Abstract
First revealed in cancer studies, HURP (hepatoma up-regulated protein) is a cell cycle-associated gene with elevated expression in the G2/M phase. Cell culture studies have revealed that HURP is an essential factor required for spindle formation and chromosome congression during mitosis. However, the function of HURP in an in vivo context has not been explored. We generated a Hurp knock-out (Hurp–/–) mouse to investigate the role of HURP in development under normal physiological conditions. Hurp–/– mice develop normally and are indistinguishable from their wild-type littermates. Interestingly, breeding experiments revealed that Hurp–/– females are completely infertile, whereas the males reproduce normally. Ovulation, fertilization, and pre-implantation embryo development are normal; however, the Hurp–/– females are unable to form implantation sites due to an inability to undergo the decidual reaction. This is caused by a defect in endometrial stromal proliferation that leads to implantation failure. Additionally, HURP expression in the uterus coincides with the implantation stage and can be induced by estrogen treatment. Our results demonstrate for the first time that HURP affects endometrial stromal proliferation during implantation but is dispensable during normal development in mice; specifically, HURP has an essential function in uterine biology.
First revealed in cancer studies, HURP (hepatoma up-regulated protein) is a cell cycle-associated gene with elevated expression in the G2/M phase (1–3). HURP is a microtubule-associated protein and an essential component of the Ran-dependent complex involved in bipolar spindle assembly (4–6). HURP binds to microtubules through its N-terminal domain, and the phosphorylation of HURP by Aurora A regulates the activity and accessibility of HURP to bind to microtubules (7). Specifically, HURP plays a crucial role during chromosome congression, which is the process whereby microtubules attach to the kinetochore of chromosomes and move the chromosomes to the metaphase plate during mitosis; depletion of HURP in cells results in chromosome misalignment at the metaphase plate (8, 9). Although cell culture studies have revealed that HURP is an essential factor required for mitosis, the role of HURP in development under normal physiological conditions has not been explored. In this study, we applied a mouse genetics approach and discovered, serendipitously, that HURP deficiency leads to female infertility caused by implantation failure. Implantation of embryos into the uterus is a sophisticated and important process for viviparous animals. In mice, decidualization, which is characterized by endometrial stromal proliferation and differentiation, is crucial for the establishment of a fetal-maternal interface during implantation (10–12). However, the molecular mechanisms by which cell cycle events govern decidualization are poorly understood. Here, we demonstrated that HURP is an essential cell cycle regulator that is needed for successful stromal proliferation during the process of decidualization to transform the uterine endometrium into a receptive state for blastocyst implantation in mice. Most important, this is a newly discovered molecular influence of HURP on implantation and represents an example whereby a deficiency in a cell cycle-associated gene exhibits phenotypic effects specifically on certain tissues, in this case, the endometrial stroma, but seems to be dispensable in normal cell proliferation during mouse development.
EXPERIMENTAL PROCEDURES
Generation of Hurp Knock-out Mice—An insertion targeting vector was used to disrupt the Hurp gene in embryonic stem cells as described under supplemental “Methods” and in Fig. S2A.
Estrogen Treatment and Induction of Artificial Decidualization—Ovariectomized females were injected with estrogen (E2)2 or an oil (vehicle) control. Mice were killed at 2, 24, and 72 h after injection. For artificial induction of decidual reaction, ovariectomized females were treated with three daily injections of E2. After 2 days of rest, mice were treated with daily injections of progesterone (P4) and E2. Six hours after the P4 and E2 injection on day 3, one uterine horn was infused with 30 μlof sesame oil to induce decidualization. The daily injections of P4 and E2 were continued until the day of sampling. Uterine weights of the oil-infused and control horns were measured on day 5 after oil infusion.
Mating, Embryo Collection, and Implantation Sites—Female mice were bred with wild-type fertile males for uterus sampling at different pregnancy stages. Virginal plugs were checked in the morning before 10:00. Sampling of the pregnant uteri was carried out between 11:00 and 13:00. On day 4 of pregnancy (day 1 = vaginal plug), uteri were flushed with M2 medium to recover the pre-implantation blastocysts (13). The collected blastocysts were examined under a dissecting microscope (Leica). Implantation sites on day 6 of pregnancy were visualized by intravenous injection of Chicago Blue B dye (1% in phosphate-buffered saline; 0.1 ml/mouse) through tail veins (14). The number of implantation sites as demarcated by distinct blue bands was then recorded.
Immunohistochemistry, DNA Synthesis, and Mitotic Index—Antibodies against bromodeoxyuridine (BrdUrd; Dako M0744) and phosphohistone H3 (pH3; Upstate 06-570) were used for DNA synthesis and mitotic index analyses. Rabbit anti-mouse HURP polyclonal antibody was generated and used for HURP immunohistochemistry (IHC).
Detailed experimental procedures for RNA analysis, artificial decidualization, and IHC are described in the supplemental “Methods.”
RESULTS AND DISCUSSION
Hurp Expression Coincides with Cell Proliferation in Wild-type Mice—Multiple tissues of Northern blot analysis showed that a higher level of Hurp mRNA was expressed in the testis, spleen, and thymus. Lower expression of Hurp mRNA was detected in the colon, ovary, and small intestine (supplemental Fig. S1A). To study the relationship between Hurp expression and cell proliferation in an in vivo context, we examine the temporal expression pattern of Hurp in the embryonic and postnatal tissues and used the different developmental stages of livers as the example. In the fetal liver, a high expression level of Hurp mRNA was sustained until embryonic day 15.5. The signal declined gradually thereafter in the embryonic liver and in the postnatal newborn until expression was barely detectable in the adult liver (supplemental Fig. S1B). Interestingly, the temporal expression pattern of Hurp coincided with the expression of the proliferation marker Pena (supplemental Fig. S1B), implying that Hurp expression correlates with the ongoing cell proliferation of hepatocytes in mouse liver. Although most hepatocytes are arrested at the quiescent stage in the adult liver, quiescent hepatocytes are able to re-enter the cell cycle during liver regeneration, which is inducible by partial hepatectomy (15, 16). Our data indeed demonstrate that the expression of Hurp mRNA and protein can be transiently induced at 2 days post-hepatectomy, which coincides with the G2/M phase during hepatocyte cell cycle progression, and then the level rapidly decreases to the basal level after 3 days post-hepatectomy (supplemental Fig. S1, C and D).
Normal Development without Overt Phenotype in Hurp–/– Mice—To study the role of Hurp involved in development under physiological conditions, we generated Hurp knock-out mice using gene targeting technology in embryonic stem cells (supplemental Fig. S2A). Southern blot and Northern blot analyses demonstrated that the Hurp gene was disrupted with undetectable mRNA expression in the homozygous knock-out (Hurp–/–) mice (supplemental Fig. S2, B and C). Hurp–/– mice, derived from a Hurp+/– heterozygous cross, were born at the expected Mendelian ratio (supplemental Table 1). Both male and female Hurp–/– mice are phenotypically normal, with no observable changes in morphology, growth curve, and behavior. Previous studies have identified HURP as a component of the mitotic apparatus involved in spindle formation and chromosome congression using different approaches, including proteomics study of HeLa cells (4), biochemical fractionation of microtubule-associated proteins from Xenopus eggs (5), and functional genomic screen of mitotic regulators in human tumor cells (6). However, our gene knock-out experiment revealed that HURP is dispensable in normal mouse development from the embryonic stage to adulthood. This could be due to redundancy whereby another yet-to-be identified gene product compensates for the loss of HURP in the knock-out mice.
Hurp Deficiency Leads to Female Infertility in Mice—To detect possible reproductive phenotypes, we performed a 6-month breeding study of Hurp–/– males and females mated with fertile Hurp+/– females and males, respectively. Interestingly, we found that the Hurp–/– females were completely infertile, with no pups at all being born over the 6 months of the breeding period, whereas the Hurp–/– males were fertile, showing no significant difference compared with the fertile Hurp+/– males (supplemental Table 1). To explore the underlying cause of this female infertility and to delineate whether HURP plays a critical role in female reproduction including ovarian and uterine functions, we first analyzed the early pregnancy events. The pre-implantation blastocysts recovered from the wild-type and Hurp–/– females mated with wild-type fertile males were examined. We found that the number of morphologically normal blastocysts recovered from the Hurp–/– females was comparable with that of wild-type females when examined on day 4 of pregnancy (Fig. 1A and supplemental Table 2); this indicated that ovulation, fertilization, and the preimplantation development of blastocysts were all normal in the Hurp–/– female. In addition, hematoxylin and eosin staining of tissue sections revealed a normal histology of Hurp–/– ovary, which contained different stages of oocytes and corpus luteum similar to those in the normal female mice (supplemental Fig. S3). Together, these results indicate that the complete infertility observed in Hurp–/– females was not due to disruption of ovarian function or an absence of fertilization, but was due to either defective implantation and/or pregnancy failure following implantation. In mice, blastocyst attachment to the uterine luminal epithelium initiates the implantation process and is accompanied by increased endometrial vascular permeability at the implantation sites, which can be visualized by the Chicago Blue B method (14). Using this staining method, we found that, although wild-type females (n = nine mice) had an expected number of implantation sites when examined on day 6 of pregnancy, the uteri of Hurp–/– females (n = 12 mice) showed no sign of implantation (Fig. 1B). This result clearly indicates that the Hurp–/– uterus is defective with respect to blastocyst implantation and implies an essential role for the HURP protein specifically in uterine receptivity.
FIGURE 1.
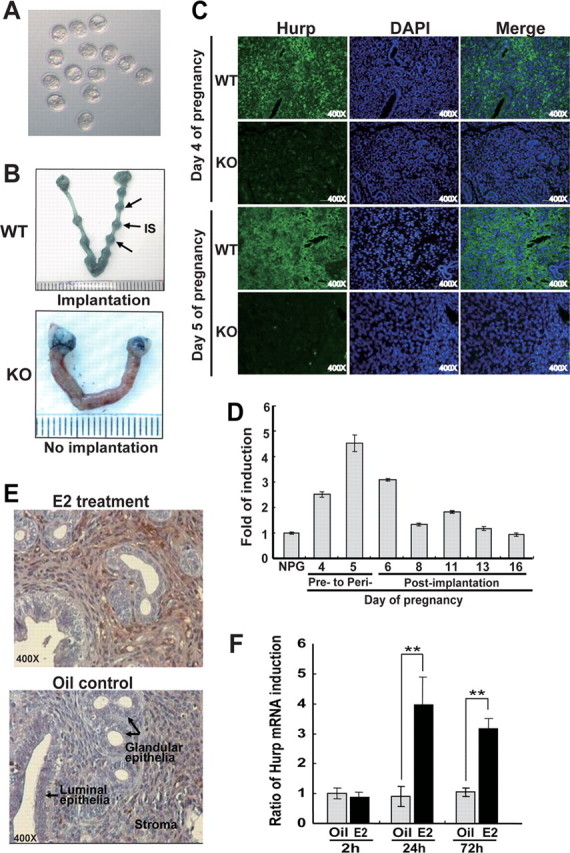
Hurp–/– females are completely infertile and unable to form implantation sites (A and B) and expression of Hurp in the pregnant uteri of wild-type mice and ovariectomized mice injected with E2 (C–F). A, representative photograph of the morphologically normal blastocysts (embryonic day 3.5) recovered from two Hurp–/– female mice on day 4 of pregnancy. B, representative photographs of wild-type uteri with implantation sites (IS; indicated by arrows) and Hurp–/– uteri without implantation sites on day 6 of pregnancy (day 1 = vaginal plug). Implantation sites were visualized by intravenous injection of Chicago Blue B dye. The wild-type and Hurp–/– females were plugged by fertile wild-type male mice (wild-type females, n = 9; and Hurp–/– females, n = 12). C, IHC staining of HURP protein in the uterine sections prepared from wild-type (WT) and Hurp–/– (knock-out (KO)) females on days 4 and 5 of pregnancy. Nuclei were counterstained blue with 4′,6-diamidino-2-phenylindole (DAPI). Original magnification × 400. D, elevated levels of Hurp mRNA detected in the pregnant uteri at the pre- to peri-implantation stages of pregnancy using real-time RT-PCR analyses. There was a peak induction of Hurp mRNA expression on day 5 of pregnancy, which decreased thereafter to a basal level at a late pregnant stage. NPG, non-pregnant. E, representative photomicrographs of IHC staining of HURP protein at 72 h. This was induced in the endometrial stromal cells of the ovariectomized females that had received an intraperitoneal injection of E2. F, quantification of Hurp mRNA expression in the uteri of the ovariectomized mice that had received intraperitoneal injections of E2 or oil (vehicle) by real-time quantitative RT-PCR analyses. The analyses were carried out at 2, 24, and 72 h after injection. Wild-type C57BL/6 mice were used for the E2 treatment experiments. The amount of total input cDNA was normalized using hypoxanthine-guanine phosphoribosyltransferase as an internal control. Three mice per group were used for these experiments. **, p < 0.005.
Hurp Expression in the Endometrial Stroma Coincides with the Implantation Stage and Can Be Induced by Estrogen Treatment—The crucial role of HURP in implantation was reinforced by studying the expression pattern of HURP in the pregnant uteri of normal mice. This expression pattern coincides with an increased stromal cell proliferation on days 4 and 5 of pregnancy (described below in Fig. 2), and this also coincides with the pre- to peri-implantation stages initiated by blastocyst attachment to the uterine luminal epithelium. In addition, IHC staining of HURP protein using a rabbit polyclonal antibody revealed the presence of strong expression of HURP in the stromal cells of the uterine endometrium (Fig. 1C). To study the temporal expression of Hurp mRNA, we performed realtime reverse transcription (RT)-PCR analyses using total RNA isolated from non-pregnant and pregnant uteri of wild-type mice at various stages of pregnancy. Our results showed that Hurp mRNA was induced on day 4 of pregnancy, and peak induction of Hurp mRNA expression was detected on day 5 of pregnancy; it decreased thereafter to a basal level during the late pregnant stage (Fig. 1D). The specificity of the antibodies against mouse HURP protein was confirmed by Western blotting using cellular extracts of regenerating livers (supplemental Fig. S1D). Moreover, our IHC staining of the uterine sections demonstrated the absence of HURP protein in the Hurp–/– uteri (Fig. 1C). To elucidate whether HURP can be induced by ovarian steroids such as E2, which is a mitogen for the uterine endometrium during implantation, we performed ovariectomy and E2 treatment experiments on wild-type adult mice. Uterine tissues were obtained from the ovariectomized mice that had received intraperitoneal injections of E2 or oil (vehicle) for 2, 24, and 72 h. We found that at 24 and 72 h following the injection of E2 into these mice, both protein and mRNA expression of Hurp was significantly induced in the endometrial stroma as detected by IHC staining and real-time RT-PCR, respectively (Fig. 1, E and F). Quantification revealed that there were 4- and 3-fold increases in the Hurp mRNA levels at 24 and 72 h after E2 treatment, respectively (Fig. 1F). This suggests that Hurp is an E2-responsive gene, the induction of which is regulated by the E2 signaling pathway in the stromal cells. Alternatively, this induction could be due to the mitogenic action of E2 on stromal proliferation, with up-regulation of Hurp expression being a consequence of cell cycle progression.
FIGURE 2.
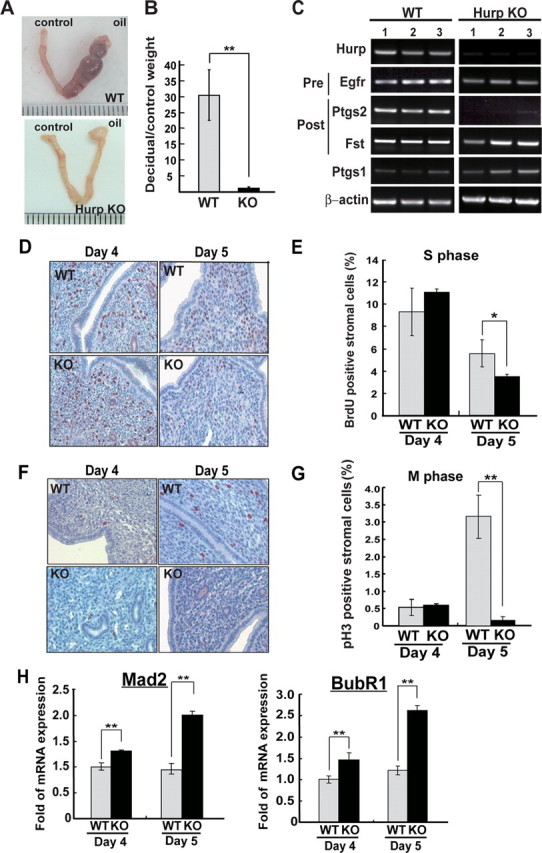
Defective decidualization of theHurp–/– uteri due to impaired cell proliferation of the endometrial stroma during implantation. A, representative photographs of gross morphology of the decidual response in the wild-type (WT) and Hurp–/– (knock-out (KO)) uteri 5 days after the decidual stimulus. B, ratios of the weights of the decidual horn to the control horn measured on day 5 after the decidual stimulus (wild-type females, n = 4; Hurp–/– females, n = 6). **, p < 0.005. C, RT-PCR analyses of genes associated with pre- and post-implantation stages of pregnancy. Total RNA samples isolated from uteri 5 days after the decidual stimulus were used for RT-PCR analyses. D, representative photomicrographs of the S phase cells as monitored by BrdUrd incorporation into the replicating DNA molecules. The uterine sections were prepared from wild-type and Hurp–/– females on days 4 and 5 of pregnancy. Original magnification × 400. E, quantification of BrdUrd (BrdU)-positive cells. About 1000 stromal cells in random fields of BrdUrd-staining slides were examined for the presence of BrdUrd-positive cells for each mouse. F, representative photomicrographs of the mitotic cells as monitored by pH3 staining of the pregnant uteri prepared from wild-type and Hurp–/– mice on days 4 and 5 of pregnancy. Original magnification × 400. G, quantification of pH3-positive mitotic cells. About 1000 stromal cells in random fields of uterine sections were examined for the presence of pH3-positive cells for each mouse. The mean for each time point is expressed as a percentage of total stromal cells counted and is shown as the mean ± S.D. H, quantification of the mRNA levels of Mad2 and BubR1 using real-time quantitative RT-PCR. The amount of total input cDNA was normalized using hypoxanthine-guanine phosphoribosyltransferase as an internal reference. Three to five mice per group were used for these experiments. *, p < 0.05; **, p < 0.005.
Defective Decidualization of the Hurp–/– Uterus during Implantation—Decidualization is a process of stromal transformation that is characterized by endometrial stromal proliferation and differentiation to form morphologically and functionally distinct cells. This process is critical for the establishment of successful pregnancy (10). To determine whether the infertility of Hurp–/– females is caused by impaired decidualization of the uterine stroma, we performed artificial induction of the decidual reaction (17). Ovariectomized wild-type and Hurp–/– females were first treated with E2 and P4. A decidualization reaction was then induced in one of the two uterine horns by an intraluminal infusion of oil, and the other horn was left unstimulated as a control. Uterine weight and gross morphology of the stimulated and control uterine horns were examined on day 5 after oil infusion. As shown in Fig. 2A, a robust reaction of decidualization was observed in the wild-type female. In contrast, the Hurp–/– uteri failed to show any significant induction of decidualization upon identical stimulation. The decidual reaction was quantified by measuring the ratio of the weight of the stimulated uterine horn to the control horn. Our results clearly indicate that the stimulated horn of the Hurp–/– uteri failed to increase in size compared with wild-type uteri (Fig. 2, A and B). To elucidate the decidualization defect in the Hurp–/– females, we studied the differential expression of genes crucial for the pre-implantation and post-implantation stages (10, 18). Total RNA samples isolated from the stimulated uterine horns of wild-type and Hurp–/– females were subjected to RT-PCR analysis. This RT-PCR experiment revealed that gene expression related to the pre-implantation stage of uterine function, such as the Egfr gene, showed no obvious differences. However, for the Hurp–/– uteri, there was a significant decrease in gene expression crucial to the peri- and post-implantation stages, such as the Ptgs2 gene (Fig. 2C). To further investigate the defective gene induction during decidualization, we performed an Affymetrix microarray analysis. Three independent microarray experiments were carried out using three RNA samples isolated from three individual mice of each group to obtain reproducible data. This analysis revealed significant differences between the wild-type and Hurp–/– uteri. In terms of cell cycle-associated gene expression, in the Hurp–/– uteri, a dramatic decrease in various G2/M markers such as Ccnb1 (cyclin B1) and Ccnb2 (cyclin B2) was noted, but this did not occur with G1 and S phase markers (supplemental Fig. S4A). This suggests cell cycle progress through the G1 and S phases but the presence of an impairment at the G2/M phase. In addition, gene products important to implantation and decidualization, such as the prostaglandin biosynthesis enzymes (19, 20) and decidual prolactins (21, 22), were significantly decreased or absent in the Hurp–/– uteri (supplemental Fig. S4, B and C). Together, the results of the artificial decidualization experiments indicate that Hurp–/– females are unable to form implantation sites due to an inability to undergo the decidual reaction, and the later is likely to be due to a defect associated with cell proliferation, which leads to blockage of the further differentiation and development of the endometrium that supports embryo implantation.
Impaired Stromal Proliferation Leads to Failure of the Decidual Reaction in the Hurp–/– Uteri—Because Hurp was originally identified as a cell cycle-regulated gene, the expression of which is up-regulated during the G2/M phase, we speculated that endometrial stromal proliferation may be affected in the Hurp–/– uteri and that this leads to a failure of the decidualization. To further illustrate the defect in stromal proliferation, we monitored the S and M phase cells by BrdUrd incorporation into the replicating DNA and IHC staining of pH3, respectively, using uterine samples prepared from wild-type and Hurp–/– females naturally mated with wild-type males. On day 4 of pregnancy, there was a comparably high percentage of S phase cells detected in the stromata of the Hurp–/– and wild-type uteri; only a few M phase cells were present in these uterine samples (Fig. 2, D–G). On day 5 of pregnancy, an elevated percentage of M phase cells was detected in the wild-type stroma. However, there was a significantly decreased percentage or near absence of M phase stromal cells detected in the Hurp–/– uteri (Fig. 2, D–G), which indicates that there was an impairment of cell cycle progression and that this would seem to caused by a block before the mitotic stage. This blockage further impaired cell progression into the next cell cycle as illustrated by the decreased S phase cells in the Hurp–/– stroma compared with the wild-type control on day 5 of pregnancy (Fig. 2, E and G). Thus, the Hurp deficiency causes a pre-mitotic block leading to impaired cell cycle progression of the endometrial stroma in the pregnant uteri. Previous cell culture studies revealed that HURP depletion leads to chromosome misalignment at the metaphase plate (4–6). In our mouse study, we have never seen stromal cells with visible condensed chromosomes in the uterine samples of Hurp knock-out mice; this may be attributable to cell cycle arrest at the pre-mitotic phase. However, we did find that HURP protein co-localized with condensed chromosomes in the wild-type uterine samples (supplemental Fig. S5). This observation is consistent with the role of HURP being involved in stabilizing and targeting K-fibers to the kinetochore of chromosomes (8, 9).
To further determine whether the mitotic spindle checkpoint is activated in the Hurp–/– stroma rendering the cell cycle arrest, we examined the expression levels of Mad2 and BubR1, which are key components of the mitotic checkpoint complex (23, 24). As expected, significantly higher levels of Mad2 and BubR1 were detected in the Hurp–/– uteri compared with wild-type controls; this was revealed as 2.1- and 2.2-fold increases in the mRNA levels of Mad2 and BubR1, respectively, on day 5 of pregnancy (Fig. 2H). These results indicate that, in the absence of HURP, checkpoint signaling is activated possibly because of lack of a functional mitotic apparatus in the stromal cells and that this causes an arrest of cell cycle progression; this activation of the mitotic checkpoint phenomenon is similar to that observed previously in HURP-depleted HeLa cells (6).
In this study, we demonstrated that Hurp deficiency in mice leads to female infertility caused by impaired cell cycle progression of the endometrial stroma, which is arrested at the premitotic phase. The lack of completion of stromal cell proliferation at an early stage of decidualization thus abrogates the latter events of stromal differentiation that follow the proliferation. This knock-out mouse model thus provides a useful system that allows the study of the in vivo effects of HURP on uterine physiology and the genetic pathways of HURP-mediated regulation of endometrial proliferation. This could be relevant to humans. We anticipate that a more thorough understanding of the molecular mechanisms that regulate uterine receptivity and implantation is of clinical relevance to the improvement of infertility-related medical treatments and may lead to the development of a novel contraceptive method.
Supplementary Material
This work was supported by National Research Program for Genomic Medicine Grants 95HC007 and NSC96-2752-B-010-004-PAE (to T.-F. T.), CMRPD160471 (to C.-K. C.), and NSC96-2320-B-031-001-MY2 (to C.-W. Y.) from the National Science Council and by a grant from the Ministry of Education “Aim for the Top University Plan.” Work performed in the Microarray and Gene Expression Analysis Core Facility of the National Yang-Ming University Genome Research Center was supported by the National Research Program for Genomic Medicine, National Science Council. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental “Methods,” Figs. S1–S5, Tables 1 and 2, and additional references.
Footnotes
The abbreviations used are: E2, estrogen; P4, progesterone; BrdUrd, bromodeoxyuridine; pH3, phosphohistone H3; IHC, immunohistochemistry; RT, reverse transcription.
References
- 1.Bassal, S., Nomura, N., Venter, D., Brand, K., McKay, M. J., and van der Spek, P. J. (2001) Genomics 77 5–7 [DOI] [PubMed] [Google Scholar]
- 2.Tsou, A. P., Yang, C.-W., Huang, C. Y., Yu, R. C., Lee, Y. C., Chang, C. W., Chen, B. R., Chung, Y. F., Fann, M. J., Chi, C. W., Chin, J. H., and Chou, C. K. (2003) Oncogene 22 298–307 [DOI] [PubMed] [Google Scholar]
- 3.Yu, C. T., Hsu, J. M., Lee, Y. C., Tsou, A. P., Chou, C.-K., and Huang, C. Y. (2005) Mol. Cell. Biol. 25 5789–5800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silljé, H. H., Nagel, S., Körner, R., and Nigg, E. A. (2006) Curr. Biol. 16 731–742 [DOI] [PubMed] [Google Scholar]
- 5.Koffa, M. D., Casanova, C. M., Santarella, R., Köcher, T., Wilm, M., and Mattaj, I. W. (2006) Curr. Biol. 16 743–775 [DOI] [PubMed] [Google Scholar]
- 6.Wong, J., and Fang, G. (2006) J. Cell Biol. 173 879–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong, J., Lerrigo, R., Jang, C.-Y., and Fang, G. (2008) Mol. Biol. Cell 19 2083–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanderson, H. S., and Clarke, P. R. (2006) Curr. Biol. 16 R466–R468 [DOI] [PubMed] [Google Scholar]
- 9.Wilde, A. (2006) J. Cell Biol. 173 829–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dey, S. K., Lim, H., Das, S. K., Reese, J., Paria, B. C., Daikoku, T., and Wang, H. (2004) Endocr. Rev. 25 341–373 [DOI] [PubMed] [Google Scholar]
- 11.Wang, H., and Dey, S. K. (2006) Nat. Rev. Genet. 7 185–199 [DOI] [PubMed] [Google Scholar]
- 12.Lee, K. Y., Jeong, J. W., Tsai, S. Y., Lydon, J. P., and DeMayo, F. J. (2007) Trends Endocrinol. Metab. 18 234–239 [DOI] [PubMed] [Google Scholar]
- 13.Hogan, B., Beddington, R., Costantini, F., and Lacy, E. (1994) Manipulating the Mouse Embryo: A Laboratory Manual, 2nd Ed., Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- 14.Tranguch, S., Cheung-Flynn, J., Daikoku, T., Prapapanich, V., Cox, M. B., Xie, H., Wang, H., Das, S. K., Smith, D. F., and Dey, S. K. (2005) Proc. Natl. Acad. Sci. U. S. A. 102 14326–14331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Greene, A. K., and Puder, M. (2003) J. Investig. Surg. 16 99–102 [PubMed] [Google Scholar]
- 16.Fausto, N., Campbell, J. S., and Riehle, K. J. (2006) Hepatology 43 S45–S53 [DOI] [PubMed] [Google Scholar]
- 17.Lee, K. Y., Jeong, J. W., Wang, J., Ma, L., Martin, J. F., Tsai, S. Y., Lydon, J. P., and DeMayo, F. J. (2007) Mol. Cell. Biol. 27 5468–5478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hewitt, S. C., Deroo, B. J., Hansen, K., Collins, J., Grissom, S., Afshari, C. A., and Korach, K. S. (2003) Mol. Endocrinol. 17 2070–2083 [DOI] [PubMed] [Google Scholar]
- 19.Lim, H., Paria, B. C., Das, S. K., Dinchuk, J. E., Langenbach, R., Trzaskos, J. M., and Dey, S. K. (1997) Cell 91 197–208 [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto, H., Ma, W. G., Daikoku, T., Zhao, X., Paria, B. C., Das, S. K., Trzaskos, J. M., and Dey, S. K. (2002) J. Biol. Chem. 277 29260–29267 [DOI] [PubMed] [Google Scholar]
- 21.Bole-Feysot, C., Goffin, V., Edery, M., Binart, N., and Kelly, P. A. (1998) Endocr. Rev. 19 225–268 [DOI] [PubMed] [Google Scholar]
- 22.Bao, L., Tessier, C., Prigent-Tessier, A., Li, F., Buzzio, O. L., Callegari, E. A., Horseman, N. D., and Gibori, G. (2007) Endocrinology 148 2326–2334 [DOI] [PubMed] [Google Scholar]
- 23.Yu, H. (2006) J. Cell Biol. 173 153–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen, R. H. (2007) J. Biomed. Sci. 14 475–479 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


