Abstract
Extracellular heat shock proteins (HSPs) can stimulate antigen-specific immune responses. Using recombinant human (rhu)Hsp70, we previously demonstrated that through complex formation with exogenous antigenic peptides, rhuHsp70 can enhance cross-presentation by antigen-presenting cells (APCs) resulting in stronger T cell stimulation. T cell stimulatory activity has also been described for mycobacterial (myc)Hsp70. MycHsp70-assisted T cell activation has been reported to act through the binding of mycHsp70 to chemokine receptor 5 (CCR5), calcium signaling, phenotypic maturation, and cytokine secretion by dendritic cells (DCs). We report that highly purified rhuHsp70 and mycHsp70 proteins both strongly enhance cross-presentation of exogenous antigens. Augmentation of cross-presentation was seen for different APCs, irrespective of CCR5 expression. Moreover, neither of the purified Hsp70 proteins induced calcium signals in APCs. Instead, calcium signaling activity was found to be caused by contaminating nucleotides present in Hsp70 protein preparations. These results refute the hypothesis that mycHsp70 proteins require CCR5 expression and calcium signaling by APCs for enhanced antigen cross-presentation for T cell stimulation.
Recent evidence has demonstrated that heat shock proteins (HSPs),2 in addition to their roles as chaperones, can stimulate antigen-specific immune responses (1). This immune stimulatory activity is thought to be activated after HSPs are released from cells either through necrosis or induced secretion (2–5). In their role as molecular chaperones, most HSPs exist in complexes with hydrophobic polypeptides. The contact of extracellular HSP:peptide complexes with dendritic cells (DCs) is thought to result in the efficient transfer of the chaperoned peptides into the antigen-presentation pathway of DCs allowing antigen-specific T cell activation.
Mechanistically, HSP binding to surface receptors and HSP-induced signaling events are thought to be involved in antigen transfer to DCs and subsequent T cell stimulation. In this regard, a number of potential HSP receptors have been reported (6–9). Among these, CD40 and chemokine receptor 5 (CCR5) represent two interesting possibilities as these molecules are intricately linked to T cell and DC biology (10, 11). In this regard, CCR5 has been reported to act as a pattern recognition receptor for mycobacterial (myc)Hsp70 thus facilitating mycobacterial defense through mycHsp70-induced DC activation and subsequent T cell stimulation. The binding of mycHsp70 to CCR5, calcium signaling in myeloid DCs, phenotypic maturation, and cytokine secretion of DCs have been reported to underlie mycHsp70-assisted T cell activation (12, 13).
We have previously demonstrated that CCR5 can mediate unique signaling events (14, 15). In addition, we have shown that human Hsp70 from melanoma cells can chaperone tumor-associated peptides (tyrosinase) and deliver them to DCs allowing cross-presentation and T cell stimulation (16). Recently, we used recombinant human (rhu)Hsp70 to define in more detail the critical parameters for the induction of immune effector responses (17). Highly purified and endotoxin-depleted rhuHsp70 was found to efficiently deliver exogenous peptides to DCs resulting in T cell activation, provided that peptide and Hsp70 had formed stable complexes before exposure to DCs. Phenotypic maturation or cytokine secretion of DCs was not observed and not required for the rhuHsp70-mediated enhancement of cross-presentation and T cell stimulation.
Here we used highly purified rhuHsp70 and mycHsp70 proteins and examined whether mycHsp70 also enhances cross-presentation of exogenous antigenic protein sequences and tested whether calcium signaling or CCR5 were required for Hsp70-assisted antigen presentation and T cell stimulation.
MATERIALS AND METHODS
Reagents and Peptides—ADP, ionomycin, suramin, Escherichia coli-derived LPS (strain 0111:B4), and reagents for calcium signaling (Indo-I AM, Pluronic F-127, DMSO) were purchased from Sigma. All buffers and solutions were prepared using aqua ad injectabilia (Braun Melsungen, Germany). Peptides were purchased from Biosyntan GmbH (Berlin, Germany). The peptides were synthesized as hybrids of the nonameric HLA-A2 binding motif with the N-terminal octameric sequence HWDFAWPW (here named pep70) (pep70-MART-1: GSGHWDFAWPWGSGLAGIGILTV).
Recombinant Hsp70 Proteins—Recombinant human (rhu)-Hsp70 protein was either purchased from Stressgen, Victoria, BC, Canada (ESP-555) or was prepared in our own laboratory as described (17). After ADP affinity chromatography, buffer was exchanged using PD10 columns followed by extensive dialysis (48 h, 5000× buffer volume) and diafiltration (Vivaspin 20, 30,000 MWCO PES, Sartorius Stedim Biotech). To assess the influence of nucleotide carryover, rhuHsp70 preparations were used after PD10 column and diafiltration with a less intensive dialysis step (Hsp70/nc+). Endotoxin was depleted, as described (17). Only proteins with endotoxin below 0.5 EU/mg rhuHsp70 were used.
Full-length mycHsp70 was obtained from Lionex Diagnostics & Therapeutics GmbH, Braunschweig, Germany (Product: M. tuberculosis Hsp70, LRP 0003.3; batch: 07-1 lot2). The cDNA coding for the C-terminal substrate binding domain of mycHsp70, mycHsp70-(359–625), kindly provided by P. J. Lehner, Cambridge (UK), was subcloned into the vector pET21d (Novagen/EMD Chemicals, Gibbstown, NJ). C-terminally His6-tagged recombinant protein was produced in E. coli strain Rosetta2(DE3) (Invitrogen, Karlsruhe, Germany) and purified using Ni-nitrilotriacetic acid-agarose (Qiagen, Hilden, Germany) as described (18). Endotoxin depletion was performed as described (17).
APCs and Cell Cultures—DCs were differentiated from monocytes of PBMC of healthy donors (CD14+ magnetic bead selection, Miltenyi) using IL4/GM-CSF exactly as described (17). Cells were used without further maturation (i.e. non-matured IL4/GM-CSF-differentiated myeloid DCs). The local ethics commission approved these studies, and donors gave informed consent. Human B-lymphoblastoid B cell line (B-LCL L721.45) and CTL A42-MART (HLA-A2-restricted Melan-A/MART-1-peptide (LAGIGILTV)-specific) were cultured as described (17).
Cross-presentation—Cross-presentation was performed exactly as described (17). Briefly, in parallel wells of a 96-well cell culture plate identical amounts of pep70-MART-peptide (70 nm) alone or together with rhuHsp70 (1 μm) or mycHsp70-(359–625) (1 μm) were incubated in HKM buffer (total volume of 30 μl) for 4 h at room temperature for complex formation. B-LCL 721.45 and CTL A42-MART were added resulting in a 1:7 dilution of rhuHsp70 and peptide. The concentrations declared in text/figure correspond to the final concentrations in the APC/T cell mixture. After 24 h at 37 °C, supernatants were harvested, and IFN-γ was measured by ELISA (BD OptEIA™, BD Biosciences Pharmingen). Control samples, containing all components except the peptide, were used to determine IFN-γ background, which was subtracted from the experimental samples.
Flow Cytometry—Surface expression of molecules was determined by flow cytometry. 0.1 × 106 cells (DCs or B-LCL) after a 48-h incubation with 300 nm rhuHsp70, LPS (1 μg/ml) or without stimulus, were stained with specific antibodies (anti-human CD80, CD86, CD83, CD40 (all BD Biosciences Pharmingen, Heidelberg, Germany), anti-CCR5 antibody (R&D systems), anti-human CD38 (Immunotech, Hamburg, Germany), or IgG control antibodies (BD Biosciences) diluted in phosphate-buffered saline containing 2% fetal calf serum. After 30 min of incubation on ice, cells were washed and fixed with 1% PFA. Immunofluorescence analyses employed the FACSCalibur and Cell Quest software (Becton Dickinson).
Cytokine Quantitation—Non-matured IL-4/GM-CSF-differentiated myeloid DCs were incubated with 300 nm rhuHsp70, LPS (1 μg/ml), or without stimuli for 48 h at 37 °C. Cytokines in the supernatants were measured using the Bio-Plex Cytokine 12-Plex Assay (Bio-Rad), and concentrations were calculated using the Bio-Plex Manager™ software program.
Intracellular Calcium Analysis—Calcium analysis was performed as described, and changes in intracellular calcium were measured with MoFlo using Summit software (Dako-Cytomation) (17). Ionomycin (250 ng/ml), ADP solutions, mycHsp70-(359–625) (1 μm), rhuHsp70 (300 nm), mycHsp70 Lionex (1 μm), or the corresponding protein-depleted solutions (volumes corresponding to the 300 nm rhuHsp70 or 1 μm mycHsp70 protein solutions, respectively) were added 1 min after the cells were measured without stimulation for background establishment. The protein-depleted solutions were obtained by removing protein through filtration of the stock preparation through a PES membrane with 30-kDa molecular weight cutoff (Vivaspin 20, 30.000 MWCO PES). For inhibition, DCs were preincubated with 250 μm suramin for 5 min. As control, the buffers without stimulating agents were measured and did not result in a calcium signal.
RESULTS
Calcium Signals in Myeloid DCs Are Induced by Contamination of rhuHsp70 and mycHsp70 Preparations—Published studies (12, 13, 19) have suggested that mycHsp70 induces a calcium signal in DCs resulting in DC maturation and antigen-specific T cell activation. Our previous results using highly purified rhuHsp70 had shown that rhuHsp70 enhances cross-presentation and T cell stimulation without changing the DC phenotype (17). We therefore compared and contrasted our rhuHsp70 to the same mycHsp70 preparation used in the published studies (12, 13, 19) for its ability to induce calcium signals in DCs.
Hsp70 proteins, mycobacterial, and human, recombinantly expressed in E. coli, are generally purified using ATP affinity chromatography, ATP elution, and buffer exchange (20). Nucleotides are documented inducers of calcium signals in immune cells (21–23). Therefore, we suspected that residual ATP or ADP could have caused the reported calcium effect.
To control for possible artifacts evoked by contaminants in the protein solution, we prepared a protein-depleted solution by separating the mycHsp70 protein from the protein solution by centrifugation through a membrane with a 30-kDa cutoff. SDS-PAGE and silver staining confirmed that the protein-depleted solution had no detectable Hsp70 protein or protein fragments. (Fig. 1A shows the result for mycHsp70; similar results were seen for rhuHsp70; not shown.) Testing for calcium signaling we observed that the mycHsp70 protein-depleted solution induced a calcium signal in DCs that was essentially equivalent to that seen with the complete commercial mycHsp70 protein preparation (Fig. 1A).
FIGURE 1.
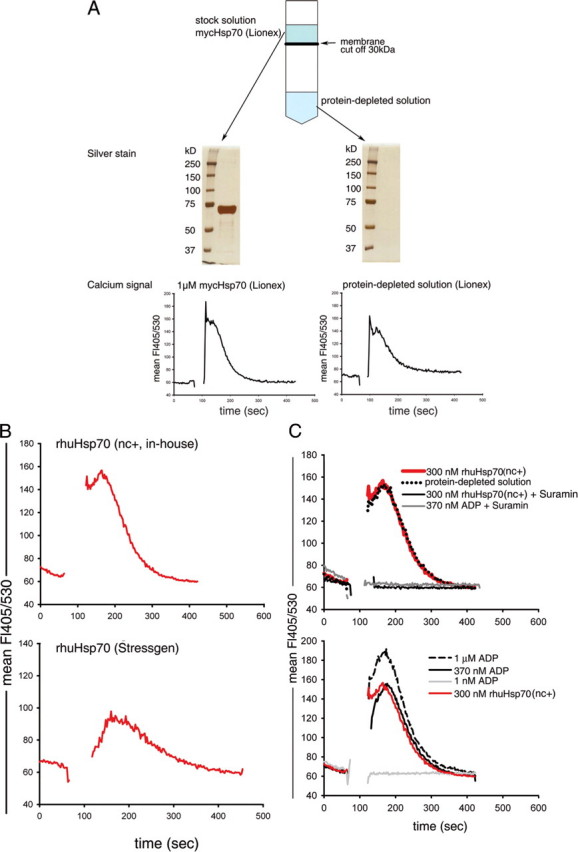
Calcium signal in DCs is caused by contamination of rhuHsp70 and mycHsp70 preparation. A, generation of the protein-depleted solution (schematic) and confirmation of protein depletion by SDS-PAGE and silver stain (mycHsp70). For measurement of intracellular calcium, DCs were stained with the calcium-sensitive dye Indo-I and stimulated with 1 μm mycHsp70 protein solution or an equal volume of the mycHsp70-depleted solution. B, DCs were stimulated with 300 nm less dialyzed, nucleotide-containing rhuHsp70 (Hsp70/nc+) or 300 nm rhuHsp70 (Stressgen). C, DCs were stimulated with 300 nm less dialyzed, nucleotide-containing rhuHsp70 (Hsp70/nc+), protein-depleted solution or titered concentrations of an ADP solution. For inhibition, DCs were preincubated with suramin.
We then used an in-house rhu-Hsp70 preparation, which had undergone a less rigorous dialysis after ATP affinity chromatography, as well a commercially available human Hsp70 (Stressgen (ESP-555). Our less dialyzed rhuHsp70(nc+) preparation induced a calcium flux comparable in magnitude to the commercial mycHsp70 (Fig. 1B), the commercial rhuHsp70 (Stressgen) induced a lower signal. We then depleted the rhuHsp70 protein from the less dialyzed protein solution as performed for mycHsp70 and tested the resultant protein-free solution for its ability to induce a calcium flux in DCs. As was found for the mycHsp70 preparation, the protein-depleted solution of rhuHsp70 triggered identical calcium responses as seen with the protein-containing solution (Fig. 1C, upper panel: red line versus dotted black line). Next, we tested titered solutions of commercial ADP and observed dose-dependent calcium signals (Fig. 1C, lower panel). A 370 nm ADP solution, which corresponded to the calculated amount of residual free nucleotide in our less dialyzed rhu-Hsp70, gave an identical signal (Fig. 1C, lower panel: black line versus red line). At 370 nm ADP, the calcium response of DCs was not in saturation (Fig. 1C, lower panel: 370 nm versus 1 μm ADP), yet the magnitude of the response to 300 nm rhuHsp70 was not higher than that achieved with the protein-free solution (Fig. 1C, upper panel) suggesting that ADP/ATP nucleotides within that Hsp70 preparation were responsible for the calcium influx with no or minimal contribution of the rhuHsp70 protein itself. Consistent with this interpretation, we found that the response induced by this less dialyzed (nucleotide-containing) rhuHsp70 protein preparation was completely blocked by the purinoreceptor antagonist, suramin, as was the response to the ADP solution (Fig. 1C, upper panel).
To confirm the conclusion that the mycHsp70 and rhuHsp70 proteins themselves do not cause calcium signals in DCs, we used an extensively dialyzed preparation of rhuHsp70, and the C-terminal substrate binding domain of mycHsp70, mycHsp70-(359–625) previously described as conferring biologic activity with regards to calcium signaling, antigen binding, and T cell activation (19). Importantly, this protein lacks the ATP binding domain and is therefore not purified via ATP-agarose. Neither the rhuHsp70 nor the mycHsp70-(359–625) induced a calcium signal in DCs (Fig. 2A).
FIGURE 2.
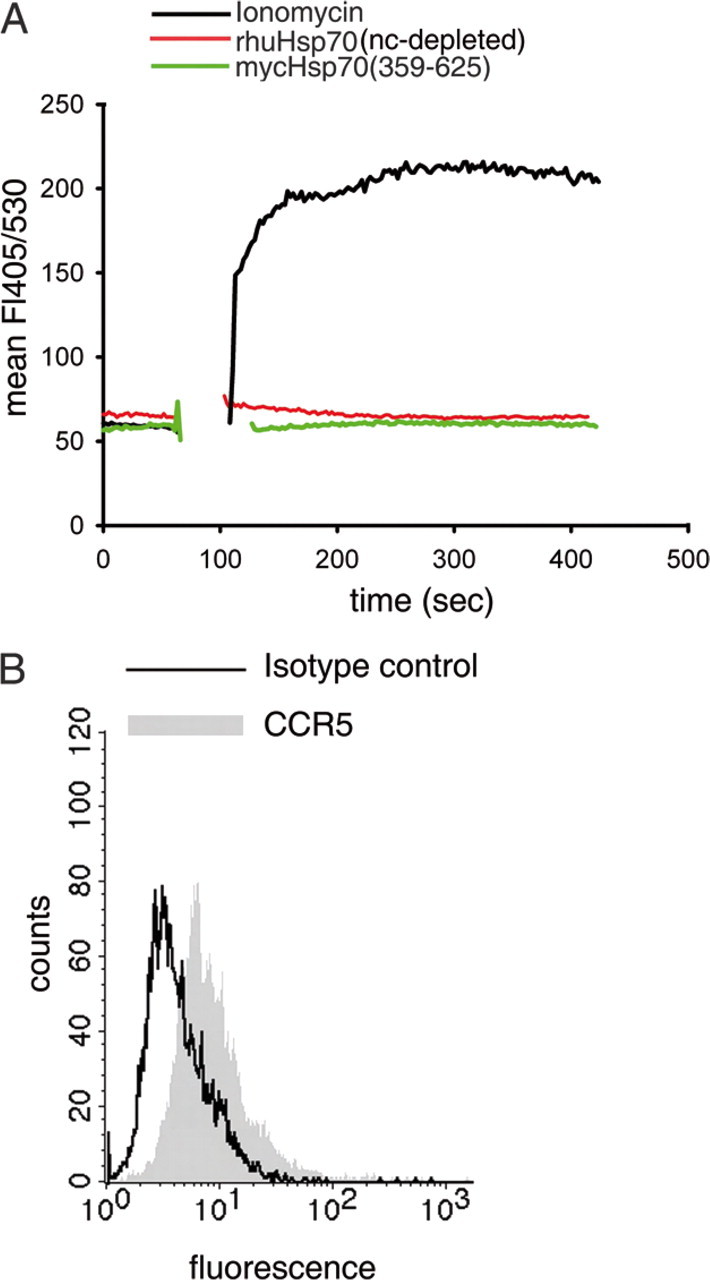
Highly purified, intensively dialyzed rhuHsp70 and mycHsp70-(359–625) do not induce a calcium signal in DCs. A, DCs were stimulated with intensively dialyzed 300 nm rhuHsp70 (red), 1 μm mycHsp70(359–625 (green), or 250 ng/ml ionomycin (black). B, CCR5 surface expression of DCs (black line: isotype control).
Because mycHsp70-associated calcium signals have been reported to be mediated through CCR5 binding, we also measured the level of surface CCR5 expression of our DCs by fluorescent-activated cell sorting (FACS) analysis. As shown in Fig. 2B, the DCs used in these experiments were CCR5-positive excluding the possibility that an absence of CCR5 could have caused the failure to induce a calcium signal.
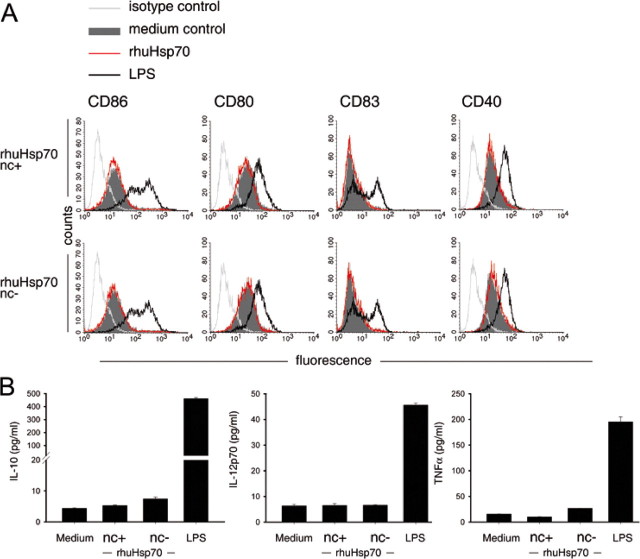
Additionally, we analyzed surface marker expression and cytokine secretion of DCs before and after incubation with rhuHsp70. Two rhuHsp70 preparations were compared: one was strongly dialyzed and therefore depleted of nucleotides (no calcium signaling activity), the other was less dialyzed and contained nucleotides (calcium signaling activity). All Hsp70 preparations were carefully endotoxin-depleted and induced no changes in DC phenotype or cytokine secretion (see Ref. 17). LPS was used as a control. As shown in Fig. 3A, the rhuHsp70 preparations did not change surface markers CD86, CD80, CD40, or CD83, regardless whether they contained nucleotides and had calcium signaling activity or were highly purified and did not cause calcium signals. LPS up-regulated these markers. Similarly, neither the nucleotide-containing nor the nucleotide-depleted rhuHsp70 preparation in its endotoxin-depleted form modulated the cytokine or chemokine profile of DCs, while LPS induced the secretion these factors (Fig. 3B; IL-10, IL-12p70, and TNF-α are shown as representative examples. IL-6, GM-CSF, CCL5/RANTES were also unchanged; not shown). These results indicate that low concentrations of nucleotides, as present in less dialyzed rhuHsp70 preparations, which cause calcium signals in DCs, do not change phenotypic markers or cytokine secretion, while surface markers and cytokine/chemokine secretion can be induced by LPS consistent with its TLR signaling activity (see references in Ref. 17).
FIGURE 3.
Nucleotide contamination does not cause changes in DC phenotype or cytokine/chemokine secretion. Non-matured IL-4/GM-CSF myeloid DCs were incubated in medium or with 300 nm of less dialyzed nucleotide-containing or nucleotide-depleted rhuHsp70 (all with endotoxin <0.5 EU/mg) or LPS (1 μg/ml) for 48 h and analyzed for surface marker expression (A) or cytokine/chemokine secretion (B). A, flow cytometry histograms for isotype (light gray line); for DCs incubated in medium (gray-filled histogram); for DCs incubated with rhuHsp70 (red line); for DCs incubated with LPS (black line). B, cytokine secretion of DCs after incubation in medium, with nucleotide-containing (nc+)rhuHsp70 or with nucleotide-depleted (nc-)rhuHsp70.
We did not perform these analyses with mycHsp70, because the available commercial source of mycHsp70 is reported to be contaminated by flagellin (24), which is known to up-regulate phenotypic markers and induce cytokine and chemokine secretion through TLR5 (25). Because we lacked the tools to measure or remove flagellin from protein preparations it would have been beyond our scope to distinguish mcyHsp70 effects from those of potential flagellin contamination.
Human and Mycobacterial Hsp70 Enhance Cross-presentation without Involving Calcium Signals or CCR5—CCR5 binding and calcium signaling were reported to be closely linked to the T cell stimulatory activity of mycHsp70 (12, 13). Given that the calcium signals of mycHsp70 or huHsp70 were caused by nucleotide contamination of the protein preparations, we then asked whether in the absence of calcium signals, it was possible to observe mycHsp70-mediated antigen cross-presentation and T-cell activation.
To address this question we tested the non-signaling preparation of mycHsp70-(325–625) in our cross-presentation system (17) and compared its ability to activate T cells to the dialyzed non-signaling rhuHsp70. The key elements of the system are highly purified Hsp70 proteins, the antigenic peptide pep70-MART-1 and the respective antigen-specific CTL. APCs were incubated in parallel settings with identical concentrations of peptide either with or without the addition of Hsp70. Peptide and Hsp70 are incubated to allow complex formation. The efficacy of peptide cross-presentation was quantified by the ability of APCs to induce an IFN-γ response in antigen-specific CTL as described (17). As APCs we selected the CCR5-negative B cell line LCL721.45 to evaluate in parallel the importance of CCR5 expression. As shown in Fig. 4, a 2-fold higher IFN-γ response was obtained when peptide was delivered to the APCs as a complex with either mycHsp70-(325–625) or rhuHsp70. These results demonstrate that mycHsp70 enhances antigen cross-presentation similar to rhuHsp70, and neither rhuHsp70 nor mycHsp70 requires CCR5 or calcium for this process.
FIGURE 4.
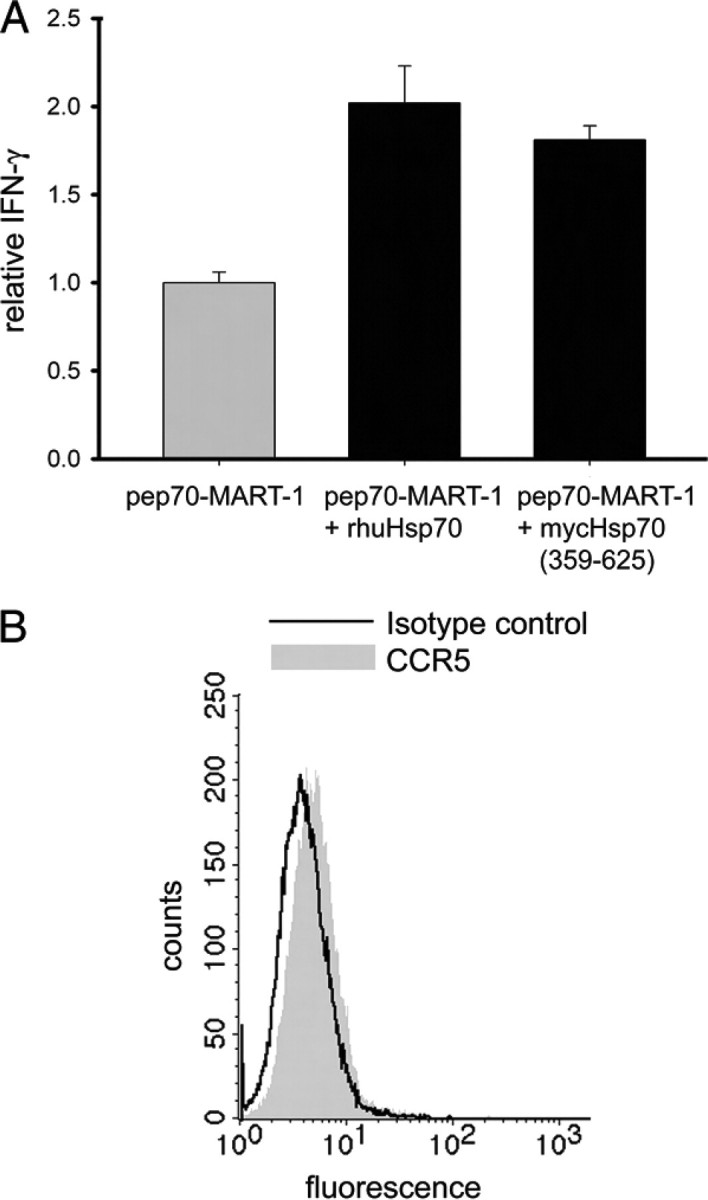
Endotoxin-depleted and highly dialyzed (nucleotide-depleted) rhuHsp70 and mycHsp70-(359–625) enhance antigen cross-presentation resulting in T cell stimulation. A, B-LCL 721.45 were incubated with equal amounts of pep70-MART-1 peptide alone or complexed to rhu-Hsp70 or to mycHsp70-(359–625). The efficacy of peptide cross-presentation was determined by the extent of A42-MART CTL activation resulting in IFN-γ secretion. The relative IFN-γ release was calculated using the IFN-γ release achieved with pep70-MART-1 peptide alone as in Ref. 1. Bars are the mean of triplicates with error bars representing the mean deviation. B, absence of CCR5 surface expression of B-LCL 721.45 (black line: isotype control).
DISCUSSION
Here we demonstrate that highly purified rhuHsp70 protein and the C-terminal mycHsp70 fragment, mycHsp70-(325–625) do not induce calcium signals in APCs nor do they require CCR5 or calcium signals to enhance exogenous antigen cross-presentation for T cell stimulation. Importantly, we found that nucleotides and possibly other, yet to be identified, contaminants within recombinant human and mycobacterial Hsp70 preparations (including those of commercial suppliers) cause calcium signals in myeloid DCs.
As reported previously (17), the Hsp70-mediated activity to stimulate antigen-specific T cell responses is strictly dependent on the ability of Hsp70 to form complexes with the exogenous peptide without requiring Hsp70-induced DC phenotypic or secretory changes. The now observed independency of calcium signaling further strengthens the conclusion that the ability of Hsp70s (human and bacterial alike) to activate adaptive immune responses may simply be an extension of their biochemical activity as molecular chaperones and there is no need to assign new activities to Hsp70 proteins to explain this immunological activity. A similar conclusion was reached by others (26–28).
Endotoxins were the first identified contaminants of Hsp70 and other HSP preparations (29–31). Unnoticed as contaminants, their stimulatory effects on DCs had led to the wrong conclusion that HSP proteins themselves have DC stimulatory capacity. Today HSP protein preparations are generally controlled for endotoxin contamination. Nevertheless, these early reports obtained with uncontrolled HSP preparations are still being used to support the postulate that HSPs have innate immune stimulatory capacity (for examples, see references 22, 43, 44 in Ref. 32, reference 5 in Ref. 33, and reference 61 in Ref. 34).
New contaminants with bioactivity on DCs and other APCs have recently emerged. Nucleotides, as detailed in this report, can now be added to the list. Nucleotides have been shown to cause calcium signals in DCs (17, 21–23). Thus, previously reported calcium signals observed in experiments using Hsp70 (12, 13, 35) need to be carefully reconsidered.
An additional example is flagellin, which was recently shown to “contaminate” microbial Hsp70, but less so Hsp70 from eukaryotic sources (24). Flagellin is a TLR5 agonist and can efficiently stimulate DCs to up-regulate phenotypic markers, to secrete chemokines and cytokines (25). Importantly, microbial Hsp70 proteins are considered to be better immune adjuvants than eukaryotic Hsp70 given that microbial Hsp70 were found to “stimulate” DC maturation and chemokine secretion even after endotoxin depletion (36). While this was thought to be related to their pathogen-derived nature, the recent discovery of flagellin in mycHsp70 preparations suggests that this interpretation may need to be reconsidered.
As chaperones, HSPs have a high intrinsic binding capacity for hydrophobic protein sequences, nucleotides and endotoxins (37–39). Thus, HSP protein preparations are prone to “contaminations” with proteins and non-proteinous substances, some of which may be difficult to remove. High standards of purification, stringent analyses of all recombinant HSP protein products for by-products and carefully controlled experimentation are indispensable to allow a precise attribution of biological function to HSP proteins and to pave the way for the utilization of their immunologic activity.
Acknowledgments
We acknowledge the excellent technical assistance of K. Nispel, A. Brandl, and D. Neumann, and thank Prof. Dr. Mahavir Singh of Lionex Diagnostics & Therapeutics GmbH for discussion.
This work was supported in part by Grants SFB455 (to E. N. and R. D. I.) and SFB-TR36 (to P. J. N.) from the Deutsche Forschungsgemeinschaft and EU Grant INNOCHEM (to P. J. N.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
The abbreviations used are: HSP, heat shock protein family; APC, antigen-presenting cell; CCR, chemokine receptor; CTL, cytotoxic T lymphocyte; DC, dendritic cell; HLA, human leukocyte antigen; Hsp70, stress-inducible member of the 70-kD family; LCL, lymphoblastoid cell line; myc, mycobacterial; rhu, recombinant human; MART-1, melanoma antigen recognized by T cells-1; nc, nucleotide; pep70, peptide sequence binding to Hsp70; IFN, interferon; LPS, lipopolysaccharide.
References
- 1.Calderwood, S. K., Theriault, J. R., and Gong, J. (2005) Eur. J. Immunol. 35 2518-2527 [DOI] [PubMed] [Google Scholar]
- 2.Basu, S., Binder, R. J., Suto, R., Anderson, K. M., and Srivastava, P. K. (2000) Int. Immunol. 12 1539-1546 [DOI] [PubMed] [Google Scholar]
- 3.Lancaster, G. I., and Febbraio, M. A. (2005) J. Biol. Chem. 280 23349-23355 [DOI] [PubMed] [Google Scholar]
- 4.Lang, A., Benke, D., Eitner, F., Engel, D., Ehrlich, S., Breloer, M., Hamilton-Williams, E., Specht, S., Hoerauf, A., Floege, J., von Bonin, A., and Kurts, C. (2005) J. Am. Soc. Nephrol. 16 383-391 [DOI] [PubMed] [Google Scholar]
- 5.Asea, A. (2007) J. Biosci. 32 579-584 [DOI] [PubMed] [Google Scholar]
- 6.Becker, T., Hartl, F. U., and Wieland, F. (2002) J. Cell Biol. 158 1277-1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binder, R. J., Vatner, R., and Srivastava, P. (2004) Tissue Antigens 64 442-451 [DOI] [PubMed] [Google Scholar]
- 8.Basu, S., Binder, R. J., Ramalingam, T., and Srivastava, P. K. (2001) Immunity 14 303-313 [DOI] [PubMed] [Google Scholar]
- 9.Calderwood, S. K., Mambula, S. S., Gray, P. J., Jr., and Theriault, J. R. (2007) FEBS Lett. 581 3689-3694 [DOI] [PubMed] [Google Scholar]
- 10.Kim, C. H., Nagata, K., and Butcher, E. C. (2003) J. Immunol. 171 152-158 [DOI] [PubMed] [Google Scholar]
- 11.Castellino, F., Huang, A. Y., Altan-Bonnet, G., Stoll, S., Scheinecker, C., and Germain, R. N. (2006) Nature 440 890-895 [DOI] [PubMed] [Google Scholar]
- 12.Floto, R. A., MacAry, P. A., Boname, J. M., Mien, T. S., Kampmann, B., Hair, J. R., Huey, O. S., Houben, E. N., Pieters, J., Day, C., Oehlmann, W., Singh, M., Smith, K. G., and Lehner, P. J. (2006) Science 314 454-458 [DOI] [PubMed] [Google Scholar]
- 13.Whittall, T., Wang, Y., Younson, J., Kelly, C., Bergmeier, L., Peters, B., Singh, M., and Lehner, T. (2006) Eur. J. Immunol. 36 2304-2314 [DOI] [PubMed] [Google Scholar]
- 14.Mack, M., Kleinschmidt, A., Bruhl, H., Klier, C., Nelson, P. J., Cihak, J., Plachy, J., Stangassinger, M., Erfle, V., and Schlondorff, D. (2000) Nat. Med. 6 769-775 [DOI] [PubMed] [Google Scholar]
- 15.Weber, C., Weber, K. S., Klier, C., Gu, S., Wank, R., Horuk, R., and Nelson, P. J. (2001) Blood 97 1144-1146 [DOI] [PubMed] [Google Scholar]
- 16.Noessner, E., Gastpar, R., Milani, V., Brandl, A., Hutzler, P. J., Kuppner, M. C., Roos, M., Kremmer, E., Asea, A., Calderwood, S. K., and Issels, R. D. (2002) J. Immunol. 169 5424-5432 [DOI] [PubMed] [Google Scholar]
- 17.Bendz, H., Ruhland, S. C., Pandya, M. J., Hainzl, O., Riegelsberger, S., Brauchle, C., Mayer, M. P., Buchner, J., Issels, R. D., and Noessner, E. (2007) J. Biol. Chem. 282 31688-31702 [DOI] [PubMed] [Google Scholar]
- 18.Udono, H., Saito, T., Ogawa, M., and Yui, Y. (2004) Methods 32 21-24 [DOI] [PubMed] [Google Scholar]
- 19.MacAry, P. A., Javid, B., Floto, R. A., Smith, K. G., Oehlmann, W., Singh, M., and Lehner, P. J. (2004) Immunity 20 95-106 [DOI] [PubMed] [Google Scholar]
- 20.Buchberger, A., Schroder, H., Buttner, M., Valencia, A., and Bukau, B. (1994) Nat. Struct. Biol. 1 95-101 [DOI] [PubMed] [Google Scholar]
- 21.la Sala, A., Ferrari, D., Corinti, S., Cavani, A., Di Virgilio, F., and Girolomoni, G. (2001) J. Immunol. 166 1611-1617 [DOI] [PubMed] [Google Scholar]
- 22.Marteau, F., Communi, D., Boeynaems, J. M., and Suarez Gonzalez, N. (2004) J. Leukoc Biol. 76 796-803 [DOI] [PubMed] [Google Scholar]
- 23.Schnurr, M., Toy, T., Shin, A., Wagner, M., Cebon, J., and Maraskovsky, E. (2005) Blood 105 1582-1589 [DOI] [PubMed] [Google Scholar]
- 24.Ye, Z., and Gan, Y. H. (2007) J. Biol. Chem. 282 4479-4484 [DOI] [PubMed] [Google Scholar]
- 25.Means, T. K., Hayashi, F., Smith, K. D., Aderem, A., and Luster, A. D. (2003) J. Immunol. 170 5165-5175 [DOI] [PubMed] [Google Scholar]
- 26.Bausinger, H., Lipsker, D., and Hanau, D. (2002) Trends Immunol. 23 342-343 [DOI] [PubMed] [Google Scholar]
- 27.Tobian, A. A., Canaday, D. H., Boom, W. H., and Harding, C. V. (2004) J. Immunol. 172 5277-5286 [DOI] [PubMed] [Google Scholar]
- 28.Tobian, A. A., Harding, C. V., and Canaday, D. H. (2005) J. Immunol. 174 5209-5214 [DOI] [PubMed] [Google Scholar]
- 29.Bausinger, H., Lipsker, D., Ziylan, U., Manie, S., Briand, J. P., Cazenave, J. P., Muller, S., Haeuw, J. F., Ravanat, C., de la Salle, H., and Hanau, D. (2002) Eur. J. Immunol. 32 3708-3713 [DOI] [PubMed] [Google Scholar]
- 30.Wallin, R. P., Lundqvist, A., More, S. H., von Bonin, A., Kiessling, R., and Ljunggren, H. G. (2002) Trends Immunol. 23 130-135 [DOI] [PubMed] [Google Scholar]
- 31.Tsan, M. F., and Gao, B. (2004) J. Leukoc Biol. 76 514-519 [DOI] [PubMed] [Google Scholar]
- 32.Pockley, A. G., Muthana, M., and Calderwood, S. K. (2008) Trends Biochem. Sci. 33 71-79 [DOI] [PubMed] [Google Scholar]
- 33.Vega, V. L., Rodriguez-Silva, M., Frey, T., Gehrmann, M., Diaz, J. C., Steinem, C., Multhoff, G., Arispe, N., and De Maio, A. (2008) J. Immunol. 180 4299-4307 [DOI] [PubMed] [Google Scholar]
- 34.Tesniere, A., Panaretakis, T., Kepp, O., Apetoh, L., Ghiringhelli, F., Zitvogel, L., and Kroemer, G. (2008) Cell Death Differ 15 3-12 [DOI] [PubMed] [Google Scholar]
- 35.Asea, A., Kraeft, S. K., Kurt-Jones, E. A., Stevenson, M. A., Chen, L. B., Finberg, R. W., Koo, G. C., and Calderwood, S. K. (2000) Nat. Med. 6 435-442 [DOI] [PubMed] [Google Scholar]
- 36.Javid, B., MacAry, P. A., and Lehner, P. J. (2007) J. Immunol. 179 2035-2040 [DOI] [PubMed] [Google Scholar]
- 37.Mayer, M. P., and Bukau, B. (2005) Cell Mol. Life Sci. 62 670-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Habich, C., Kempe, K., van der Zee, R., Rumenapf, R., Akiyama, H., Kolb, H., and Burkart, V. (2005) J. Immunol. 174 1298-1305 [DOI] [PubMed] [Google Scholar]
- 39.Triantafilou, M., and Triantafilou, K. (2002) Trends Immunol. 23 301-304 [DOI] [PubMed] [Google Scholar]