Abstract
Human axillary odor is formed by the action of Corynebacteria on odorless axilla secretions. Sulfanylalkanols, 3-methyl-3-sulfanylhexan-1-ol in particular, form one key class of the odoriferous compounds. A conjugate with the dipeptide Cys-Gly has been reported as the secreted precursor for 3-methyl-3-sulfanylhexan-1-ol. Here, we confirm the Cys-Gly-(S) conjugate as the major precursor of this odorant, with lower levels of the Cys-(S) conjugate being present in axilla secretions. The enzymatic release of 3-methyl-3-sulfanylhexan-1-ol from the Cys-Gly-(S) conjugate by the axilla isolate Corynebacterium Ax20 was thus investigated. Cellular extracts of Ax20 released 3-methyl-3-sulfanylhexan-1-ol from the Cys-Gly-(S) conjugate and from the Cys-(S) conjugate, whereas the previously isolated C-S lyase of this bacterial strain was only able to cleave the Cys-(S) conjugate. o-Phenanthroline blocked the release from the Cys-Gly-(S) conjugate but did not affect cleavage of the Cys-(S) conjugate, indicating that in a first step, a metal-dependent dipeptidase hydrolyzes the Cys-Gly bond. This enzyme was purified by four chromatographic steps and gel electrophoresis, and the partial amino acid sequence was determined. The corresponding gene was cloned and expressed in Escherichia coli. It codes for a novel dipeptidase with a high affinity toward the Cys-Gly-(S) conjugate of 3-methyl-3-sulfanylhexan-1-ol. Co-incubating either the synthetic Cys-Gly-(S) conjugate or fresh axilla secretions with both the C-S lyase and the novel dipeptidase did release 3-methyl-3-sulfanylhexan-1-ol, proving that the sequential action of these two enzymes from the skin bacterium Corynebacterium Ax20 does release the odorant from the key secreted precursor.
The skin in human armpits contains a dense arrangement of sweat glands. Volatile substances evaporating from these areas make a key contribution to human body odor. However, sweat secreted from apocrine glands in these skin areas is initially odorless, and since the work of Shelley et al. (1), it is known that skin bacteria release the odoriferous principles from non-smelling substrates present in these secretions. Indeed, the axilla is a skin region colonized by an unusually dense bacterial population, with a species composition dominated by the two genera Staphylococcus and Corynebacterium (2, 3). Most individuals carry a flora that is dominated by either one of these two genera, and there is a strong correlation between a high population of Corynebacteria and strong axillary odor formation (2, 4). Subjects whose axillary skin is mainly colonized by Staphylococci emit only low levels of odor. Based on this fundamental work, axilla secretions contain non-odoriferous precursors that are transformed into the volatile substances by bacterial enzymes mainly present in Corynebacteria and to a lesser extent in Staphylococci.
Early studies on the chemistry of human axilla odors identified the odoriferous steroids 5α-androst-16-en-3-one (5, 6) and 5α-androst-16-en-3α-ol (7) in human axilla secretions. Later, Zeng et al. (8) reported that short, branched fatty acids make a major contribution to the axilla odor with (E)-3-methyl-2-hexenoic acid being the key component. In our previous work, we had shown that a broad diversity of other unsaturated or hydroxylated odorant acids is present in hydrolyzed axilla secretions and that all these acids are released from the glands in the form of odorless glutamine conjugates (9, 10). We had isolated a specific zinc-dependent aminoacylase from the axilla isolate Corynebacterium striatum Ax20, which catalyzes the release of the odoriferous principles from these glutamine conjugates (9).
The third and most recently discovered class of human axilla odorants are volatile sulfanylalkanols (11–13), with 3-methyl-3-sulfanylhexan-1-ol (3M3SH)2 as the quantitatively dominating compound within this structural class. This compound can be released both from a Cys-(S) conjugate and from axilla secretions by a C-S lyase cloned from Corynebacterium Ax20, indicating that Cys-(S) conjugates could be physiological precursors for this compound class (12). However, it was later shown that a Cys-Gly-(S) conjugate of 3M3SH is secreted by human subjects (14) (for structures, see Fig. 1) and that bacterial cultures of Staphylococcus haemolyticus can release 3M3SH from this dipeptide precursor. In a recent patent application, this activity was attributed to a β-lyase, but the corresponding enzyme was neither isolated from S. haemolyticus, nor has it been characterized (15). Thus, the enzymatic release of the key axilla odorant 3M3SH by skin bacteria from the physiological dipeptide precursor has not yet been deciphered. Here, we report the isolation and the characterization of a novel specific dipeptidase and the corresponding gene from the axilla isolate Corynebacterium Ax20. We show that the secreted Cys-Gly-(S) conjugate of 3M3SH first needs to be cleaved by this dipeptidase and only afterward becomes a substrate of the previously reported C-S lyase, which finally releases 3M3SH.
FIGURE 1.
Proposed scheme for the release of 3M3SH from the Cys-Gly-(S) conjugate.
EXPERIMENTAL PROCEDURES
Materials—Unless otherwise noted, all chemicals were purchased from Fluka (Buchs, Switzerland). (Z)-protected amino acids and peptides as enzyme substrates were from Aldrich (Buchs, Switzerland) and from Senn Chemicals (Dielsdorf, Switzerland). All columns and chromatography resins were from Amersham Biosciences (Otelfingen, Switzerland) with the exception of Ni-NTA agarose purchased from Qiagen (Hombrechtikon, Switzerland). (S)-(1-(2-hydroxyethyl)-1-methylbutyl)-l-cysteinylglycine (Cys-Gly-(S) conjugate) was synthesized by the method described by Starkenmann et al. (14). 3M3SH, its Cys-(S) conjugate, and Gln conjugates of carboxylic acids as reference substrates were synthesized as described before (9, 10, 12). The recombinant C-S lyase from Corynebacterium Ax20 was expressed and purified on a Ni-NTA-affinity column (12). Axilla secretions of individual donors were sampled on cotton pads fixed in the axillary region during physical exercise (10).
Bacterial Strains—Isolation and characterization of axilla bacteria were described previously (9). The bacterial strains were grown on tryptic soy agar supplemented with 0.01% Tween 80 as lipid source. For enzyme purification or enzyme assays, axilla bacteria were grown in Mueller-Hinton broth supplemented with 0.01% Tween 80. E. coli strain TOP10, used for the expression of recombinant enzymes, was grown in LB broth.
LC-MS Analysis of Axilla Secretions—The aqueous fraction of axilla secretions was fractionated over a Superdex peptide 10/300 GL column using (NH4)2CO3 (100 mm) as elution buffer, and the single fractions were analyzed with LC-MS with the method laid out in Natsch et al. (10). In brief, a Finnigan LCQ mass spectrometer operated in the atmospheric pressure chemical ionization mass spectrometry mode and equipped with a Flux Rheos 2000 HPLC pump was used, and HPLC separation was performed on a C18 RP column modified for proteins and peptides (Grace Vydac, Hesperia, CA). The mobile phase consisted of H2O (A) and MeOH (B) each containing 1% HOAc (v/v).
GC-FPD Analysis for Release of 3M3SH—The Cys- and Cys-Gly-(S) conjugates of 3M3SH or the aqueous fraction of axilla secretions were incubated with the recombinant enzymes or bacterial extracts for 2 h. The aqueous phase (500 μl) was extracted with 250 μl of methyl-tert-butyl-ether, and 6 μl were injected in the splitless pulse-pressure mode onto a SPW1-sulfur column (Supelco, Bellefonte, PA) mounted on an Agilent GC 6890N (Agilent, Wilmington, DE) system with a flame photometric detector specific for sulfur chemicals. The temperature program was set to 2 min of initial temperature at 50 °C, heating at a rate of 10 °C/min to 240 °C, and a final 15 min at 240 °C.
Dipeptidase Activity Assay—Bacterial extracts, column fractions, or the purified peptidase were incubated with the Cys-Gly-(S) conjugate or (S)-benzyl-Cys-Gly at a final concentration of 0.5 or 1 mm and with an excess of β-lyase (10 μg/ml) in buffer A (50 mm NaCl, 50 mm NaH2PO4/K2HPO4, pH 7) in a final volume of 100 μl. Release of 3M3SH or benzylthiol was detected by adding 50 μl of a 1 mm solution of the thiol-specific fluorescent probe monobromobimane dissolved in NaCO3 buffer (100 mm, pH 8.8) and, after a 5-min reaction time, fluorescence measurement on a Flexstation (Molecular Devices, Sunnyvale, CA) with an excitation at 390 nm and emission at 478 nm. To determine enzyme kinetics, the same assay was used, but the enzyme reaction with the dipeptidase was stopped by adding EDTA (0.1 mm), and only then the β-lyase was added to release the thiol for fluorescent detection.
Thin Layer Chromatography for Peptide Cleavage—Cys- and Cys-Gly-(S) conjugates, dipeptides and acetyl ornithine (1 mm solutions) were incubated with the peptidase for 1 h. 10 μl of each reaction were spotted on TLC plates and developed with a mixture of 1-butanol, acetic acid, H2O (4:1:1) in the case of the Cys- and Cys-Gly-(S) conjugates or with H2O and 1-propanol (1:1) in the case of the dipeptides. Products were visualized by spraying a ninhydrin solution and heating the plates until the completion of the ninhydrin reaction.
Protein Determination and SDS-PAGE—Protein concentrations were determined with the Bradford reagent (Bio-Rad) using bovine serum albumin as standard. SDS-PAGE was performed according to Laemmli (16) with 5% stacking gels and 10% separation gels. Protein bands were visualized by Coomassie Blue staining.
Purification of the Peptidase—C. striatum Ax20 was selected for the purification of the enzyme responsible for the cleavage of the Cys-Gly-(S) conjugate. It was grown for 42 h, and cells from a total culture volume of four liters were washed once with buffer A and resuspended in a final volume of 5 ml of buffer A. Using glass beads (425–600 μm, Sigma), the cells were mechanically disrupted by vortexing them at maximal speed for 20 min. The lysate was cleared by centrifugation and passed through a 0.45-μm syringe filter. The extract was then sequentially run over four chromatography columns: 1) phenyl-sepharose hydrophobic interaction resin, elution with a linear gradient from 1000 to 0 mm (NH4)2SO4 in Buffer A; 2) Mono Q strong anion exchange column on the fast protein liquid chromatography system, elution with a gradient from 0 to 800 mm KCl in buffer A; 3) Mono P weak anion exchange column on the fast protein liquid chromatography system, elution with a gradient from 0 to 800 mm KCl in 50 mm bis-Tris buffer (pH 6.5); 4) Superdex 200 gel filtration column, elution with buffer A. After each column separation, active fractions (determined by the fluorescent assay) were pooled and desalted by dilution/ultrafiltration. Fractions of the final Superdex gel filtration step were run on a 10% SDS-PAGE gel. The single protein band common to the active fractions was isolated and subjected to tryptic digestion, and the sequence of internal peptides was determined with LC-electrospray mass ionization-tandem MS analysis (Genomic Center, University of Zürich, Switzerland).
Molecular Biology Methods—Chromosomal DNA of Ax20 was obtained from cell lysates by proteinase K digestion and subsequent extraction with cetyltrimethylammonium bromide/NaCl and chloroform/isoamylacetate (17). Based on the partial amino acid sequences of the purified enzyme, degenerated primers were designed to amplify genomic DNA fragments. Standard PCR conditions were used according to the manufacturer (Taq polymerase, Sigma, Buchs, Switzerland). The amplified DNA was submitted to Microsynth (Balgach, Switzerland) for sequence analysis. Based on the obtained partial sequence, specific nested oligonucleotides were designed to clone the upstream and downstream regions. Chromosomal DNA of Ax20 was digested with SmaI and PvuII and ligated to the GenomeWalker Adaptor (Clontech). The upstream and downstream regions were then amplified as described in the instructions to the Universal GenomeWalker™ kit (Clontech Laboratories) and sequenced. The resulting open reading frame was amplified from chromosomal DNA of Ax20 by PCR using the specific primers 5′-CGA CAT GCC ATG GGC AGC AAC GAC AAG GCA GCA ACC AGC-3′ and 5′-CGA CAT AAG CTT TTT CCC GTA GGT GAG CAG GAA T-3′. The amplified DNA fragment was digested with NcoI and HindIII and ligated into the vector pBAD/myc-HisA (Invitrogen, Groningen, The Netherlands) predigested with the same enzymes. The resulting plasmid pBAD/mycHis-tpdA was transformed into the host strain E. coli TOP10 (Invitrogen). This strain was grown in LB broth until it reached an A600 of 0.5. The culture was supplemented with arabinose (0.2% final concentration) to induce gene expression, further incubated for 4 h, and harvested by centrifugation, and the cells were disrupted using a French Press. The His-tagged recombinant peptidase was finally purified using a Ni-NTA column according to the manufacturer's instructions.
RESULTS
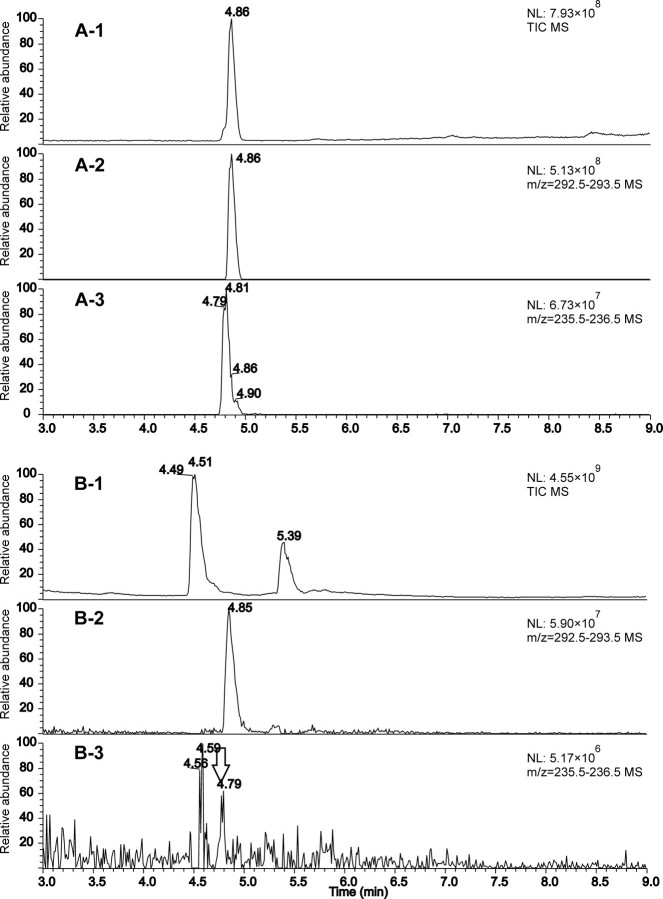
LC-MS Analysis of Axilla Fractions to Detect Amino Acid Conjugates of 3M3SH—Aqueous fractions of axilla secretions obtained by gel filtration were analyzed by LC-MS in comparison with synthetic Cys- and Cys-Gly-(S) conjugates of 3M3SH. In axilla secretions, a significant peak at retention time 4.85 min was observed on the extracted mass trace m/z 293, corresponding to the protonated Cys-Gly-(S) conjugate (Fig. 2, A-2 and B-2). This peak can only be detected in axilla secretions on the selected ion trace, whereas the total ion current (TIC) trace contains two dominant peaks (Fig. 2, B-1), which correspond to the two key Gln conjugates of carboxylic acids reported before (9), as verified based on MS analysis and comparison with synthetic references (data not shown). The Cys-(S) conjugate of 3M3SH yields an 8-fold lower signal intensity in LC-MS analysis as compared with the Cys-Gly-(S) conjugate (compare the different normalization level NL in Fig. 2, A-2 and A-3), probably due to less efficient ionization of the Cys-(S) conjugate. In axilla secretions, on the m/z trace 236, only a very minor peak at the correct retention time was observed, putatively corresponding to the Cys-(S) conjugate (indicated in Fig. 2, B-3 by an arrow). Indeed, the on-line MS2 spectrum of this peak showed the same base ion at m/z 122 as the MS2 spectrum of the synthetic reference sample. This ion presumably corresponds to the protonated free Cys (supplemental Fig. S1). Nevertheless, even considering the lower response factor for the Cys-(S) conjugate, this compound, albeit clearly present, is the minor component, and thus, the Cys-Gly-(S) conjugate of 3M3SH indeed is the main precursor present in axilla secretions.
FIGURE 2.
LC-atmospheric pressure chemical ionization (+)-MS analysis of synthetic Cys- and Cys-Gly-(S) conjugates of 3M3SH (A) and unhydrolyzed axilla secretions fractionated by gel filtration (B). A, a solution containing each 100 μm of the Cys- and Cys-Gly-(S) conjugates of 3M3SH. B, the fraction eluting after 20.5 ml from a Superdex peptide 10/300 GL column loaded with pooled axilla secretions from two donors. A-1 and B-1, total ion current (TIC) chromatograms (mass range m/z 80–600); A-2 and B-2, extracted mass chromatograms of m/z 293 ([M+H]+ of the Cys-Gly-(S) conjugate); A-3 and B-3, extracted mass chromatograms of m/z 236 ([M+H]+ of the Cys-(S) conjugate). The largest peak in each chromatogram is normalized to 100, and the normalization level NL is indicated in the graphs.
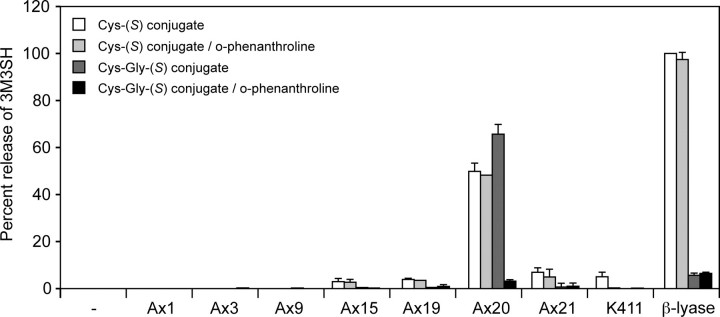
3M3SH Is Released from the Cys-Gly-(S) Conjugate by the Sequential Action of a Metallopeptidase and a β-Lyase—The fact that mainly the Cys-Gly-(S) conjugate and only small amounts of the Cys-(S) conjugate can be found in fresh sweat suggests that 3M3SH is directly released either by a β-lyase in axilla bacteria from the Cys-Gly-(S) conjugate or from a Cys-(S) conjugate that has been generated from the Cys-Gly-(S) conjugate by hydrolysis of the Cys-Gly peptide bond. To test the two hypotheses, the synthetic Cys- and the Cys-Gly-(S) conjugates of 3M3SH were incubated with the purified β-lyase from the axilla strain Ax20 or with total cell extracts of several bacterial strains isolated from the axilla. As shown in Fig. 3, the purified β-lyase and the Ax20 extract released significant amounts of 3M3SH from the Cys-(S) conjugate, and this could not be blocked by the metallopeptidase inhibitor o-phenanthroline. When incubated with the Cys-Gly-(S) conjugate, only the Ax20 extract but not the recombinant β-lyase released a significant amount of 3M3SH. Interestingly, this reaction could be blocked by o-phenanthroline, suggesting that the strain Ax20 harbors an o-phenanthroline-sensitive dipeptidase that hydrolyzes the Cys-Gly-(S) conjugate, thereby producing the substrate for the β-lyase. Extracts of Staphylococcus and Micrococcus strains did not cleave either of the two conjugates, whereas other tested Corynebacteria showed only weak β-lyase activity (Fig. 3). Corynebacterium jeikeium K411 is the only axilla isolate whose genome had been sequenced (19). After a 2-h incubation, no significant cleavage of the Cys-Gly-(S) conjugate by this strain was observed; however, after prolonged incubation it slowly cleaved this substrate (data not shown). The structures of the conjugates along with these proposed reactions are shown in Fig. 1.
FIGURE 3.
Release of 3M3SH from synthetic conjugates by bacterial extracts and recombinant β-lyase. Total cell extracts (0.25 mg of protein/ml) of bacterial strains isolated from axillary skin and β-lyase (0.005 mg/ml) were incubated with the Cys- or the Cys-Gly-(S) conjugates (0.5 mm) of 3M3SH for 2 h. Where indicated, o-phenanthroline was added at a final concentration of 0.5 mm. Released 3M3SH was detected using the fluorescent dye monobromobimane. Species assignment is as follows: Ax1, Staphylococcus capitis; Ax6, Staphylococcus epidermidis; Ax9, Micrococcus luteus; Ax15, C. jeikeium; Ax19, C. jeikeium; Ax20, C. striatum; Ax21, Corynebacterium bovis; and K411, C. jeikeium.
Purification of the Novel Metallopeptidase and Cloning of the Corresponding Gene—The unknown metallopeptidase cleaving the Cys-Gly-(S) conjugates of 3M3SH was then purified from cellular extracts of C. striatum Ax20 by activity-guided fractionation as described under “Experimental Procedures.” Two columns were tested for the first purification step, a Mono-Q and a phenyl-Sepharose column. From both columns, the metallopeptidase activity eluted as a single peak. Also, from all subsequent column fractionations, only a single peak of activity was recovered, indicating that only one key enzyme is involved in the hydrolysis of the Cys-Gly-(S) conjugate. Fractions of the last purification step were analyzed by SDS-PAGE. Three different polypeptides were left in the active fractions. Comparison of the relative peptidase activity and the intensity of the bands resulted in a clear candidate protein, which had an apparent mass of ∼50 kDa (see supplemental Fig. S2 in the supporting information). The candidate protein was excised and submitted to a tryptic digest and sequence analysis, leading to the amino acid sequence of several internal peptides.
A data base search with the obtained peptide sequences revealed homology to putative peptidases, suggesting that the analyzed protein could indeed be responsible for the cleavage of the Cys-Gly bond. Based on four peptide sequences, degenerated primers were designed, and a total of 20 primer combinations were used for PCR amplification with chromosomal DNA of Ax20 as template. Two primer combinations led to specific products of 281 and 272 bp, respectively. The obtained PCR products were sequenced, and based on these partial sequences, oligonucleotides were designed to clone the upstream and downstream regions by genome walking using libraries generated from PvuII and SmaI digests of chromosomal Ax20 DNA. An upstream fragment of 600 bp and a downstream fragment of 2400 bp were obtained. Within these sequenced regions, open reading frames coding for the N-terminal part and the C-terminal part were identified. Finally, the complete open reading frame was amplified by PCR using Ax20 genomic DNA as template, and it was cloned into the bacterial expression vector pBAD/mycHisA. The sequence was deposited in the GenBank™ under accession number EU311559. The gene was named tpdA, which stands for “thiol precursor dipeptidase.”
Sequence Comparison with Related Proteins—The protein deduced from the open reading frame has a high homology to a large number of putative bacterial peptidases belonging to the M20 family of metallopeptidases. Closely related genes, for which no function has been identified yet, exist in the genomes of most other members of the class of actinobacteria. The closest homologues in E. coli are the succinyl-diaminopimelate desuccinylase and the acetyl-ornithine deacetylase (18), two related proteins also belonging to the M20 family of peptidases, which are involved in the biosynthesis of lysine and arginine, respectively. The sequence alignment of the dipeptidase with these genes of known function, with the closest relatives from Corynebacterium diphteriae and C. jeikeium K411 (19) and with the carboxypeptidase G2 from Pseudomonas aeruginosa (18, 20), is shown in supplemental Fig. S3 in the supporting information.
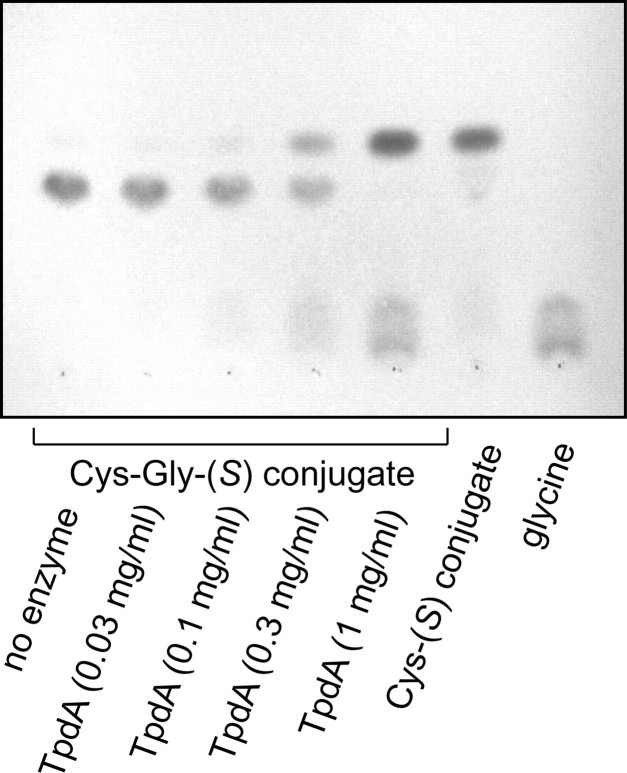
Characterization of the Pure Recombinant Enzyme—Transformants of E. coli strain TOP10 harboring the plasmid pBAD/mycHis-tpdA expressed high levels of the His-tagged recombinant protein, which was purified to >95% purity using a Ni-NTA column. To demonstrate that the isolated protein indeed catalyzes the hydrolysis of the peptide bond of the Cys-Gly-(S) conjugate, this substrate was incubated with the purified protein, and the reaction products were separated by TLC. As shown in Fig. 4, the Cys-Gly-(S) conjugate was indeed hydrolyzed to the Cys-(S) conjugate and glycine, and the same reaction was performed by the TpdA with (S)-benzyl-Cys-Gly as a substrate (data not shown).
FIGURE 4.
Cleavage of the Cys-Gly-(S) conjugate by TpdA. The Cys-Gly-(S) conjugate of 3M3SH (1 mm) was incubated with increasing amounts of the TpdA for 1 h. 10 μl of each reaction were spotted on a TLC plate and developed with 1-butanol:acetic acid:H2O (4:1:1).
Having shown this, we tried to find additional substrates for the identified enzyme. A series of 24 dipeptides was incubated for 1 h with the peptidase and then analyzed by TLC. The peptidase did hydrolyze a relatively broad range of dipeptides, but it was not able to cleave acidic amino acids from the C terminus (Table 1). In addition, the peptidase did not hydrolyze dipeptides with glycine in the second position unless a bulky hydrophobic residue was present in the N-terminal position. Thus, although (S)-substituted Cys-Gly conjugates are very good substrates, unsubstituted Cys-Gly is not cleaved.
TABLE 1.
Substrate specificity of TpdA and the native enzyme preparation
| Substrate | Recombinant TpdA | Native enzyme preparationb |
|---|---|---|
| Dipeptides and derivatives | ||
| (S) 3M3SH-Cys-Gly | ++ | ++ |
| (S) Benzyl-Cys-Gly | +++ | +++ |
| Cys-Gly | - | - |
| Gly-Gly | - | ND |
| Lys-Gly | - | ND |
| Phe-Gly | ++ | ++ |
| Trp-Leu | +++ | +++ |
| Glu-Trp | +++ | ++ |
| Ile-Asn | +++ | ND |
| Leu-Ala | +++ | ND |
| Leu-Asn | +++ | ND |
| Val-Lys | +++ | ND |
| Ala-Ala | ++ | ++ |
| Ser-Leu | ++ | ND |
| Thr-Gln | ++ | ND |
| Val-Gln | ++ | ND |
| Ile-Leu | + | + |
| Trp-Val | + | ND |
| Ala-Glu | - | - |
| Arg-Glu | - | ND |
| Asp-Asp | - | - |
| Asp-Glu | - | ND |
| Glu-Asp | - | ND |
| Glu-Glu | - | - |
| Ile-Trp | - | - |
| Ile-Val | - | ND |
|
Val-Glu
|
-
|
ND
|
| Acyl-amino acids | ND | |
| Acetyl-ornithine | - | ND |
| Nα-Lauroyl-Gln | + | ND |
| Nα-(E)-3-methyl-2-hexenoyl-glutamine | +/- | ND |
| Nα-3-methyl-3-hydroxy-hexanoyl-glutamine | +/- | ND |
a 1 mm substrate was incubated with different concentrations of TpdA for 1 h. - indicates no hydrolysis; +/- indicates traces of product detected with 1 μg/ml enzyme; >20% of substrate hydrolyzed with 1 μg/ml enzyme; ++ indicates >20% of substrate hydrolyzed with 0.3 μg/ml enzyme; +++ indicates >20% of substrate hydrolyzed with 0.1 μg/ml enzyme; ++++ indicates >20% of substrate hydrolyzed with 0.03 μg/ml enzyme.
1 mm substrate was incubated with different dilutions of the native enzymatic preparation obtained after the fourth column (Superdex gel filtration). - indicates no hydrolysis; +/- indicates traces of product detected with 1:1 dilution; + indicates >20% of substrate hydrolyzed with 1:1 dilution; ++ indicates >20% of substrate hydrolyzed with 1:3 dilution; +++ indicates >20% of substrate hydrolyzed with 1:10 dilution; ++++ indicates >20% of substrate hydrolyzed with 1:30 dilution. The native preparation used had an apparent purity of 30%. ND, not determined.
To verify that the recombinant enzyme indeed has the same substrate specificity as the native enzyme, the purified fraction after the last purification step (Superdex gel filtration) was incubated with 13 of the potential substrates, and the substrate specificity was compared with the recombinant enzyme. As illustrated in Table 1, column 3, the specificity of both preparations is essentially the same.
As mentioned above, the closest homologues in E. coli are the succinyl-diaminopimelate desuccinylase and the acetyl-ornithine deacetylase. To test whether the isolated peptidase also harbors deacylase activity, it was incubated with acetyl-l-ornithine, but no deacylation was detected. Nα-Acyl-Gln conjugates for odorant acids are secreted in the axilla and are substrates of the recently identified enzyme Nα-acyl-Gln-aminoacylase also belonging to the M20 class of peptidases (9). Since two dipeptides with Gln in second positions served as substrates for the dipeptidase, we tested whether the Nα-acyl-Gln conjugates can also serve as substrates for TpdA. Interestingly, we found that these glutamine conjugates are indeed hydrolyzed by the peptidase to a certain extent (Table 1).
The Cys-Gly-(S) conjugates of 3M3SH and benzylthiol were finally used to determine enzyme kinetics. The non-physiological benzylthiol conjugate was chosen as an alternative substrate for analysis of enzyme activity as it is the simplest Cys-Gly conjugate available. A Km value of 0.045 mm and a Vmax of 0.023 mmol ·min–1 ·mg–1 was obtained for the hydrolysis of the Cys-Gly-(S) conjugate of 3M3SH. For the hydrolysis of the Cys-Gly-(S) conjugate of benzylthiol, we determined a Km value of 0.20 mm and a Vmax of 0.19 mmol ·min–1 ·mg–1(data not shown). These data correlate with the results listed in Table 1, suggesting that the dipeptidase cleaves the benzylthiol precursor more efficiently at high substrate concentrations. At low substrate concentrations, as found in the secreted axilla sweat, the dipeptidase shows a significantly higher affinity for the physiological Cys-Gly-(S) conjugate of 3M3SH.
The homologous gene jk0266 from Corynebacterium jeikeium K411 (19) was also cloned and expressed. It cleaves the Cys-Gly-(S) conjugate of benzylthiol with a Km value of 0.28 mm andavmax of 0.16 mmol ·min–1 ·mg–1, but the Cys-Gly-(S) conjugate of 3M3SH was cleaved with a 25-fold lower vmax by this related enzyme as compared with TpdA. Thus, not all Corynebacterium strains isolated from the axillary skin have an enzyme perfectly adapted to this substrate (data not shown).
The recombinant TpdA was incubated with the metal chelating agents o-phenanthroline and pyridine-2,6-dicarboxylic acid. The IC50 of both these compounds is 15 μm. This confirms the conclusion from the results in Fig. 3, namely that the responsible enzyme for the cleavage of the Cys-Gly conjugates is a o-phenanthroline sensitive metallopeptidase.
Release of 3M3SH from Axilla Secretions by the Recombinant Enzymes—To finally prove the involvement of the novel dipeptidase in the release of 3M3SH from axilla secretions, a pooled axilla secretion sample from two donors was split into four equal portions and left untreated (A) or treated with the dipeptidase (B), the β-lyase (C), both the dipeptidase and the β-lyase (D). The samples were analyzed by GC-FPD, and the resulting chromatograms as compared with synthetic 3M3SH. The untreated and the dipeptidase-treated samples contained no 3M3SH, whereas the β-lyase did only release a small quantity of 3M3SH (0.5 μg/ml; indicated in Fig. 5C by an arrow). The combined action of both enzymes released a 14-fold higher quantity of 3M3SH as compared with the incubation with the β-lyase only (7 μg/ml; Fig. 5D). In addition, several further small peaks for sulfur-containing compounds were formed by the combined enzyme treatment, indicating that further sulfur volatiles could be present in axilla secretions as Cys-Gly-(S) conjugates and are released by the same two enzymes.
FIGURE 5.
GC-FPD analysis of axilla secretions treated with the TpdA and the β-lyase. The aqueous fraction from axilla secretions pooled from two donors was split into four portions, treated with the enzymes (10 μg/ml) for 2 h, extracted with solvent, and analyzed with GC with a sulfur specific detector. A, untreated sample; B, sample treated with the dipeptidase TpdA; C, sample treated with the β-lyase; D, sample treated with TpdA and β-lyase; and E, 2 ppm of synthetic 3M3SH as reference.
DISCUSSION
Based on the fact that a C-S lyase from Corynebacterium Ax20 can release 3M3SH both from a synthetic Cys-(S) conjugate and from axilla secretions, we had proposed that a Cys-(S) conjugate might be the key secreted precursor (12). However, the conjugate with the dipeptide Cys-Gly was later reported as the key precursor for 3M3SH (14). The presented LC-MS data now confirm the Cys-Gly-(S) conjugate as the major precursor of 3M3SH, but, in combination with the LC-MS2 analysis, they clearly show that the Cys-(S) conjugate is also present, albeit at lower levels. This reminds of the biochemical precursors for the key odorant 3-methyl-3-sulfanyl-butan-1-ol in cat urine. Cat urine contains as a precursor the unusual amino acid felinine ((S)-(1,1-dimethyl-3-hydroxypropyl)-cysteine (21)), and it was later shown that in addition to the Cys-(S) conjugate felinine, cat urine also contains the corresponding Cys-Gly-(S) conjugate (22). Interestingly, in cats, unlike in most other mammals, urine contains a higher level of protein, and it was shown that this protein level is mainly due to a single protein named Cauxin (carboxylesterase-like urinary excreted protein (23)), which cleaves the Cys-Gly-(S) conjugate and releases felinine (24). Therefore the cleavage of this precursor is performed by a carboxypeptidase that is physiologically secreted in the cat.
Here, we show that on the human skin, the cleavage of the Cys-Gly-(S) conjugate can be performed by skin commensal microorganisms rather than by a secreted human enzyme. The novel dipeptidase reported from the skin isolate Corynebacterium Ax20 has a very high affinity to the secreted Cys-Gly-(S) conjugate, but it also cleaves a range of dipeptides. The fact that the dipeptidase has a low Km for the Cys-Gly-(S) conjugate could indicate that the skin bacteria have adapted their enzyme specifically for this type of substrate present in the natural habitat of this bacterial strain. On the other hand, the Vmax for the physiological substrate is relatively low. It is noteworthy that even if the enzymatic cleavage is relatively slow, it may quickly yield a perceivable quantity of the odorant since the human nose is very sensitive to 3M3SH. We had measured the sensory threshold (12) to be 1 pg/liter air, indicating that 0.007 nmol is perceivable in 1 m3 air.
We could detect cleavage of the Cys-Gly-(S) conjugate only in strains of Corynebacteria, which is in agreement with the well known fact that human subjects with a high population of Corynebacteria can form axillary malodor (2, 25). However, Starkenmann et al. (14) have reported that an isolate of S. haemeolyticus is releasing 3M3SH from the Cys-Gly-(S) conjugate. Thus, it appears that members of different bacterial genera present on axillary skin have adopted their enzymes to these secreted substrates.
Based on homology searches, the novel thiol precursor dipeptidase TpdA belongs to the M20 class of peptidases. Most bacterial species sequenced in recent years do contain a homologue of this gene. However, no function has been associated to the 75 most closely related sequences investigated. The closest relatives with a known function are the two enzymes DapE and ArgE involved in the biosynthesis of lysine and arginine, respectively, which were characterized in E. coli and other bacteria (18, 26, 27), and the carboxypeptidase G2 form P. aeriginosa. Interestingly, among the five residues involved in zinc binding in carboxypeptidase G2 (28), four are also conserved in TpdA.
The final proof that both enzymes, the novel thiol precursor dipeptidase and the β-lyase, are needed to cleave the Cys-Gly-(S) conjugate comes from co-incubations of either the synthetic compound or the axilla secretions with both enzymes. From axilla secretions, the β-lyase alone can release low quantities of 3M3SH, as reported earlier (12), which is in agreement with the LC-MS finding that low levels of the Cys-(S) conjugate are present in these secretions; however, much larger quantities of 3M3SH are released by the joint action of the β-lyase and the dipeptidase.
Interestingly, the dipeptidase also has a certain affinity toward the Nα-acyl-Gln conjugates of odorant acids in the axilla reported before (9). On the other hand, the previously isolated enzyme Nα-acyl-Gln-aminoacylase, which also belongs to the M20 class of peptidases and which is specific for these Gln conjugates, has a very low but detectable catalytic activity cleaving the Cys-Gly-(S) conjugate (data not shown). Thus, the two related metallopeptidases involved in the formation of these very different structural classes of key axilla odorants have a certain cross-specificity. In the pharmaceutical field, it was shown that dual metallopeptidase inhibitors can be developed, e.g. for the two metallopeptidases angiotensin converting enzyme and neutral endopeptidase, which both are involved in neuropeptide processing and regulation of blood pressure (29). Since two metallopeptidases with a certain cross-specificity both are involved at key stages of axilla odor formation, the medicinal chemistry approach of designing dual metallopeptidase inhibitors could, in the future, be applied to develop next generation ingredients for cosmetic deodorants.
The dipeptidase identified in this work and the previously identified Nα-acyl-Gln-aminoacylase and β-lyase enzymes are all involved in the release of odorants from precursors. This release is time-dependent, and thus, fresh sweat secreted during physical exercise initially is odorless. It is a common observation that under certain conditions of nervous tension (and thus, maybe under a specific hormonal regulation), an immediately perceivable body odor is formed. This very particular odor might be directly secreted without bacterial/enzymatic action on the axilla secretions being necessary. The chemical nature and the origin of this specific odor cannot yet be explained by the detailed analytical and enzymatic studies presented here or published previously, and thus, forms a potential subject of further research.
Supplementary Material
Acknowledgments
We give our thanks to F. Flachsmann for synthesizing reference compounds, H. Gfeller for LC-MS analysis, B. Schilling for critical discussions, and Dr. Peter Hunziker (Functional Genomics Center Zurich, Zürich, Switzerland) for amino acid sequence analysis.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
The nucleotide sequence(s) reported in this paper has been submitted to the GenBank™/EBI Data Bank with accession number(s) EU311559.
The on-line version of this article (available at http://www.jbc.org) contains three supplemental figures.
Footnotes
The abbreviations used are: 3M3SH, 3-methyl-3-sulfanylhexan-1-ol; GC-FPD, gas-chromatography with flame photometric detection; LC, liquid chromatography; MS, mass spectrometry; MS2, two-stage mass analysis; HPLC, high pressure liquid chromatography; Ni-NTA, nickel-nitrilotriacetic acid.
References
- 1.Shelley, W. B., Hurley, H. J., and Nichols, A. C. (1953) Arch. Dermatol. Syphilol. 68 430–446 [DOI] [PubMed] [Google Scholar]
- 2.Leyden, J. J., McGinley, K. J., Hoelzle, E., Labows, J. N., and Kligman, A. M. (1981) J. Investig. Dermatol. 77 413–416 [DOI] [PubMed] [Google Scholar]
- 3.Shehadeh, N., and Kligman, A. (1963) J. Investig. Dermatol. 41 1–5 [PubMed] [Google Scholar]
- 4.Jackman, P. J., and Noble, W. C. (1983) Clin. Exp. Dermatol. 8 259–268 [DOI] [PubMed] [Google Scholar]
- 5.Claus, R., and Alsing, W. (1976) J. Endocrinol. 68 483–484 [DOI] [PubMed] [Google Scholar]
- 6.Bird, S., and Gower, D. B. (1981) J. Steroid Biochem. 14 213–219 [DOI] [PubMed] [Google Scholar]
- 7.Brooksbank, B. W. L., Brown, R., and Gustafsson, J. A. (1974) Experientia (Basel) 30 864–865 [DOI] [PubMed] [Google Scholar]
- 8.Zeng, X. N., Leyden, J. J., Lawley, H. J., Sawano, K., Nohara, I., and Preti, G. (1991) J. Chem. Ecol. 17 1469–1492 [DOI] [PubMed] [Google Scholar]
- 9.Natsch, A., Gfeller, H., Gygax, P., Schmid, J., and Acuña, G. (2003) J. Biol. Chem. 278 5718–5727 [DOI] [PubMed] [Google Scholar]
- 10.Natsch, A., Derrer, S., Flachsmann, F., and Schmid, J. (2006) Chemistry & Biodiversity 3 1–20 [DOI] [PubMed] [Google Scholar]
- 11.Troccaz, M., Starkenmann, C., Niclass, Y., van de Waal, M., and Clark, A. J. (2004) Chemistry & Biodiversity 1 1022–1035 [DOI] [PubMed] [Google Scholar]
- 12.Natsch, A., Schmid, J., and Flachsmann, F. (2004) Chemistry & Biodiversity 1 1058–1072 [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa, Y., Yabuki, M., and Matsukane, M. (2004) Chemistry & Biodiversity 1 2042–2050 [DOI] [PubMed] [Google Scholar]
- 14.Starkenmann, C., Niclass, Y., Troccaz, M., and Clark, A. J. (2005) Chemistry & Biodiversity 2 705–716 [DOI] [PubMed] [Google Scholar]
- 15.Starkenmann, C., Clark, A., Troccaz, M., and Niclass, Y. (March 8, 2006) PCT International Patent Application WO 2006/079934 A2
- 16.Laemmli, U. K. (1970) Nature 227 680–685 [DOI] [PubMed] [Google Scholar]
- 17.Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Smith, J. A., and Struhl, K. (1995) Current Protocols in Molecular Biology, John Wiley and Sons, New York
- 18.Boyen, A., Charlier, D., Charlier, J., Sakanyan, V., Mett, I., and Glansdorff, N. (1992) Gene (Amst.) 116 1–6 [DOI] [PubMed] [Google Scholar]
- 19.Tauch, A., Kaiser, O., Hain, T., Goesmann, A., Weisshaar, B., Albersmeier, A., Bekel, T., Bischoff, N., Brune, I., Chakraborty, T., Kalinowski, J., Meyer, F., Rupp, O., Schneiker, S., Viehoever, P., and Puehler, A. (2005) J. Bacteriol. 187 4671–4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Minton, N. P., Atkinson, T., Bruton, C. J., and Sherwood, R. F. (1984) Gene (Amst.) 31 31–38 [DOI] [PubMed] [Google Scholar]
- 21.Westall, R. G. (1953) Biochem. J. 55 244–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hendriks, W. H., Harding, D. R. K., and Rutherfurd-Markwick, K. J. (2004) Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 139B 245–251 [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki, M., Kamiie, K., Soeta, S., Taira, H., and Yamashita, T. (2003) Biochem. J. 370 101–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyazaki, M., Yamashita, T., Suzuki, Y., Saito, Y., Soeta, S., Taira, H., and Suzuki, A. (2006) Chem. Biol. 13 1071–1079 [DOI] [PubMed] [Google Scholar]
- 25.Taylor, D., Daulby, A., Grimshaw, S., James, G., Mercer, J., and Vaziri, S. (2003) Int. J. Cosmet. Sci. 25 137–145 [DOI] [PubMed] [Google Scholar]
- 26.Bouvier, J., Richaud, C., Higgins, W., Bogler, O., and Stragier, P. (1992) J. Bacteriol. 174 5265–5271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McGregor, W. C., Swierczek, S. I., Bennett, B., and Holz, R. C. (2005) J. Am. Chem. Soc. 127 14100–14107 [DOI] [PubMed] [Google Scholar]
- 28.Rowsell, S., Pauptit, R. A., Tucker, A. D., Blow, D. M., and Brick, P. (1997) Structure (Lond.) 5 337–347 [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez, W., Fournie-Zaluski, M., Turcaud, S., Roques, B., and Michel, J. (1996) Cardiovasc. Drug Rev. 14 166–184 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.