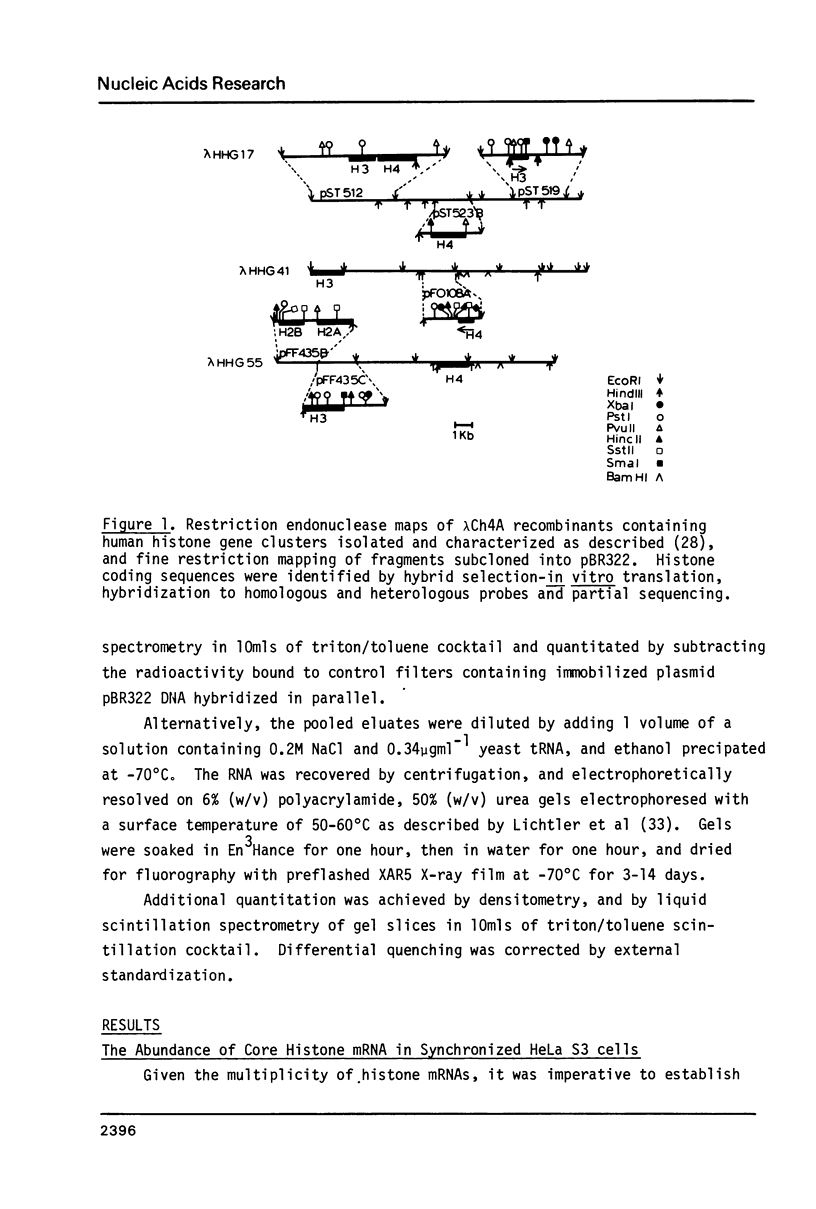
Abstract
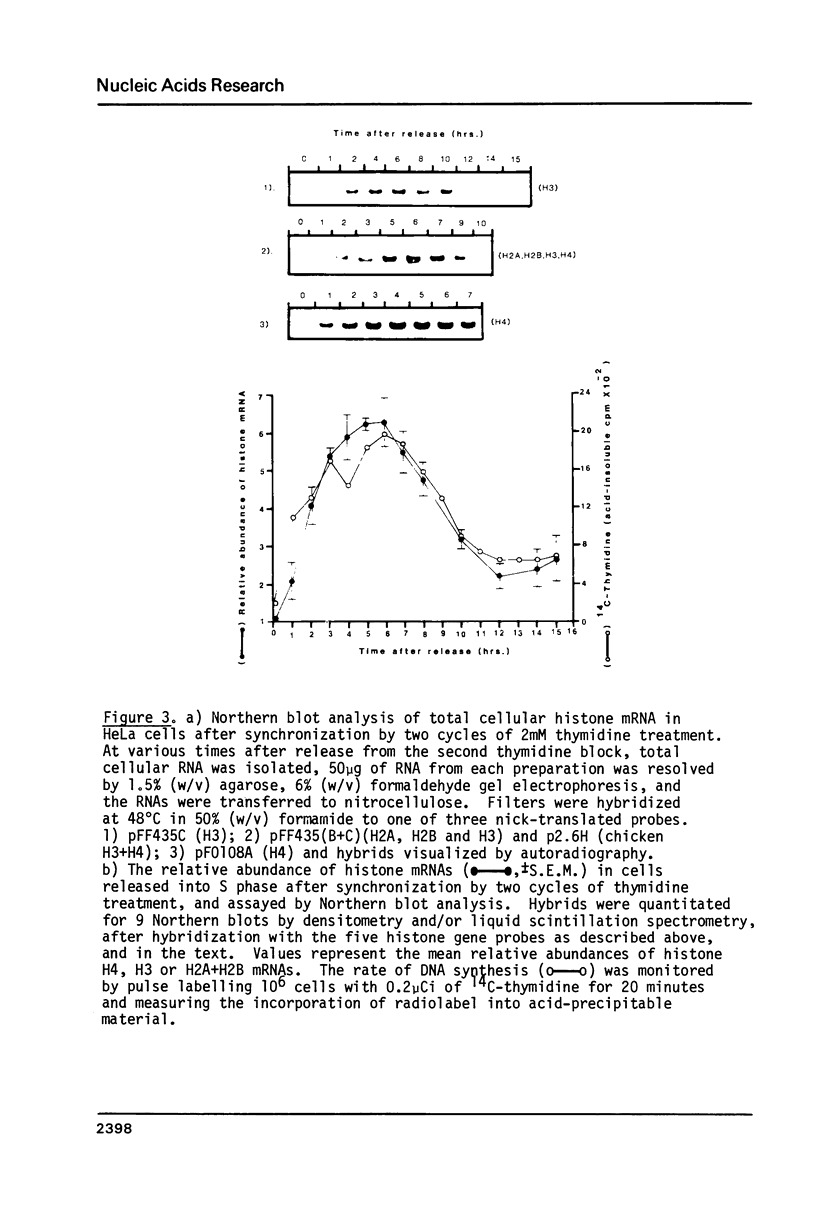
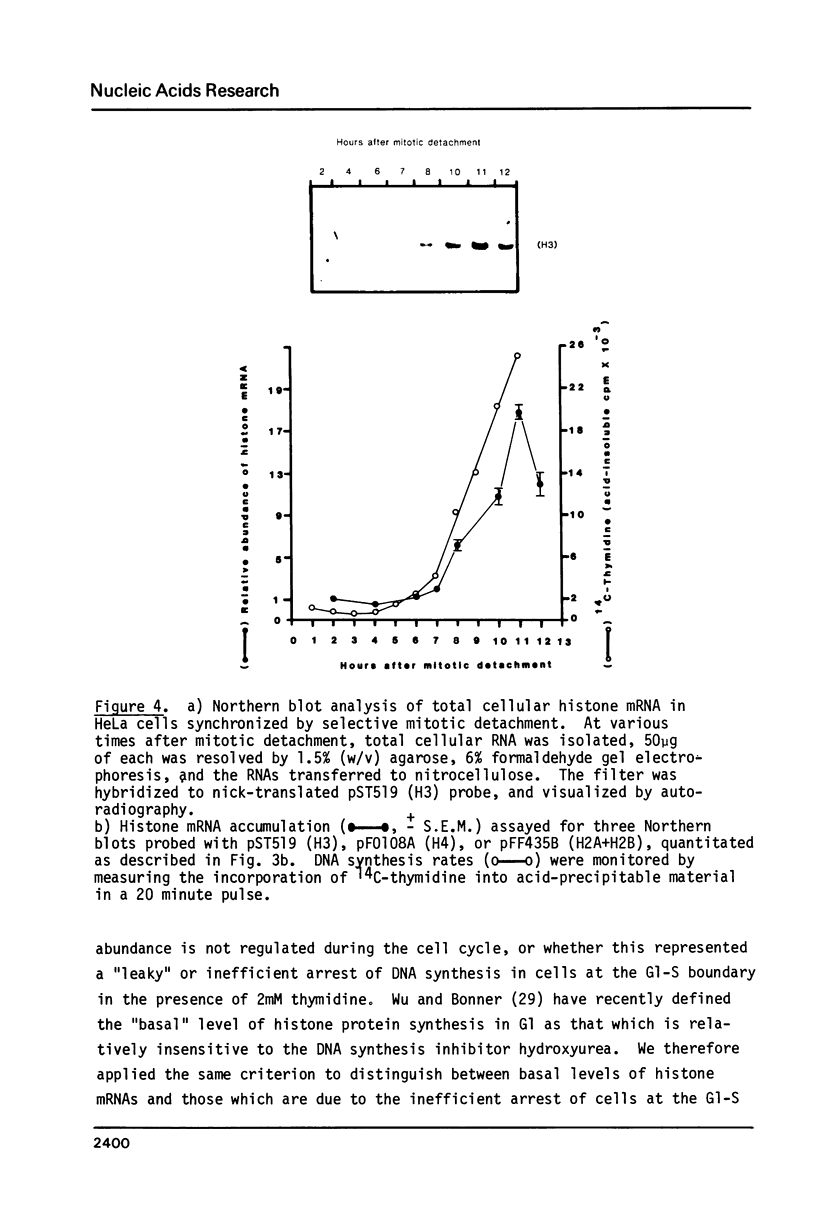
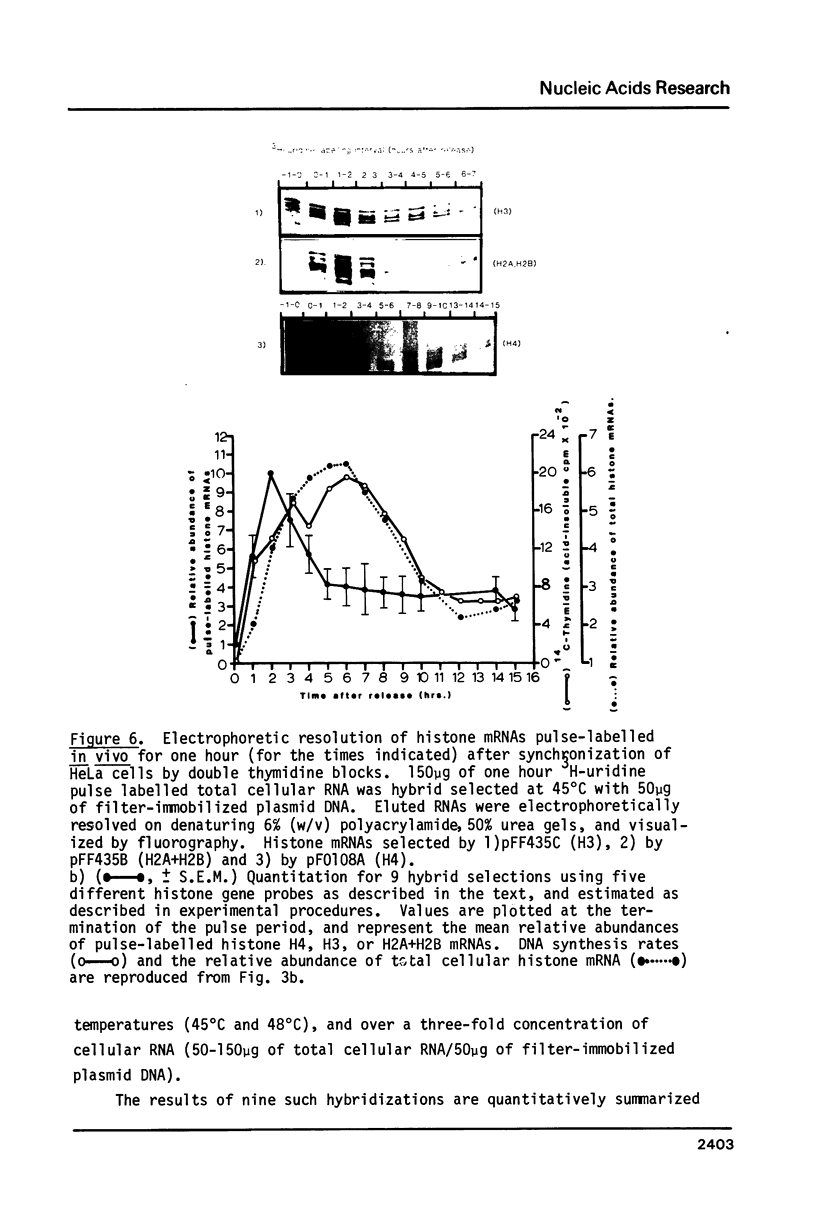
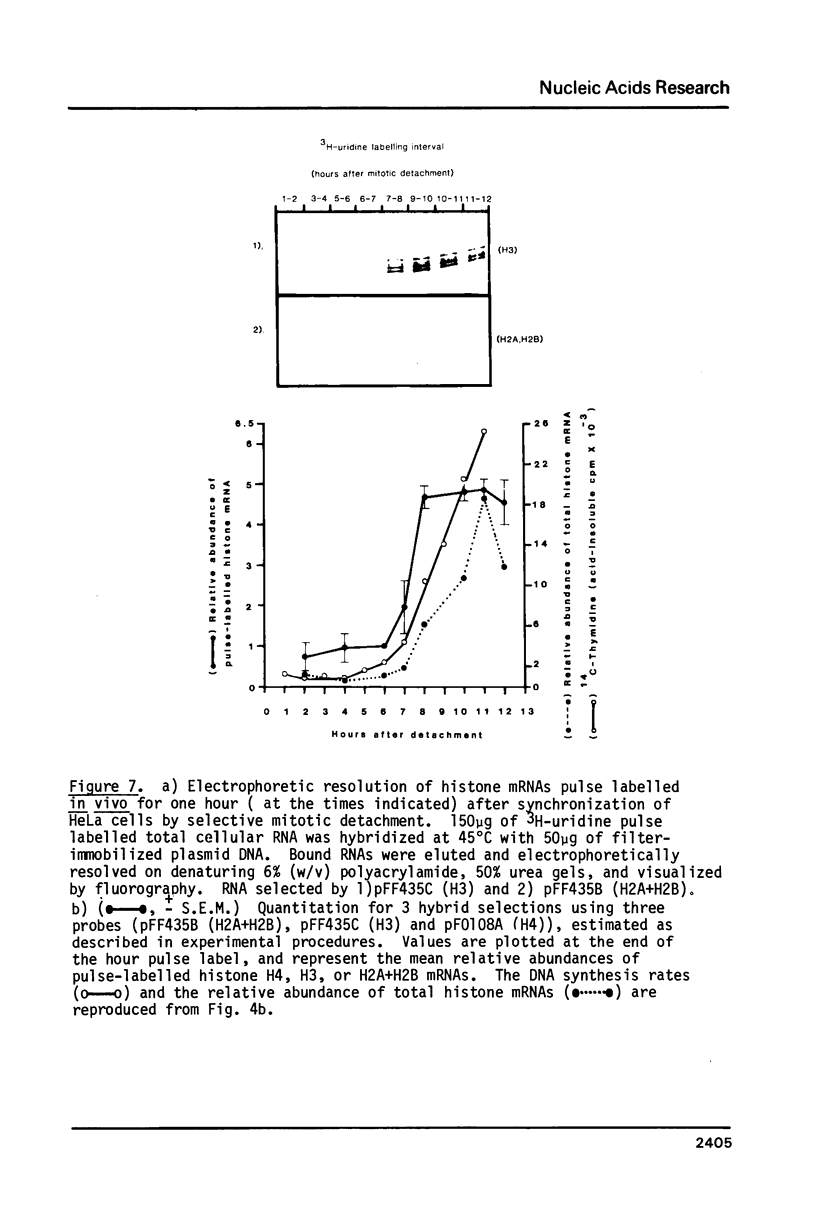
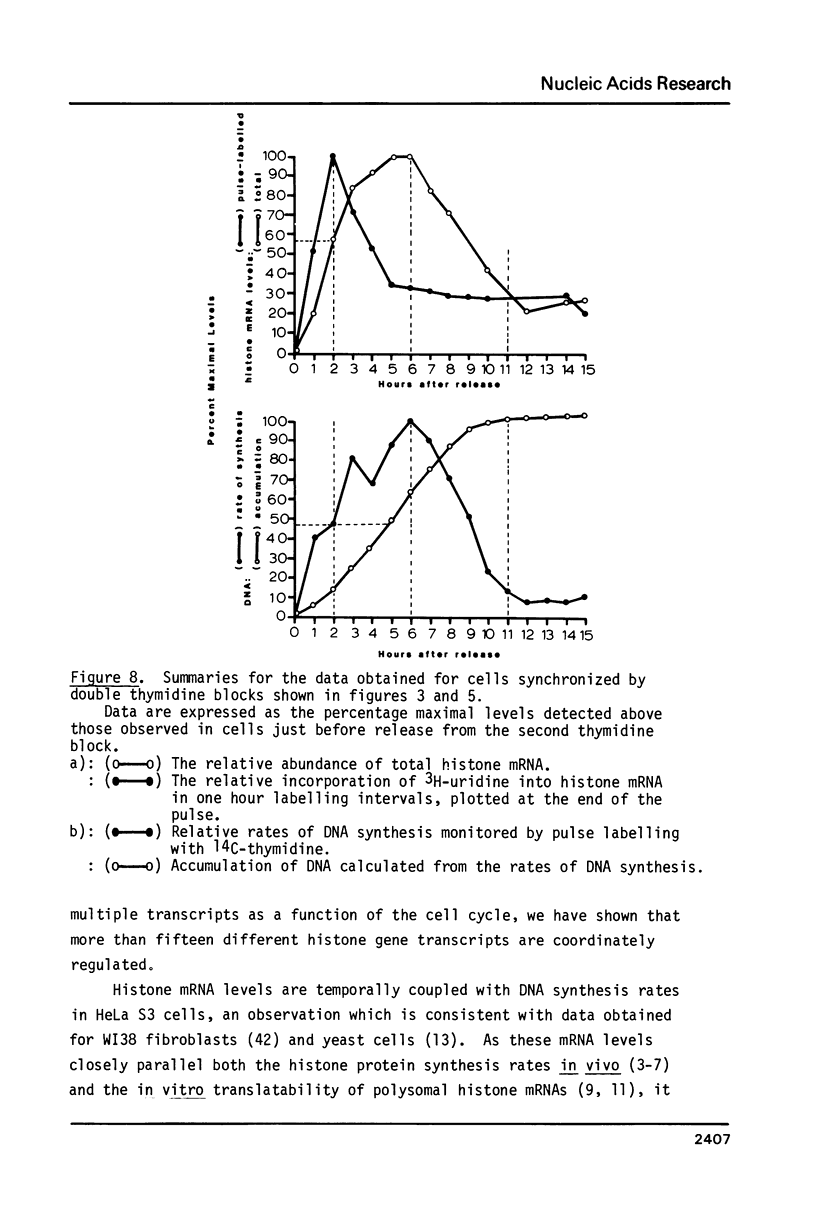
Core histone gene expression in HeLa S3 cells has been examined as a function of the cell cycle using cloned human histone gene probes. Total cellular histone mRNAs were analyzed by Northern blot analysis, and their relative abundance shown to be temporally coupled to DNA synthesis rates in S phase. The in vivo incorporation of 3H-uridine into at least fifteen heterologous histone mRNAs (in one hour pulse intervals at various times in the cell cycle), was monitored by hybrid selection. Hybridized RNAs were eluted and resolved electrophoretically to give both a quantitative and qualitative assay for multiple mRNA species. Maximal incorporation of 3H-uridine into histone mRNAs precedes their maximal accumulation, indicating that transcriptional regulation is predominant in early S phase. The turnover of histone mRNAs in late S occurs in the presence of a reduced apparent transcription rate, indicating that post-transcriptional regulation is predominant in late S. All the detected multiple histone mRNAs are coordinately regulated during the HeLa cell cycle.
Full text
PDF




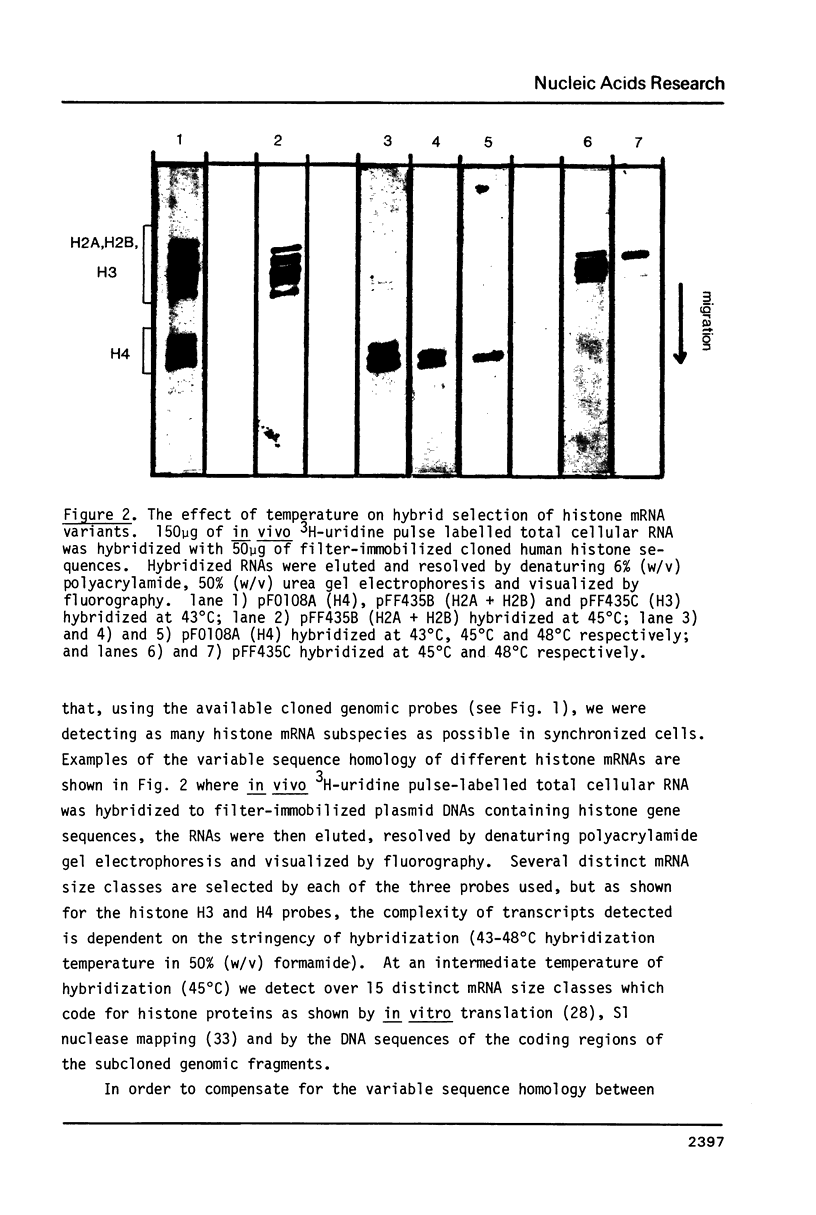








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D., Woodland H. R. Histone synthesis in early amphibian development: histone and DNA syntheses are not co-ordinated. J Mol Biol. 1974 Sep 15;88(2):263–285. doi: 10.1016/0022-2836(74)90481-1. [DOI] [PubMed] [Google Scholar]
- Borun T. W., Gabrielli F., Ajiro K., Zweidler A., Baglioni C. Further evidence of transcriptional and translational control of histone messenger RNA during the HeLa S3 cycle. Cell. 1975 Jan;4(1):59–67. doi: 10.1016/0092-8674(75)90134-8. [DOI] [PubMed] [Google Scholar]
- Borun T. W., Scharff M. D., Robbins E. Rapidly labeled, polyribosome-associated RNA having the properties of histone messenger. Proc Natl Acad Sci U S A. 1967 Nov;58(5):1977–1983. doi: 10.1073/pnas.58.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breindl M., Gallwitz D. Effects of cordycepin, hydroxyurea and cycloheximide on histone mRNA synthesis in synchronized HeLa cells. Mol Biol Rep. 1974 Feb;1(5):263–268. doi: 10.1007/BF00417581. [DOI] [PubMed] [Google Scholar]
- Childs G., Maxson R., Kedes L. H. Histone gene expression during sea urchin embryogenesis: isolation and characterization of early and late messenger RNAs of Strongylocentrotus purpuratus by gene-specific hybridization and template activity. Dev Biol. 1979 Nov;73(1):153–173. doi: 10.1016/0012-1606(79)90144-1. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Delegeane A. M., Lee A. S. Coupling of histone and DNA synthesis in the somatic cell cycle. Science. 1982 Jan 1;215(4528):79–81. doi: 10.1126/science.7053561. [DOI] [PubMed] [Google Scholar]
- Detke S., Lichtler A., Phillips I., Stein J., Stein G. Reassessment of histone gene expression during cell cycle in human cells by using homologous H4 histone cDNA. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4995–4999. doi: 10.1073/pnas.76.10.4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. D., Dodgson J. B. Histone genes are clustered but not tandemly repeated in the chicken genome. Proc Natl Acad Sci U S A. 1981 May;78(5):2856–2860. doi: 10.1073/pnas.78.5.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groppi V. E., Jr, Coffino P. G1 and S phase mammalian cells synthesize histones at equivalent rates. Cell. 1980 Aug;21(1):195–204. doi: 10.1016/0092-8674(80)90127-0. [DOI] [PubMed] [Google Scholar]
- Heintz N., Zernik M., Roeder R. G. The structure of the human histone genes: clustered but not tandemly repeated. Cell. 1981 Jun;24(3):661–668. doi: 10.1016/0092-8674(81)90092-1. [DOI] [PubMed] [Google Scholar]
- Hentschel C. C., Birnstiel M. L. The organization and expression of histone gene families. Cell. 1981 Aug;25(2):301–313. doi: 10.1016/0092-8674(81)90048-9. [DOI] [PubMed] [Google Scholar]
- Hereford L. M., Osley M. A., Ludwig T. R., 2nd, McLaughlin C. S. Cell-cycle regulation of yeast histone mRNA. Cell. 1981 May;24(2):367–375. doi: 10.1016/0092-8674(81)90326-3. [DOI] [PubMed] [Google Scholar]
- Hereford L., Bromley S., Osley M. A. Periodic transcription of yeast histone genes. Cell. 1982 Aug;30(1):305–310. doi: 10.1016/0092-8674(82)90036-8. [DOI] [PubMed] [Google Scholar]
- Hieter P. A., Hendricks M. B., Hemminki K., Weinberg E. S. Histone gene switch in the sea urchin embryo. Identification of late embryonic histone messenger ribonucleic acids and the control of their synthesis. Biochemistry. 1979 Jun 26;18(13):2707–2716. doi: 10.1021/bi00580a004. [DOI] [PubMed] [Google Scholar]
- Isenberg I. Histones. Annu Rev Biochem. 1979;48:159–191. doi: 10.1146/annurev.bi.48.070179.001111. [DOI] [PubMed] [Google Scholar]
- Jacobs-Lorena M., Gabrielli F., Borun T. W., Baglioni C. Studies on the translational control of histone synthesis. I. Translation of histone messenger RNA by heterologous cell-free systems prepared from cells inactive in DNA synthesis. Biochim Biophys Acta. 1973 Oct 12;324(2):275–281. [PubMed] [Google Scholar]
- Kunkel N. S., Weinberg E. S. Histone gene transcripts in the cleavage and mesenchyme blastula embryo of the sea urchin, S. purpuratus. Cell. 1978 Jun;14(2):313–326. doi: 10.1016/0092-8674(78)90117-4. [DOI] [PubMed] [Google Scholar]
- Lichtler A. C., Sierra F., Clark S., Wells J. R., Stein J. L., Stein G. S. Multiple H4 histone mRNAs of HeLa cells are encoded in different genes. Nature. 1982 Jul 8;298(5870):195–198. doi: 10.1038/298195a0. [DOI] [PubMed] [Google Scholar]
- Lifton R. P., Goldberg M. L., Karp R. W., Hogness D. S. The organization of the histone genes in Drosophila melanogaster: functional and evolutionary implications. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1047–1051. doi: 10.1101/sqb.1978.042.01.105. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marashi F., Baumbach L., Rickles R., Sierra F., Stein J. L., Stein G. S. Histone proteins in HeLa S3 cells are synthesized in a cell cycle stage specific manner. Science. 1982 Feb 5;215(4533):683–685. doi: 10.1126/science.7058333. [DOI] [PubMed] [Google Scholar]
- Moll R., Wintersberger E. Synthesis of yeast histones in the cell cycle. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1863–1867. doi: 10.1073/pnas.73.6.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newrock K. M., Cohen L. H., Hendricks M. B., Donnelly R. J., Weinberg E. S. Stage-specific mRNAs coding for subtypes of H2A and H2B histones in the sea urchin embryo. Cell. 1978 Jun;14(2):327–336. doi: 10.1016/0092-8674(78)90118-6. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA turnover in mouse L cells. J Mol Biol. 1973 Oct 5;79(4):681–696. doi: 10.1016/0022-2836(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickles R., Marashi F., Sierra F., Clark S., Wells J., Stein J., Stein G. Analysis of histone gene expression during the cell cycle in HeLa cells by using cloned human histone genes. Proc Natl Acad Sci U S A. 1982 Feb;79(3):749–753. doi: 10.1073/pnas.79.3.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins E., Borun T. W. The cytoplasmic synthesis of histones in hela cells and its temporal relationship to DNA replication. Proc Natl Acad Sci U S A. 1967 Feb;57(2):409–416. doi: 10.1073/pnas.57.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffner W., Kunz G., Daetwyler H., Telford J., Smith H. O., Birnstiel M. L. Genes and spacers of cloned sea urchin histone DNA analyzed by sequencing. Cell. 1978 Jul;14(3):655–671. doi: 10.1016/0092-8674(78)90249-0. [DOI] [PubMed] [Google Scholar]
- Shephard E. A., Phillips I., Davis J., Stein J. L., Stein G. S. Evidence for the resumption of DNA replication prior to histone synthesis in HeLa cells after release from treatment with hydroxyurea. FEBS Lett. 1982 Apr 19;140(2):189–192. doi: 10.1016/0014-5793(82)80891-0. [DOI] [PubMed] [Google Scholar]
- Sierra F., Leza A., Marashi F., Plumb M., Rickles R., Van Dyke T., Clark S., Wells J., Stein G. S., Stein J. L. Human histone genes are interspersed with members of the Alu family and with other transcribed sequences. Biochem Biophys Res Commun. 1982 Jan 29;104(2):785–792. doi: 10.1016/0006-291x(82)90706-9. [DOI] [PubMed] [Google Scholar]
- Sierra F., Lichtler A., Marashi F., Rickles R., Van Dyke T., Clark S., Wells J., Stein G., Stein J. Organization of human histone genes. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1795–1799. doi: 10.1073/pnas.79.6.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Spalding J., Kajiwara K., Mueller G. C. The metabolism of basic proteins in HeLa cell nuclei. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1535–1542. doi: 10.1073/pnas.56.5.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl H., Gallwitz D. Fate of histone messenger RNA in synchronized HeLa cells in the absence of initiation of protein synthesis. Eur J Biochem. 1977 Jan;72(2):385–392. doi: 10.1111/j.1432-1033.1977.tb11263.x. [DOI] [PubMed] [Google Scholar]
- Trostle-Weige P. K., Meistrich M. L., Brock W. A., Nishioka K., Bremer J. W. Isolation and characterization of TH2A, a germ cell-specific variant of histone 2A in rat testis. J Biol Chem. 1982 May 25;257(10):5560–5567. [PubMed] [Google Scholar]
- Tullis R. H., Rubin H. Calcium protects DNase I from proteinase K: a new method for the removal of contaminating RNase from DNase I. Anal Biochem. 1980 Sep 1;107(1):260–264. doi: 10.1016/0003-2697(80)90519-9. [DOI] [PubMed] [Google Scholar]
- Wallis J. W., Hereford L., Grunstein M. Histone H2B genes of yeast encode two different proteins. Cell. 1980 Dec;22(3):799–805. doi: 10.1016/0092-8674(80)90556-5. [DOI] [PubMed] [Google Scholar]
- Wilson M. C., Melli M. Determination of the number of histone genes in human DNA. J Mol Biol. 1977 Mar 5;110(3):511–535. doi: 10.1016/s0022-2836(77)80110-1. [DOI] [PubMed] [Google Scholar]
- Wu R. S., Bonner W. M. Separation of basal histone synthesis from S-phase histone synthesis in dividing cells. Cell. 1981 Dec;27(2 Pt 1):321–330. doi: 10.1016/0092-8674(81)90415-3. [DOI] [PubMed] [Google Scholar]
- Zernik M., Heintz N., Boime I., Roeder R. G. Xenopus laevis histone genes: variant H1 genes are present in different clusters. Cell. 1980 Dec;22(3):807–815. doi: 10.1016/0092-8674(80)90557-7. [DOI] [PubMed] [Google Scholar]
- van Dongen W., de Laaf L., Zaal R., Moorman A., Destrée O. The organization of the histone genes in the genome of Xenopus laevis. Nucleic Acids Res. 1981 May 25;9(10):2297–2311. doi: 10.1093/nar/9.10.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]