This study demonstrates that IFN-γ–inducible chemokines and CXCR3 are key components of the ocular surface inflammatory response in dry eye syndrome. CXCL11 may be a major chemoattractant for effector T cells involved in the pathophysiology of dry eye.
Abstract
Purpose.
To investigate the expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome.
Methods.
Thirty-three patients with dry eye (16 with and 17 without Sjögren's syndrome) and 15 control subjects were recruited. The concentrations of CXCL9, -10, and -11 in tears were measured with enzyme-linked immunosorbent assays. The correlation between chemokine levels and tear film and ocular surface parameters was analyzed. The expression of CXCL9, -10, -11, and CXCR3 in the conjunctiva was evaluated by using immunohistochemistry. Flow cytometry was performed to count CXCR3+ cells and CXCR3+CD4+ cells in the conjunctiva.
Results.
The concentrations of CXCL9, -10, and -11 were 1,148 ± 1,088, 24,338 ± 8,706, and 853 ± 334 pg/mL, in the patients with dry eye, and 272 ± 269 (P = 0.01), 18,149 ± 5,266 (P = 0.02), and 486 ± 175 (P < 0.01) pg/mL in the control subjects, respectively. The concentrations significantly increased in tears of the patients with Sjögren's syndrome compared with those of the patients with non-Sjögren's dry eye (P < 0.05). CXCL10 levels correlated significantly with basal tear secretion, and CXCL11 levels correlated significantly with basal tear secretion, tear clearance rate, keratoepitheliopathy score, and goblet cell density (P < 0.05). Staining for CXCL9, -10, -11, and CXCR3 increased in patients with dry eye, especially in the patients with Sjögren's syndrome. Flow cytometry demonstrated an increased number of CXCR3+ and CXCR3+CD4+ cells in all the patients with dry eye.
Conclusions.
Expression of CXCL9, -10, -11, and CXCR3 increased in the tear film and ocular surface of patients with dry eye syndrome, especially in those with Sjögren's syndrome. CXCL11 levels correlated significantly with various tear film and ocular surface parameters. (ClinicalTrials.gov number, NCT00991679.)
Dry eye syndrome is a multifactorial disease of the tears and ocular surface, resulting in symptoms of ocular discomfort, visual disturbance, and tear film instability, with potential damage to the ocular surface.1 It is caused by aqueous-deficient conditions, including Sjögren's syndrome, non-Sjögren's dry eye, and evaporative conditions.
The pathogenesis of dry eye syndrome has not been clearly established. However, there is increasing evidence to suggest that it is associated with ocular surface inflammation and that the inflammatory component contributes to the dry eye symptoms as well as the disease process itself. Immunopathologic changes in the conjunctival epithelium of dry eyes include inflammatory cell infiltration; increased expression of immune activation and adhesion molecules, such as HLA-DR, intercellular adhesion molecule (ICAM)-1, and CD 40; elevated levels of inflammatory cytokines or chemokines, such as interleukin (IL)-1, -6, and -8 and tumor necrosis factor (TNF)-α; and increased expression of chemokine receptor CCR5.2–6 The ocular surface in dry eye syndrome also manifests increased concentration and activity of matrix metalloproteinases (MMPs), such as MMP-9, and increased apoptosis.7–9
Chemokines are low-molecular-mass (7- to 12-kDa) secreted proteins that play an important role in the recruitment of leukocytes to sites of inflammation.10,11 They are produced by a variety of cell types and are divided into four subgroups based on the position of the first two cysteines at the N-terminal region of the protein: CXC (α), CC (β), C (γ), and CXXXC (δ).12 CXC chemokines can be further divided into two groups. The first group, termed ELR+, contains a glutamic acid, leucine, and arginine tripeptide motif. The ELR motif has high affinity for CXCR1 and -2 chemokine receptors on neutrophils. The second group of CXC chemokines lacks the ELR motif and has a high affinity for the CXCR3 receptor found on activated T cells and NK cells.11–13 Members of the ELR– chemokine subset, which are readily induced by interferon (IFN)-γ, include monokine induced by interferon-γ (MIG/CXCL9), interferon-γ–inducible protein 10 kDa (IP-10/CXCL10), and interferon-inducible T-cell α chemoattractant (I-TAC/CXCL11). Chemokines are involved in specific attraction and expansion of T-cell subtypes based on their complement of chemokine receptors.14,15 Chemokine receptors CCR5 and CXCR3 are expressed primarily on T cells that mediate type 1 inflammatory responses, whereas CCR3 and -4 are expressed primarily on T cells that mediate type 2 responses.6,16–18
Although there is considerable evidence of the roles of chemokines and chemokine receptors in the pathogenesis of many acute and chronic inflammatory diseases, such as asthma and rheumatoid arthritis, very little is known about chemokines and their receptors in dry eye syndrome.19 Experimental studies have established that human corneal keratocytes and epithelial cells have the potential to produce ELR− chemokines, such as CXCL9, -10, and -11.20,21 In a mouse model of experimental dry eye, desiccating stress potently stimulated the expression of T-helper type 1 (Th-1) cell–attracting chemokines and their receptors on the ocular surface of C57BL/6 mice.22 In human studies, CCR5 expression has been shown to increase in the conjunctival epithelium of patients with dry eye syndrome.6,18
In the present study, we investigated the CXCL9, -10, and -11 levels in tears of patients with Sjögren's syndrome and those with non-Sjögren's dry eye, and compared the results with those of normal control subjects. We also analyzed the correlation between CXCL9, -10, and -11 levels and tear film and ocular surface parameters in patients with dry eye syndrome. The relative expressions of these chemokines and their receptor CXCR3 were evaluated in conjunctival biopsy specimens using immunohistochemistry and flow cytometry.
Materials and Methods
We prospectively investigated the expression of CXCL9/MIG, CXCL10/IP-10, CXCL11/I-TAC, and CXCR3 in the tear film and conjunctiva of patients with dry eye and normal control subjects. All subjects were recruited from the Department of Ophthalmology at Chonnam National University Hospital. Informed consent was obtained from each subject enrolled. Institutional review board/ethics committee approval was obtained from the Chonnam National University Medical School Institutional Review Board. The study protocol adhered to the guidelines of the Declaration of Helsinki.
Patient Selection
Patients with dry eye syndrome who had had symptoms for more than 3 months, low tear film break-up time (BUT, ≤7 seconds), low Schirmer test result (<10 mm), low tear clearance rate (<8×), and positive fluorescein or rose bengal vital staining (≥3) and were not treated with anti-inflammatory agents such as topical cyclosporine or steroids were included in the study. The diagnosis of Sjögren's syndrome was based on the criteria proposed by the American-European Consensus Group23 and included the following: (1) ocular symptoms, (2) oral symptoms, (3) ocular signs (Schirmer 1 test score ≤5 mm or rose bengal score ≥4), (4) focal sialoadenitis in minor salivary gland, (5) objective evidence of salivary gland involvement, and (6) presence of serum autoantibodies (antibodies to Ro and/or La antigens). The remaining patients with dry eye, with symptoms and positive findings in tear function tests or staining scores, received a diagnosis of non-Sjögren's dry eye. Individuals who had an active ocular infection or inflammation not associated with dry eye, drug toxicity, contact lens wear, ocular allergy, ocular surgery within the past 3 months, or lid or lash abnormalities were excluded from the study.
Thirty-three patients with dry eye (27 women and 6 men; mean age, 48.23 ± 16.61 years; range, 24–77) were recruited for the study. Sixteen patients (14 women and 2 men; mean age, 49.9 ± 14.6 years; range, 30–74) had Sjögren's syndrome, and 17 (13 women and 4 men; mean age, 47.3 ± 17.8 years; range, 24–77) had non-Sjögren's dry eye. Fifteen volunteers (11 women and 4 men; mean age, 43.0 ± 14.9 years; range, 28–67) participated as control subjects.
Tear Collection
Basal tear samples were collected atraumatically from the inferior tear meniscus of both eyes by using glass capillary tubes (Corning, Inc., Corning, NY) or micropipettes (Eppendorf, Hamburg, Germany). Care was taken to avoid touching the corneal and conjunctival surfaces. Thirty-microliter tear samples were obtained and diluted with phosphate-buffered saline (PBS). Tear samples were placed in microtubes (Eppendorf) and stored at −70°C until further examination.
Measurement of MIG, IP-10, and I-TAC Levels in Tears
The CXCL9, -10, and -11 concentrations were measured with commercial enzyme-linked immunosorbent assay (ELISA) kits (Quantikine; R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. The sensitivities of the CXCL9, -10, and -11 concentrations were higher than 3.84, 1.67, and 13.90 pg/mL, respectively.
Evaluation of Tear Film and Ocular Surface
Corneal sensitivity, tear film BUT, basal tear secretion, tear clearance rate, keratoepitheliopathy scoring, and conjunctival goblet cell density were evaluated. All examinations were performed by the same researcher.
Corneal sensitivity was measured with a Cochet-Bonnet esthesiometer (Luneau, Chartres, France). The tip of the fully extended nylon filament was applied perpendicularly to the central corneal surface and advanced steadily. When the subject felt its presence, the length of the filament was recorded in millimeters.
Tear film BUT and Schirmer test results with topical anesthesia were measured as previously described.24 The tear clearance rate was determined 5 minutes after instillation of a 10-μL drop of 0.5% fluorescein and 0.4% oxybuprocaine hydrochloride into the conjunctival sac. A standard Schirmer test strip was then placed for another 5 minutes. The intensity of the staining was compared with a color standard. The tear clearance rate was determined based on the rate at which the color of the fluorescein dye faded (graded as 1, 0.5, 0.25, 0.125, 0.0625, 0.03125, 0.015625, 0.0078125, or 0.0039062) and was represented in logarithmic form.25
After the cornea was stained with 1% fluorescein dye, the severity of keratoepitheliopathy was scored by multiplying the area score by the density score of staining. The staining area was graded on a numerical scale of 0 to 3, with 0 representing no punctate staining and 1, less than one third; 2, one third to two thirds; and 3, more than two thirds staining. The staining density was also graded on a numerical scale of 0 to 3, with 0 representing no punctate staining; 1, sparse density; 2, moderate density; and 3, high density with overlapping lesions.26
Impression cytology was performed from the lower bulbar conjunctiva by using strips of cellulose acetate filter paper (MFS membrane filters; Advantec MFS, Dublin, CA) according to a previously reported method.27 Specimens were fixed in absolute alcohol, stained with periodic acid-Schiff, and photographed at a magnification of ×400. Goblet cell density was calculated as the number of cells per square millimeter.
Immunohistochemistry
Immunohistochemistry was performed to evaluate the relative expression of CXCL9, -10, and -11 and to detect and count cells that stained positively for the chemokine receptor CXCR3 in the conjunctiva. Conjunctival biopsy specimens were obtained from the lower temporal bulbar conjunctiva in all the patients with dry eye and the control subjects (n = 6). The conjunctival tissues were excised and immersed in 4% paraformaldehyde fixative overnight at 4°C. The tissue blocks were washed, dehydrated, embedded in paraffin, cut at 3 μm, and mounted.
After fixation, endogenous peroxidases were quenched with 0.3% H2O2 in PBS for 10 minutes. The sections were sequentially blocked with avidin/biotin block (Vector Laboratories, Burlingame, CA) for 10 minutes each. After the sections were blocked with 20% normal serum in PBS for 45 minutes, mouse monoclonal anti-CXCL9/MIG antibody (R&D Systems), mouse monoclonal anti-CXCL10/IP-10 antibody (R&D Systems), mouse monoclonal anti-CXCL11/I-TAC antibody (R&D Systems), and rabbit polyclonal anti-CXCR3 (R&D Systems) were applied separately and incubated for 1 hour at RT. After the sections were washed, they were incubated with biotinylated secondary antibodies. The samples were then incubated with 3,3′-diaminobenzidine (NovaRed; Vector Laboratories) peroxidase substrate and counterstained with Mayer's hematoxylin. Secondary antibody alone and appropriate anti-human isotypes (R&D Systems) were used in control experiments.
Staining intensities of CXCL9, -10, and -11 were graded on a numerical scale of 0 to 3: 0, no different from the secondary antibody control; 1, slightly greater than the secondary antibody control; 2, moderate staining; and 3, intense staining.22,28 CXCR3-positive cells were counted over a length of at least 500 μm in the conjunctival epithelium with image-analysis software (Media Cybernetics, Silver Spring, MD).22,29 The results were expressed as the number of positive cells per linear 50 μm.
Flow Cytometry
Flow cytometry was performed to count the number of CXCR3+ cells from the conjunctiva, as previously described, with modifications.30 Conjunctival cells were harvested from the conjunctival biopsy specimens from each group (n = 6), which were dipped in PBS. The tissues were teased with scissors and shaken at 37°C for 60 minutes with collagenase type D (Roche Applied Science, Indianapolis, IN). After incubation, the tissues were disrupted by being ground with a syringe plunger and passed through a cell strainer. The cells were centrifuged at 1500 rpm for 7 minutes, resuspended in PBS with 1% bovine serum albumin, washed, stained with trypan blue, and counted. The samples were incubated with phycoerythrin-conjugated mouse monoclonal anti-human CXCR3 antibody (R&D Systems) and isotype control antibody at 37°C for 30 minutes.
Two-color flow cytometry was performed to clarify the phenotype of CXCR3+ cells, by using anti-CXCR3 and anti-CD4 antibodies. Phycoerythrin-conjugated mouse monoclonal anti-human CXCR3 antibody (R&D Systems) and fluorescein-conjugated mouse monoclonal anti-human CD4 antibody (BD Bioscience, San Jose, CA) were used. Flow cytometry was then performed (FACSCalibur with CellQuest software; BD Bioscience).
Statistical Analysis
The t-test and ANOVA with post hoc test were used to compare the results between groups. Spearman correlation coefficients were calculated for the correlation between MIG, IP-10, and I-TAC levels and tear film and ocular surface parameters. P < 0.05 was considered statistically significant.
Results
Demographics and results of tear film and ocular surface parameters in the control subjects and patients with dry eye syndrome are presented in Table 1.
Table 1.
Demographics and Results of Tear Film and Ocular Surface Parameters in Control Subjects and Patients with Dry Eye Syndrome
| Control Group (n = 15) | Dry Eye Group (n = 33) |
||
|---|---|---|---|
| Non-SS | SS | ||
| Age, y | |||
| Mean | 43.0 ± 14.9 | 48.2 ± 16.6 | |
| 47.3 ± 17.8 | 49.9 ± 14.6 | ||
| Range | 28–67 | 24–77 | |
| 24–77 | 30–74 | ||
| Sex (M/F) | 4/11 | 6/27 | |
| 4/13 | 2/14 | ||
| Tear film and ocular surface parameters | |||
| Corneal sensitivity, mm | 58.5 ± 2.4 | 55.7 ± 2.6 | |
| 55.1 ± 2.8 | 56.3 ± 2.6 | ||
| Tear film break-up time, s | 12.4 ± 2.4 | 3.7 ± 2.5* | |
| 4.4 ± 2.4 | 2.7 ± 1.4† | ||
| Basal tear secretion, mm | 12.0 ± 3.0 | 5.2 ± 2.5* | |
| 6.4 ± 1.7 | 3.8 ± 1.8† | ||
| Tear clearance rate, (Log2)−1 | 5.6 ± 0.8 | 2.2 ± 0.8* | |
| 2.5 ± 1.2 | 1.9 ± 0.9† | ||
| Keratoepitheliopathy score | 0.1 ± 0.3 | 5.6 ± 3.6* | |
| 4.3 ± 2.4 | 6.9 ± 2.1† | ||
| Goblet cell density, cells/mm2 | 357.6 ± 33.1 | 155.2 ± 36.6* | |
| 202.1 ± 32.6 | 101.5 ± 28.5† | ||
Non-SS, non-Sjögren's syndrome; SS, Sjögren's syndrome.
P < 0.05 compared with normal control.
P < 0.05 compared with non-Sjögren's syndrome.
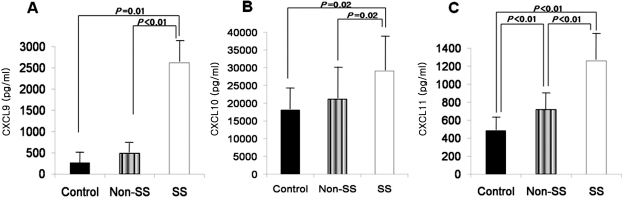
The mean levels of CXCL9 in tears were 272 ± 269 pg/mL in the control subjects and 1148 ± 1088 pg/mL in both groups of patients with dry eye (P = 0.01): 491 ± 235 pg/mL in the patients with non-Sjögren's dry eye and 2626 ± 621 pg/mL in the patients with Sjögren's syndrome. The CXCL9 levels were higher in the patients with Sjögren's syndrome than in those with non-Sjögren's dry eye (P < 0.01) or the control subjects (P = 0.01). However, there was no significant difference in CXCL9 levels between the patients with non-Sjögren's dry eye and the control subjects (Fig. 1A).
Figure 1.
CXCL9 (A), -10 (B), and -11 (C) levels in tears of control subjects, patients with non-Sjögren's dry eye (non-SS), and those with Sjögren's syndrome (SS).
The mean levels of CXCL10 in tears were 18,149 ± 5,266 pg/mL in the control subjects and 24,338 ± 8,706 pg/mL in the patients with dry eye (P = 0.02): 21,173 ± 7,773 pg/mL in the patients with non-Sjögren's dry eye and 29,086 ± 8,148 pg/mL in the patients with Sjögren's syndrome. The tear levels of CXCL10 were higher in the patients with Sjögren's syndrome than in those with non-Sjögren's dry eye (P = 0.02) or the control subjects (P = 0.02). However, there was no significant difference in CXCL10 levels between the patients with non-Sjögren's dry eye and the control subjects (Fig. 1B).
The mean levels of CXCL11 in tears were 486 ± 175 pg/mL in the control subjects and 853 ± 334 pg/mL in the patients with dry eye (P < 0.01): 717 ± 205 pg/mL in the patients with non-Sjögren's dry eye and 1261 ± 324 pg/mL in the patients with Sjögren's syndrome. The tear levels of CXCL11 were higher in the patients with Sjögren's syndrome than in the patients with non-Sjögren's dry eye (P < 0.01) and the control subjects (P < 0.01). The CXCL11 levels in the patients with non-Sjögren's dry eye were higher than in the control subjects (P < 0.01; Fig. 1C).
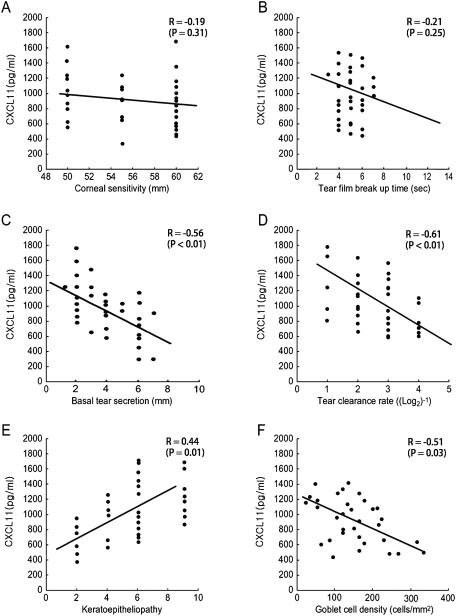
The correlation between CXCL9, -10, and -11 levels in tears and tear film and ocular surface parameters in patients with dry eye were evaluated. There was no significant correlation between CXCL9 concentration and any clinical parameter. CXCL10 showed significant correlation with basal tear secretion (R = −0.47, P = 0.03) only. On the other hand, the CXCL11 level correlated significantly with basal tear secretion (R = −0.56, P < 0.01), tear clearance rate (R = −0.61, P < 0.01), keratoepitheliopathy score (R = 0.44, P = 0.01), and goblet cell density (R = −0.51, P = 0.03), but not with tear film BUT (R = −0.19, P = 0.31) or corneal sensitivity (R = −0.21, P = 0.25; Fig. 2).
Figure 2.
Correlation between tear CXCL11 level and tear surface parameters including corneal sensitivity (A), tear film BUT (B), basal tear secretion (C), tear clearance rate (D), keratoepitheliopathy score (E), and conjunctival goblet cell density (F).
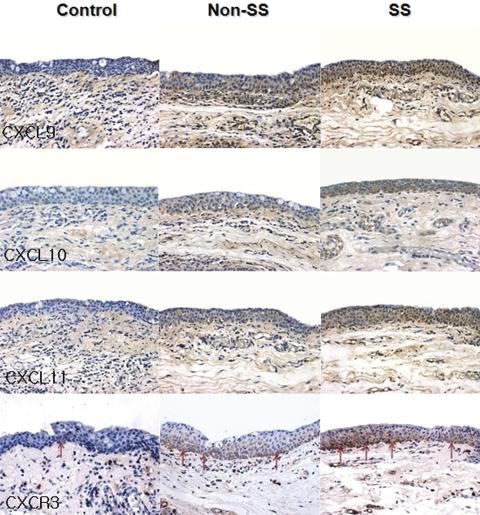
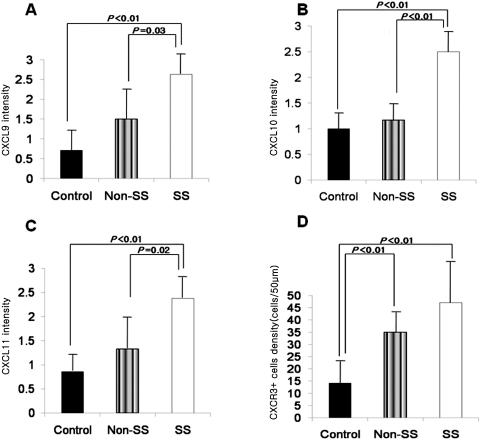
Immunohistochemical staining showed higher levels of CXCL9, -10, and -11 in the conjunctival epithelium of patients with dry eye than that of the control subjects. The density of CXCR3+ cells was also higher in the basal layer of the epithelium and subepithelial stroma of patients with dry eye than in that of the control subjects (Fig. 3). Staining with the secondary antibody control was negative. The mean staining intensities of CXCL9 were 0.71 ± 0.49 in the control subjects, 1.50 ± 0.61 (P = 0.14 compared with the control group) in the patients non-Sjögren's dry eye, and 2.63 ± 0.48 (P < 0.01 compared with the control group; P < 0.03 compared with the non-Sjögren's dry eye group) in the patients with Sjögren's syndrome. The mean staining intensities of CXCL10 were 1.00 ± 0.58 in the control subjects, 1.17 ± 0.37 (P = 0.73 compared with the control group) in the patients with non-Sjögren's dry eye, and 2.50 ± 0.53 in the patients with Sjögren's syndrome (P < 0.01 compared with the control group; P < 0.01 compared with the non-Sjögren's dry eye group). The mean staining intensities of CXCL10 were 0.86 ± 0.38, 1.33 ± 0.56 (P = 0.45 compared with the control group), and 2.38 ± 0.44 (P < 0.01 compared with the control group; P < 0.02 compared with the non-Sjögren's dry eye group) in the control subjects, the patients with non-Sjögren's dry eye, and the patients with Sjögren's syndrome, respectively (Figs. 4A–C). The mean density of CXCR3+ cells was 14.14 ± 10.67 cells/50 μm in the control subjects, 35.00 ± 9.12 cells/50 μm in the patients with non-Sjögren's dry eye (P < 0.01 compared with the control group), and 47.09 ± 18.31 cells/50 μm in the patients with Sjögren's syndrome (P < 0.01; Fig. 4D).
Figure 3.
Immunohistochemistry for CXCL9, -10, and -11, and CXCR3 in the conjunctiva of the control subjects, the patients with non-Sjögren's dry eye (non-SS), and the patients with Sjögren's syndrome (SS).
Figure 4.
Mean immunostaining intensity for CXCL9 (A), -10 (B), and -11 (C) and mean density of CXCR3+ cells (D) in the conjunctiva.
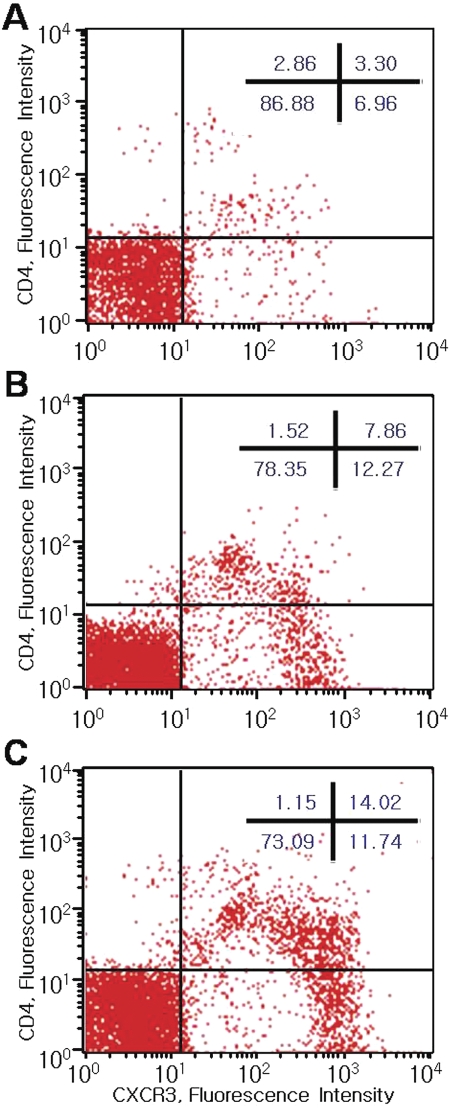
The mean percentages of CXCR3+ cells in flow cytometry were 6.9% ± 2.4% in the control group, 21.0% ± 8.3% in the non-Sjögren's dry eye group (P = 0.02 compared with the control group), and 31.6% ± 12.3% in the Sjögren's syndrome group (P < 0.01). The mean percentages of CXCR3+CD4+ cells were 3.2% ± 1.8% in the control group, 9.1% ± 3.7% in the non-Sjögren's dry eye group (P = 0.04 compared with the control group), and 13.5% ± 6.2% in the Sjögren's syndrome group (P < 0.01). However, there was no significant difference between the non-Sjögren's and the Sjögren's dry eye groups. The mean percentages of CXCR3+CD4− cells were 7.2% ± 2.9% in the control group, 11.4% ± 4.3% in the non-Sjögren's dry eye group (P = 0.09 compared with the control group), and 10.8% ± 4.0% in the Sjögren's syndrome group (P = 0.13). Histograms of the percentages of CXCR3+ and CD4+ cells in representative samples from each group are shown in Figure 5.
Figure 5.
Two-color flow cytometry showing CXCR3+ and CD4+ cells in the conjunctiva of the control subjects (A), the patients with non-Sjögren's dry eye (B), and the patients with Sjögren's syndrome (C).
Discussion
Several pathologic findings in the ocular surface epithelium indicate that dry eye is associated with ocular surface inflammation. Pflugfelder et al.5 reported that the levels of IL-1α, -6, and -8 and TNF-α, and transforming growth factor-(TGF-β1) RNA transcripts were significantly increased in the conjunctival epithelium of patients with Sjögren's syndrome compared with normal subjects. IL-1α and TNF-α are the primary proinflammatory cytokines released into corneal tissue at sites of injury or disease. These proinflammatory mediators activate synthesis of several ELR+ α-chemokines that play an important role in chemoattracting neutrophils to sites of inflammation.
IFN-γ, mainly produced by activated T lymphocytes and NK cells, is a pleiotropic cytokine involved in the regulation of nearly all phases of the immune and inflammatory responses. It has recently been proposed that IFN-γ stimulates the production of CXCL9, -10, and -11 in the ductal epithelium of the salivary glands of patients with Sjögren's syndrome and that CXCL9, -10, and -11 are involved in the accumulation of T-cell infiltrates in the salivary glands of these patients.31,32 De Paiva et al.33 reported that experimental dry eye promotes migration of CD4+ T cells and IFN-γ+ cells into goblet cell zones of the conjunctiva and increases the concentration of IFN-γ in tears. They suggested that IFN-γ plays a pivotal role in promoting conjunctival squamous metaplasia in dry eye. Furthermore, McInnis et al.20,21 reported that human corneal epithelial cells and keratocytes can synthesize the ELR– α-chemokines essential in chemoattracting activated T-cell subsets to sites of inflammation in response to proinflammatory mediators, including IFN-γ.
Chemokines are known to be produced by a variety of cell types in response to inflammatory cytokines, and they operate through regulated expression of specific chemokine receptors.34 Chemokines play a role in leukocyte movement, inflammation, response to infection, angiogenesis, tumor growth, and stem cell proliferation.11 We demonstrated in a prior study the effects of desiccating stress on the expression of chemokines and their receptors by the ocular surface tissues of mice.22 In the C57BL/6 mouse, which has a predilection for a Th-1 immune response to proinflammatory stress, there was a significant increase in concentrations of macrophage inflammatory protein (MIP)-1α and -1β, MIG, and IP-10 proteins in the corneal epithelium and conjunctiva. Desiccating stress also increased MIP-1α and -1β and CCR5 transcripts in the cornea and conjunctiva and RANTES, MIG, IP-10, and CXCR3 transcripts in the conjunctiva of C57BL/6 mice.
The tear film layer plays a critical role in the innate and acquired host defense systems. Increased chemokine levels have been demonstrated in tears of closed eyes. Sack et al.35 used a membrane-bound antibody array system (mass spectrometric analysis) and found that several CXC chemokines (IL-8, epithelial neutrophil–activating protein [ENA]-78, IP-10, and growth-related oncogene [GRO]), as well as two CC chemokines (monocyte chemoattractant protein [MCP]-1 and MIP-1β), accumulates in closed-eye tear fluid. To our knowledge, no studies have been performed to describe ELR– CXC chemokines in the setting of human dry eye disease.
In the present study, we investigated the expression of IFN-γ–inducible ELR– CXC chemokines (CXCL9, -10, and -11) and their receptor CXCR3 in the tear film and ocular surface of patients with dry eye. The CXCL9, -10, and -11 levels significantly increased in tears of the patients with dry eye compared with those in the control subjects. These levels were higher in the patients with Sjögren's syndrome than those with non-Sjögren's dry eye. Immunohistochemical staining for CXCL9, -10, -11, and CXCR3 supported these results. The chemokine staining increased in the conjunctival epithelium, and CXCR3+ cells were detected distinctly in the nearby basal epithelium and subepithelial stroma of the conjunctiva in the patients with dry eye. Flow cytometry showed more CXCR3+ cells in the conjunctiva in the patients with dry eye than in the control subjects. Using two-color flow cytometry, we also demonstrated CXCR3+CD4+ cells in the ocular surface, which increased in non-Sjögren's and Sjögren's dry eye. Although statistically insignificant, a considerable amount of CXCR3+CD4- cells was also detected in the conjunctiva of the patients with dry eye. Because the population of NK cells is very low among total lymphocytes, we suppose that CXCR3+CD4− cells are predominantly CD8+ cells. The findings suggest that these IFN-γ–inducible chemokines and CXCR3 are key components of the ocular surface inflammatory response in dry eye syndrome.
Among these chemokines, CXCL11 is known to be the most potent inducer of CXCR3 internalization and the physiologic inducer of CXCR3 internalization after T-cell contact with activated endothelial cells.36 Moreover, CXCL11 has a significantly higher affinity for CXCR3 than CXCL9 or -10 does, which is demonstrated by transient mobilization of intercellular calcium as well as by chemotactic migration in both activated T cells and CXCR3+ cells.37 In this study, in contrast to CXCL9 and -10, the CXCL11 levels correlated significantly with various tear film and ocular surface parameters, such as basal tear secretion, tear clearance rate, keratoepitheliopathy, and conjunctival goblet cell density. Therefore, our results as well as those already reported suggest that CXCL11 is a major chemoattractant for effector T cells involved in the pathophysiology of immune inflammatory disorders such as dry eye syndrome. Measurement of CXCL11 levels in tears may help to define disease progression in patients with dry eye and to evaluate the therapeutic efficacy of immunosuppressive agents such as cyclosporine eye drops for dry eye.
In our study, low levels of the chemokines were detected in the tear film and ocular surface of the healthy control subjects. The expression of the chemokines in normal subjects may be important in host immune defense and immune maintenance on the ocular surface. Increased expression of the Th-1 chemokines and their receptor CXCR3 in the patients with dry eye supports the current concepts that dry eye disease can be caused by inflammation of the ocular surface and lacrimal gland and that CD4+ T cells are the main effector cells. The higher expression in patients with Sjögren's syndrome correlates with the more severe inflammation in this group. It has been known that both Sjögren's and non- Sjögren's dry eyes have conjunctival inflammation manifested by T-cell infiltration and immune inflammatory marker expression.38 Although there were differences in tear CXCL11 level and CXCR3+ cell density between the non- Sjögren's syndrome and control groups in our study, the CXCL9 and -10 levels in the non-Sjögren's syndrome group were not significantly higher than those in the control group. This discrepancy can be explained by relatively lower sensitivity and affinity of CXCL9 and -10 for T cells compared with CXCL11, as mentioned.
A limitation of our study is that the staining intensity for chemokines was graded rather subjectively. Differences in chemokine expression between the aqueous-deficient and the evaporative types in the non-Sjögren's dry eye group were not evaluated. In addition, except for CD4+ T cells, the presence of the other phenotypes of CXCR3+ cells was not determined in the conjunctiva. Flow cytometry from whole conjunctival tissues analyzed the CXCR3+ or CD4+ cells in the stroma as well as the epithelium and may not exactly reflect the population of the epithelial immune cells.
In conclusion, the expression of CXCL9, -10, and -11 and CXCR3 increased in the tear film and on the ocular surface of patients with dry eye, especially in those with Sjögren's syndrome. CXCL11 levels correlated significantly with various tear film and ocular surface parameters.
Footnotes
Disclosure: K.-C. Yoon, None; C.-S. Park, None; I.-C. You, None; H.-J. Choi, None; K.-H. Lee, None; S.-K. Im, None; H.-Y. Park, None; S.C. Pflugfelder, None
References
- 1. Lemp MA, Baudouin C, Baum J, et al. The definition of classification of dry eye diseases: report of the Definition and Classification Subcommittee of the International Dry Eye Work Shop 2007. Ocul Surf. 2007; 5: 75–92 [DOI] [PubMed] [Google Scholar]
- 2. Raphael M, Bellefqih S, Piette JC, Le Hoang P, Debre P, Chomette G. Conjunctival biopsy in Sjögren's syndrome: correlations between histological and immunohistochemical features. Histopathology. 1988; 13: 191–202 [DOI] [PubMed] [Google Scholar]
- 3. Jones DT, Monroy D, Ji Z, Atherton SS, Pflugfelder SC. Sjögren's syndrome: cytokine and Epstein-Barr viral gene expression within the conjunctival epithelium Invest Opthalmol Vis Sci. 1994; 35: 3493–3504 [PubMed] [Google Scholar]
- 4. Pflugfelder SC, Huang AJ, Feuer W, Chuchovski PT, Pereira IC, Tseng SC. Conjunctival cytologic features of primary Sjögren's syndrome. Ophthalmology. 1990; 97: 985–991 [DOI] [PubMed] [Google Scholar]
- 5. Pflugfelder SC, Jones D, Ji Z, Afonso A, Monroy D. Altered cytokine balance in the tear fluid and conjunctiva of patients with Sjögren's syndrome keratoconjunctivitis sicca. Curr Eye Res. 1999; 19: 201–211 [DOI] [PubMed] [Google Scholar]
- 6. Gulati A, Sacchetti M, Bonini S, Dana R. Chemokine receptor CCR5 expression in conjunctival epithelium of patients with dry eye syndrome. Arch Ophthalmol. 2006; 124: 710–716 [DOI] [PubMed] [Google Scholar]
- 7. Pflugfelder SC, Farley W, Luo L, et al. Matrix metalloproteinase-9 knockout confers resistance to corneal epithelial barrier disruption in experimental dry eye. Am J Pathol. 2005; 166: 61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corrales RM, Stern ME, De Paiva CS, Welch J, Li DQ, Pflugfelder SC. Desiccating stress stimulates expression of matrix metalloproteinases by the corneal epithelium. Invest Ophthalmol Vis Sci. 2006; 47: 3293–3302 [DOI] [PubMed] [Google Scholar]
- 9. Yeh S, Song XJ, Farley W, Li DQ, Stern ME, Pflugfelder SC. Apoptosis of ocular surface cells in experimentally induced dry eye. Invest Ophthalmol Vis Sci. 2003; 44: 124–129 [DOI] [PubMed] [Google Scholar]
- 10. Sallusto F, Baggiolini M. Chemokines and leukocyte traffic. Nat Immunol. 2008; 9: 949–952 [DOI] [PubMed] [Google Scholar]
- 11. Moser B, Wolf M, Walz A, Loestscher P. Chemokines: multiple levels of leukocyte migration control. Trends Immunol. 2004; 25: 75–84 [DOI] [PubMed] [Google Scholar]
- 12. Bacon K, Baggiolini M, Broxmeyer H, et al. IUIS/WHO Subcommittee on Chemokine Nomenclature: chemokine/chemokine receptor nomenclature. J Interferon Cytokine Res. 2002; 22: 1067–1068 [DOI] [PubMed] [Google Scholar]
- 13. Balestrieri ML, Balestrieri A, Mancini FP, Napoli C. Understanding the immunoangiostatic CXC chemokine network. Cardiovasc Res. 2008; 78: 250–256 [DOI] [PubMed] [Google Scholar]
- 14. Colantonio L, Recalde H, Sinigaglia F, D'Ambrosio D. Modulation of chemokine receptor expression and chemotactic responsiveness during differentiation of human naive T cells into Th1 or Th2 cells. Eur J Immunol. 2002; 32: 1264–1273 [DOI] [PubMed] [Google Scholar]
- 15. Wallace GR, John Curnow S, Wloka K, Salmon M, Murray PI. The role of chemokines and their receptors in ocular disease. Prog Retin Eye Res. 2004; 23: 435–448 [DOI] [PubMed] [Google Scholar]
- 16. Turner JE, Steinmetz OM, Stahl RA, Panzer U. Targeting of Th1-associated chemokine receptors CXCR3 and CCR5 as therapeutic strategy for inflammatory diseases. Mini Rev Med Chem. 2007; 7: 1089–1096 [DOI] [PubMed] [Google Scholar]
- 17. Annunziato F, Maggi E, Romagnani S, Manetti R. Chemoattractant receptors expressed on type 2 T cells and their role in disease. Int Arch Allergy Immunol. 2001; 125: 273–279 [DOI] [PubMed] [Google Scholar]
- 18. Baudouin C, Liang H, Bremond-Gignac D, et al. CCR 4 and CCR 5 expression in conjunctival specimens as differential markers of T(H1)/T(H)2 in ocular surface disorders. J Allergy Clin Immunol. 2005; 116: 614–619 [DOI] [PubMed] [Google Scholar]
- 19. Gerard C, Rollins BJ. Chemokine and disease. Nat Immunol. 2001; 2: 108–115 [DOI] [PubMed] [Google Scholar]
- 20. McInnis KA, Britain A, Lausch RN, Oakes JE. Synthesis of α-chemokines IP-10, I-TAC, and MIG are differentially regulated in human corneal keratocytes. Invest Ophthalmol Vis Sci. 2005; 46: 1668–1674 [DOI] [PubMed] [Google Scholar]
- 21. McInnis KA, Britain A, Lausch RN, Oakes JE. Human corneal epithelial cells synthesize ELR- α-chemokines in response to proinflammatory mediators. Ocul Immunol Inflamm. 2007; 15: 295–302 [DOI] [PubMed] [Google Scholar]
- 22. Yoon KC, De Paiva CS, Qi H, et al. Expression of Th-1 chemokines and chemokine receptors on the ocular surface of C57BL/6 mice: effects of desiccating stress. Invest Ophthalmol Vis Sci. 2007; 48: 2561–2569 [DOI] [PubMed] [Google Scholar]
- 23. Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria of Sjögren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002; 61: 554–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology. 2001; 108: 586–592 [DOI] [PubMed] [Google Scholar]
- 25. Yoon KC, Heo H, Im SK, You IC, Kim YH, Park YG. Comparison of autologous serum and umbilical cord serum eye drops for dry eye syndrome. Am J Ophthalmol. 2007; 144: 86–92 [DOI] [PubMed] [Google Scholar]
- 26. Yoon KC, Jeong IY, Park YG, Yang SY. Interleukin-6 and tumor necrosis factor-alpha levels in tears of patients with dry eye syndrome. Cornea. 2007; 26: 431–437 [DOI] [PubMed] [Google Scholar]
- 27. Yoon KC, Im SK, Park YG, Jung YD, Yang SY, Choi J. Application of umbilical cord serum eyedrops for the treatment of dry eye syndrome. Cornea. 2006; 25: 268–272 [DOI] [PubMed] [Google Scholar]
- 28. Liu Z, Carvajal M, Carothers Carraway CA, Carraway KL, Pflugfleder SC. Increased expression of the type 1 growth factor receptor family in the conjunctival epithelium of patients with keratoconjunctivitis sicca. Am J Ophthalmol. 2000; 129: 472–480 [DOI] [PubMed] [Google Scholar]
- 29. Yoon KC, De Paiva CS, Chen Z, et al. Desiccating environmental stress exacerbate autoimmune lacrimal keratoconjunctivitis in non-obese diabetic mice. J Autoimmun. 2008; 30: 212–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yoon KC, Heo H, Kang IS, et al. Effect of topical cyclosporine A on herpes stromal keratitis in a mouse model. Cornea. 2008; 27: 454–460 [DOI] [PubMed] [Google Scholar]
- 31. Ogawa N, Ping L, Zhenjun L, Takada Y, Sugai S. Involvement of the interferon-γ-induced T cell-attracting chemokines, interferon-γ-inducible 10-kd protein (CXCL10) and monokine induced by interferon-γ (CXCL9), in the salivary gland lesions of patients with Sjögren's syndrome. Arthritis Rheum. 2002; 46: 2730–2741 [DOI] [PubMed] [Google Scholar]
- 32. Ogawa N, Kawanami T, Shimoyama K, Ping L, Sugai S. Expression of interferon-inducible T cell alpha chemoattractant (CXCL11) in the salivary glands of patients with Sjögren's syndrome. Clin Immunol. 2004; 112: 235–238 [DOI] [PubMed] [Google Scholar]
- 33. De Paiva CS, Villarreal AL, Corrales RM, et al. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-γ. Invest Ophthalmol Vis Sci. 2007; 48: 2553–2560 [DOI] [PubMed] [Google Scholar]
- 34. Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000; 18: 217–242 [DOI] [PubMed] [Google Scholar]
- 35. Sack RA, Conradi L, Krumholz D, Beaton A, Sathe S, Morris C. Membrane array characterization of 80 chemokines, cytokines, and growth factors in open- and closed-eye tears: angiogenin and other defense system constituents. Invest Ophthalmol Vis Sci. 2005; 45: 1228–1238 [DOI] [PubMed] [Google Scholar]
- 36. Colvin RA, Campanella GS, Sun J, Luster AD. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function. J Biol Chem. 2004; 279: 30219–30227 [DOI] [PubMed] [Google Scholar]
- 37. Cole KE, Strick CA, Paradis TJ, et al. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998; 187: 2009–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjogren's and non-Sjogren's patients with dry eye. Invest Ophthalmol Vis Sci. 2002; 43: 2609–2614 [PubMed] [Google Scholar]