Abstract
In contrast to compact myelin, the series of paranodal loops located in the outermost lateral region of myelin is non-compact; the intracellular space is filled by a continuous channel of cytoplasm, the extracellular surfaces between neighboring loops keep a definite distance, but the loop membranes have junctional specializations. Although the proteins that form compact myelin have been well studied, the protein components of paranodal loop membranes are not fully understood. This report describes the biochemical characterization and expression of Opalin as a novel membrane protein in paranodal loops. Mouse Opalin is composed of a short N-terminal extracellular domain (amino acid residues 1–30), a transmembrane domain (residues 31–53), and a long C-terminal intracellular domain (residues 54–143). Opalin is enriched in myelin of the central nervous system, but not that of the peripheral nervous system of mice. Enzymatic deglycosylation showed that myelin Opalin contained N- and O-glycans, and that the O-glycans, at least, had negatively charged sialic acids. We identified two N-glycan sites at Asn-6 and Asn-12 and an O-glycan site at Thr-14 in the extracellular domain. Site-directed mutations at the glycan sites impaired the cell surface localization of Opalin. In addition to the somata and processes of oligodendrocytes, Opalin immunoreactivity was observed in myelinated axons in a spiral fashion, and was concentrated in the paranodal loop region. Immunogold electron microscopy demonstrated that Opalin was localized at particular sites in the paranodal loop membrane. These results suggest a role for highly sialylglycosylated Opalin in an intermembranous function of the myelin paranodal loops in the central nervous system.
The myelin sheath is a multilamellar membrane structure formed by myelin-forming glial cells, oligodendrocytes in the central nervous system (CNS)4 and Schwann cells in the peripheral nervous system (PNS); it tightly wraps and electrically insulates axons with gaps at regular intervals (nodes of Ranvier), between which nerve impulses rapidly jump from node to node (saltatory conduction). The lamellar compaction of myelin is formed by the tight apposition of cytoplasmic face to cytoplasmic face and extracellular face to extracellular face of myelin membranes, which is mediated by two major protein constituents, myelin basic protein (MBP) (1,2) and proteolipid protein (PLP) (3,4), in CNS myelin. By contrast, the outermost lateral myelin regions are composed of a series of non-compact myelin loops, called paranodal loops, which flank the node and closely appose the axolemma via specialized septate-like junctions. The intracellular sides of paranodal loops are filled with channels of cytoplasm, whereas the extracellular faces of paranodal loop membranes maintain a defined distance, but have inter-membranous interactions via junctional specializations, including tight (5–8), gap (9–14), and adherens (15–19) junctions. These junction structures between paranodal loops are thought to be involved in the diffusion barrier between the periaxonal space and the loops, the integrity of the loops, direct communication between the loops, and intracellular signaling between the loops (20–23). However, the protein composition and function of paranodal loop membranes are not yet fully understood, especially in CNS myelin.
We recently identified a mouse gene called Opalin (oligodendrocytic paranodal loop protein), whose expression is specific to the brain, predominantly localized in many white matter-rich regions and up-regulated during the postnatal developmental stages (24), in the course of the Cerebellar Development Transcriptome Database (CDT-DB) project. Phylogenetic genome analysis showed that Opalin is a new class of genes that are found across mammalian species, but not in non-mammalian species with myelinated axons (24). Porcine (referred to as gene 83.5 (tmp83.5) (25)) and human (referred to as HTMP10 in Ref. 26) homologues of Opalin were reported to encode a brain-specific putative transmembrane protein. GeneChip microarray analysis showed that the rat homologue is up-regulated during the differentiation of oligodendrocyte cultures (27). Our previous study also indicated that Opalin gene expression specific to oligodendrocytes is regulated, at least in part, by intronic enhancer elements including the binding sites for myelin transcription factor 1 and cAMP-response element-binding protein (24). These previous studies suggested that Opalin protein plays a unique role in mammalian myelin. However, the structure and function of Opalin protein and its expression in the brain have been largely unknown.
In this study, we analyzed the structural features, developmental expression, and cellular localization of mouse Opalin. We showed that the level of Opalin was drastically increased at the postnatal myelination stage and that it predominantly distributes throughout the myelin-rich regions of the CNS, but not the PNS of mice. Opalin is a type 1 transmembrane protein consisting of a short N-terminal extracellular domain and a long C-terminal cytoplasmic domain. The native myelin Opalin contained large glycan moieties in the N-terminal domain. We identified two Asn residues for N-glycosylation and one Thr residue for O-glycosylation by enzymatic deglycosylation and site-directed amino acid substitution experiments. Interestingly, the O-glycans, at least, contained negatively charged sialic acids. Heterologous expression of mutated Opalin lacking these glycans suggested that glycosylation is required for the specific cell surface localization of Opalin. Moreover, we demonstrated that Opalin is specifically localized to the membranes of paranodal and inner loops. Our study suggests a role of the sialylglyco-transmembrane protein Opalin in an inter-membranous function of the paranodal loops of mammalian CNS myelin.
EXPERIMENTAL PROCEDURES
Animals—Mice (ICR and C57BL/6J), rabbits (New Zealand White), and rats (Wistar) were purchased from Nihon SLC (Hamamatsu, Japan) and used according to protocols approved by the Animal Care and Use Committee of RIKEN and the Iwate Medical University.
Cloning and Sequencing of Opalin cDNA—The first clone (clone ID number CD02046, see the CDT-DB; DDBJ/GenBank™/EMBL accession number BP428301) was isolated by fluorescent differential display analysis of the cerebellar transcriptome of mice during the postnatal developmental stages, as described in a previous study (28). Full-length Opalin cDNAs were cloned by screening full-length cDNA libraries, generated from poly(A)+ mRNAs isolated from mouse cerebella at P21 using the oligo-capping method (29), for sequence homology to clone CD02046. The full-length cDNA sequence was determined using the dideoxynucleotide termination method with a DNA sequencer (ABI 3730xl, Applied Biosystems Inc.). Opalin cDNA was cloned into the mammalian expression vectors pcDNA3 (Invitrogen) and pEGFP-N3 (Clontech Laboratories, Inc.) to generate site-directed glycosylation mutants and C-terminal epidermal growth factor protein fusion constructs, respectively. Venus, a YFP derivative (30), was used to study the expression of Opalin in primary oligodendrocyte cultures. Site-directed mutagenesis of Opalin was performed by means of a two-step PCR, as described previously (31).
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)—cDNAs were prepared from total RNAs obtained from mouse cerebella at embryonic day 18 and postnatal days (P)0, P3, P7, P12, P21, and P56, to analyze expression patterns during postnatal cerebellar development, and from whole brain, thymus, lung, heart, liver, spleen, kidney, and testis at P21, to analyze tissue distribution patterns. PCRs were performed in a PCR system (GeneAmp PCR system 9700, ABI). The forward and reverse primers used for the amplification of each gene were: Opalin, 5′-CTGCCTCTCACTCAACATCA-3′ and 5′-GCTGGATCAAAGTAAACAGC-3′, respectively; PLP/DM20, 5′-GGCAGATCTTTGGCGACTAC-3′ and 5′-AGCATTCCATGGGAGAACAC-3′, respectively; MBP, 5′-CTTCGCCACAATAACGTGAG-3′ and 5′-TGCTTCTGTCCAGCCATACT-3′, respectively; myelin oligodendrocyte glycoprotein (MOG), 5′-GCAGGTCTCTGTAGGCCTTG-3′ and 5′-CAGTTGAGGGAGGGAAATCA-3′, respectively; and glyceraldehyde-3-phosphate dehydrogenase, 5′-GCCATCAACGACCCCTTCATTGACCTC-3′ and 5′-GCCATGTAGGCCATGAGGTCCACCAC-3′, respectively.
In Situ Hybridization—In situ hybridization brain histochemistry was performed as described in previous studies (32, 33). Paraffin sections (6 μm) through P21 mouse brains were hybridized with 2 μg/ml Opalin riboprobes labeled with digoxigenin-11-UTP (Roche Diagnostics) at 60 °C overnight, and hybridization signals were detected using a digoxygenin detection kit (Roche Diagnostics).
Antibodies—An anti-mouse Opalin antibody was raised in female New Zealand White rabbits (Nihon-SLC) by subcutaneous injection of an antigenic synthetic peptide consisting of the C-terminal 16 amino acid residues (from amino acid 128 to 143, ERRRGLWWLVPSLSLE) of Opalin following an N-terminal Cys residue, which was used to conjugate this peptide to keyhole limpet hemocyanin (catalog number H7017, Sigma). The Opalin antibody was purified by affinity chromatography on an antigenic peptide-coupled column (HiTrap NHS-activated HP column, catalog number 17-0716-01, GE Healthcare), and used at a concentration of 1 μg/ml. The specificity of the anti-Opalin antibody was proven by a preabsorption test with the antigenic peptide, specific recognition of expressed recombinant proteins, and similarity in the developmental, cellular, and tissue distribution patterns between immunoreactive protein and mRNA (for example, Western blot patterns versus RT-PCR patterns; immunohistochemistry patterns versus in situ hybridization patterns). The following antibodies were also used: anti-calbindin D-28K (1:1000) (mouse monoclonal CB-955, catalog number C9848), anti-neurofilament 160 (1:500) (mouse monoclonal NN18, catalog number N-5264), and anti-pan voltage-dependent Na+ channel (1:250) (mouse monoclonal K58/35, catalog number S8809) from Sigma; anti-myelin-associated glycoprotein (MAG) (1:250) (mouse monoclonal, catalog number MAB1567), anti-O4 (1:200) (mouse monoclonal, catalog number MAB345), and anti-ZO-1 (1:250) (mouse monoclonal, catalog number MAB1520) from Chemicon (Temecula, CA); anti-MBP (1:1000) (rabbit polyclonal, catalog number 16141) from IBL (Gunma, Japan); anti-E-cadherin (1:250) (mouse monoclonal, catalog number C20820) from Transduction Laboratories (Lexington, KY); and anti-caspr (1:250) (mouse monoclonal) was a kind gift from Dr. E. Peles (34). The secondary antibodies used in Western blot analyses were horseradish peroxidase (HRP)-conjugated anti-rabbit IgG (H+L) (1:2000) (catalog number NA9340, GE Healthcare UK Ltd.) and HRP-conjugated anti-mouse IgG (H+L) (1:2000) (catalog number NA9310, GE Healthcare UK Ltd.). The secondary antibodies used in immunohistochemical analyses were Alexa Fluor 488-conjugated anti-rabbit antibody and Alexa Fluor 594-conjugated anti-mouse IgG (H+L) antibody (1:1000) (catalog numbers A11029 and A11005, respectively; Molecular Probes).
Subcellular Fractionation—Under deep anesthesia with diethyl ether, brains were dissected from the mice (ICR, Nihon-SLC) and homogenized in an ice-cold Teflon-glass Potter homogenizer containing 9 volumes (w/v) of homogenizing buffer (0.32 m sucrose, 5 mm Tris-HCl, pH 7.4, and protease inhibitor mixture (1 mm phenylmethylsulfonyl fluoride, 10 μm pepstatin A, 10 μm leupeptin)) as described previously (35). The homogenates were centrifuged at 1,000 × g for 10 min at 4 °C to obtain precipitate fraction 1 (ppt1). The supernatants were centrifuged at 105,000 × g for 1 h at 4 °C to obtain precipitate fractions 2 + 3 (ppt2 + 3), and the supernatant (sup3) was used as the cytosolic fraction. ppt1 and ppt2 + 3 were re-suspended in suspension buffer (20 mm Tris-HCl, pH 7.4, 150 mm NaCl, 1 mm dithiothreitol, and protease inhibitor mixture), and the protein concentration was measured using a BCA protein assay kit (Pierce).
The crude myelin fraction and pure myelin fraction were prepared essentially as described (36). Briefly, mouse brains were homogenized with the Dounce homogenizer in 14 volumes (w/v) of ice-cold 0.32 m sucrose, 5 mm Tris-HCl (pH 7.4) and protease inhibitor mixture (1× Complete, EDTA-free; Roche Diagnostics). The homogenates (15 ml) were layered over 20 ml of 0.85 m sucrose and 5 mm Tris-HCl (pH 7.4) in a Beckman Coulter SW28 rotor and centrifuged for 30 min at 75,000 × g at 4 °C. The crude myelin layer at the interface of the two sucrose solution was collected (∼5 ml), resuspended with ice-cold deionized water to a final volume of 30 ml, and recentrifuged in a SW28 rotor at 75,000 × g for 15 min at 4 °C. The pellet was resuspended and homogenized with the Dounce homogenizer in 40 ml of ice-cold deionized water, and centrifuged at 12,000 × g for 13 min at 4 °C. The resultant pellet was again homogenized and recentrifuged at 12,000 × g as described above. The crude myelin membrane pellet was homogenized in 15 ml of ice-cold 0.32 m sucrose and 5 mm Tris-HCl (pH 7.4), layered over 20 ml of 0.85 m sucrose and 5 mm Tris-HCl (pH 7.4), and centrifuged in a SW28 rotor at 75,000 × g as described above. The resultant pellet was again homogenized and recentrifuged in a SW28 rotor as described above. The pure myelin membrane pellet was resuspended in 10 mm Tris-HCl (pH 7.4) and protease inhibitor mixture.
Western Blot Analysis—Protein lysates were separated by SDS-PAGE and electroblotted onto nitrocellulose filter membranes (Hybond-ECL, catalog number RPN2020D, GE Healthcare) as described previously (35). The blots were processed at room temperature by immersing for 1 h in blocking buffer consisting of 5% (w/v) skim milk (Snow Brand, Sapporo, Japan) in 1× PBS containing 0.1% (v/v) Tween 20 (PBS-T), then exposing them to primary antibody in PBS-T for 1 h, and, finally, exposing them to HRP-conjugated secondary antibody for 1 h. After washing with PBS-T, bound antibody was detected using the ECL Plus Western blotting detection reagent (catalog number RPN2106, GE Healthcare) and x-ray film (MXJB Film, catalog number 864–8651, Eastman Kodak).
Immunohistochemistry—C57BL/6J mice (Nihon SLC) anesthetized with diethyl ether were transcardially perfused with PBS and then with 4% paraformaldehyde in PBS. Their brains were dissected out, postfixed in 4% paraformaldehyde at 4 °C for 1 h, and cryoprotected by immersion in 15% sucrose in PBS overnight at 4 °C. After embedding in Tissue-Tek OCT compound (Sakura Finetechnical, Tokyo, Japan), the brains were frozen in dry ice powder and cut into 10–16 μm sagittal sections at –18 °C using a cryostat (CM1850; Leica Microsystems). The sections were then air-dried for 1 h, rinsed three times in PBS, and treated with methanol at –20 °C for 20 min followed by three washes with PBS at 4 °C for 10 min each. After blocking with 10% normal donkey serum (catalog number D9663, Sigma) in 0.2% Triton X-100 and PBS, the sections were reacted with primary antibody at 4 °C overnight, then rinsed in 0.2% Triton and PBS, and allowed to react with Alexa Fluor- or HRP-conjugated secondary antibody in 5% serum, 0.2% Triton, and PBS at room temperature for 1 h, before being rinsed again in PBS. To visualize actin filaments, sections were also stained with Alexa Fluor 647-phalloidin (1:50 dilution, catalog number A22287, Invitrogen). Immunostained sections were mounted using Vectorshield mounting medium (Vector, Burlingame, CA) and examined using an epifluorescence microscope (Eclipse E800; Nikon) equipped with a cooled CCD camera (Spot; Diagnostic Instruments Inc., Sterling Heights, MI), or a confocal laser microscope (LSM 510 META; Carl Zeiss, Oberkochen, Germany). Digital images were processed using Adobe Photoshop 6.0 software.
Electron Microscopy—Immunogold electron microscopy of sections stained with anti-Opalin antibody was carried out essentially as described previously (37). Briefly, the optic nerves of two-week-old ICR mice (Nihon SLC) were fixed with PLP fixative (4% paraformaldehyde, 10 mm sodium periodate, 7.5 mm lysine-HCl, 100 mm phosphate buffer, pH 7.4) and then infused with 20% polyvinylpyrrolidone, 1.8 m sucrose in 0.1 m phosphate buffer (pH 7 h at room temperature, then overnight at 4 °C. The infused specimens were frozen at –185 °C in a rapid-freezing apparatus (KF-80, Leica) and cut on an ultramicrotome (UCT or UC6, Leica) equipped with a cryo-attachment (FCS or FC6, Leica). The resulting ultrathin sections were mounted on carbon-coated grids according to methods described previously (38); after immunoreacting sections with 1 μg/ml anti-Opalin antibody for 24 h at 4 °C, they were incubated with colloidal gold (5 nm)-conjugated secondary anti-rabbit IgG (GE Healthcare) for 2 h at room temperature (37). Immunogold-labeled sections were examined using an electron microscope (H-7100 or H-7650, Hitachi Ltd., Tokyo, Japan).
Enzymatic Deglycosylation—Protein samples were incubated in 1% SDS, 144 mm 2-mercaptoethanol, and protease inhibitor mixture at 37 °C for 30 min before treatment with glycosidase(s) in 20 mm sodium phosphate buffer (pH 7.2), 0.5% Non-idet P-40, 20 mm EDTA, 1 mm 2-mercaptoethanol, and the protease inhibitor mixture as follows. N-Glycosidase F (from Flavobacterium meningosepticum), O-Glycosidase (from Diplococcus pneumoniae), and neuraminidase (from Arthrobacter ureafaciens) were purchased from Roche Diagnostics GmbH. Deglycosylation was performed essentially according to the protocol provided by the manufacturer. N-Linked glycans were removed by treatment with 30 units/ml N-glycosidase F overnight at 37 °C. O-Linked glycans were removed by incubation with 20 milliunits/ml O-glycosidase, overnight at 37 °C. Sialic acids were removed by incubation with 0.4 units/ml neuraminidase overnight at 37 °C. To remove all of these glycans, proteins were treated with 30 units/ml N-glycosidase F, 20 milliunits/ml O-glycosidase, and 0.4 units/ml neuraminidase overnight at 37 °C. Each of these reactions was performed in sodium phosphate buffer (20 mm, pH 7.2).
In Vitro Transcription/Translation—In vitro transcription/translation of Opalin was performed using TnT rabbit reticulocyte lysate in the presence or absence of canine pancreatic microsomes (Promega) according to the protocol provided by the manufacturer.
Cell Culture and Transfection—Transfection of COS7, HeLa, and Madin-Darby canine kidney cells was carried out using Lipofectoamine 2000 reagent (Invitrogen), essentially as described in a previous report (32). Primary cultures of oligodendrocytes were prepared from embryonic rat cerebra, essentially as described previously (39).
RESULTS
Opalin Expression Is Up-regulated in White Matter-rich Regions during Postnatal Mouse Brain Development—The cloned mouse Opalin full-length cDNA comprises 892 nucleotide residues with an open reading frame of 143 amino acids (aa) (calculated molecular mass = ∼15,833 Da, pI = 4.88) (Fig. 1A). The predicted Opalin protein has a transmembrane domain (31–53 aa) flanked by a short N-terminal domain (1–30 aa) with two N-linked glycosylation consensus sites (Asn-6 and Asn-12), and a long C-terminal domain (54–143 aa) with six consensus sites for protein phosphorylation (Fig. 1A). A DNA data base search indicated that Opalin is identical to the gene-encoding transmembrane protein 10 (Tmem10), and that homologues are present only in mammals (24). The amino acid sequence of Opalin in all eight mammalian species analyzed (mouse, rat, pig, cattle, dog, orangutan, chimpanzee, and human) was evolutionarily well conserved, especially the two N-linked glycosylation sites and the putative transmembrane domain, although there was a mouse-specific insertion (Asp-66 in mouse Opalin) and a primate-specific deletion (Ser/Gly-21 in non-primate Opalin) (supplemental Fig. S1).
FIGURE 1.
Structure of mouse Opalin protein and mRNA expression. A, schematic representation of mouse Opalin (1–143 aa). The predicted transmembrane domain (31–53 aa) is shown in the closed box. Two N-linked glycosylation sites at Asn-6 (N6) and Asn-12 (N12) are indicated by open triangles and an O-linked glycosylation site at Thr-14 (T14) is indicated by a closed triangle. Six predicted protein phosphorylation sites are indicated by vertical bars with single-letter amino acid positions: Thr-59 (T59) (cAMP-dependent protein kinase and protein kinase C), Tyr-78 (Y78) (insulin receptor), S90 (mitogen-activated protein kinase, cdc2, and cyclin-dependent kinase), Thr-92 (T92) (protein kinase A), Tyr-106 (Y106) (epidermal growth factor receptor and INSR), and Tyr-121 (Y121) (spleen tyrosine kinase and INSR). Three deletion mutants (dC, dIC, and dN) contained parts of the full-length structure (FL) (see supplemental Fig. S2). dC has a deletion of the C-terminal 38 aa (and thus comprises residues 1 to 115), dN has a deletion of the N-terminal 26 (and thus comprises residues 27 to 143), and dIC has a deletion of the intracellular domain from 55 to 115 aa (and thus comprises residues 1 to 54 and 116 to 143). B, RT-PCR analysis of developmental time series expression. Expression of Opalin, PLP/DM20 (two splice variants, upper band; PLP, lower band, DM20), MBP, MOG, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNAs during the postnatal stages of mouse cerebellar development are shown. Opalin mRNA expression is up-regulated in the postnatal mouse cerebellum, similar to the expression of myelin-related genes. C, RT-PCR analysis of tissue specificity. Expression of Opalin mRNA in the brain, thymus, lung, heart, liver, spleen, kidney, and testis of mice at P21 is shown. Opalin mRNA expression is brain specific. D, in situ hybridization analysis of spatial cellular expression. Expression of Opalin mRNAs in sagittal brain sections of P21 mice is shown. Opalin mRNA is concentrated in white matter-rich regions, including the optic chiasm, corpus callosum, anterior commissure, and the white matter layer of the cerebellar cortex. ox, optic chiasm; cc, corpus callosum; df, dorsal fornix; ac, anterior commissure; ML, molecular layer; GL, granular layer; WM, white matter.
Developmental expression analysis by RT-PCR showed that the level of Opalin mRNA increased steeply after the first postnatal week in mouse cerebellum, comparable with the expression profile of the myelin-related genes PLP/DM20, MBP, and myelin oligodendrocyte glycoprotein (MOG) (Fig. 1B). Tissue expression analysis by RT-PCR indicated that Opalin mRNA was selectively expressed in the mouse brain at P21, and not expressed in any of the other tissues tested (thymus, lung, heart, liver, spleen, kidney, and testis) (Fig. 1C). In situ hybridization analysis revealed that Opalin mRNA was predominantly expressed in white matter-rich regions of P21 mouse brains, including the optic chiasm, corpus callosum, fornix, anterior commissure, and cerebellar white matter layer (Fig. 1D). The cellular localization pattern of mouse Opalin mRNA in P21 brains was similar to that in P56 brains registered in the Allen Brain Atlas in situ hybridization data base.
Opalin Is Specifically Localized in CNS Myelin—To examine the expression of mouse Opalin protein in the nervous system, we generated an in situ hybridization specificity, see “Experimental Procedures”) and performed a subcellular fractionation assay followed by immunoblotting. In cerebellar fractions prepared from postnatal mice at P1, 7, 14, and 21, Opalin was abundantly detected in the precipitate microsomal fractions (ppt2 + 3) of P14 and P21 cerebella as multiple major bands with approximate molecular masses between 33.5 and 38.5 kDa (Fig. 2A). Crude and pure myelin fractions, both of which were rich in MBP, contained more Opalin than the ppt2 + 3 fractions (Fig. 2B). Moreover, Opalin was present in the brain and spinal cord, but was undetectable in the sciatic nerve (Fig. 2C). Thus, mouse Opalin is a myelin protein expressed in the CNS, but not in the PNS.
FIGURE 2.
Western blot analysis of Opalin protein in subcellular fractions of the mouse nervous system. A, Western blot analysis of subcellular fractions from postnatal mouse cerebellum at P1, P7, P14, and P21 with the anti-Opalin antibody. Opalin, with a molecular mass of ∼33–36 kDa, is detected in precipitated membrane-rich fractions between P14 and P21. ppt1, precipitate nuclear fraction; ppt2 + 3, precipitate microsomal fraction; sup3, soluble cytosolic fraction. Molecular mass standards are indicated on the left. B, Western blot analysis of myelin fractions with the anti-Opalin antibody. Equal amounts of protein samples were loaded in each lane. Opalin was more enriched in the crude and pure myelin fractions than in ppt2 + 3, similar to the myelin marker MBP (bottom panel). C, Western blot analysis of CNS and PNS myelin fractions with the anti-Opalin antibody. Opalin protein is concentrated in CNS myelin fractions (from whole brain or spinal cords) and not in PNS myelin fractions (from sciatic nerves), whereas MBP is abundant in both fractions (bottom panel). The anti-MBP antibody reacts with multiple splice variants. Molecular mass standards are indicated on the left.
We next analyzed the cellular distribution of Opalin in the CNS. Opalin immunoreactivity (Fig. 3, A–E) was observed in many myelin-rich regions throughout the mouse brain (at P21), including the corpus callosum, anterior commissure, lateral olfactory tract, optic tract, internal capsule, fimbria of the hippocampus, habenular commissure, fasciculus retroflexus, mammillothalamic tract, cerebellar peduncle, and olivocerebellar tract, as well as the white matter of the cerebral cortex, caudate-putamen, nucleus accumbens, globus pallidus, thalamus, cerebellum, brain stem, and spinal cord; its distribution was almost the same as that of MBP immunoreactivity (Fig. 3, F–J).
FIGURE 3.
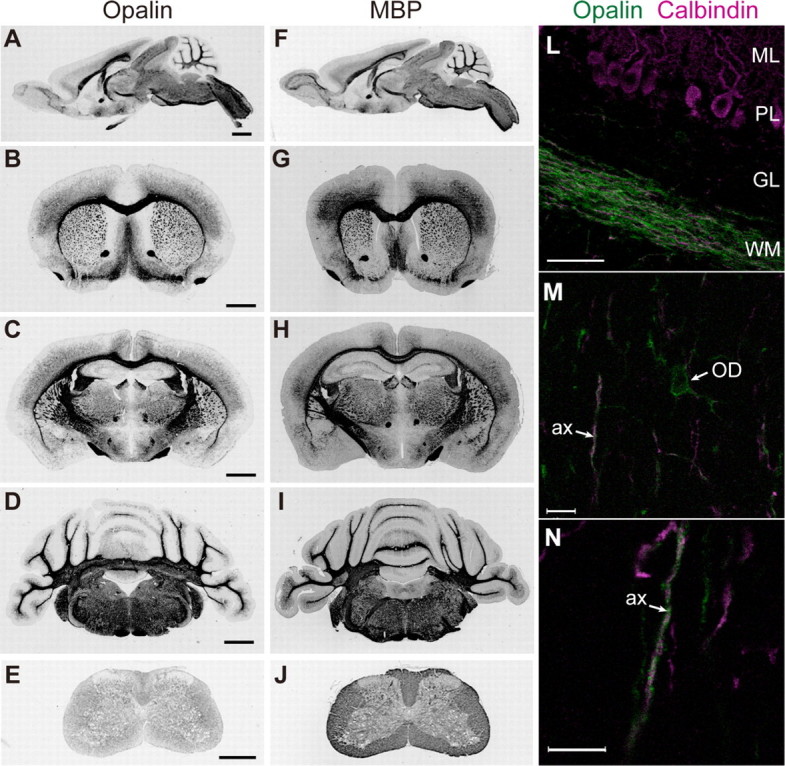
Immunohistochemical localization analysis of Opalin in mouse brains. A–J, sagittal (A and F) and coronal (B–E and G–J) sections of P21 mouse brains were immunoreacted with anti-Opalin antibody (A–E) and anti-MBP antibody (F–J) and detected by HRP-DAB stain. B and G, regions including the frontal cortex and caudate-putamen; C and H, regions including the cerebral cortex, hippocampus and thalamus; D and I, regions including the cerebellum and brain stem; E and J, spinal cords. Opalin is predominantly localized in white matter-rich regions. L–N, sagittal sections of P14 mouse cerebellum were double-immunostained with anti-Opalin (green) and anti-calbindin (magenta) antibodies and were observed by fluorescent confocal microscopy. M and N, higher magnifications of the granular layer of the cerebellum. Opalin immunoreactivity is found in soma and process of oligodendrocytes (M) and is also localized in myelinating axons in a spiral fashion (N). ML, molecular layer; PL, Purkinje cell layer; GL, granular layer; WM, white matter; OD, oligodendrocyte cell body; ax, axon of Purkinje cells. Scale bars, 1 mm in A–D; 0.5 mm in E; 50 μm in L; 10 μm in M and N.
Immunohistochemical analysis showed a fibrous pattern of Opalin immunoreactivity densely localized within the white matter of the P21 mouse cerebellum (Fig. 3L). In the granular layer, where oligodendrocytes are more sparsely distributed than in the white matter, Opalin immunoreactivity was observed in the cell bodies and processes of oligodendrocytes (Fig. 3M) and around the calbindin-immunopositive axons of Purkinje cells in a spiral-like fashion (Fig. 3, M and N), indicating that Opalin is localized to myelin enwrapping the axons.
Opalin Is Highly Glycosylated and Sialylated—Western blotting analysis of Opalin in myelin fractions revealed a broad banding pattern within ∼33.5–38.5 kDa, as shown in Figs. 2, A–C, and 4A (lane 1) despite the fact that its calculated molecular mass based on the primary sequence is ∼16 kDa. Opalin contains two predicted N-glycan sites at Asn-6 and Asn-12 (Fig. 1A). These results suggested a post-translational modification of myelin Opalin. To determine whether the endogenous myelin Opalin contained N-glycans, O-glycans, and/or sialic acids, we performed an enzymatic deglycosylation analysis of myelin fractions using N-glycosidase F, O-glycosidase, neuraminidase, or combinations of these enzymes, followed by immunoblotting with the anti-Opalin antibody (Fig. 4A). After deglycosylation with O-glycosidase (lane 2), neuraminidase (lane 4), and N-glycosidase (lane 6), the mass of the Opalin-immunoreactive band notably decreased in comparison with that in untreated myelin fractions (lane 1), indicating that myelin Opalin contains O-linked and N-linked glycan and sialic acid moieties. The Opalin band in samples triple-digested with N-glycosidase F, O-glycosidase, and neuraminidase migrated faster, to an apparent molecular mass of 18.5–19.5 kDa (lane 8). This electrophoretic mobility after triple digestion was close to that deduced from the primary sequence, but still slower, possibly due to the effect(s) of other modifications (such as linkage to glucosaminoglycan chains or phosphorylation) and/or a general feature commonly observed in transmembrane proteins containing hydrophobic membrane-spanning domains. Opalin glycosylation was also supported by an in vitro transcription-translation assay using canine pancreatic microsomes, which are capable of processing post-translational modifications, including glycosylation (data not shown).
FIGURE 4.

Enzymatic deglycosylation analysis of myelin Opalin and identification of N- and O-glycosylation sites at Asn-6, Asn-12, and Thr-14 residues of Opalin by site-directed amino acid substitutions. A, enzymatic deglycosylation of myelin fractions prepared from P23 mouse brains with N-glycosidase F (N-Glyc), O-glycosidase (O-Glyc), and neuraminidase (NA) followed by immunoblotting with anti-Opalin antibody. Faster mobility on SDS-PAGE upon deglycosylation treatment indicates the glycosylation of myelin Opalin; Opalin contains both N-linked and O-linked glycans, both types of which are sialylated. B, Western blot analysis of protein lysates from COS7 cells transfected with the Opalin wild-type (wt), Asn-6 to Gln substitution mutant (N6Q), Asn-12 to Gln substitution mutant (N12Q), Thr-12 to Ala substitution mutant (T14A), N6Q/N12Q double mutant, N6Q/N12Q/T14Q triple mutant, and vector alone. Myelin, myelin fraction of mouse brains. Opalin is N-glycosylated at Asn-6 and Asn-12 and O-glycosylated at Thr-14. The N6Q/N12Q/T14A mutant migrates along with N6Q/N12Q on SDS-PAGE, suggesting that the N6Q/N12Q mutation affects the O-glycosylation pattern. Enzymatic deglycosylation experiments indicated that the N6Q/N12Q/T14A mutant expressed in COS7 cells still contains glycans at other site(s) (supplemental Fig. S2). Molecular mass standards (in kDa) are shown on the left.
After double digestion of myelin fractions with O-glycosidase and neuraminidase (lane 3), the Opalin band migrated faster (26.0–30.5 kDa) than after single digestion with either O-glycosidase (lane 2; 32.0–36.5 kDa) or neuraminidase (lane 4; 27.0–31.5 kDa). The O-glycosidase used is known to cleave O-linked glycans, but not sialylated O-glycans. Thus, these results indicated that Opalin contains O-glycans attached to sialic acids.
It was noteworthy that digestion with N-glycosidase generated multiple Opalin immunoreactive bands (major band at 20.5–22.0 kDa and minor bands at 23.0 and 24.0–26.5 kDa) (lane 6). In addition, double digestion with N-glycosidase and neuraminidase generated Opalin bands at 19.5–22.0 kDa (lane 5), whereas double digestion with N-glycosidase and O-glycosidase showed mainly three bands (lane 7) including the 18.5–19.5 kDa band that was similar in size to the triple-digested Opalin band (lane 8). Although it remains elusive whether N-glycans have sialic acids, these digestion patterns indicated that O-glycans are heterogeneous in sialylation; that is, myelin Opalin has O-glycans with and without sialic acids. Moreover, these multiple binding patterns suggested heterogeneity in the number and/or chain length of these N- and O-glycans.
Opalin Expressed in COS7 Cells Has Two N-Glycan Chains at Asn-6 and Asn-12 and a Sialylated O-Glycan Chain at Thr-14—To identify the N-linked glycosylation sites in Opalin, we constructed three mutants carrying amino acid substitutions of the consensus Asn to Gln at Asn-6 and Asn-12 residues (Fig. 1A), singly or in combination, and named them single mutant N6Q, single mutant N12Q, and double mutant N6Q/N12Q (Fig. 4B). Wild-type Opalin exogenously expressed in COS7 cells (lane 2) was hyperglycosylated in comparison with myelin Opalin (lane 1). This finding is probably related to the evidence that different cell types have different N-glycosylation patterns, including the degree and linkage of sialylation (40). In this heterologous expression system, the single mutants N6Q (lane 3) and N12Q (lane 4) migrated faster in an SDS-PAGE system than wild-type Opalin (lane 2), and the double-mutant N6Q/N12Q (lane 8) migrated faster than the single mutants. These results indicated that Opalin is a type I transmembrane protein and is N-glycosylated at both Asn-6 and Asn-12 residues of the N-terminal extracellular domain.
Enzymatic deglycosylation experiments showed that Opalin also contains small O-linked oligosaccharides (Fig. 4A, lanes 2–4). We substituted Ala for the Thr-14, Ser-15, and Thr-20 residues, potential sites for mucin-type O-glycosylation (T14A, S15A, and T20A, respectively). As shown in Fig. 4B, the T14A mutant (lane 5) migrated faster than wild-type Opalin (lane 2) in SDS-PAGE, but neither S15A (lane 6) nor T20A (lane 7) mutants exhibited drastic changes in their mobility, indicating that Opalin is also O-glycosylated at Thr-14. The triple mutant N6Q/N12Q/T14A (lane 9), which has three mutations at two N- and one O-glycosylation sites, migrated to almost the same position as the double mutant N6Q/N12Q (lane 8), which might be explained by the possibility that a deficiency in N-glycosylation indirectly affects the O-glycosylation pattern of the N6Q/N12Q mutant. We also examined by enzymatic deglycosylation if the N6Q/N12Q/T14A had still more glycosylation, and indicated that the N6Q/N12Q/T14A mutant expressed in COS7 cells still contained sialyl O-glycan chains (supplemental Fig. S2).
Sialylated Glycans Are Important for the Cell Surface Expression of Opalin—To evaluate the functional significance of Opalin glycosylation, we made glycan mutants fused with green fluorescent protein (GFP) at their C termini and heterologously expressed them in HeLa cells (Fig. 5). Opalin-GFP fluorescence was mostly localized in the cell periphery and cell-cell contact sites (Fig. 5A), and was detected in spike-like protrusions (Fig. 5H,I). Some of the Opalin-GFP fluorescence was colocalized with phallodin-labeled actin filaments (Fig. 5, H and I). By contrast, in the glycan mutants N6Q, N12Q, T14A, N6Q/N12Q, and N6Q/N12Q/T14A, high GFP fluorescence intensities were observed in the cytoplasmic space, namely the endoplasmic reticulum-Golgi network (data not shown), rather than at the cell surface. It is noteworthy that the glycan mutants, especially the N6Q/N12Q/T14A triple mutant, tended to induce large membrane protrusions (arrows in Fig. 5F), within which they were colocalized with phalloidin labels (data not shown).
FIGURE 5.

Opalin is localized to the cell surface and cell-cell contact sites in HeLa cells but the mutants lacking the glycan sites are mislocalized to the endoplasmic reticulum-Golgi network. C-terminal GFP fusion constructs of Opalin (A), N6Q (B), N12Q (C), T14A (D), N6Q/N12Q (E), N6Q/N12Q/T14A (F), and GFP alone (G) were transfected into HeLa fibroblasts. Panels A–G show GFP fluorescent images (green) in a 2-μm optical slice taken with a confocal microscope equipped with the ×63 objective lens. Panels H and I show GFP (green) and phalloidin-actin filament (magenta) fluorescent images of a 1-μm confocal optical slice around the bottom of the cells, observed using the ×100 objective lens. Wild-type Opalin was predominantly localized in spike-like protrusions (H and I) as well as the cell periphery and cell-cell contact sites (A) and was partly colocalized with phallodin-labeled actin filaments. On the other hand, the glycan mutants were concentrated in the endoplasmic reticulum-Golgi networks (stained with marker proteins, data not shown) rather than at the cell surface (B–F). In addition, these mutants, especially the N6Q/N12Q/T14A triple mutant, tended to induce large membrane protrusions (arrows in C–F). Scale bars, 10 μm in panels G and H and 5 μm in panel I.
To further analyze the structure-function relationship of Opalin in terms of its subcellular localization, we constructed three deletion mutants: dC, with a deletion of the C-terminal intracellular domain (116–143 aa); dN, with a deletion of the N-terminal extracellular domain (1–26 aa); and dIC, with a deletion of the intracellular domain (55–115 aa) (Figs. 1A and supplemental S3). The dC mutant was localized in the cell periphery and microspike-like protrusions (supplemental Fig. S4, B, F, and J), whereas the dN mutant, lacking Asn-6, Asn-12, and Thr-14, was mainly localized in the cytoplasm and failed to achieve its specific cell surface localization (supplemental Fig. S4, C, G, and K), suggesting that the glycosylated N-terminal domain of Opalin is critical for targeting to the cell surface and spike-like protrusions in HeLa cells. Interestingly, the dIC mutant, lacking five of six putative phosphorylation sites, also remained in the cytoplasm (supplemental Fig. S4, D, H, and L) and exhibited almost the same intracellular distribution pattern as the dN mutant (supplemental Fig. S4, C, G, and K).
To examine the effect of mutation of glycosylation sites in oligodendrocytes, we made two C-terminal Venus (a brighter derivative of YFP) (30) fusion constructs, Opalin-Venus and N6Q/N12Q-Venus, and transfected them into rat oligodendrocyte primary cultures (Fig. 6A). In oligodendrocytes immunopositive for O4 (a marker for oligodendrocytes and their progenitors) and extending multiple thick processes (39), Opalin-Venus was localized around the cell bodies, but was also found in the membranous extensions between processes, whereas N6Q/N12Q-Venus was distributed in the cell bodies and processes in a punctate pattern. These observations suggested that the sialylglycans of Opalin are involved in its specific intracellular localization in oligodendrocytes.
FIGURE 6.

Localization of transfected Opalin-Venus and N6Q/N12Q-Venus in primary cultured oligodendrocytes and immunolocalization of native Opalin in myelinating axons of spinal cords. A, Opalin-Venus and N6Q/N12Q-Venus were transfected into primary oligodendrocyte cultures prepared from rat cerebrum. Venus fluorescence was observed in O4-immunopositive cells extending multiple thick processes (data not shown), most of which were oligodendrocytes, as described previously (39). Opalin-Venus was localized in soma, processes, and thin membranous structures between processes. On the other hand, N6Q/N12Q-Venus was punctately distributed throughout the oligodendrocytes. B and C, the native Opalin in the myelinating axons of spinal cords from 8-week-old mice was immunolocalized together with neurofilament 160 (B, left panels), MAG (B, right), Na+ channel (C, left), and caspr (C, right). Opalin immunoreactivity localized to myelin-wrapped neurofilament-immunopositive axons in a spiral-like fashion was only partly colocalized with MAG immunoreactivity. Opalin immunoreactivity was more concentrated around the regions flanking the node. Scale bars, 5 μm.
Opalin Is Localized in the Paranodal Loops of Myelinated Axons—Immunohistochemistry of mouse cerebellum indicated that Opalin is localized in myelin-wrapped Purkinje cell axons (Fig. 3, L, M, and N). To examine the membrane localization of native Opalin in the polarized domains of myelin structures, we performed double immunostaining of mouse spinal cord sections with the anti-Opalin antibody and marker protein antibodies (Fig. 6B). Opalin immunoreactivity was localized in myelin membrane-wrapped axons immunostained for neurofilaments in a spiral fashion (Fig. 6B), as observed in the axons of Purkinje cells (Fig. 3N). Opalin immunoreactivity partially overlapped that of MAG, a marker for the inner loop membrane (41). Intense immunoreactivities for Opalin often flanked the nodes, which immunoreacted with anti-pan Na+ channels (Fig. 6C). Moreover, these myelin Opalin immunoreactivities apposed to the nodes were partly colocalized with the axonal immunoreactivities for caspr, a marker for axons forming axoglial junctions with myelin paranodal loops (Fig. 6C).
To localize Opalin in the myelin membrane, we carried out an immunogold electron microscopy analysis of mouse optic nerves. In transverse sections, Opalin immunogold was specifically detected around the inner loop (adaxonal) region, whereas none was detected in the compact myelin lamellae, the outer loop (abaxonal) region, or the axo-glial junctions (Fig. 7A). In longitudinal sections, Opalin immunogold was localized at specific sites in the paranodal loop region, where it was distributed in the cytoplasm immediately below the paranodal loop membrane (Fig. 7, B and C), consistent with the intracellular position of the antigenic epitope (the C terminus of Opalin) recognized by the antibody used. It is noteworthy that a pair of immunogold particles was often observed between adjacent loop membranes (Fig. 7C). No immunogold was detected in the axo-glial junctions of paranodes.
FIGURE 7.

Opalin is localized at specific sites in the paranodal and inner loop regions of mouse optic nerves. Immunogold electron microscopic analysis of the optic nerves of 2-week-old mice. A, Opalin immunogold particles in a transverse section through the optic nerve. Inset, magnification of the inner mesaxon area in the boxed area. Opalin immunogold particles are localized around the inner loop (or adaxonal) membrane (or inner mesaxon), and none are seen in the compact myelin or axo-glial junctions. The dotted line in the inset indicates the axo-glial junction. B and C, Opalin immunogold particles in longitudinal sections of optic nerves. Opalin immunogold particles are localized on the cytoplasmic side immediately below the paranodal loop membranes. Pairs of immunogold particles are often observed on apposing membranes. Arrows indicate the position of the immunogold particles. Ax, axon; CM, compact myelin; IL, inner loop; PN, paranode. Scale bars, 0.1 μm.
Paranodal loops themselves have been shown to form autotypic junctions like junctional specializations between polarized epithelial cells (20–22). Interestingly, exogenously expressed Opalin was preferentially localized near the adherens junctional protein E-cadherin at cell-cell contact sites between Madin-Darby canine kidney epithelial cells (supplemental Fig. S5A), whereas it was not colocalized near the tight junctional protein ZO-1 (supplemental Fig. S5B).
DISCUSSION
In this study, we demonstrate that mouse Opalin protein is a type 1 transmembrane sialylglycoprotein and an oligodendrocyte-specific component of myelin membranes. We identified three glycosylation sites, two N-glycan sites, Asn-6 and Asn-12, and one O-glycan site at Thr-14, in the short N-terminal extracellular domain. At least a fraction of O-glycan chains contained sialic acids. In addition to its localization in the somata and processes of oligodendrocytes, Opalin is concentrated on the membranes of the paranodal loops and inner loops of myelin, but is not located in the outer loops. The sialylglycans of Opalin are required for its localization in the cell periphery and cell-cell contact sites. These results suggest that Opalin is involved in an intermembranous function between non-compact cytoplasm-filled myelin loops in mammalian CNS myelin.
Our study indicates that myelin Opalin undergoes a high degree of post-translational modification by glycosylation. Enzymatic deglycosylation experiments showed that myelin Opalin contains sialylated glycans. We identified two N-glycan sites at Asn-6 and Asn-12 residues and one O-glycan site at Thr-14. However, whether myelin Opalin is actually N- and O-glycosylated at these sites remains to be studied.
It is an intriguing feature that myelin Opalin has glycans with sialic acids. Opalin mutants lacking sialylglycans failed to localize at specific cell surfaces in primary cultured oligodendrocytes and induced actin-rich membrane protrusions in HeLa cells. These results suggest that the sialylated glycans of Opalin are required for targeting to specific cell surfaces, and that Opalin may be involved in actin-cytoskeletal signaling. Deletion of the C-terminal intracellular domain (55–115 aa), which is predicted to have several protein phosphorylation consensus sites, also resulted in an impaired localization pattern, suggesting a possible involvement of the intracellular domain in specific cell surface localization. Sialic acids, which have a negatively charged carboxyl group, provide hydrophilicity to cell surfaces and regulate many cellular interactions (42). Attachment of sialic acids also modulates protein functions; for example, the channel gating of the voltage-dependent Na+ channel is modulated by sialylation of either the α subunit or β1 subunit (43,44). Neural cell adhesion molecule has large, hydrated and negatively charged polysialic acid moieties that are crucial for modulating the distance between cells; through them, neural cell adhesion molecule regulates cell migration, axon guidance and targeting, and neural plasticity (45). There are many sialic acid-binding proteins, including the protein family of sialic acid-binding immunoglobulin-like lectins (siglecs). MAG, a siglec expressed in oligodendrocytes and Schwann cells, recognizes sialic acid residues of gangliosides abundantly expressed on the neuron surface, and is involved in the long-term stability of myelin (46, 47). Considering these previous reports on the functional importance of sialic acids, sialylglycosylated Opalin may also play a role in cell recognition or signaling on paranodal loop membranes.
In addition to the somata and processes of oligodendrocytes, Opalin immunoreactivity was observed in myelinated axons in a spiral fashion, was more concentrated at the paranodal loops, and was also present in the inner loops (inner mesaxons). However, Opalin was not present in outer loops (outer mesaxons) or axoglial junctions. The paranodal loops are known to contain specific membrane protein components constituting three autotypic junctional specializations, tight, gap, and adherens junctions; in the CNS paranodal loops, claudin-11/OSP (5, 6) and connexin-32 (13) form tight junctions and gap junctions, respectively; in the PNS paranodal loops, claudin-19 (8) and claudin-1 (18) form tight junctions and connexin-32 (10) forms gap junctions. Although E-cadherin was localized to adherens junctions of the PNS paranodal loops (15–19), no adherens junctional proteins have been identified in the CNS paranodal loops to date. The present study showed that Opalin exogenously expressed in polarized epithelial Madin-Darby canine kidney cells was localized near E-cadherin at cell-cell contact sites, but was not colocalized with ZO-1 (a tight junctional protein present in the PNS paranodal loops and inner mesaxons). These results suggest that Opalin may be associated with a specific component(s), for example, adherens junctional proteins, present in the paranodal loop membranes. Moreover, our immunogold electron microscopic study demonstrated that a pair of Opalin immunogold particles often localized to apposing paranodal loop membranes, which implies a homophilic interaction of Opalin via a head-to-head transmembrane topology between two opposing loop membranes.
In conclusion, our study demonstrates that the Opalin gene encodes a type-1 transmembrane sialylglycoprotein that is phylogenetically unique to the paranodal loops of mammalian CNS myelin. The short extracellular domain of Opalin has N- and O-glycan chains exposed to the intermembranous space between paranodal loops, and a fraction of the O-glycans is sialylated. The long intracellular domain contains many consensus sites for protein phosphorylation. We hypothesize that sialylglycosylated Opalin plays a role in intermembranous recognition or intracellular signaling in the cytoplasm-filled paranodal loops.
Supplementary Material
Acknowledgments
We thank Dr. K. Ishida (Iwate Medical University) for excellent technical assistance, and Dr. Tamao Endo (Tokyo Metropolitan Institute of Gerontology), Dr. Jun Aruga (RIKEN BSI), Dr. Yoshio Hirabayashi (RIKEN BSI), Dr. Tetsushi Sadakata (RIKEN BSI), and Dr. Yo Shinoda (RIKEN BSI) for fruitful comments. We also thank Dr. Elior Peles (The Weizmann Institute of Science) for providing the anti-caspr antibody.
This work was supported by Grants-in-Aid for Scientific Research and for Advanced Medical Science Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT) and the Japan Society for the Promotion of Science (JSPS), and by the Institute of Physical and Chemical Research (RIKEN). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S5.
Footnotes
The abbreviations used are: CNS, central nervous system; PNS, peripheral nervous system; MBP, myelin basic protein; PLP, proteolipid protein; CDT-DB, Cerebellar Development Transcriptome Database; P, postnatal day; RT, reverse transcription; GFP, green fluorescent protein; MOG, myelin oligodendrocyte glycoprotein; MAG, myelin-associated glycoprotein; siglec, sialic acid-binding immunoglobulin-like lectin; HRP, horseradish peroxidase; PBS, phosphate-buffered saline; aa, amino acid(s).
References
- 1.Roach, A., Boylan, K., Horvath, S., Prusiner, S. B., and Hood, L. E. (1983) Cell 34 799–806 [DOI] [PubMed] [Google Scholar]
- 2.Matthieu, J. M., Roch, J. M., Omlin, F. X., Rambaldi, I., Almazan, G., and Braun, P. E. (1986) J. Cell Biol. 103 2673–2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boison, D., and Stoffel, W. (1994) Proc. Natl. Acad. Sci. U. S. A. 91 11709–11713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rosenbluth, J., Nave, K. A., Mierzwa, A., and Schiff, R. (2006) Glia 54 172–182 [DOI] [PubMed] [Google Scholar]
- 5.Morita, K., Sasaki, H., Fujimoto, K., Furuse, M., and Tsukita, Sh. (1999) J. Cell Biol. 145 579–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gow, A., Southwood, C. M., Li, J. S., Pariali, M., Riordan, G. P., Brodie, S. E., Danias, J., Bronstein, J. M., Kachar, B., and Lazzarini, R. A. (1999) Cell 99 645–659 [DOI] [PubMed] [Google Scholar]
- 7.Tiwari-Woodruff, S. K., Buznikov, A. G., Vu, T. Q., Micevych, P. E., Chen, K., Kornblum, H. I., and Bronstein, J. M. (2001) J. Cell Biol. 153 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyamoto, T., Morita, K., Takemoto, D., Takeuchi, K., Kitano, Y., Miyakawa, T., Nakayama, K., Okamura, Y., Sasaki, H., Miyachi, Y., Furuse, M., and Tsukita, Sh. (2005) J. Cell Biol. 169 527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergoffen, J., Scherer, S. S., Wang, S., Scott, M. O., Bone, L. J., Paul, D. L., Chen, K., Lensch, M. W., Chance, P. F., and Fischbeck, K. H. (1993) Science 262 2039–2042 [DOI] [PubMed] [Google Scholar]
- 10.Scherer, S., Deschenes, S. M., Xu, Y.-t., Grinspan, J. B., Fischebeck, K. H., and Paul, D. L. (1995) J. Neurosci. 15 8281–8294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleopa, K. A., Orthmann, J. L., Enriquez, A., Paul, D. L., and Scherer, S. S. (2004) Glia 47 346–357 [DOI] [PubMed] [Google Scholar]
- 12.Li, X., Ionescu, A. V., Lynn, B. D., Lu, S., Kamasawa, N., Morita, M., Davidson, K. G., Yasumura, T., Rash, J. E., and Nagy, J. I. (2004) Neuroscience 126 611–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamasawa, N., Sik, A., Morita, M., Yasumura, T., Davidson, K. G. V., Nagy, J. I., and Rash, J. E. (2005) Neuroscience 136 65–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Orthmann-Murphy, J. L., Freidin, M., Fischer, E., Scherer, S. S., and Abrams, C. K. (2007) J. Neurosci. 27 13949–13957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fannon, A. M., Sherman, D. L., Ilyina-Gragerova, G., Brophy, P. J., Friedrich, V. L., and Colman, D. R. (1995) J. Cell Biol. 129 189–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menichella, D. M., Arroyo, E. J., Awatramani, R., Xu, T., Baron, P., Vallat, J. M., Balsamo, J., Lilien, J., Scarlato, G., Kamholz, J., Scherer, S. S., and Shy, M. E. (2001) Mol. Cell. Neurosci. 18 606–618 [DOI] [PubMed] [Google Scholar]
- 17.Young, P., Boussadia, O., Berger, P., Leone, D. P., Charnay, P., Kemler, R., and Suter, U. (2002) Mol. Cell. Neurosci. 21 341–351 [DOI] [PubMed] [Google Scholar]
- 18.Poliak, S., Matlis, S., Ullmer, C., Scherer, S. S., and Peles, E. (2002) J. Cell Biol. 159 361–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tricaud, N., Perrin-Tricaud, C., Bruses, J. L., and Rutishauser, U. (2005) J. Neurosci. 25 3259–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedraza, L., Huang, J. K., and Colman, D. R. (2001) Neuron 30 335–344 [DOI] [PubMed] [Google Scholar]
- 21.Spiegel, I., and Peles, E. (2002) Mol. Membr. Biol. 19 95–101 [DOI] [PubMed] [Google Scholar]
- 22.Salzer, J. L. (2003) Neuron 40 297–318 [DOI] [PubMed] [Google Scholar]
- 23.Nagy, J. I., Dudek, F. E., and Rash, J. E. (2004) Brain Res. Brain Res. Rev. 47 191–215 [DOI] [PubMed] [Google Scholar]
- 24.Aruga, J., Yoshikawa, F., Nozaki, Y., Sakaki, Y., Toyoda, A., and Furuichi, T. (2007) J. Neurochem. 102 1533–1547 [DOI] [PubMed] [Google Scholar]
- 25.Bangsow, T., Schepelmann, S., Martin, C., May, M., Oberthür, A., Perl, S., Knüpfer, E., Zinke, H., and Gassen, H. G. (1998) Eur. J. Biochem. 256 24–35 [DOI] [PubMed] [Google Scholar]
- 26.Nobile, C., Hinzmann, B., Scannapieco, P., Siebert, R., Zimbello, R., Perez-Tur, J., Sarafidou, T., Moschonas, N. K., French, L., Deloukas, P., Ciccodicola, A., Gesk, S., Poza, J. J., Lo Nigro, C., Seri, M., Schlegelberger, B., Rosenthal, A., Valle, G., Lopez de Munain, A., Tassinari, C. A., and Mihcelucci, R. (2002) Gene (Amst.) 282 87–94 [DOI] [PubMed] [Google Scholar]
- 27.Dugas, J. C., Tai, Y. C., Speed, T. P., Ngai, J., and Barres, B. A. (2006) J. Neurosci. 26 10967–10983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shiraishi, Y., Mizutani, A., Bito, H., Fujisawa, K., Narumiya, S., Mikoshiba, K., and Furuichi, T. (1999) J. Neurosci. 19 8389–8400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suzuki, Y., Yoshitomo-Nakagawa, K., Maruyama, K., Suyam, A., and Sugano, S. (2000) Gene (Amst.) 200 149–156 [DOI] [PubMed] [Google Scholar]
- 30.Nagai, T., Ibata, K., Park, E. S., Kubota, M., Mikoshiba, K., and Miyawaki, A. (2002) Nat. Biotechnol. 20 87–90 [DOI] [PubMed] [Google Scholar]
- 31.Yoshikawa, F., Morita, M., Monkawa, T., Michikawa, T., Furuichi, T., and Mikoshiba, K. (1996) J. Biol. Chem. 271 18277–18284 [DOI] [PubMed] [Google Scholar]
- 32.Sadakata, T., Mizoguchi, A., Sato, Y., Katoh-Semba, R., Fukuda, M., Mikoshiba, K., and Furuichi, T. (2004) J. Neurosci. 24 43–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang, J., Sakai, R., and Furuichi, T. (2006) Mol. Biol. Cell 17 3187–3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poliak, S., Gallan, L., Martinez, R., Custer, A., Einheber, S., Salzer, J. L., Trimmer, J. S., Shrager, P., and Peles, E. (1999) Neuron 24 1037–1047 [DOI] [PubMed] [Google Scholar]
- 35.Yoshikawa, F., Iwasaki, H., Michikawa, T., Furuichi, T., and Mikoshiba, K. (1999) J. Biol. Chem. 274 328–334 [DOI] [PubMed] [Google Scholar]
- 36.Norton, W. T. (1974) Methods Enzymol. 31 435–444 [DOI] [PubMed] [Google Scholar]
- 37.Akagi, T., Ishida, K., Hanasaka, T., Hayashi, S., Watanabe, M., Hashikawa, T., and Tohyama, K. (2006) J. Neurosci. Methods 153 276–282 [DOI] [PubMed] [Google Scholar]
- 38.Tokuyasu, K. T. (1989) Histochem. J. 21 163–171 [DOI] [PubMed] [Google Scholar]
- 39.Itoh, K. (2002) Brain Res. Prot. 10 23–30 [DOI] [PubMed] [Google Scholar]
- 40.Otto, V. I., Schürpf, T., Folkers, G., and Cummings, R. D. (2004) J. Biol. Chem. 279 35201–35209 [DOI] [PubMed] [Google Scholar]
- 41.Trapp, B. D., Andrews, S. B., Cootauco, C., and Quarles, R. (1989) J. Cell Biol. 109 2417–2426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varki, A. (2007) Nature 446 1023–1029 [DOI] [PubMed] [Google Scholar]
- 43.Recio-Pinto, E., Thornhill, W. B., Duch, D. S., Levinson, S. R., and Urban, B. W. (1990) Neuron 5 675–684 [DOI] [PubMed] [Google Scholar]
- 44.Johnson, D., Montpetit, M. L., Stocker, P. J., and Bennett, E. S. (2004) J. Biol. Chem. 279 44303–44310 [DOI] [PubMed] [Google Scholar]
- 45.Rutishauser, U., and Landmesser, L. (1996) Trends Neurosci. 19 422–427 [DOI] [PubMed] [Google Scholar]
- 46.Crocker, P. R. (2002) Curr. Opin. Struct. Biol. 12 609–615 [DOI] [PubMed] [Google Scholar]
- 47.Filbin, M. T. (2003) Nat. Rev. Neurosci. 4 1–11 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.




