygen species as well as MMPs, providing a cellular mechanism that links smoking with inflammation in COPD (2). It was recently proposed that lowered activity of histone deacetylases (HDACs), which are suppressors of inflammatory genes, accounts for the persistent activation of macrophages in COPD patients (5).
The tetraspanin proteins include at least 33 members, including CD9, CD63, CD81, CD82, and CD151 in mammals. They are characterized by the structure that spans the plasma membrane four times and have a propensity to form complexes with each other and with other functional molecules, including integrins, signaling proteins, and membrane-anchored growth factors at specialized membrane microdomains. As organizer of these multimeric complexes, tetraspanins regulate cell morphology, motility, invasion, fusion, and signaling (6). It has been increasingly recognized that tetraspanin-integrin complexes also regulate the production of MMPs, particularly in tumor cells. Treatment of a breast cancer cell line with anti-tetraspanin monoclonal antibodies (mAbs) stimulated production of MMP-2 and formation of invasive protrusions (7). CD9 expression inhibited integrin-dependent morphologic differentiation and MMP-2 production of small cell lung cancer cells via the phosphatidylinositol 3-kinase/Akt pathway (8). Overexpression of CD81 or CD82 reduced cell motility and MMP-9 activity in multiple myeloma cell lines (9). However, their role in motility and MMP production of macrophage has yet to be studied.
In this study, our in vitro experiments show that CD9 and CD81, the two widely distributed and closely correlated tetraspanins, are down-regulated in smoking-related inflammatory response of macrophages and that ablation of their function suppresses cell motility and increases the production of MMPs. Moreover, in vivo experiments using CD9/CD81 double-knock-out (DKO) mice displayed accumulation of macrophages and increased activities of MMPs in the mutant lung. The DKO mice progressively developed pulmonary emphysema, weight loss, and osteopenia, a phenotype akin to human COPD.
EXPERIMENTAL PROCEDURES
Immunoblotting—A mouse macrophage line, RAW264.7, and a human alveolar epithelial cell line, A549, were serum-starved for 24 h and treated with 10 ng/ml trichostatin A (TSA; Wako Pure Chemical Industries, Osaka, Japan) or 0.1% cigarette smoke extract (CSE) as described previously (10) for 48 h. In some experiments, 10 μm theophylline (Wako Pure Chemical Industries) or 1 μm dexamethasone (Sigma) were co-added with TSA into the culture. Cells were lysed in lysis buffer containing 1% Brij99, 25 mm HEPES (pH 7.5), 150 mm NaCl, 5 mm MgCl2, 2 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, and 10 μg/ml leupeptin. Cell lysates were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and probed with rat anti-mouse CD9 (KMC8) and integrin β1 (KMI6) mAbs (BD Biosciences) and hamster anti-mouse CD81 mAb (Eat2; AbD Serotec, Oxford, UK). For A549 lysates, mouse anti-human CD9 (MM2/57; BIOSOURCE) and CD81 (JS64; Immunotech, Marseille, France) mAbs were used.
Treatment of RAW264.7 Cells with mAbs or Small Interfering RNA (siRNA) Transfection against CD9 and CD81—To test the effects of function-inhibitory mAbs to CD9 (KMC8) and CD81 (2F7; Southern Biotechnology, Birmingham, AL), RAW264.7 cells were cultured in DMEM containing 0.1% FBS for 24 h in the absence or presence of 20 μg/ml of IgG (isotype matched with KMC8), KMC8, 2F7, and KMC8 plus 2F7. mRNA was extracted, and expressions of MMP-2, MMP-9, MMP-12, tissue inhibitor of metalloproteinase (TIMP)-1, and TIMP-2 were evaluated by reverse transcription (RT)-PCR as described previously (11, 12). Culture supernatants were studied for MMP-9 activity in gelatin zymography. The activities were quantified on a FluorChem using software AlphaEase (Alpha Innotech, San Leandro, CA). In a cell migration assay, RAW264.7 cells (5 × 104) suspended in serum-free DMEM and preincubated with the mAbs were applied to the upper chamber of fibronectin (FN)-precoated Transwells. DMEM containing 10% FBS was added to the lower chamber. After 4 h, cells migrating to the lower surface were counted with Diff-Quick stain (International Reagents, Hyogo, Japan). For siRNA transfection, RAW264.7 cells were transfected with mixture siRNAs against mouse CD9 or CD81 (B-Bridge International, Sunnyvale, CA) or control mixture RNAs (B-Bridge International) using Lipofectamine 2000 (Invitrogen). Gene silencing effects were confirmed by immunoblotting. The cells were cultured for 24 h in serum-free DMEM, and the culture supernatants were studied for MMP-9 activity by gelatin zymography.
Mice—The generation of CD9/CD81 DKO mice was described previously (13). These mice were backcrossed into the C57BL/6J background. The genotyping of littermates was achieved by PCR analysis. All animal experiments were performed with age- and sex-matched littermate controls using at least three animals at each time point. The mice were maintained in a specific pathogen-free facility, and all animal procedures were performed in accordance with the Osaka University guidelines on Animal Care.
Histology and Histomorphometric Analysis of the Lung—Lungs were inflated to 25 cm of water pressure with 10% buffered neutral formalin via an intratracheal cannula and embedded in paraffin. Parasagittal 5-μm-thick sections were stained with hematoxylin and eosin. Elastica-van Gieson stain for elastin, Masson's trichrome stain for collagen, and Alcian blue/periodic acid-Schiff (PAS) stain for mucus-secreting cells were also performed. Airspace size was quantified by calculating the mean chord length using the NIH Image software (14). Briefly, a minimum of 10 fields from each mouse lung was randomly acquired and visualized using the program Scion Image (Scion, Frederick, MD). The images were subject to sequential logical image match and operations with a horizontal and vertical grid. At least 200 measurements per field were made, and the length of the lines overlying airspace was averaged as the mean chord length.
Bronchoalveolar Lavage and Gelatin Zymography—Lungs of anesthetized mice were subjected to lavage with 3 volumes of 1 ml of phosphate-buffered saline containing 0.1% bovine serum albumin. Collected cells in the BALF were centrifuged onto Cytospin slides and visualized by Diff-Quick stain. Total cell counts and their subsets were determined using a hemocytometer. The supernatants of BALF were concentrated 10-fold using Centricon 10 filtration units (Millipore, Bedford, MA). Samples containing an equal amount of protein were electrophoresed in 10% zymogram gelatin gels (NOVEX, Carlsbad, CA). Gels were washed twice in 2.5% Triton X-100, incubated for 24 h with 40 mm Tris/HCl (pH 7.5), 10 mm CaCl2, and 1 μm ZnCl2, and stained with Coomassie Blue. Gelatinolytic activities were quantified on a FluorChem using software AlphaEase. The identical gels were treated in the presence of 0.01 m EDTA in parallel, and metal dependence, which is characteristic of MMPs, was confirmed by disappearance of lytic bands.
Peripheral Quantitative Computed Tomography (pQCT) Analysis—Femurs were fixed with 10% buffered formalin and measured using an XCT Research SA (Stratec Medizintechnik, Pforzheim, Germany) as described previously (15). The contour of the total bone was determined automatically with the pQCT software algorithm. The cortical and trabecular parameters were obtained at the diaphysis and 2 mm from distal epiphysis, respectively. The total mineral content and strength strain index were determined as described previously (16).
RESULTS
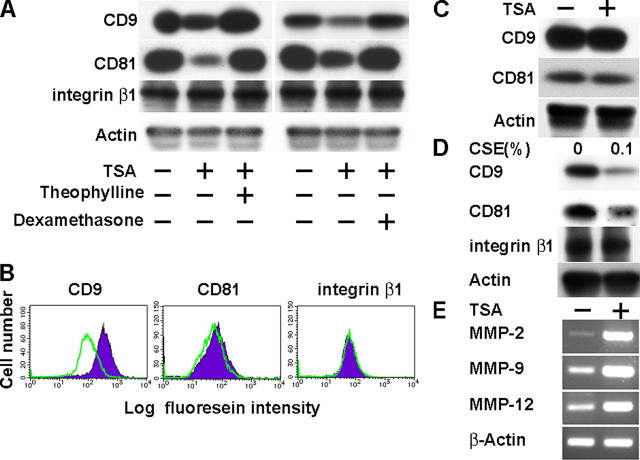
CD9 and CD81 Are Down-regulated by Smoking-related Inflammatory Stimuli in RAW264.7 Macrophages—Cigarette smoke reduces the expression and activity of HDACs in macrophages of COPD patients, resulting in amplification of pro-inflammatory gene transcription (5). To examine the effect of lowered HDAC activities on CD9 and CD81 expression, RAW264.7 macrophages were cultured in the presence of a HDAC inhibitor, TSA. As shown in Fig. 1A, treatment of RAW264.7 with TSA induced a decrease in protein levels of CD9 and CD81. The effects were reversed by the addition of a low concentration of HDAC activators, theophylline and dexamethasone (17), suggesting that their down-regulation was specific to HDAC inactivation and not because of nonspecific cytotoxicity of 10 ng/ml TSA. As a control, we tested the expression of integrin β1 but found no change in the presence of these agents (Fig. 1A). Consistent with the immunoblotting data, flow cytometry showed that TSA decreases surface expressions of CD9 and, to a lesser extent, CD81 while not affecting integrin β1 (Fig. 1B). The inhibitory effect of TSA was not observed in an alveolar epithelial cell line, A549 (Fig. 1C). We also examined the effect of IFN-γ and TNF-α, which are pro-inflammatory cytokines elevated in smoke-induced inflammation (18), on RAW264.7. As shown in supplemental Fig. 1, although TNF-α had no effect, IFN-γ dose-dependently suppressed the expressions of CD9 and CD81. Finally, we directly treated RAW264.7 cells with CSE (10) and observed that 0.1% CSE similarly reduced CD9 and CD81 but not integrin β1 in immunoblotting (Fig. 1D) and flow cytometry (data not shown). These results suggest that cigarette smoke may induce macrophages to down-regulate CD9 and CD81 expression in a cell-autonomous manner.
FIGURE 1.
TSA or CSE down-regulates CD9 and CD81 while up-regulating MMPs in RAW264.7 macrophages. A, RAW264.7 cells were cultured in the absence or presence of TSA, theophylline, and dexamethasone for 48 h. Expressions of CD9, CD81, and integrin β1 were examined by immunoblotting using whole cell lysates. Anti-actin blots confirm equal amounts of protein loaded in each lane. B, RAW264.7 was cultured in the absence (filled histograms) or presence (open histograms) of TSA. Surface expressions of CD9, CD81, and integrin β1 were analyzed by flow cytometry. C, A549 alveolar epithelial cells were cultured in the absence or presence of TSA. CD9 and CD81 were immunoblotted. D, RAW264.7 was cultured in the absence or presence of 0.1% CSE for 48 h. CD9, CD81, and integrin β1 were immunoblotted. E, RAW264.7 was cultured in the absence or presence of TSA. Expressions of MMP-2, MMP-9, and MMP-12 were analyzed by RT-PCR. β-Actin amplification was internal control.
MMP production of macrophages is an essential part of pathophysiological mechanisms of COPD (1, 2). To examine the effect of HDAC inactivation, expressions of MMP-2, MMP-9, and MMP-12 were analyzed by RT-PCR. As shown in Fig. 1E, these MMPs were up-regulated with the addition of TSA. Based on these results, we hypothesized that the decrease of CD9 and CD81 may be upstream events to the increase of MMPs in macrophages stimulated with cigarette smoke.
mAbs or siRNA Transfection against CD9 and CD81 Enhance MMP Production and Suppress Cell Motility of RAW264.7—To examine if the loss of CD9 and CD81 function is causal to the up-regulation of MMPs, we treated RAW264.7 with function-inhibitory mAbs (13). Co-addition of anti-CD9 mAb, KMC8, and anti-CD81 mAb, 2F7, to RAW264.7 promoted the transcription of MMP-2 and MMP-9, although MMP-12 was unchanged (Fig. 2A). Up-regulation of TIMP-1 was also observed; this might be a counteraction against overproduction of MMP-9 (19). In gelatin zymography using culture supernatant, the addition of KMC8 or 2F7 increased gelatinolytic activity of MMP-9 compared with control, and the combination of KMC8 and 2F7 had an additive effect, suggesting a coordinate role of CD9 and CD81 in MMP-9 production (Fig. 2B). We failed to detect MMP-2 activity (data not shown) despite its transcription (Fig. 2A), possibly because of its defective activation in this cell line (20). MMP-9 activity was also examined by knockdown of CD9 or CD81 with siRNA transfection. As shown in Fig. 2C, protein levels of CD9 or CD81 were successfully lowered relative to control, and MMP-9 activity was increased by 75 and 49% in densitometry, respectively.
FIGURE 2.
mAbs or siRNA to CD9 and CD81 enhance MMP production and suppress motility of RAW264.7 cells. A, RAW264.7 cells were cultured for 24 h in the absence (-) or presence of KMC8 plus 2F7 (+). mRNA was extracted, and RT-PCR was performed for expressions of MMPs and TIMPs. B, RAW264.7 cells were cultured for 24 h in the absence (-) or presence of the indicated mAbs. MMP-9 activity in culture supernatant was examined by gelatin zymography (lower) and quantified by densitometry (upper). The gelatin zymography is from one representative of three similar experiments. C, RAW264.7 was transfected with siRNAs against CD9 or CD81. Decrease in CD9 or CD81 was shown in immunoblotting (upper). MMP-9 activity in supernatants of 24-hour culture was examined by gelatin zymography (lower). D, RAW264.7 cells were applied into the upper chamber of FN-precoated Transwells in the absence (-) or presence of the indicated mAbs. DMEM containing 10% FBS was added to the lower chamber. After 4 h, cells migrating to the lower surface of the membrane were counted after Diff-Quick stain. Bars represent the mean ± S.E. *, p < 0.05 versus IgG; **, p < 0.05 versus KMC8. KMC8, anti-CD9; 2F7, anti-CD81; HMβ1-1, anti-integrin β1.
One of major tetraspanin functions is to regulate cell motility by forming complexes with integrins (21). In fact, we previously demonstrated that CD9 and CD81 complex with β1 and β2 integrins in blood monocytes (13). To study effects of the anti-tetraspanin mAbs on RAW264.7 motility, a migration assay was performed using Transwells (Fig. 2D). Although these mAbs did not affect cell proliferation and adhesion (data not shown), KMC8 and 2F7 suppressed migration of RAW264.7 cells on FN. Co-addition of anti-CD9 and anti-CD81 mAbs revealed an additive effect. The migration was dependent on β1 integrins because it was almost completely blocked by anti-integrin β1 mAb. Thus, CD9 and CD81 appeared to regulate integrin-dependent motility as well as MMP production in RAW264.7 macrophages.
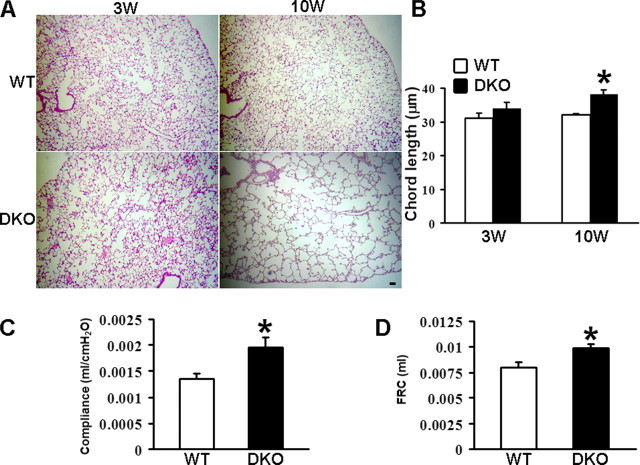
CD9/CD81 DKO Mice Spontaneously Develop Pulmonary Emphysema—CD9/CD81 DKO mice were generated in our previous work, which reported that the fusion of mononuclear phagocytes were accelerated in these mice (13). Remarkably, we also found that the DKO mice developed pulmonary emphysema; the mutant lung showed enlargement of airspace and infiltration of inflammatory cells with age (Fig. 3, A and B, and supplemental Fig. 2). As shown in chord length measurement, the airspace size of CD9/CD81 DKO mice was not different from wild-type (WT) littermates at 3 weeks of age but significantly increased at 10 weeks (Fig. 3B). CD9 and CD81 single-KO mice also displayed mild focal airspace enlargement in close examination (supplemental Fig. 2A), but their chord lengths at 10 weeks were not significantly different from WT (data not shown). We next assessed the DKO lung using a whole body plethysmograph. When compared with the WT lung, static compliance (Fig. 3C) and functional residual capacity (Fig. 3D) were significantly increased, indicating physiological impairment of lung function.
FIGURE 3.
CD9/CD81 DKO mice develop pulmonary emphysema. A, histological lung sections from DKO mice and WT littermates at 3 and 10 weeks of age were stained with hematoxylin and eosin. Bar, 50 μm. B, chord length measured from the WT and DKO lungs. C and D, functional tests of the lung. Lung compliance (C) and functional residual capacity (FRC) (D) of mice at 23 weeks of age were measured with a whole body plethysmograph. Values were normalized to body weight. At least three mice were used for each group. Bars represent the mean ± S.E. *, p < 0.05 versus WT.
It is believed that elastin degradation and remodeling processes occur within human emphysematous lungs (22). To investigate the pathogenesis of the emphysema of DKO lungs in more detail, we did additional staining and ultrastructural studies of histological sections. As shown in Fig. 4, A and B, elastin fibers appeared to be disrupted, and their network was lost in Elastica-van Gieson stain. Masson's trichrome stain of the enlarged alveolar region revealed abnormal deposition of collagen, suggesting the occurrence of a repair process (Fig. 4, C and D) (23). Of note, in the terminal bronchiole, Alcian blue/PAS stain detected secretory cell metaplasia of the epithelium (Fig. 4F), a finding not present in the WT bronchiole (Fig. 4E). In electron microscopy, alveolar macrophages developed many lysosomes and vacuoles (Fig. 4G). Some were multinucleated and contained intracytoplasmic needle-shaped inclusions (Fig. 4H), which appeared to be phagocytosed collagen as shown previously in destructive process of animal models of emphysema (24). These changes were again not observed in WT macrophages (Fig. 4I). There were also abnormal findings in the alveolar septa of the DKO lung. Type II epithelial cells were hypertrophic and contained many lamellar bodies (Fig. 4J). Localized increase of elastin and collagen fibers in alveolar walls (Fig. 4K), presumably representing structural remodeling (22), was noted when compared with WT (Fig. 4L). Collectively, these finding suggest that alveolar destruction and remodeling process were ongoing in the emphysematous lung of DKO mice.
FIGURE 4.
Histological lung sections from DKO mice and WT littermates. A–F, Elastica-van Gieson stain for elastin (dark blue) (A and B), Masson's trichrome stain for collagen (blue) (C and D), and Alcian blue/PAS stain (E and F) of lung sections from WT (A, C, and E) and DKO (B, D, and F) mice at 10 weeks of age. Arrowheads in B and D indicate sparse elastin fibers and abnormal deposition of collagen, respectively. Bars, 50 μm. G–L, a macrophage with many lysosomes and vacuoles (G), a macrophage containing needle-shaped inclusions (H), a hypertrophic type II cells accumulating lamellar bodies (J), and increased elastin and collagen fibers (K), were shown in electron microscopy of an alveolar region of DKO mice. A macrophage (I) and elastin and collagen (L) in an alveolar region of a WT littermate were also shown as control. El, elastin; Col, collagen. Bars, 1 μm.
It was previously proposed that apoptosis of septal epithelial and endothelial cells is part of the pathogenesis of emphysema (25). To examine if cell apoptosis contributes to emphysema of the DKO lung, lung sections were stained by terminal deoxynucleotidyltransferase-mediated dUTP nick-end labeling (supplemental Fig. 3A), and immunoblotting for active caspase-3 was done using whole lung lysates (supplemental Fig. 3B). However, no increase of apoptosis was observed in the DKO lung. We additionally induced apoptosis of bone marrow-derived macrophages (BMDMs) isolated from WT and DKO mice by depriving FBS in culture, but detected no significant difference (supplemental Fig. 3C).
Accumulation of Macrophages and Elevation of MMP Activities in the Lung of DKO Mice—Inflammation and protease overactivity are essential causal factors for emphysema lungs in humans (2). To examine if the double deletion of CD9 and CD81 leads to these pathogenic conditions in vivo, inflammatory cells and MMP activities in the DKO lung were evaluated by BALF analysis. As shown in Fig. 5A, a larger number of inflammatory cells were isolated from the DKO lung than from WT, and this was because of an increase of macrophages. BALF from CD9 and CD81 single-KO mice contained slightly increased numbers of cells, but they were not significantly different from WT (supplemental Fig. 2B). Gelatin zymography showed that the BALF supernatants from DKO mice contained increased proteolytic activities of MMP-2 and MMP-9 (Fig. 5B). Also, macrophages isolated from the BALF were strongly stained with anti-MMP-9 antibody especially at the cell periphery, when compared with WT macrophages (Fig. 5C). We failed to detect obvious difference in the staining with anti-MMP-2 antibody (data not shown). Increase of the MMP proteins was also confirmed in lysates of the entire lung of DKO mice (Fig. 5D).
FIGURE 5.
Accumulation of macrophages and elevation of MMP activities in the DKO lung. A, cells isolated from BALF of DKO mice and WT littermates at 10 weeks of age were visualized using Diff-Quick stain, and the number of total cells (TC), macrophages (Mφ), and lymphocytes (Ly) was determined. B, MMP activities in BALF from individuals of DKO mice and WT littermates were examined by gelatin zymography (upper) and quantified by densitometry (lower). Bars represent the mean ± S.E. *, p < 0.05 versus WT. C, macrophages from the BALF were stained with anti-MMP-9 antibody. Bar, 100 μm. D, expressions of MMP-2 and MMP-9 were examined by immunoblotting using lung homogenate protein from individuals of WT and DKO mice. Anti-actin blots confirm equal amounts of protein loaded in each lane.
Macrophages from DKO Mice Are Less Motile and Produce More MMPs than WT—To confirm that motility and MMP production of macrophages in CD9/CD81 DKO mice are intrinsically altered like mAb- or siRNA-treated RAW264.7 cells (Fig. 2), we isolated and cultured BMDMs from DKO mice and compared with WT macrophages in vitro. Cell proliferation or cell adhesion onto FN or Matrigel was not different as shown in Fig. 6, A and B, respectively. However, when migration and random motility were examined in assays using Transwells (Fig. 6C) and a time-lapse video microscope (Fig. 6D and supplemental video 1), respectively (26), those of DKO macrophages were markedly decreased compared with WT cells. Also, expressions of MMP-2 and MMP-9, but not that of MMP-12, were increased (Fig. 6E), similarly to mAb- or siRNA-treated RAW264.7 cells. As a control, we isolated lung fibroblasts from WT and DKO mice and examined MMP-2 and MMP-9 production in gelatin zymography, but we found no differences (data not shown).
FIGURE 6.
BMDMs from DKO mice are less motile and produce more MMPs than WT. A, BMDMs from DKO mice or WT littermates at 10 weeks of age were cultured in DMEM containing 20% FBS and 30% L929 supernatant. Cell proliferation was quantified using Cell Counting Kit-8. B, BMDMs in serum-free DMEM were cultured on FN- or Matrigel-precoated wells for 1.5 h. After unattached cells were removed, adherent cells were quantified with Cell Counting Kit-8. C, BMDMs in serum-free DMEM were applied to the upper chamber of Transwells that were precoated with FN or Matrigel. DMEM containing 10% FBS was added to the lower chamber. After 4 h, cells migrating to the lower surface were counted with Diff-Quick stain. D, BMDMs were plated onto Matrigel-precoated dishes. Images were acquired every minute for 90 min, and tracks (right) and distances (left) of random motility were determined using Scion Image tools. Values represent the mean ± S.E. *, p < 0.05 versus WT. E, mRNA was extracted from BMDMs, and expressions of MMP-2, MMP-9, and MMP-12 were analyzed by RT-PCR. β-Actin amplification was internal control.
Analysis of Gene Expression in CD9/CD81 DKO Macrophages—To gain further information on altered macrophage function of DKO mice, we compared gene expression profiles of BMDMs isolated from WT and DKO mice with oligonucleotide arrays consisting of more than 20,000 mouse genes. The supplemental Tables 1 and 2 list the top 70 genes up-regulated or down-regulated preferentially in the DKO macrophages, respectively. Interestingly, fold differences of the down-regulated genes were higher than those of the up-regulated, also indicating that suppressed genes were much more than induced genes. The induced genes included proteases (carboxypeptidase A5; cathepsin E; cathepsin H; MMP-9; arginyl aminopeptidase (aminopeptidase B)), adhesion-related proteins (cortactin; syndecan 1; glypican 1; integrin αX), and pro-inflammatory cytokine receptors (CD74 (macrophage migration inhibitory factor receptor); colony-stimulating factor 2 receptor). Meanwhile, the suppressed genes included an antioxidant protein (ceruloplasmin), a protease inhibitor (serine peptidase inhibitor, clade B, members 5 (Maspin)), and extracellular matrix proteins (integrin-binding sialoprotein; secreted acidic cysteine-rich glycoprotein (osteonectin); biglycan; matrilin 3). It is of note that fucosyltransferase 8 was listed as a down-regulated gene in the DKO macrophages, because the deficiency of this glycosyltransferase was recently reported to impair α3β1 integrin-mediated cell migration and cause pulmonary emphysema in mice (27). Expressions of other tetraspanins, including CD37, CD53, CD82, and CD151, were unremarkably changed (data not shown).
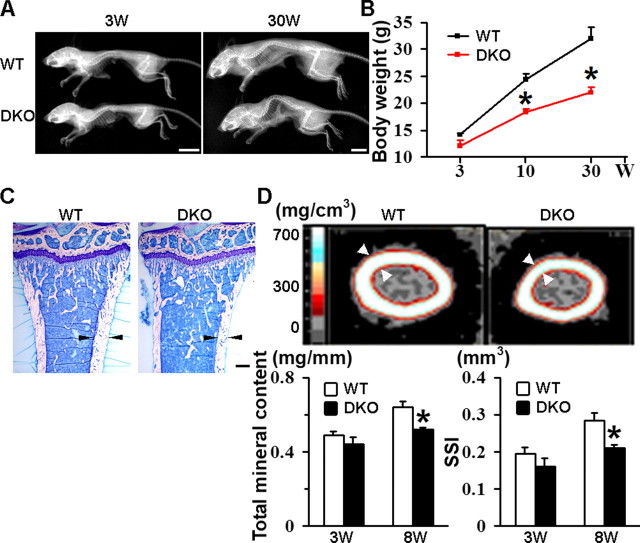
CD9/CD81 DKO Mice Also Display Body Weight Loss, Kyphosis, and Osteopenia—With aging, the DKO mice could be discriminated from WT (Fig. 7A) and CD9 or CD81 single-KO littermates (data not shown) by progressive kyphotic appearance, as shown in whole body radiographs. Moreover, weight loss became evident over time when compared with WT mice (Fig. 7B). Histological sections of proximal tibia displayed that, although the trabecular bone appeared normal, the cortical bone of DKO mice was thinner than WT (Fig. 7C). Images of femoral diaphysis in pQCT analysis also showed a decrease in cortical bone thickness and age-dependent reduction in total mineral content and strength strain index (Fig. 7D). Decrease in these parameters was not observed in CD9 or CD81 single-KO mice (data not shown). To investigate the pathophysiology of the osteopenic phenotype in more detail, histomorphometric analysis was done with the proximal tibia of DKO mice (supplemental Fig. 4). Consistently with the histological appearance of the trabecular bone (Fig. 7C), there were no significant differences in trabecular bone volume and trabecular thickness (supple-mental Fig. 4A). The number of osteoclasts in the DKO mice was increased by 60% when compared with WT (supplemental Fig. 4B). This seems to reflect elevated fusogenic capacity of DKO macrophages in vitro (supplemental Fig. 4C) and consistent with our previous observation (13). However, eroded surface was decreased (supplemental Fig. 4B), suggesting that osteoclast activity was unexpectedly impaired. Meanwhile, osteoblast activity was likewise reduced. The number of osteoblasts and the surface of osteoblasts were decreased, and their function in matrix production was damaged as indicated by the decreased thickness of newly deposited matrix (osteoid thickness) (supplemental Fig. 4, D and E). To perform kinetic analysis of bone formation and mineralization, mice were intraperitoneally injected twice with calcein at a 3-day interval. Compared with WT, the distance of two labeled bands was shorter in the DKO mice (supplemental Fig. 4F), resulting in decreased mineral apposition rate and bone formation rate in trabecular dynamic histomorphometry (supplemental Fig. 4G). These results indicate that, although osteoclastogenesis is accelerated, both osteolytic and osteoblastic activities were impaired and that decrease of bone formation rather exceeded that of bone resorption.
FIGURE 7.
CD9/CD81 DKO mice display body weight loss, kyphosis, and osteopenia. A, whole body radiographs of WT and DKO mice at 3 and 30 weeks of age. Bars, 10 mm. B, time course of body weight of WT and DKO littermates. C, toluidine blue stain of longitudinal sections of proximal tibia at 8 weeks of age. Note that cortical bone of the DKO mouse is thinner than that of the WT littermate (arrowheads). Bar, 250 μm. D, pQCT images of femoral diaphysis at 8 weeks of age (upper). Mineral densities are shown as different colors according to the standard mineral density gradients. Note the reduction in cortical thickness of the DKO mouse (arrowheads). Total mineral content and strength strain index (SSI) were also determined (lower). Trabecular parameters were not different between WT and DKO mice (data not shown). Values represent the mean ± S.E. *, p < 0.05 versus WT.
We further analyzed histological sections of muscle, skin, eye, heart, aorta, liver, and kidney, but no obvious difference was observed between WT and DKO mice. Hemoglobin and the number of leukocytes and platelets determined in peripheral blood analysis were normal. Serum levels of total protein, albumin, cholesterol, triglyceride, calcium, and creatinine were also not different (data not shown). Thus, the phenotype unlikely to be caused by malnutrition, and the CD9/CD81 DKO mouse is not a premature aging model such as klotho mice, which exhibits ectopic calcifications, arteriosclerosis, skin atrophy, and cardiac dysfunction in addition to pulmonary emphysema and osteopenia (28).
DISCUSSION
At specialized membrane microdomains, tetraspanins facilitate the formation of multimolecular complexes and regulate cell morphology, motility, invasion, fusion, and signaling. Based on these characteristics, they are also referred to as molecular facilitator or organizer (6). Tetraspanins have been extensively studied in cancer biology. They affect tumor cell motility in vitro and tumor metastasis in vivo most likely by regulating function of integrins and production of MMPs (7–9, 29). However, it has not been investigated if tetraspanins play a role in motility and MMP production of macrophages. Using a mAb- or siRNA-treated macrophage line, RAW264.7 (Fig. 2), and primary DKO macrophages (Fig. 6), we have shown that dysfunction of CD9 and CD81 suppresses cell motility and promoted the production of MMP-2 and MMP-9 in vitro. CD9/CD81 double deletion also caused increase of macrophages and elevation of MMP-2 and MMP-9 activities in the mouse lung in vivo (Fig. 5). Thus, co-deficiency of CD9 and CD81 function seems to be sufficient for macrophages to increase MMP production as well as to suppress cell motility. Suppressed motility of macrophages does not necessarily contradict with their infiltration into the lung. For example, macrophages from SH2-containing inositol-5-phosphatase 1 (SHIP1) KO mice, which revealed massive infiltration of macrophages in alveolar air spaces, exhibited defects in motility in vitro (30). In these mice, decreased efflux of macrophages from the airspace, rather than their increased influx into the airspace, might contribute to macrophage accumulation. The expression of MMPs is regulated by multiple factors, including growth factors and their receptors, cell adhesion molecules, and GTPases (31). We speculate that CD9 and CD81 coordinately assemble these factors in their own microdomains and thereby indirectly control the production of MMPs (6).
Expression of inflammatory genes is determined by a balance between histone acetylation, which activates transcription, and deacetylation, which switches off transcription. In humans, cigarette smoke suppresses the activity of HDAC in alveolar macrophages, and this was correlated with increased expression of inflammatory genes, including TNF-α and IL-8 in these cells. There was also a reduction in HDAC activity in peripheral lung and alveolar macrophages from COPD patients, and this was correlated with disease severity (5). We cultured RAW264.7 cells in the presence of CSE, the HDAC inhibitor TSA, and pro-inflammatory cytokines IFN-γ and TNF-α, and all these treatments but TNF-α down-regulated the protein levels of both CD9 and CD81 (Fig. 1 and supplemental Fig. 1). These results raise an hypothesis that cigarette smoke reduces the HDAC activity, switching on inflammatory genes and leading to the down-regulation of CD9 and CD81 in macrophages. The resultant insufficiency of CD9 and CD81 functions may be part of important mechanisms that cause or exacerbate the accumulation of alveolar macrophages and their overproduction of MMPs in cigarette smokers.
CD9 single-KO mice are infertile because of impaired oocyte fusion with sperm (32). CD81 single-KO mice show likewise impaired oocyte fertilization (33) in addition to altered immune response (34, 35). KO mice of single tetraspanin have so far resulted in relatively mild phenotype. One possible reason is that the loss of a single tetraspanin may be compensated by other tetraspanins (21). In this study, double deletion of CD9 and CD81 in mice leads to the lung phenotype morphologically and functionally mimicking the emphysematous lung in human COPD (Fig. 3). The CD9/CD81 DKO mice displayed age-dependent progression of airspace enlargement. Secretory cell metaplasia, a finding similar to goblet cell metaplasia in cigarette smokers (36), was also observed. It is unlikely that the emphysema resulted from defective alveolization, because mouse alveolization is completed by 3 weeks (37). Moreover, the elastin/collagen staining and ultrastructural studies of histological sections suggested that alveolar destruction and remodeling process were ongoing in the DKO lung (Fig. 4). Thus, together with the findings of macrophage infiltration and elevated MMP activities in the lung (Fig. 5), it appears that inflammatory and elastolytic processes caused emphysema in the DKO mice. In the gene expression profile of DKO macrophages, multiple proteases, including MMP-9, were induced (supplemental Table 1), whereas the antioxidant protein ceruloplasmin, the protease inhibitor Maspin, and multiple extracellular matrix proteins were suppressed (supplemental Table 2). Although these differential gene expressions remain to be validated with quantitative PCR, the profile was consistent with the hypothesis that the imbalances of proteases/antiproteases and oxidants/antioxidants were present in the DKO macrophages as proposed previously for molecular mechanisms in macrophages orchestrating the progression of emphysema (2).
Common extrapulmonary effects of COPD include weight loss and osteoporosis, mechanisms of which have been poorly understood (38, 39). Interestingly, the CD9/CD81 DKO mice displayed age-dependent weight loss and osteopenia (Fig. 7). The number of osteoclasts was increased because of the enhanced fusion of their progenitors of the macrophage lineage, but the osteopenia was caused not by increased bone resorption but by decreased bone formation (supplemental Fig. 4), indicating low turnover osteopenia. This type of osteopenia occurs as an aging process in humans (40), and it has been proposed that there are clear parallels between the pathophysiological responses to aging and those involved in COPD (41). To assess the function of the DKO osteoblasts in vitro, we isolated and cultured bone marrow cells in the presence of ascorbic acid and β-glycerophosphate and stained mineralized nodules with alizarin red (42). However, preliminary results failed to reveal obvious differences between WT and DKO mice (data not shown). Possibly, the impaired bone formation in DKO mice might be attributed not to primary defects of osteoblasts but to their microenvironmental factors such as hormones, cytokines, growth factors, MMPs, and extracellular matrix proteins (43, 44). Dysfunction of other cell lineage may also be indirectly involved in impaired osteoblast function in vivo. For example, the defective production of osteonectin and biglycan in bone marrow macrophages, as shown in our gene expression analysis (supplemental Table 2), might damage osteoblast functions in the DKO mice as described previously (45, 46).
Cigarette smoking is known as a common risk factor for COPD and osteoporosis, which are both characterized by net loss of lung or bone tissue mass (47), and the phenotype of DKO mice has raised a possibility that these two pathological conditions might share common mechanisms involving tetraspanins. Further studies using human samples are needed because there are considerable differences in physiology and anatomy between mice and humans (48).
In conclusion, this study has shown that tetraspanins CD9 and CD81, which regulate motility and MMP production of macrophages, are down-regulated by smoking-related pro-inflammatory stimuli. Double deficiency of these tetraspanins causes age-related progression of pulmonary emphysema and osteopenia in mice. Given that tetraspanins function as molecular facilitator, the loss of CD9 and CD81 could lead to disordered organization of molecules that otherwise associate with these tetraspanins. Such defective molecular organization might be a part of mechanisms underlying the lung disease and osteoporosis in human COPD.
Supplementary Material
Acknowledgments
We thank T. Miyazaki (University of Texas Southwestern Medical Center, Dallas) for providing CD81 KO mice; M. Kobayashi (Osaka University) for assistance in mouse genotyping; Y. Tomita and S. Furue (Shionogi and Co., Ltd., Osaka, Japan) for assistance in chord length measurement; T. Yoneda (Osaka University Graduate School of Dentistry, Osaka, Japan) for helpful comments on bone analysis; T. Ito and K. Ozawa (Osaka University Graduate School of Medicine) for helpful comments on kidney and skin histology, respectively; Y. Satou (Teijin Pharma Ltd.) and M. Hamaoka (Osaka University) for technical assistance; and Y. Habe for secretarial assistance.
This work was supported by a grant from Takeda Science Foundation, Osaka, Japan (to I. T.), and a grant from “Kansai Biomedical Cluster” Project in Saito, Japan, which is promoted by the Knowledge Cluster Initiative of the Ministry of Education, Culture, Sports, Science and Technology, Japan (to I. T.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Experimental Procedures, Figs. 1–4, Tables 1 and 2, and Video 1.
Footnotes
The abbreviations used are: COPD, chronic obstructive pulmonary disease; MMP, matrix metalloproteinase; BALF, bronchoalveolar lavage fluid; HDAC, histone deacetylase; KO, knock-out; DKO, double-knock-out; TSA, trichostatin A; CSE, cigarette smoke extract; TIMP, tissue inhibitor of metalloproteinase; FN, fibronectin; BMDM, bone marrow-derived macrophage; pQCT, peripheral quantitative computed tomography; IFN, interferon; mAb, monoclonal antibody; siRNA, small interfering RNA; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; WT, wild type; RT, reverse transcription; TNF-α, tumor necrosis factor-α; PAS, periodic acid-Schiff.
References
- 1.Shapiro, S. D. (2002) Biochem. Soc. Trans. 30 98-102 [DOI] [PubMed] [Google Scholar]
- 2.Barnes, P. J., Shapiro, S. D., and Pauwels, R. A. (2003) Eur. Respir. J. 22 672-688 [DOI] [PubMed] [Google Scholar]
- 3.Finlay, G. A., O'Driscoll, L. R., Russell, K. J., D'Arcy, E. M., Masterson, J. B., FitzGerald, M. X., and O'Connor, C. M. (1997) Am. J. Respir. Crit. Care Med. 156 240-247 [DOI] [PubMed] [Google Scholar]
- 4.Ohnishi, K., Takagi, M., Kurokawa, Y., Satomi, S., and Konttinen, Y. T. (1998) Lab. Investig. 78 1077-1087 [PubMed] [Google Scholar]
- 5.Barnes, P. J. (2006) Chest 129 151-155 [DOI] [PubMed] [Google Scholar]
- 6.Hemler, M. E. (2005) Nat. Rev. Mol. Cell Biol. 6 801-811 [DOI] [PubMed] [Google Scholar]
- 7.Sugiura, T., and Berditchevski, F. (1999) J. Cell Biol. 146 1375-1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito, Y., Tachibana, I., Takeda, Y., Yamane, H., He, P., Suzuki, M., Minami, S., Kijima, T., Yoshida, M., Kumagai, T., Osaki, T., and Kawase, I. (2006) Cancer Res. 66 9557-9565 [DOI] [PubMed] [Google Scholar]
- 9.Tohami, T., Drucker, L., Shapiro, H., Radnay, J., and Lishner, M. (2007) FASEB J. 21 691-699 [DOI] [PubMed] [Google Scholar]
- 10.Hoshino, S., Yoshida, M., Inoue, K., Yano, Y., Yanagita, M., Mawatari, H., Yamane, H., Kijima, T., Kumagai, T., Osaki, T., Tachiba, I., and Kawase, I. (2005) Biochem. Biophys. Res. Commun. 329 58-63 [DOI] [PubMed] [Google Scholar]
- 11.Shibata, Y., Zsengeller, Z., Otake, K., Palaniyar, N., and Trapnell, B. C. (2001) Blood 98 2845-2852 [DOI] [PubMed] [Google Scholar]
- 12.Wang, Z., Zheng, T., Zhu, Z., Homer, R. J., Riese, R. J., Chapman, H. A., Jr., Shapiro, S. D., and Elias, J. A. (2000) J. Exp. Med. 192 1587-1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takeda, Y., Tachibana, I., Miyado, K., Kobayashi, M., Miyazaki, T., Funakoshi, T., Kimura, H., Yamane, H., Saito, Y., Goto, H., Yoneda, T., Yoshida, M., Kumagai, T., Osaki, T., Hayashi, S., Kawase, I., and Mekada, E. (2003) J. Cell Biol. 161 945-956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng, T., Zhu, Z., Wang, Z., Homer, R. J., Ma, B., Riese, R. J., Jr., Chapman, H. A., Jr., Shapiro, S. D., and Elias, J. A. (2000) J. Clin. Investig. 106 1081-1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu, W., Toyosawa, S., Furuichi, T., Kanatani, N., Yoshida, C., Liu, Y., Himeno, M., Narai, S., Yamaguchi, A., and Komori, T. (2001) J. Cell Biol. 155 157-166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lind, P. M., Lind, L., Larsson, S., and Orberg, J. (2001) Bone (Elmsford) 29 265-270 [DOI] [PubMed] [Google Scholar]
- 17.Ito, K., Lim, S., Caramori, G., Cosio, B., Chung, K. F., Adcock, I. M., and Barnes, P. J. (2002) Proc. Natl. Acad. Sci. U. S. A. 99 8921-8926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park, G. Y., Park, J. W., Jeong, D. H., and Jeong, S. H. (2003) Chest 123 475-480 [DOI] [PubMed] [Google Scholar]
- 19.Cawston, T., Carrere, S., Catterall, J., Duggleby, R., Elliott, S., Shingleton, B., and Rowan, A. (2001) Novartis Found. Symp. 234 205-228 [DOI] [PubMed] [Google Scholar]
- 20.Ogawa, K., Chen, F., Kuang, C., and Chen, Y. (2004) Biochem. J. 381 413-422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hemler, M. E. (2003) Annu. Rev. Cell Dev. Biol. 19 397-422 [DOI] [PubMed] [Google Scholar]
- 22.Finlay, G. A., O'Donnell, M. D., O'Connor, C. M., Hayes, J. P., and FitzGerald, M. X. (1996) Am. J. Pathol. 149 1405-1415 [PMC free article] [PubMed] [Google Scholar]
- 23.Jeffery, P. K. (2001) Am. J. Respir. Crit. Care Med. 164 S28-S38 [DOI] [PubMed] [Google Scholar]
- 24.Lucattelli, M., Cavarra, E., de Santi, M. M., Tetley, T. D., Martorana, P. A., and Lungarella, G. (2003) Eur. Respir. J. 22 728-734 [DOI] [PubMed] [Google Scholar]
- 25.Kasahara, Y., Tuder, R. M., Cool, C. D., Lynch, D. A., Flores, S. C., and Voelkel, N. F. (2001) Am. J. Respir. Crit. Care Med. 163 737-744 [DOI] [PubMed] [Google Scholar]
- 26.Takeda, Y., Kazarov, A. R., Butterfield, C. E., Hopkins, B. D., Benjamin, L. E., Kaipainen, A., and Hemler, M. E. (2007) Blood 109 1524-1532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang, X., Inoue, S., Gu, J., Miyoshi, E., Noda, K., Li, W., Mizuno-Horikawa, Y., Nakano, M., Asahi, M., Takahashi, M., Uozumi, N., Ihara, S., Lee, S. H., Ikeda, Y., Yamaguchi, Y., Aze, Y., Tomiyama, Y., Fujii, J., Suzuki, K., Kondo, A., Shapiro, S. D., Lopez-Otin, C., Kuwaki, T., Okabe, M., Honke, K., and Taniguchi, N. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 15791-15796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nabeshima, Y. (2002) Ageing Res. Rev. 1 627-638 [DOI] [PubMed] [Google Scholar]
- 29.Chirco, R., Liu, X. W., Jung, K. K., and Kim, H. R. (2006) Cancer Metastasis Rev. 25 99-113 [DOI] [PubMed] [Google Scholar]
- 30.Nishio, M., Watanabe, K., Sasaki, J., Taya, C., Takasuga, S., Iizuka, R., Balla, T., Yamazaki, M., Watanabe, H., Itoh, R., Kuroda, S., Horie, Y., Forster, I., Mak, T. W., Yonekawa, H., Penninger, J. M., Kanaho, Y., Suzuki, A., and Sasaki, T. (2007) Nat. Cell Biol. 9 36-44 [DOI] [PubMed] [Google Scholar]
- 31.Greenlee, K. J., Werb, Z., and Kheradmand, F. (2007) Physiol. Rev. 87 69-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyado, K., Yamada, G., Yamada, S., Hasuwa, H., Nakamura, Y., Ryu, F., Suzuki, K., Kosai, K., Inoue, K., Ogura, A., Okabe, M., and Mekada, E. (2000) Science 287 321-324 [DOI] [PubMed] [Google Scholar]
- 33.Rubinstein, E., Ziyyat, A., Prenant, M., Wrobel, E., Wolf, J. P., Levy, S., Le Naour, F., and Boucheix, C. (2006) Dev. Biol. 290 351-358 [DOI] [PubMed] [Google Scholar]
- 34.Maecker, H. T., and Levy, S. (1997) J. Exp. Med. 185 1505-1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyazaki, T., Muller, U., and Campbell, K. S. (1997) EMBO J. 16 4217-4225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright, J. L., and Churg, A. (2002) Chest 122 S301-S306 [Google Scholar]
- 37.Amy, R. W., Bowes, D., Burri, P. H., Haines, J., and Thurlbeck, W. M. (1977) J. Anat. 124 131-151 [PMC free article] [PubMed] [Google Scholar]
- 38.Gross, N. J. (2001) Curr. Opin. Pulm. Med. 7 84-92 [DOI] [PubMed] [Google Scholar]
- 39.Agusti, A. (2007) Proc. Am. Thorac. Soc. 4 522-525 [DOI] [PubMed] [Google Scholar]
- 40.Manolagas, S. C., and Jilka, R. L. (1995) N. Engl. J. Med. 332 305-311 [DOI] [PubMed] [Google Scholar]
- 41.Tuder, R. M., Yoshida, T., Arap, W., Pasqualini, R., and Petrache, I. (2006) Proc. Am. Thorac. Soc. 3 503-510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inoue, K., Mikuni-Takagaki, Y., Oikawa, K., Itoh, T., Inada, M., Noguchi, T., Park, J. S., Onodera, T., Krane, S. M., Noda, M., and Itohara, S. (2006) J. Biol. Chem. 281 33814-33824 [DOI] [PubMed] [Google Scholar]
- 43.Ortega, N., Behonick, D., Stickens, D., and Werb, Z. (2003) Ann. N. Y. Acad. Sci. 995 109-116 [DOI] [PubMed] [Google Scholar]
- 44.Hadjidakis, D. J., and Androulakis, I. I. (2006) Ann. N. Y. Acad. Sci. 1092 385-396 [DOI] [PubMed] [Google Scholar]
- 45.Xu, T., Bianco, P., Fisher, L. W., Longenecker, G., Smith, E., Goldstein, S., Bonadio, J., Boskey, A., Heegaard, A. M., Sommer, B., Satomura, K., Dominguez, P., Zhao, C., Kulkarni, A. B., Robey, P. G., and Young, M. F. (1998) Nat. Genet. 20 78-82 [DOI] [PubMed] [Google Scholar]
- 46.Delany, A. M., Amling, M., Priemel, M., Howe, C., Baron, R., and Canalis, E. (2000) J. Clin. Investig. 105 915-923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agusti, A. G., Noguera, A., Sauleda, J., Sala, E., Pons, J., and Busquets, X. (2003) Eur. Respir. J. 21 347-360 [DOI] [PubMed] [Google Scholar]
- 48.Brusselle, G. G., Bracke, K. R., Maes, T., D'Hulst, A. I., Moerloose, K. B., Joos, G. F., and Pauwels, R. A. (2006) Pulm. Pharmacol. Ther. 19 155-165 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.