One of the most striking features of the vertebrates is that their retinal structures are remarkably similar, as recognized by Ramon y Cajal over 100 years ago.1 All vertebrates have two types of photoreceptors, rods and cones, with a ubiquitous molecular cascade underlying phototransduction in the outer segments of both cell types.2,3 Five major types of second-/third-order retinal neurons are found in all vertebrate species: bipolar cells (BCs, including the hyperpolarizing and depolarizing BCs, HBCs, and DBCs), horizontal cells (HCs), amacrine cells (ACs), interplexiform cells (IPCs), and ganglion cells (GCs).4 The neuronal somas are located in three nuclear layers, and their axonal and dendritic processes form complex and orderly networks of chemical and electrical synapses in two (outer and inner) plexiform layers (OPL and IPL).1 During the past few decades, detailed studies on synaptic connectivity and functional pathways in the retina have been performed in many species, including fish, amphibians, reptiles, mammals, and humans. Results from these studies indicate that retinal synapses are organized in an intricate and orderly fashion, to efficiently process visual signals,4,5 and that synaptic pathways in the retina are arranged according to several general, cross-species principles.6,7 These principles dictate how retinal neurons are connected, synapses are formed, and various synaptic pathways are used to process different attributes of visual stimuli. Since not every type of retinal cell in every vertebrate is equally accessible for experimentation, identifying cross-species, general synaptic principles is important, not only to unravel how the first stages of the visual system operate, but also to facilitate our understanding of retinal function and dysfunction in less accessible animals, especially in humans.
In this article, I shall discuss several general principles of synaptic organization in the vertebrate retina and their roles in visual information processing. Moreover, I will briefly mention several species-specific variations in retinal synaptic organization, but because of the space limitation, I will focus on the mammalian-specific AII amacrine cell (AIIAC) pathway.
General Principles of Synaptic Organization in Vertebrate Retinas
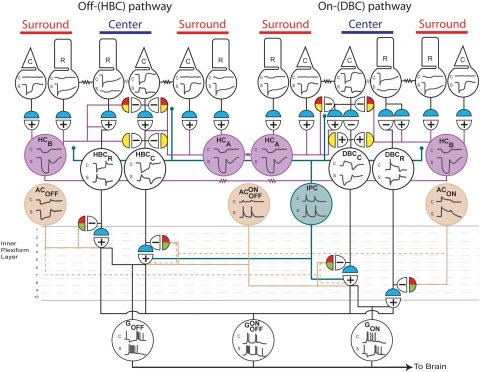
Synaptic pathways mediating signal transmission in all vertebrate retinas follow a very similar plan. When light falls on the retina, its energy is transduced into electrical signals in the form of photoreceptor membrane hyperpolarization.8 Photoreceptor signals are transmitted and processed by the retinal network whose synaptic pathways project signals in three directions. The radial pathways include several parallel photoreceptor→BC→GC synaptic channels; the lateral pathways include the HC→BC (outer retina) and the AC→BC/GC (inner retina) synapses; and the feedback pathways are the HC→cone (outer retina), AC→BC (inner retina), IPC→photoreceptor/BC/HC (from inner to outer retina), and the centrifugal fiber→IPC/AC (brain to inner retina) synapses.4,6,9 The radial synapses are primarily glutamatergic, most of which are mediated by sign-preserving ionotropic AMPA/kainate receptors, with the exception of the photoreceptor-DBC synapses that are mediated by sign-inverting mGluR6 receptors.10–12 In the lateral pathways, most of the AC output synapses are GABAergic or glycinergic,13 whereas the HC output synapses are more complex, although subpopulations of HCs in some vertebrates are found to be GABAergic.14 In the feedback pathways, AC→BC synapses are GABAergic/glycinergic, IPC→photoreceptor/BC/HC synapses are either dopaminergic or glycinergic,15–17 and synaptic mechanisms underlying the HC→cone synapses remain unclear (but see discussion later). In addition to the chemical synaptic pathways, retinal neurons are extensively coupled by electrical synapses.9,18–20 These chemical and electrical synapses form an orderly network whose major components are preserved during evolution, and thus the same set of organizational principles are used by virtually all vertebrates to process retinal signals. These general principles are described in the following five sections. A summary schematic diagram on major chemical and electrical synapses mediating the HBC/OFF-GC pathways and the DBC/ON-GC pathways is given in Figure 1, and it will be used as a general map for my discussion on synaptic organization of the vertebrate retina.
Figure 1.
Summary of the major synapses that mediate the CSARF of BCs and GCs in the retina. Left: the OFF- or HBC-pathway; right: the ON- or DBC-pathway. Top traces: the voltage response to center illumination; bottom traces: the response to surround illumination. In the postsynaptic semicircles, +, sign-preserving chemical synapses; −, sign-inverting chemical synapses; zigzags, electric synapses. Neurotransmitter color code in the presynaptic semicircles: blue: glutamatergic; red: GABAergic; green: glycinergic; and yellow: unspecified. R, rod; C, cone; HC, horizontal cell (A-type: HCA and B-type: HCB); HBCR, HBCC, DBCR, and DBCC are rod- and cone-dominated hyperpolarizing and depolarizing BCs; AON, sustained ON amacrine cell; AON-OFF, transient ON-OFF amacrine cell; AOFF, sustained OFF amacrine cell; IPC, interplexiform cell; GON, sustained ON ganglion cell; GON-OFF, ON-OFF ganglion cell; GOFF, sustained OFF ganglion cell; OPL, outer plexiform layer; IPL, inner plexiform layer. For animation of the center-surround signaling pathways, please visit http://neuro.neusc.bcm.tmc.edu/wu/resources.html.
Cell–Cell Coupling
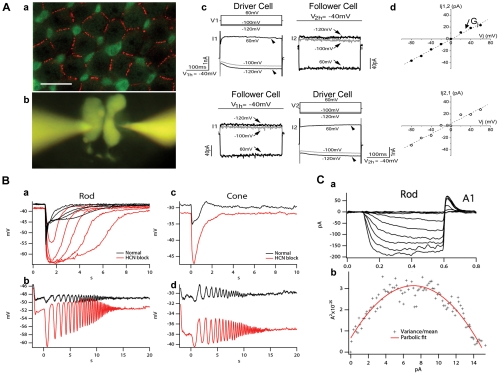
Electrical synapses (coupling) play a major role in cell–cell communication in the vertebrate retina. There are two types of cell–cell coupling. The first is homocellular coupling (between cells of the same kind), and the second is heterocellular coupling (between different types of cells).20 In the vertebrate retina, homocellular coupling is strong and robust, and it is present in all major types of retinal neurons, such as rod–rod,21 cone–cone,22,23 HC–HC,18,24 BC–BC,25 AC–AC,19 and GC–GC.26 Most of the homocellular gap junctions are linear and symmetrical, and they are made of homologous gap junction proteins, such as connexin36 for rod–rod and cone–cone and AIIAC–AIIAC coupling.27,28 Patterns of connexin36-labeled gap junctions between rods and between rod and cones in the salamander retina are given in Figure 2Aa. Moreover, homocellular gap junctions are frequency-independent over a wide range, but cell–cell signal spread is low-pass filtered by the passive membrane of the coupled network.21 An example of homocellular coupling is given in Figures 2Ab–d in which current flow through the gap junctions between a pair of rods in the salamander retina were recorded under voltage-clamped conditions. The current–voltage relations of the gap junction show that rod–rod coupling is symmetrical and linear, with an average junctional conductance of 500 pS.21 Similar symmetrical, linear gap junctions have been reported between cones in ground squirrel and monkey retinas.22,23
Figure 2.
Electrical coupling and HCN1 channels in salamander photoreceptors. (Aa) Confocal image of a salamander flatmount retina (with the focal plane at the level of the distal region of rod cell bodies) double labeled with anti-Cx35/36 (red) and recoverin (green). Recoverin differentially labeled rods (r, weak green) and cone inner segments (c, strong green). The strong Cx35/36 punctate labeling on membrane contacts outlined the mosaic of the rod network in the field. Scale bar, 20 μm. (Ab) A pair of rods simultaneously patch clamped and filled with Lucifer yellow through two recording pipettes. (Ac) Simultaneous dual whole-cell voltage clamp recordings from a pair of neighboring rods. The membrane potential of two rods was held at −40 mV. Upper panel: when a series of voltage step commands (V1) (from −120 mV to 60 mV with an increment of 20 mV) were applied to cell 1 (driver cell), the voltage activated current responses (I1) (arrowheads) were recorded in cell 1 (left panel) and the junctional currents of the opposite polarity (I2) (arrows) were recorded in cell 2 (follower cell, right panel). Lower panel: switching the position of driver/follower cells. (Ad) Relations of transjunctional current (Ij) and transjunctional voltage (Vj) obtained in upper and lower panels in (Ac). The junctional conductance (Gj) measured in either direction is 500 pS. (Reprinted from Zhang J, Wu SM. Physiological properties of rod photoreceptor electrical coupling in the tiger salamander retina. J Physiol. 2005;564:849–86. © 2005 by The Physiological Society.) (Ba) Rod responses to flashes of increasing light intensity in normal Ringer's (black traces) and in the presence of 100 μM HCN channel blocker ZD 7288 (red traces). (Bb) Rod response to a frequency-chirped light stimulus (chirped sine wave-modulated light ranged from 0.5 to 5 Hz over the course of 20 seconds) in normal Ringer's (black) and in 100 μM ZD 7288 (red). (Bc, Bd) Cone responses to flashes of increasing light intensity (Bc) and to frequency-chirped light stimulus (Bd) in normal Ringer's (black traces) and in the presence of 100 μM ZD 7288 (red traces). (Ca) Whole-cell recording of HCN channels from a rod to hyperpolarizing voltage steps with an extracellular solution containing TEA, cobalt and barium so that all other ionic currents other than Ih were blocked. (Cb) Variance versus mean plot computed from an ensemble of whole-cell Ih current. The slope of the variance-mean plot at 0 mean current gives an estimate of single channel conductance of 663 ± 71 fS, and the peak of the hyperbolic curve gives an estimate of the total number of HCN1 channels per rod of 2214 ± 986. (Part C reprinted with permission from Barrow AJ, Wu SM. Low-conductance HCN1 ion channels augment the frequency response of rod and cone photoreceptors. J Neurosci. 2009;29:5841–5853. © 2009 by the Society for Neuroscience.)
A major function of homocellular coupling is to increase the cells' receptive field size. The dendritic diameter of an HC ranges from 30 to 150 μm, but the receptive field of these cells typically range from 500 to 2000 μm.24 In the salamander retina, A-type HCs exhibit a narrower average receptive field diameter (∼500 μm) than do the B-type HCs (>1500 μm), and the strength of coupling (indicated by the number of neurobiotin-filled cells) is much weaker in A-type HCs than in the B-type.24 Since a major function of HCs is to mediate lateral signal transmission, homocellular HC coupling increases the receptive fields and the cells' capacity to aggregate lateral signals in the retina. In addition, it has been shown that some BCs are homocellularly coupled, enabling them to have receptive field centers substantially larger than their dendritic fields25 (see Fig. 4). Another important function of homocellular coupling is that it improves the signal-to-noise ratio of the coupled cells by spatially averaging visual signals over some lateral distance.29 This averaging is especially important for the rods, which in dark-adapted conditions detect small signals generated from dim stimuli images under a voltage noise background.30
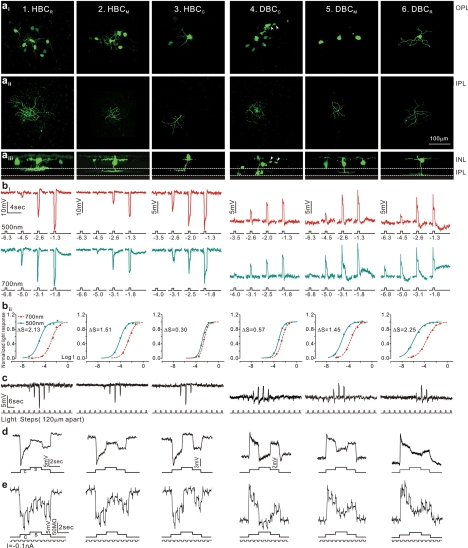
Figure 4.
Morphology, light responses, and receptive fields of six types of bipolar cells in the tiger salamander retina. (a) Fluorescent micrographs of a neurobiotin-filled HBCR (column 1), a HBCM (column 2), HBCC (column 3), a DBCC (column 4), a DBCM (column 5), and a DBCR (column 6) viewed with a confocal microscope at the outer INL/OPL level (ai), the IPL level (aii), and with z-axis rotation (aiii). Calibration bar, 100 μm. (bi) BC voltage responses to 500-nm and 700-nm light steps of various intensities. (bii) Response-intensity (V-Log I) curves of the responses to 500-nm and 700-nm lights. ΔS (spectral difference, see Fig. 3A) of the 6 BCs are 2.13, 1.51, 0.30, 0.57, 1.45, and 2.25. (c) Measurements of BC receptive field center diameters (RFCD) by recording voltage responses to a 100-μm-wide light bar moving stepwise (with 120-μm step increments) across the receptive field. (d) Voltage responses of the 6 types of BCs elicited by a center light spot (300 μm) and a surround light annulus (700 μm inner diameter, 2000 μm outer diameter). The surround light annulus was of the same intensity (700 nm, −2) for all 6 cells whereas the intensity of the center light spot was adjusted so that it allowed the annulus to produce the maximum response. (e) Voltage responses of the 6 types of BCs elicited by a center light spot and a surround light annulus (same as in d), and by a train of −0.1-nA/200-msec current pulses passed into the cell by the recording microelectrode through a bridge circuit. (Reprinted with permission from Zhang A-J, Wu SM. Receptive fields of retinal bipolar cells are mediated by heterogeneous synaptic circuitry. J Neurosci. 2009;29:789–797. © 2009 by The Society for Neuroscience.)
Heterocellular coupling is found between selected populations of retinal neurons, including rod–cone, HCB–DBCC, and AIIAC–DBCC1 pairs,25,31,32 and between certain types of GCs and ACs.33 Rod–cone coupling has been found in many cold-blooded vertebrates34,35 and several mammals,28,36 and it is much weaker than the homocellular rod–rod and cone–cone coupling.34 Rod and cone photoreceptors operate in different ranges of luminance and they exhibit different spectral sensitivities.37 Rod–cone coupling helps to broaden the operating ranges and spectral sensitivity spans of both photoreceptors.37 Moreover, under conditions when one type of photoreceptor is suppressed (e.g., rod response in the presence of background light), its output synapse can be used to transmit signals from the other type of photoreceptors (e.g., cones). Such “synaptic sharing” reduces the amount of required neural hardware and thus makes signal processing in the outer retina more “economical.”38
Another example of heterocellular coupling is the AIIAC–DBCC electrical synapse in the mammalian inner retina.39 In mammals, the primary rod signaling channel is the rod→DBCR→AIIAC pathway,40 and rod signals in AIIACs “piggyback” on the cone signaling pathways via the heterocellular AIIAC–DBCC electrical synapse41 (described later). The AIIAC–DBCC electrical synapse is believed to be mediated by heterogenous gap junction proteins (connexin36 at the AIIAC side and connexin45 at the DBCC side42). Electrical properties of the AIIAC–DBCC gap junction channels have been characterized by a dual patch study in the rat retina32 and light-evoked signals from DBCCs to AIIACs have been examined by using pharmacologic tools, mice that lack connexin36, and mice that lack DBCRs.43
Why does the retina have many small cells that are electrically coupled rather than having larger cells? One answer is that most, if not all, electrical synapses can be modulated by lighting conditions, the circadian clock, and various neurotransmitter/modulators.44–46 For example, photoreceptor coupling in certain vertebrates is regulated by D1 and D2 receptor agonists,47 and rod–cone coupling is enhanced by background light44 and modulated by the circadian clock.46 These findings suggest that the cell–cell coupling in the retina is not static; its strength can be regulated by various lighting conditions and chemical microenvironments. It is therefore advantageous for the retina to use small electrically coupled cells instead of larger cells to process spatial information, as electrical coupling allows plasticity and adjustability in retinal cells in the spatial domain.
Voltage-Dependent Channels Shape Retinal Voltage Responses
Most retinal neurons (except ganglion cells) exhibit graded voltage responses to light, and these voltage responses do not necessarily follow the waveform of their generator current sources (e.g., photocurrents in rods and cones and the postsynaptic currents in higher-order neurons).48–50 Instead, graded voltage responses are often “shaped” by voltage- and time-dependent ion currents.51 Light-evoked voltage changes activate (or deactivate) voltage-gated, time-dependent channels, leading to additional voltage changes with time courses determined by the channel kinetics. Examples include voltage-gated cation currents (e.g., Ih, IKx, and ICa) in photoreceptors, and INa and ICa in certain types of BCs and ACs.51–56 These currents make the voltage responses more transient and modify the frequency responses and kinetics of the retinal neurons. In this section, I will focus on two voltage-dependent currents in rod and cone photoreceptors: Ih, the hyperpolarization-gated current, and IKx, the x-type potassium current.57
We found that Ih in both rods and cones is mediated by HCN1 (hyperpolarization-activated cyclic nucleotide-gated channel isoform 1) channels.58 Ih current makes the initial phase of the light response of rod and cone photoreceptors more transient, an effect similar to that of a high-pass filter (Fig. 2B). In both classes of photoreceptors, HCN1 channels serve to increase the natural frequency response of single cells by modifying the input of the photocurrent, which is limited in its frequency response by the speed of a molecular signaling cascade. In doing so, HCN1 channels form the first stage of many systems in the retina that augment the speed of the visual response, allowing the animal to perceive visual stimuli that change more quickly than the speed of the underlying photocurrent decay (Fig. 2B). HCN1 channels in rods and cones have a small single-channel current that is below the thermal noise threshold of measuring electronics. We therefore used nonstationary fluctuation analysis (NSFA) to estimate the single-channel conductance of HCN1 channels, revealing a conductance of approximately 650 fS in both rod and cone photoreceptors (Fig. 2C).58
In addition to the HCN1 channels that mediate the Ih, the IKx performs signal filtering in rod photoreceptors. This current is known to be mediated by potassium channels and has similarities to the neuronal M current and EAG potassium channels.57 Although it is known that in filtering the light response of rods, Ih and IKx undergo complementary conductance changes, the qualities and significance of these changes are not clear. We demonstrated that the filtering effect of HCN1 channels in salamander rods is intact when IKx is blocked. Using a simulation analysis of the rod light response, we predicted the magnitude and time course of the conductance changes by both currents, showing that they largely cancel each other.59 We have indicated that the membrane conductance of individual rods is another mechanism that modulates signal spread in the rod network independent of gap junction coupling. From this idea and our simulation analysis, we propose that one purpose of the opposing conductance changes by Ih and IKx may be to optimize the lateral propagation of signals through gap junctions in the rod network.59
Feedback Synapses
Feedback synapses have been found at almost all levels in the visual pathway, and they are normally made from cells with relatively broad receptive fields to cells with narrower receptive fields at one or more upstream levels (Fig. 1). In the outer retina, HCs send sign-inverting signals to cone photoreceptors9; three synaptic mechanisms have been proposed for the HC feedback actions on cones: The first theory is that HCs release an inhibitory neurotransmitter (GABA in several species) in darkness that opens chloride channels in cones and that surround annular light stimuli hyperpolarizes the HCs, suppresses feedback transmitter release, depolarizes the cones, and the HBCs, and hyperpolarizes the DBCs.60,61 The second theory is that surround light hyperpolarizes HCs, resulting in an inward current through hemichannels in their dendrites near the cones, charging the cone membrane and modulating calcium currents in cones, increasing their calcium-dependent glutamate release, which depolarizes the HBCs and hyperpolarizes the DBCs.62,63 The third theory is that surround-induced HC hyperpolarization elevates the pH in the HC-cone synaptic cleft, leading to an increase in calcium current in cones and a higher rate of glutamate release that depolarizes the HBCs and hyperpolarizes the DBCs.64,65 It is possible that different species under different conditions favor different feedback synaptic mechanisms, and/or different types of HC-cone synapses in the same animal may use one or more of these three HC-cone feedback mechanisms. This idea is supported by a study demonstrating that the responses of salamander GCs to dim surround stimuli are sensitive to GABA blockers and those responding to bright surround stimuli are sensitive to carbenoxolone, a gap junction/hemichannel blocker.66
ACs are involved in another interesting feedback system in the retina. They make GABAergic or glycinergic feedback synapses on BC axon terminals in the IPL13 that are also thought to mediate the antagonistic surround responses of BCs and GCs67–69 (Fig. 1). Moreover, since many ACs exhibit transient voltage responses at the onset or offset of a light step, or transient responses to changes of light, the AC→BC feedback synapses have been proposed to contribute to motion detection and direction selectivity in the visual world.70
Interplexiform cells (IPCs) received inputs from BC axons and AC dendrites in the IPL and send output signals to photoreceptors, HCs, and BCs in the OPL.15,16 In fish and new world monkeys, dopamine is the IPC neurotransmitter that has been found to uncouple HCs45 and regulate glutamate receptor efficacy in second-order retinal neurons.71 Glycine is the IPC neurotransmitter in some vertebrates, and it modulates calcium currents in photoreceptors,72 gates chloride channels in BC dendrites16 and regulates HC response kinetics73 in the outer retina.
The HC-cone and AC–BC feedback synapses act as negative feedback circuits for the photoreceptor and BC output synapses, respectively. According to the principles of system analysis, negative feedback loops improve the reliability, signal-to-noise ratio, linearity, response bandwidth, and stability of the forward signals, but at the cost of overall gain.74 In the retina, the photoreceptor→BC→GC synaptic pathway is the direct and express route for signal transmission from rods and cones to the brain, and the negative feedback synapses of the outer and inner retina, found in most, if not all vertebrates, are used as the general strategy for improving the performance and accuracy of this pathway.
Since retinal feedback synapses are made from cells with wide receptive fields, they are used to process spatial information. The basic unit for spatial information encoding in the visual system is the center-surround antagonistic receptive field (CSARF) organization,75 and the HC-cone and AC-BC feedback synapses are two major contributors of the antagonistic surround responses in retinal BCs and GCs25 (see Fig. 1 and the section on the Center-Surround Receptive Field). Moreover, negative-feedback synapses have been proposed to mediate color opponency in retinal neurons: light of one wavelength elicits responses of opposite polarity to responses to light of other wavelengths.76,77 Therefore feedback synapses in the retina are crucial for color vision in animals and humans.
In most vertebrates, different types of retinal neurons operate in different light-intensity (dynamic) ranges. The dynamic ranges of photoreceptors and HCs, for example, are wider than those of BCs, and BC dynamic ranges are wider than those of GCs.78,79 Since photoreceptor-BC and BC–GC synaptic transmissions are limited within windows of presynaptic voltages,78,80,81 a tonic negative feedback signal shifts the dynamic ranges of the postsynaptic cells toward a functional setpoint.82 Under physiological conditions, for instance, a steady background illumination tonically activates the HC-cone feedback synapses, which shift the operating range of BCs to the right in the intensity axis so that brighter light is necessary to generate a given BC response.79 Such synaptic arrangement facilitates greater simultaneous contrast in the visual system: The brighter the background (ambient) light, the brighter the stimulus needed to generate a given response.82 Moreover, this feedback-induced operation range shift demonstrates how synapses may perform signal “multiplication/division”: When one adds a background light to a test light flash, the two responses in a BC do not add, rather, the background light makes the flash response smaller (division) by shifting the BC dynamic range through reducing cone responses via the HC-cone feedback synapses.
Rod/Cone Inputs to BCs and Rules for Correlating BC Responses and Axonal Morphology
BCs are the central neurons of the retina, and they carry rod and cone photoreceptor signals to ACs and GCs in the IPL.4 Previously, a simple dichotomy between cold-blooded and mammalian vertebrates was thought to exist, based on anatomic evidence: BCs in lower vertebrates received mixed rod/cone inputs and in mammals received segregated inputs from rods and cones.83,84 However, recent physiological studies have revealed that BCs in dark-adapted salamander retinas can be rod or cone dominated,50 and that in the mouse retina some cone-BCs receive direct rod inputs and some rod-BCs receive direct cone inputs.85 Thus, a general rule appears to hold for both cold-blooded and mammalian vertebrates: Some BCs receive synaptic inputs predominantly from rods, some predominantly from cones, and others receive mixed rod/cone inputs of various proportions. Although rod–cone coupling contributes to mixing rod/cone inputs in BCs, direct rod-BC and cone-BC chemical synapses play major roles in rod/cone signals in BCs because rod–cone coupling is generally weak compared with rod–rod and cone–cone coupling34 (see the section on Cell-Cell Coupling), and recent results have shown that rod inputs to cone BCs and cone inputs to rod BCs persist in mice whose gap junction channels between rods and cones are deleted.43,86
Based on studies of BC physiological properties in various species, vertebrate retinas have six major types of BCs: the rod (or rod-dominated), cone (or cone-dominated), and mixed (rod/cone) depolarizing and hyperpolarizing BCs (DBCR, DBCC, DBCM, HBCR, HBCC, and HBCM). Each carries a characteristic set of light-response attributes and projects them to the inner retina through axons that terminate at segregated regions (strata) of the IPL.87 Such stratum-by-stratum projection of light response attributes was investigated by a large-scale voltage clamp study of the salamander BC responses and morphology (Fig. 3A).49 This study revealed several rules for the function–morphology relationships of retinal BCs that are applicable to several other cold-blooded and mammalian vertebrates.88,89 Figure 3B illustrates that seven types of mouse BCs follow the same rules in correlating the relative rod/cone inputs with levels of axon terminal endings in the IPL.85 These rules are: (1) Cells with axon terminals in strata 1 to 5 (sublamina A) are HBCs (with outward light-evoked cation currents [ΔIC]) and those in strata 6 to 10 (sublamina B) are DBCs (with inward ΔIC). This agrees with the sublamina A/B rule observed in many vertebrate species.90–92 (2) Cells with axon terminals in strata 1, 2, and 10 are rod dominated, those in strata 4 to 8 are cone dominated, and those in strata 3 and 9 exhibit mixed rod/cone dominance. (3) Light-evoked ΔIC at light onset in rod-dominated HBCs and DBCs are sustained, that of the cone-dominated HBCs exhibit a smaller sustained outward current followed by a transient inward current at light offset, and that of the cone-dominated DBCs exhibit a sustained inward current followed by a small transient off outward current. (4) ΔICl (light-evoked chloride currents) in rod-dominated BCs are sustained ON currents, whereas those in cone-dominated BCs are transient ON-OFF currents. ΔICl in all BCs are outward, and thus they are synergistic to ΔIC in HBCs and antagonistic to ΔIC in DBCs. (5) BCs with axon terminals stratified in multiple strata exhibit combined light response properties of the narrowly monostratified cells in the same strata. (6) BCs with pyramidally branching or globular axons (mammalian rod BCs) exhibit light-response properties very similar to those of narrowly monostratified cells whose axon terminals stratify in the same stratum as the axon terminal endings of the pyramidally branching, or globular, cells.93
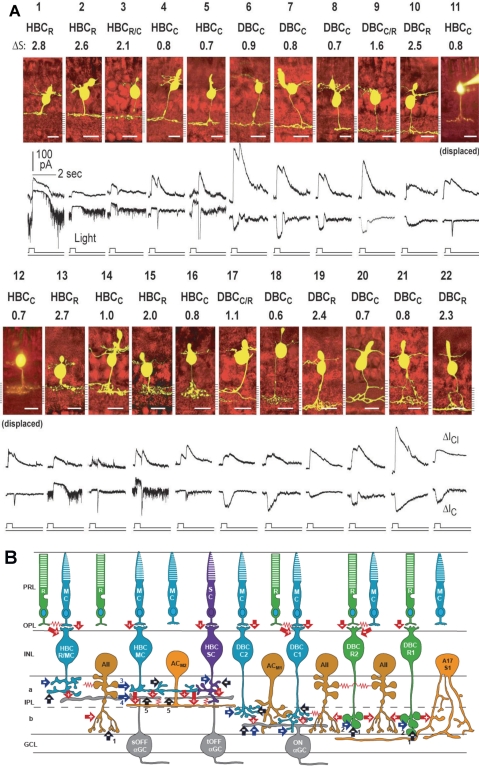
Figure 3.
(A) Stratum-by-stratum rules for correlating patterns of axon terminal ramification and physiological responses in retinal bipolar cells. 22 morphologically identified (by Lucifer yellow filling) BCs and their light-evoked excitatory cation current (ΔIC) and inhibitory chloride current (ΔICl) recorded from dark-adapted salamander retinal slices. Each cell is named according to their spectral difference (ΔS) and ΔIC polarity as rod-dominated, cone-dominated, mixed rod/cone hyperpolarizing or depolarizing bipolar cells (HBCR, HBCC, HBCR/C, DBCR, DBCC or DBCR/C). BCs with inward ΔIC are DBCs and with outward ΔIC are HBCs. The spectral difference, ΔS, is defined as S700 − S500 (where S700 and S500 are intensities in log units of 700- and 500-nm light eliciting responses of the same amplitude). Since ΔS for the salamander rods is approximately 3.4 and that for the cones is approximately 0.1, BCs with ΔS > 2.0 are rod-dominated (HBCR or DBCR), with ΔS < 1.0 are cone-dominated (HBCC or DBCC), and with 1.0 < ΔS < 2.0 are mixed rod/cone cells (HBCR/C or DBCR/C, also named HBCM or DBCM, see Fig. 4). Displaced HBCCs: HBCCs with somas displaced in the outer nuclear layer. (Modified from Pang J-J, Gao F, Wu SM. Stratum-by-stratum projection of light response attributes by retinal bipolar cells of Ambystoma. J. Physiol. 2004;558:249–262. © 2004 by The Physiological Society; and Maple BR, Zhang J, Pang J-J, Gao F, Wu SM. Characterization of displaced bipolar cells in the tiger salamander retina. Vision Res. 2005;45:697–705. © 2005 Elsevier Ltd.) (B) Schematic diagram of synaptic connections of photoreceptors, BCs, ACs, and α GCs in the mammalian retina. R, rod; MC, M-cone; SC, S-cone; HBCMC/R, mixed M-cone/rod hyperpolarizing BC; HBCMC, M-cone dominated hyperpolarizing bipolar cell; HBCSC, S-cone dominated hyperpolarizing bipolar cell; DBCC2, type 2 cone depolarizing bipolar cell; DBCC1, type 1 cone depolarizing bipolar cell; DBCR2, type 2 rod depolarizing bipolar cell; DBCR1, type 1 rod depolarizing bipolar cell. Note that BCs with the most rod inputs have axon terminal endings near the two margins of the IPL, whereas those with the most cone inputs bear axons ramifying in the central regions of the IPL, similar to the rules set forward by the salamander BCs (A). ACM1, M-cone dominated depolarizing amacrine cell; ACM2, M-cone dominated ON-OFF amacrine cell; AII, AII amacrine cell; A17/S1, A17 amacrine cell; sOFFαGC, sustained OFF αGC; tOFFαGC, transient OFF αGC; ONαGC, ON αGC; green: rods and rod BCs; blue: M-cones and M-cone BCs, purple: S-cone and S-cone BCs; light orange: GABAergic ACs; dark orange: glycinergic ACs; gray: αGCs; arrows: chemical synapses (red, glutamatergic; black, GABAergic; blue, glycinergic; +, sign-preserving; −, sign-inverting); zigzag (red): electrical synapses. PRL, photoreceptor layer; OPL, outer plexiform layer; INL, inner nuclear layer; IPL, inner plexiform layer (a, sublamina a, b, sublamina b); GCL, ganglion cell layer. Many inhibitory synapses from unspecified ACs are represented as black and blue arrows.
It is intriguing to note that, although the retina represents information about positions in the visual world in a two-dimensional sheet of neurons, it uses the orthogonal third dimension (the depth of the IPL) to represent various attributes of visual stimuli. The orderly segregation of visual information along the depth of the IPL simplifies signal integration at the AC and GC levels and facilitates orderly ramification of AC and GC dendrites in the IPL.94,95 Consequently, various parameters of visual signals can be computed effectively by synapses in the IPL in a stratum-by-stratum fashion with minimal interstrata crossovers.
Center-Surround Receptive Fields of Retinal Neurons Are Mediated by Heterogeneous Synaptic Circuitry
In addition to projecting signals to various strata of the IPL, where ACs and ganglion cells (GCs) gather their inputs, BCs with various light response attributes have different CSARF organizations.25 Figure 4 shows the morphology, patterns of dye coupling, light responses, CSARF properties, and membrane resistance changes associated with the center and surround voltage responses of the six functional types of BCs (HBCR, HBCM, HBCC, DBCC, DBCM, and DBCR) in the tiger salamander retina. These results suggest that the center and surround responses of various types of BCs in the retina are mediated by heterogeneous synaptic circuitry. The BC receptive field center diameters (RFCDs) vary with the relative rod/cone input: RFCD is larger in DBCs with stronger cone input, and it is larger in HBCs with stronger rod input. RFCD also correlates with the degree of homocellular coupling: BCs with larger RFCDs are more strongly dye coupled with neighboring cells of the same type, suggesting that BC–BC homocellular coupling significantly contributes to the BC receptive field center.25
Results in Figure 4 also show that the relative surround response strength, S/(Ct–CS) (S, Ct and CS are surround, transient center, and sustained rebound responses, respectively) of BCs vary with the relative rod/cone input: stronger surround responses for cells with more cone inputs. In the rabbit retina, it has been shown that cone BCs have much stronger surround responses than rod BCs,96 suggesting that the rod/cone-dependent heterogeneity rule for BC surround response strength also applies to other vertebrates.
Based on the surround response polarity and accompanying resistance changes shown in Figure 4, the HC-cone-BC feedback synapses may contribute to the surround responses of all six types of BCs. The negative HC-cone feedback synapses partially “turn off” the center responses by depolarizing the cones, as the membrane resistance changes associated with surround responses of all BCs are opposite to the resistance changes associated with center responses (Fig. 4e). Although all BCs share the common HC-cone-BC feedback pathway, various types of BCs use different HC and AC synaptic inputs to mediate their surround responses. It is unlikely, for example, that HBC surround responses are directly mediated by chemical synaptic inputs from hyperpolarizing lateral neurons such as HCs and ACOFFs because of resistance change mismatch, and thus HBCs may only receive surround inputs from HC–cone–HBC and ACON–HBC synapses. On the other hand, resistance analysis suggests that DBC surround responses can be mediated by HC–cone–DBC, HC–DBC, and ACOFF–DBC chemical synapses, but not the ACON–DBC synapses. Moreover, dye coupling (Fig. 4a) results indicate that DBCCs receive additional surround inputs from wide-field HCs through electrical synapses. Similar HC-DBC dye coupling has been observed in the rabbit retina,97 suggesting that such surround synaptic pathways are present in other vertebrates. Despite the heterogeneity, it is interesting to point out that a ON/OFF crossover inhibition rule applies: Cells with OFF (hyperpolarizing) responses (HCs and ACOFFs) mediate surround inhibitory inputs to ON cells (DBCs), and cells with ON (depolarizing) responses (ACONs) mediate surround inhibitory inputs to OFF cells (HBCs). ON/OFF crossover inhibition from amacrine cells to ganglion cells has been reported in salamander and mammalian retinas,98,99 and thus it may be a general rule for lateral inhibition in the vertebrate visual system.
The AC–BC contributions to BC surround responses are mediated by GABAergic or glycinergic synapses.13 GABA receptors on ACs and GCs are largely GABAA, and those on BC axon terminals are largely GABAC.100,101 Glycine receptors have been localized in ACs, GCs, and BC dendrites and in BC axon terminals,13,102 and those in the IPL are postsynaptic to glycinergic ACs, whereas those in the OPL are postsynaptic to glycinergic interplexiform cells.16,102 In the Xenopus retina, GABA suppresses the surround responses of the DBCs, but only slightly reduces the surround of the HBCs, and glycine suppresses the surround responses of both DBCs and HBCs.69 In the tiger salamander, one study shows that GABA reduces the surround responses of a subpopulation of HBCs,67 but another report reveals that application of picrotoxin and strychnine does not affect the surround responses of either DBCs or HBCs.103 Recent studies in the primate retina indicate that the HC feedback signal to cones as well as the surround responses of GCs are not sensitive to GABAergic or glycinergic agents, but are sensitive to carbenoxolone, suggesting that the surround responses in GCs are mainly mediated by HC actions on BCs in the outer retina, not by GABAergic or glycinergic AC actions in the inner retina.104,105 The reasons for these different GABA/glycine actions on surround responses are unclear. As suggested by results in Figure 4, surround responses of different functional types of BCs in the salamander retina are mediated by different combinations of synaptic circuitries: HC→cone (GABA/hemichannel/proton)→BC, HC→BC (chemical/gap junction), and AC→BC (GABA/glycine). It is conceivable that the surround responses of different BCs/GCs from different animals under different conditions are mediated by different combinations of surround synaptic pathways and thus they are sensitive to different synaptic blockers. The wide variation of synaptic circuitries underlying surround responses of various functional types of BCs allows for flexibility in function-specific modulation of BC/GC receptive fields. Hence different features of spatial and contrast information, such as rod/cone and ON/OFF signals, can be differentially modulated by different lighting and adaptation conditions.
Species-Specific Variations and the AII Amacrine Cell Pathway in Mammals
Despite the general principles in retinal synaptic organization, there are species-specific variations in these principles, as different animals must adapt to different environments and perform different survival tasks. For example, diurnal vertebrates who have to distinguish color in their living environments, such as teleost fish, turtles, and primates, have more cone types with different visual pigments and color-opponent higher-order retinal cells than the nocturnal animals, such as mice and rats.4,106 Moreover, the one-to-one cone–midget BC–midget GC synaptic pathways, which allow for high-resolution vision, are found mostly in primate foveal regions.106
One of the most prominent species-specific variations in retinal synaptic circuitry is the previously mentioned mammalian AII amacrine cell (AIIAC) pathway (Fig. 3B).39,107 In cold-blooded vertebrates, almost all BCs make direct synapses on GCs.108 In mammals, however, the rod depolarizing BCs (DBCRs), which are the main (in some species the only) BCs that carries rod signals, do not make synapses directly on GCs, but instead send signals indirectly through the AIIACs: DBCRs make chemical synapses on AIIACs, which relay the DBCR signal to cone depolarizing BCs (DBCCs) via gap junctions, and subsequently transmit the signal to the ON-center GCs through the DBCC–ONGC synapses.39,109,110 AIIACs also make glycinergic chemical synapses on cone hyperpolarizing BC (HBCC) axon terminals111 and off-center GCs,90 resulting in rod-mediated hyperpolarizing signals in OFF-GCs (Fig. 3B). Therefore the AIIAC is a crucial relay station for rod-mediated signals in the mammalian inner retina. AIIACs receive high-gain synaptic inputs from DBCRs and DBCCs,43 and they are homocellularly coupled with one another,19 thus exhibiting a very high light sensitivity: They give rise to a threshold response to a light stimulus so dim that only 1 of 1000 rods absorbs a photon.85 Hence, the mammalian retina uses AIIACs as sensitivity boosters for dim rod signals, a feature only available to mammals for facilitating night vision.
Concluding Remarks
The eye is the brain's window, yet it does much more than reproducing the outside world in electrical signals on an array of photoreceptors. The retina performs much information processing through a complex and highly organized neuronal network that contains arrays of electrical and chemical synapses as well as feedback loops that regulate gain and contrast. The retina is a part of the brain and it contains all neuronal and synaptic components found in other parts of the nervous system.4 These include various types of ion channels, neurotransmitters, and their accompanying receptors, and structural and functional elements of chemical and electrical synapses. Moreover, because the retina's natural input, light, is known and precisely controllable, synaptic pathways in the retina that encode various parameters of the natural input, such as light intensity, color, shape, contrast, and motion, have been studied in much greater detail than any other parts of the brain. Therefore, a comprehensive understanding of general principles of synaptic organization and functional pathways of the retina may promote three missions: (1) to unravel how the first synaptic network of the visual system operates and processes light images; (2) to extract crucial information on human retina function in healthy conditions and dysfunction in diseased states, as human retinas are often inaccessible for experimentation; and (3) to provide important clues and research paradigms for studying how natural input signals are processed in other parts of the brain.
Acknowledgments
I thank the ARVO Board of Trustees and the Award Committee for selecting me for the 2009 Friedenwald Award. I am most grateful to Professors John E. Dowling and Frank S. Werblin, my graduate school and postdoc mentors, for teaching me vision research and how to think about and approach science. I can never adequately express my gratitude to those who have come through my laboratory as students, postdocs, and visiting scientists, as they did all the work. Special thanks go to many colleagues, collaborators and friends in the field, to Dan B. Jones, my department chairman, for his leadership and support and to Alice McPherson for her continued support through the Retina Research Foundation. Moreover, I thank my wife Judy and two daughters Schonmei and Hanmei for their support, love, and patience. I am grateful to David Simons and Cameron Cowan, who made Figure 1, and to Roy Jacoby, Andrew Barrow, and Cameron Cowan, who proofread the manuscript and provided many insightful comments.
Footnotes
Supported by National Institutes of Health Grant EY04446 and Vision Core EY02520, the Retina Research Foundation (Houston), and Research to Prevent Blindness, Inc.
Disclosure: Wu SM, None
References
- 1. Cajal SR. La retine des vertebres. La Cellule. 1893;9:17–257 [Google Scholar]
- 2. Baylor DA. Photoreceptor signals and vision: The Proctor Lecture. Invest Ophthalmol Vis Sci. 1987;28:34–49 [PubMed] [Google Scholar]
- 3. Yau KW. Phototransduction mechanism in retinal rods and cones: The Friedenwald Lecture. Invest Ophthalmol Vis Sci. 1994;35:9–32 [PubMed] [Google Scholar]
- 4. Dowling JE. The Retina, an Approachable Part of the Brain. Boston: Harvard University Press; 1987. [Google Scholar]
- 5. Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757 [DOI] [PubMed] [Google Scholar]
- 6. Wu SM. Synaptic transmission in the outer retina. Annu Rev Physiol. 1994;56:141–168 [DOI] [PubMed] [Google Scholar]
- 7. Wu SM, Gao F, Maple BR. Integration and segregation of visual signals by bipolar cells in the tiger salamander retina. Prog Brain Res. 2001;131:125–143 [DOI] [PubMed] [Google Scholar]
- 8. Tomita T, Kaneko A, Murakami M, Pautler EL. Spectral response curves of single cones in the carp. Vision Res. 1967;7:519–531 [DOI] [PubMed] [Google Scholar]
- 9. Baylor DA, Fuortes MG, O'Bryan PM. Receptive fields of cones in the retina of the turtle. J Physiol. 1971;214:265–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Slaughter MM, Miller RF. Bipolar cells in the mudpuppy retina use an excitatory amino acid neurotransmitter. Nature. 1983;303:537–538 [DOI] [PubMed] [Google Scholar]
- 11. Slaughter MM, Miller RF. 2-Amino-4-phosphonobutyric acid: a new pharmacological tool for retina research. Science. 1981;211:182–185 [DOI] [PubMed] [Google Scholar]
- 12. Snellman J, Kaur T, Shen Y, Nawy S. Regulation of ON bipolar cell activity. Prog Retin Eye Res. 2008;27:450–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Muller JF, Marc RE. GABA-ergic and glycinergic pathways in the inner plexiform layer of the goldfish retina. J Comp Neurol. 1990;291:281–304 [DOI] [PubMed] [Google Scholar]
- 14. Zhang J, Zhang AJ, Wu SM. Immunocytochemical analysis of GABA-positive and calretinin-positive horizontal cells in the tiger salamander retina. J Comp Neurol. 2006;499:432–441 [DOI] [PubMed] [Google Scholar]
- 15. Dowling JE, Ehinger B. Synaptic organization of the amine-containing interplexiform cells of the goldfish and Cebus monkey retinas. Science. 1975;188:270–273 [DOI] [PubMed] [Google Scholar]
- 16. Maple BR, Wu SM. Glycinergic synaptic inputs to bipolar cells in the salamander retina. J Physiol. 1998;506:731–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Marc RE, Lam DM. Glycinergic pathways in the goldfish retina. J Neurosci. 1981;1152–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kaneko A. Electrical connexions between horizontal cells in the dogfish retina. J Physiol. 1971;21395–21105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Veruki ML, Hartveit E. AII (rod) amacrine cells form a network of electrically coupled interneurons in the mammalian retina. Neuron. 2002;33:935–946 [DOI] [PubMed] [Google Scholar]
- 20. Vaney DI, Weiler R. Gap junctions in the eye: evidence for heteromeric, heterotypic and mixed-homotypic interactions. Brain Res Brain Res Rev. 2000;32:115–120 [DOI] [PubMed] [Google Scholar]
- 21. Zhang J, Wu SM. Physiological properties of rod photoreceptor electrical coupling in the tiger salamander retina. J Physiol. 2005;564:849–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li W, Devries SH. Separate blue and green cone network in the mammalian retina. Nat Neurosci. 2004;7:751–756 [DOI] [PubMed] [Google Scholar]
- 23. Hornstein EP, Verweij J, Schnapf JL. Electrical coupling between red and green cones in primate retina. Nat Neurosci. 2004;7:745–750 [DOI] [PubMed] [Google Scholar]
- 24. Zhang AJ, Zhang J, Wu SM. Electrical coupling, receptive fields, and relative rod/cone inputs of horizontal cells in the tiger salamander retina. J Comp Neurol. 2006;499:422–431 [DOI] [PubMed] [Google Scholar]
- 25. Zhang AJ, Wu SM. Receptive fields of retinal bipolar cells are mediated by heterogeneous synaptic circuitry. J Neurosci. 2009;29:789–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Volgyi B, Chheda S, Bloomfield SA. Tracer coupling patterns of the ganglion cell subtypes in the mouse retina. J Comp Neurol. 2009;512:664–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang J, Wu SM. Connexin35/36 gap junction proteins are expressed in photoreceptors of the tiger salamander retina. J Comp Neurol. 2004;470:1–12 [DOI] [PubMed] [Google Scholar]
- 28. Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simon EJ, Lamb TD, Hodgkin AL. Spontaneous voltage fluctuations in retinal cones and bipolar cells. Nature. 1975;256:661–662 [DOI] [PubMed] [Google Scholar]
- 30. Rieke F, Baylor DA. Molecular origin of continuous dark noise in rod photoreceptors. Biophys J. 1996;71:2553–2572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schwartz EA. Cones excite rods in the retina of the turtle. J Physiol. 1975;246:639–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Veruki ML, Hartveit E. Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina. J Neurosci. 2002;22:10558–10566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xin D, Bloomfield SA. Tracer coupling pattern of amacrine and ganglion cells in the rabbit retina. J Comp Neurol. 1997;383:512–528 [DOI] [PubMed] [Google Scholar]
- 34. Attwell D, Wilson M, Wu SM. A quantitative analysis of interactions between photoreceptors in the salamander (Ambystoma) retina. J Physiol. 1984;352:703–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schwartz EA. Rod-rod interaction in the retina of the turtle. J Physiol. 1975;246:617–638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hornstein EP, Verweij J, Li PH, Schnapf JL. Gap-junctional coupling and absolute sensitivity of photoreceptors in macaque retina. J Neurosci. 2005;25:11201–11209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wu SM, Yang XL. Electrical coupling between rods and cones in the tiger salamander retina. Proc Natl Acad Sci U S A. 1988;85:275–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wu SM. Signal transmission and adaptation-induced modulation of photoreceptor synapses in the retina. Prog Retin Res. 1991;10:27–44 [Google Scholar]
- 39. Strettoi E, Raviola E, Dacheux RF. Synaptic connections of the narrow-field, bistratified rod amacrine cell (AII) in the rabbit retina. J Comp Neurol. 1992;325:152–168 [DOI] [PubMed] [Google Scholar]
- 40. Soucy E, Wang Y, Nirenberg S, Nathans J, Meister M. A novel signaling pathway from rod photoreceptors to ganglion cells in mammalian retina. Neuron. 1998;21:481–493 [DOI] [PubMed] [Google Scholar]
- 41. Strettoi E, Dacheux RF, Raviola E. Cone bipolar cells as interneurons in the rod pathway of the rabbit retina. J Comp Neurol. 1994;347:139–149 [DOI] [PubMed] [Google Scholar]
- 42. Dedek K, Schultz K, Pieper M, et al. Localization of heterotypic gap junctions composed of connexin45 and connexin36 in the rod pathway of the mouse retina. Eur J Neurosci. 2006;24:1675–1686 [DOI] [PubMed] [Google Scholar]
- 43. Pang JJ, Abd-El-Barr MM, Gao F, Bramblett DE, Paul DL, Wu SM. Relative contributions of rod and cone bipolar cell inputs to AII amacrine cell light responses in the mouse retina, J Physiol. 2007;580:397–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yang XL, Wu SM. Modulation of rod-cone coupling by light. Science. 1989;244:352–354 [DOI] [PubMed] [Google Scholar]
- 45. Lasater EM, Dowling JE. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci USA. 1985;82:3025–3029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod-cone coupling. Neuron. 2008;59:790–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Krizaj D, Gabriel R, Owen WG, Witkovsky P. Dopamine D2 receptor-mediated modulation of rod-cone coupling in the Xenopus retina. J Comp Neurol. 1998;398:529–538 [PMC free article] [PubMed] [Google Scholar]
- 48. Baylor DA, Nunn BJ. Electrical properties of the light-sensitive conductance of rods of the salamander Ambystoma tigrinum. J Physiol. 1986;371:115–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pang JJ, Gao F, Wu SM. Stratum-by-stratum projection of light response attributes by retinal bipolar cells of Ambystoma, J Physiol. 2004;558:249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang XL, Wu SM. Response sensitivity and voltage gain of the rod- and cone-bipolar cell synapses in dark-adapted tiger salamander retina. J Neurophysiol. 1997;78:2662–2673 [DOI] [PubMed] [Google Scholar]
- 51. Attwell D, Wilson M. Behaviour of the rod network in the tiger salamander retina mediated by membrane properties of individual rods. J Physiol. 1980;309:287–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Attwell D, Werblin FS, Wilson M. The properties of single cones isolated from the tiger salamander retina. J Physiol. 1982;328:259–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ichinose T, Shields CR, Lukasiewicz PD. Sodium channels in transient retinal bipolar cells enhance visual responses in ganglion cells. J Neurosci. 2005;5:1856–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Yang XL, Gao F, Wu SM. Non-linear, high-gain and sustained-to-transient signal transmission from rods to amacrine cells in dark-adapted retina of Ambystoma. J Physiol. 2002;539:239–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Barnes S, Werblin F. Gated currents generate single spike activity in amacrine cells of the tiger salamander retina. Proc Natl Acad Sci USA. 1986;83:1509–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Eliasof S, Barnes S, Werblin F. The interaction of ionic currents mediating single spike activity in retinal amacrine cells of the tiger salamander. J Neurosci. 1987;7: 3512–3524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kurennyi DE, Barnes S. Regulation of M-like K+ current, IKx, by Ca(2+)-dependent phosphorylation in rod photoreceptors. Am J Physiol. 1997;272:C1844–C1853 [DOI] [PubMed] [Google Scholar]
- 58. Barrow AJ, Wu SM. Low-conductance HCN1 ion channels augment the frequency response of rod and cone photoreceptors. J Neurosci. 2009;29:5841–5853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Barrow AJ, Wu SM. Complementary conductance changes by IKx and Ih contribute to membrane impedance stability during the rod light response. Channels (Austin). 2009;3(5):301–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Murakami M, Shimoda Y, Nakatani K, Miyachi E, Watanabe S. GABA-mediated negative feedback from horizontal cells to cones in the carp retina. Jpn J Physiol. 1982;32:911–926 [DOI] [PubMed] [Google Scholar]
- 61. Wu SM. Input-output relations of the feedback synapse between horizontal cells and cones in the tiger salamander retina. J Neurophysiol. 1991;65:1197–1206 [DOI] [PubMed] [Google Scholar]
- 62. Kamermans M, Fahrenfort I, Schultz K, Janssen-Bienhold U, Sjoerdsma T, Weiler R. Hemichannel-mediated inhibition in outer retina. Science. 2001;292:1178–1180 [DOI] [PubMed] [Google Scholar]
- 63. Kamermans M, Fahrenfort I. Ephaptic interactions within a chemical synapse: hemichannel-mediated ephaptic inhibition in the retina. Curr Opin Neurobiol. 2004;14:531–541 [DOI] [PubMed] [Google Scholar]
- 64. Vessey JP, Stratis AK, Daniels BA, et al. Proton-mediated feedback inhibition of presynaptic calcium channels at the cone photoreceptor synapse. J Neurosci. 2005;25:4108–4117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hirasawa H, Kaneko A. pH changes in the invaginating synaptic cleft mediate feedback from horizontal cells to cone photoreceptors by modulating Ca2+ channels. J Gen Physiol. 2003;122:657–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Ichinose T, Lukasiewicz PD. Inner and outer retinal pathways both contribute to surround inhibition of salamander ganglion cells. J Physiol. 2005;565:517–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wu SM. Effects of gamma-aminobutyric acid on cones and bipolar cells of the tiger salamander retina. Brain Res. 1986;365:70–77 [DOI] [PubMed] [Google Scholar]
- 68. Stone S, Witkovsky P. Center-surround organization of Xenopus horizontal cells and its modification by gamma-aminobutyric acid and strontium. Exp Biol. 1987;47:1–12 [PubMed] [Google Scholar]
- 69. Stone S, Schutte M. Physiological and morphological properties of OFF- and ON-center bipolar cells in the xenopus retina. Vis Neurosci. 1991;7:363–376 [DOI] [PubMed] [Google Scholar]
- 70. Werblin FS. Response of retinal cells to moving spots: intracellular recording in Necturus maculosus. J Neurophysiol. 1970;33:342–350 [DOI] [PubMed] [Google Scholar]
- 71. Knapp AG, Dowling JE. Dopamine enhances excitatory amino acid-gated conductances in cultured retinal horizontal cells. Nature. 1987;325:437–439 [DOI] [PubMed] [Google Scholar]
- 72. Shen W, Jiang Z, Li B. Glycine input induces the synaptic facilitation in salamander rod photoreceptors. J Biomed Sci. 2008;15:743–754 [DOI] [PubMed] [Google Scholar]
- 73. Yang XL, Wu SM. Effects of prolonged light exposure, GABA, and glycine on horizontal cell responses in tiger salamander retina. J Neurophysiol. 1989;61:1025–1035 [DOI] [PubMed] [Google Scholar]
- 74. Marmarelis PZ, Naka K. Experimental analysis of a neural system: two modeling approaches. Kybernetik. 1974;15:11–26 [DOI] [PubMed] [Google Scholar]
- 75. Hubel DH, Wiesel TN. Receptive field, binocular interaction and functional architecture in the cat's visual cortex. J Physiol. 1962;160:106–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Stell WK, Lightfood DO, Wheeler TG, Leeper HF. Goldfish retina: functional polarization of cone horizontal cell dendrites and synapses. Science. 1975;190:989–990 [DOI] [PubMed] [Google Scholar]
- 77. Kaneko A, Tachibana M. Retinal bipolar cells with double colour-opponent receptive fields. Nature. 1981;293:220–222 [DOI] [PubMed] [Google Scholar]
- 78. Thibos LN, Werblin FS. The properties of surround antagonism elicited by spinning windmill patterns in the mudpuppy retina. J Physiol. 1978;278:101–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Thibos LN, Werblin FS. The response properties of the steady antagonistic surround in the mudpuppy retina. J Physiol. 1978;278:79–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Attwell D, Borges S, Wu SM, Wilson M. Signal clipping by the rod output synapse. Nature. 1987;328:522–524 [DOI] [PubMed] [Google Scholar]
- 81. Field GD, Rieke F. Nonlinear signal transfer from mouse rods to bipolar cells and implications for visual sensitivity. Neuron. 2002;34:773–785 [DOI] [PubMed] [Google Scholar]
- 82. Wu SM. Feedback connections and operation of the outer plexiform layer of the retina. Curr Opin Neurobiol. 1992;2:462–468 [DOI] [PubMed] [Google Scholar]
- 83. Lasansky A. Organization of outer synaptic layer in the retina of larval tiger salamander. Philos Trans R Soc Lond B Biol Sci. 1973;265:471–489 [DOI] [PubMed] [Google Scholar]
- 84. Boycott B, Wassle H. Parallel processing in the mammalian retina: the Proctor Lecture. Invest Ophthalmol Vis Sci. 1990;40:1313–1327 [PubMed] [Google Scholar]
- 85. Pang JJ, Gao F, Wu SM. Light-evoked current responses in rod bipolar cells, cone depolarizing bipolar cells and AII amacrine cells in dark-adapted mouse retina. J Physiol. 2004;559:123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Abd-El-Barr MM, Pennesi ME, Saszik SM, et al. Genetic dissection of rod and cone pathways in the dark-adapted mouse retina. J Neurophysiol. 2009;102(3):1945–1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wu SM, Gao F, Maple BR. Functional architecture of synapses in the inner retina: segregation of visual signals by stratification of bipolar cell axon terminals. J Neurosci. 2000;20:4462–4470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Connaughton VP, Nelson R. Axonal stratification patterns and glutamate-gated conductance mechanisms in zebrafish retinal bipolar cells. J Physiol. 2000;524:135–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Li W, Devries SH. Bipolar cell pathways for color and luminance vision in a dichromatic mammalian retina. Nat Neurosci. 2006;9:669–675 [DOI] [PubMed] [Google Scholar]
- 90. Kolb H, Famiglietti EV. Rod and cone pathways in the inner plexiform layer of cat retina. Science. 1974;186:47–49 [DOI] [PubMed] [Google Scholar]
- 91. Famiglietti EV, Jr, Kaneko A, Tachibana M. Neuronal architecture of on and off pathways to ganglion cells in carp retina. Science. 1977;198:1267–1269 [DOI] [PubMed] [Google Scholar]
- 92. Nelson R, Famiglietti EV, Jr, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978;41:472–483 [DOI] [PubMed] [Google Scholar]
- 93. Wu SM, Gao F, Pang JJ. Synaptic circuitry mediating light-evoked signals in dark-adapted mouse retina. Vision Res. 2004;44:3277–3288 [DOI] [PubMed] [Google Scholar]
- 94. Pang JJ, Gao F, Wu SM. Relative contributions of bipolar cell and amacrine cell inputs to light responses of ON, OFF and ON-OFF retinal ganglion cells. Vision Res. 2002;42:19–27 [DOI] [PubMed] [Google Scholar]
- 95. Pang JJ, Gao F, Wu SM. Segregation and integration of visual channels: layer-by-layer computation of ON-OFF signals by amacrine cell dendrites. J Neurosci. 2002;22:4693–4701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Bloomfield SA, Xin D. Surround inhibition of mammalian AII amacrine cells is generated in the proximal retina. J Physiol. 2000;523:771–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Trexler EB, Li W, Mills SL, Massey SC. Coupling from AII amacrine cells to ON cone bipolar cells is bidirectional. J Comp Neurol. 2001;437:408–422 [DOI] [PubMed] [Google Scholar]
- 98. Pang JJ, Gao F, Wu SM. Cross-talk between ON and OFF channels in the salamander retina: indirect bipolar cell inputs to ON-OFF ganglion cells. Vision Res. 2007;47:384–392 [DOI] [PubMed] [Google Scholar]
- 99. Hsueh HA, Molnar A, Werblin FS. Amacrine-to-amacrine cell Inhibition in the rabbit retina. J Neurophysiol. 2008;100:2077–2088 [DOI] [PubMed] [Google Scholar]
- 100. Lukasiewicz PD, Werblin FS. A novel GABA receptor modulates synaptic transmission from bipolar to ganglion and amacrine cells in the tiger salamander retina. J Neurosci. 1994;14:1213–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Eggers ED, Lukasiewicz PD. GABA(A), GABA(C) and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol. 2006;572:215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Wassle H, Koulen P, Brandstatter JH, Fletcher EL, Becker CM. Glycine and GABA receptors in the mammalian retina. Vision Res. 1998;38:1411–1430 [DOI] [PubMed] [Google Scholar]
- 103. Hare WA, Owen WG. Receptive field of the retinal bipolar cell: a pharmacological study in the tiger salamander. J Neurophysiol. 1996;76:2005–2019 [DOI] [PubMed] [Google Scholar]
- 104. Verweij J, Hornstein EP, Schnapf JL. Surround antagonism in macaque cone photoreceptors. J Neurosci. 2003;23:1049–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. McMahon MJ, Packer OS, Dacey DM. The classical receptive field surround of primate parasol ganglion cells is mediated primarily by a non-GABA pathway. J Neurosci. 2004;l24:3736–3745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Rodieck RW. The First Steps in Seeing. Sunderland, MA: Sinauer Associates, Inc.; 1998. [Google Scholar]
- 107. Kolb H. The architecture of functional neural circuits in the vertebrate retina: The Proctor Lecture. Invest Ophthalmol Vis Sci. 1994;35:2385–2404 [PubMed] [Google Scholar]
- 108. Wong-Riley MTT. Synaptic organization of the inner plexiform layer in the retina of the tiger salamander. J Neurocytol. 1974;3:1–33 [DOI] [PubMed] [Google Scholar]
- 109. Famiglietti EV, Jr, Kolb H. Structural basis for ON-and OFF-center responses in retinal ganglion cells. Science. 1976;194:193–195 [DOI] [PubMed] [Google Scholar]
- 110. Strettoi E, Dacheux RF, Raviola E. Synaptic connections of rod bipolar cells in the inner plexiform layer of the rabbit retina. J Comp Neurol. 1990;295:449–466 [DOI] [PubMed] [Google Scholar]
- 111. Bolz J, Wassle H, Thier P. Pharmacological modulation of on and off ganglion cells in the cat retina. Neuroscience. 1984;12:875–885 [DOI] [PubMed] [Google Scholar]