Introduction
The prevalence of pre-operative anaemia may be high among surgical patients, depending on the patients’ co-morbidities, gender, age and the underlying pathology for which they require surgery. In this regard, a recent systematic review showed that the weighted mean prevalence of anaemia was 24% in patients undergoing elective knee or hip surgery and 45% in those undergoing surgery because of a hip fracture1. In addition, it is worth noting that the proportion of older patients who undergo major orthopaedic surgery is rising progressively, and a high prevalence of anaemia (43%) was observed in a retrospective cohort of 57,636 veterans aged 65 years or older who underwent major elective and non-elective orthopaedic surgery2.
Iron deficiency and chronic inflammation (including mild to moderate renal failure), with or without iron deficiency, are the most common causes of pre-operative anaemia, although deficiencies of iron, folic acid and/or vitamin B12 without anaemia are also frequent, especially among an elderly population. Saleh et al.3 reported that 19.6% (210/1,142) of admissions for elective total knee or hip replacement were anaemic compared with local population reference ranges (males 13 g/dL; females, 11.5 g/dL), with 76 of these anaemic patients having a haemoglobin (Hb) <11 g/dL and 13 having a Hb <10 g/dL. Regarding the types of anaemia, 135 had normocytic normochromic anaemia, 49 showed hypochromic varieties of anaemia, and 26 were classified as having other types of anaemia.
Similarly, in another series of 715 patients undergoing major elective orthopaedic surgery the prevalence of pre-operative anaemia was 10.5%, because of haematinic deficiency (31%), chronic inflammation with or without iron deficiency (31%), and mixed or unknown cause (38%)4. Interestingly, iron deficiency was present in 18% of non-anaemic patients, vitamin B12 deficiency in 5%, and folate deficiency in 2%4. These deficiencies might blunt the response to erythropoiesis-stimulating agents, and delay the recovery from post-operative anaemia. In addition, almost 20% of patients had a Hb level <13 g/dL4 and it is well known that a low pre-operative Hb level is one of the major predictive factors for requiring peri-operative blood transfusion in orthopaedic surgery with moderate to high peri-operative blood loss5–7.
In this regard, a European study including almost 4,000 patients showed an inverse relationship between pre-operative Hb values and the probability of receiving allogeneic blood transfusion (e.g., 10–18% for Hb 15 g/dL, 20–30% for Hb 13 g/dL, 50–60% for Hb 10 g/dL; 70–75% for Hb 8 g/dL)8. Similarly, 30 to 70% of patients undergoing hip fracture repair received allogeneic blood transfusion peri-operatively, and the logistic regression analysis identified pre-operative Hb value as an independent predictor of the need for these transfusions9. The limited physiological reserve and the higher prevalence of unrecognised cardiovascular disease may render the elderly population vulnerable to milder degrees of anaemia when undergoing the stress of surgery. In this regard, two large retrospective cohort studies of patients who underwent major non-cardiac surgery found that the adjusted risk of 30-day post-operative mortality and cardiac morbidity begins to rise when pre-operative haematocrit levels decrease to less than 39%2,10.
On the other hand, major orthopaedic procedures are associated with a significant peri-operative blood loss. As a consequence, up to 90% of patients undergoing such procedures develop post-operative anaemia, which may be aggravated by inflammation-induced blunted erythropoiesis, especially through decreased iron availability (i.e, hepcidin-dependent down-regulation of intestinal absorption and impaired mobilisation from body stores)11. The correction of severe post-operative anaemia often requires allogeneic blood transfusion.
Orthopaedic surgical patients at risk of developing severe post-operative anaemia and receiving peri-operative allogeneic blood transfusion should, therefore, be identified, on the basis of red blood cell mass (reflected by haemoglobin concentration on the day of pre-operative assessment), the lowest haemoglobin concentration that the patient can tolerate (transfusion trigger), and the expected blood loss (e.g., using Mercuriali’s algorithm)12. Whenever clinically feasible, these patients should have their Hb level and iron status (serum iron, ferritin, and transferrin saturation index) tested preferably 30 days before the scheduled surgical procedure13. For patients older than 60 years, vitamin B12 and folic acid should also be measured13. Any deficiency should be corrected prior to surgery, and unexplained anaemia should always be considered as secondary to some other process and, therefore, elective surgery should be deferred until a diagnosis has been made13,14.
The patient’s situation
Let us analyse three possible scenarios.
- Clinical suspicion of anaemia. This suspicion can be easily confirmed or ruled out by determining Hb level, which provides a reliable indication of the presence and severity of anaemia. In this regard, Muñoz et al.15 evaluated the utility of point-of-care haemoglobin measurements with the HemoCue B-Haemoglobin for the initial diagnosis of anaemia. Results from venous and capillary samples were compared with those of standard blood laboratory tests. The results with capillary samples were highly encouraging with only one false negative. It was suggested that the use of such “office/consulting room”, point-of-care based testing could exclude patients who are non-anaemic, whereas those identified as anaemic could then have an appropriate laboratory appraisal for anaemia. Overall, this saves time and is cost-saving to the healthcare system. However, as most patients have their Hb measured when they present for assessment by the anaesthetist, the HemoCue B-Haemoglobin may be more useful in the follow-up of patients being treated for anaemia (especially in those receiving recombinant human erythropoietin, rHuEPO), and upon admission for surgery.
-
- Iron deficiency without anaemia. The downside of “in office” testing is that iron deficiency (per se) will not be detected if it is not reflected in the Hb level. It is well known that a normal Hb level does not exclude iron deficiency, because individuals with normal body iron stores must lose a large proportion of body iron before the Hb falls below the laboratory defined level of anaemia (generally, Hb <12 g/dL for women and Hb <13 g/dL for men, although higher levels were recently proposed, according to gender, age and racial origin)16.
In non-anaemic patients, the most important clinical indicator of iron deficiency is the symptom of chronic fatigue (iron is required for the enzymes involved in oxidative metabolism). However, it is of little screening value because clinicians rarely consider the presence of iron deficiency in patients who are not anaemic, and therefore iron deficiency is almost invariably diagnosed in the laboratory17.
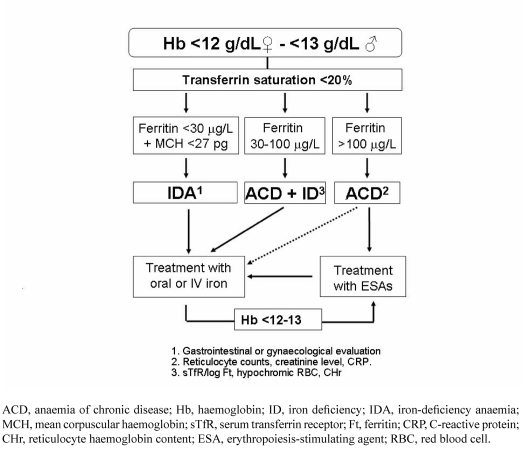
- Overt anaemia. Finally, our patient may present with overt anaemia, thus requiring directed laboratory work-up for its differential diagnosis. Laboratory tests for investigating iron deficiency fall into two categories: measurements providing evidence of iron depletion in the body (serum iron, transferrin saturation [TSAT], Ferritin [Ft], serum transferrin receptors [sTfR], ferritin index [sTfR/log Ft], hepcidin) and measurements reflecting iron-deficient red cell production (Hb, mean corpuscular volume [MCV], red cell distribution width [RDW], mean corpuscular Hb [MCH], hypochromic red cells, reticulocyte Hb content [CHr])18. The appropriate combination of these laboratory tests, plus some markers of inflammation (e.g., C-reactive protein) will help to establish a correct diagnosis of anaemia and iron deficiency status. An easy-to-follow algorithm for the diagnosis and treatment of iron-deficiency anaemia (IDA) and non-IDA is presented in Figure 111. Of course, if no alteration in iron metabolism is found, vitamin B12 and folic acid levels should also be measured.
Figure 1.
A simplified algorithm for the diagnosis of iron deficiency anaemia (modified from reference 11).
How to diagnose iron deficiency in the laboratory
Iron deficiency without anaemia
Iron deficiency can be defined by a ferritin level <15–30 ng/mL in the absence of inflammation (e.g., normal serum concentrations of C-reactive protein [CRP] and/or normal erythrocyte sedimentation rate) (absolute iron deficiency). In the presence of inflammation, iron deficiency should be defined by low transferrin saturation (TSAT <20%) and normal ferritin concentrations (>100 ng/mL) (functional iron deficiency) or intermediate ferritin values (30–100 ng/mL) (absolute iron deficiency)11.
Iron-deficiency anaemia
Patients should be considered as having IDA when they present with low Hb (men <13 g/dL and women <12 g/dL), TSAT (<20%) and ferritin concentrations (<30 ng/mL) but no signs of inflammation11. A low MCH (<27 pg) or, better still, a low CHr (<28 pg), rather than low mean MCV (<80 fL) has become the most important red cell marker for detecting iron deficiency in circulating red blood cells (Figure 1). MCV is a reliable and widely available measurement but it is a relatively late indicator in patients who are not actively bleeding. In the presence of a low MCV, the differential diagnosis must be made with thalassaemia (normal RDW). In addition, patients may present with IDA without microcytosis when there is coexisting vitamin B12 or folate deficiency, post-bleeding reticulocytosis, an initial response to oral iron treatment, alcohol intake or mild myelodysplasia.
A truncated form of the transferrin receptor can be detected in human serum. The serum concentration of this soluble form of transferrin receptor is proportional to the total amount of surface transferrin receptor (sTfR; normal median concentration: 1.2–3.0 mg/L, but its measurement is not standardised and depends on which of the three available kits is used). Although sTfR levels are usually high or very high in uncomplicated IDA, as is the ferritin index, they are not usually required for the diagnosis.
Anaemia of chronic disease
Patients should be considered to have anaemia of chronic disease (ACD), also called anaemia of inflammation, when they have: (i) evidence of inflammation (e.g., high CRP level), (ii) a Hb concentration <13 g/dL for men and <12 g/dL for women, and (iii) a low transferrin saturation (TSAT<20%), but normal or increased serum ferritin concentration (>100 ng/mL [Figure 1]). In the presence of a low serum ferritin concentration (50–100 ng/mL), confirmation of ACD will be given by the ferritin index plot as a sTfR/log ferritin ratio <2 with normal CHr11. Both ACD and functional iron deficiency are frequent among patients with inflammatory disease without apparent blood losses.
Anaemia of chronic disease with absolute iron deficiency
Patients should be suspected of anaemia of chronic disease with absolute iron deficiency (ACD/IDA) when they have: (i) a chronic inflammation, (ii) a Hb concentration <13 g/dL for men and <12 g/dL for women, and (iii) a low transferrin saturation (TSAT<20%) with a serum ferritin concentration >30 and <100 ng/mL (Figure 1). Confirmation of ACD/IDA will be given by the ferritin index plot as a sTfR/log ferritin ratio >2 with a CHr <28 pg11,19. This type of anaemia is more frequent in patients with inflammatory diseases and chronic blood losses (e.g. inflammatory bowel disease).
The regulation of iron metabolism in ACD/IDA involves the iron regulatory protein hepcidin. Hepcidin is a central regulator of iron metabolism controlling cellular iron efflux from enterocytes and macrophages. During inflammation, interleukin-6 induces hepcidin production which binds to ferroportin in enterocytes, macrophages and hepatocytes, leading to a decrease in dietary iron absorption and an increase in macrophage iron. As a consequence, inflammation causes decreased circulating iron and impaired iron distribution within the body20. Very recently, it has been shown that serum hepcidin-25 (the active form of hepcidin) levels correlated with ferritin levels and the ferritin index. The use of hepcidin-25 levels ≤4 nmol/L allows differentiation of IDA from both ACD and ACD/IDA. Furthermore, the discrimination of ACD/IDA from ACD requires combination with CHr (hepcidin-25 plot) data. Thus, the hepcidin-25 plot and the ferritin index plot showed a good correspondence in the differentiation of iron states in patients with anaemia21.
There are several other important haematological indices that may also help in the diagnosis of iron deficiency in ACD, although they are only available in specific haematology analysers. Besides CHr, hypochromic red blood cells are reported by the Bayer Advia 120 haematology analyser (normal value <5%)22. The Sysmex XE-2100 haematology analyser determines RET-Y, which can be considered as the reticulocyte haemoglobin equivalent (RET-He), as well as RBC-Y, which can be considered as the erythrocyte haemoglobin equivalent23. The Beckman-Coulter LH 750 determines the microcytic anaemia factor or microcytic factor or low haemoglobin density24. The clinical utility of these new indices in the diagnosis of iron deficiency will be evaluated in the near future.
How to manage iron deficiency in orthopaedic patients
Initiation of iron treatment and goals
According to the recommendations of the Network for the Advancement of Transfusion Alternatives (NATA) Consensus Statement on management of pre-operative anaemia and on the use of intravenous iron13,14 iron supplementation should be initiated when IDA is present. For iron deficiency without anaemia, different approaches to iron replacement should be considered and discussed with the patient. The major goal of therapy for IDA is to supply sufficient iron to increase haemoglobin levels to normal values within 4–6 weeks, and to replenish iron stores. Nevertheless, the route by which to administer the iron is still a matter of debate.
Oral iron
Oral iron supplementation is adequate in most clinical conditions. Administration of oral iron (mostly ferrous salts) in the absence of inflammation or significant ongoing blood loss can correct the anaemia, provided significant doses can be tolerated. Thus, oral iron is the conventional iron treatment because it is a low cost and non-invasive route of administration.
However, time is very important for surgical patients (e.g., Hb levels are inversely correlated with the risk for blood transfusion). Total iron deficit can be calculated using Ganzoni’s formula: Total iron deficit (mg) = Weight (kg)× [Ideal Hb-Actual Hb] (g/dL)×0.24+depot iron (500 mg).
According to this formula, a person weighing 70 kg with a Hb level of 9 g/dL would have a body iron deficit of about 1400–1600 mg. If oral iron treatment is used, it would take 2–2.5 weeks for Hb levels to increase, as long as 2 months to reach normal values, and at least 6 months to fill iron stores.
In addition, there is a large list of situations (loss of small bowel integrity, Helicobacter pylori infection, inflammation, etc.) and substances (e.g., antibiotics, antacid medications, phytates and phenolic compounds) that may interfere with the absorption of iron salts, and lead to failure of oral iron therapy11. Moreover, non-absorbed iron salts may produce a variety of highly reactive oxygen species, including hypochlorous acid, superoxides and peroxides, which may lead to digestive intolerance, causing nausea, flatulence, abdominal pain, diarrhoea or constipation, and black or tarry stools, and may be able to activate relapsed inflammatory bowel disease26,27. To avoid the risk of poisoning during oral iron therapy, other iron compounds (such as iron polymaltose which has low toxicity and meets the requirements for a food supplement) might be used instead of ferrous salt preparations26 and lower doses (e.g., 50–100 mg of elemental iron) should be recommended27. Additionally, the response and tolerance should be monitored and treatment changed to intravenous iron if necessary.
A randomised controlled trial on iron pre-load for major joint replacement showed that at least 18% of patients attending for total hip or knee replacement were anaemic and benefited significantly from pre-operative iron supplementation over 4 weeks. In addition, iron supplementation in patients without obvious anaemia protected against a fall in Hb during the immediate post-operative period, suggesting a widespread underlying depletion of iron stores in this group despite a normal Hb28.
In this regard, the implementation of a blood-saving protocol for total knee replacement, consisting of a restrictive transfusion trigger (Hb <8 g/dL) plus oral haematinics for 30–45 days prior surgery29, was found to be useful since the transfusion rate (5.8%) and the transfusion index (1.78 units per transfused patient) was reduced with respect to those in both the previous series in our institution7 and other published series8,30. Similar results were obtained by Rodgers et al31. Moreover, for patients with a pre-operative Hb <13 g/dL, this protocol seems to be as effective as other more complex and expensive protocols, involving the use of high doses of rHuEPO32.
In contrast, the results of several randomised controlled trials seem to demonstrate that the administration of oral iron for anaemia after orthopaedic surgery does not appear to be worthwhile, despite being a widespread practice, since postoperative erythropoiesis is limited by the inflammatory effects of surgery on iron metabolism33–38.
Intravenous iron
Because of the limitations of oral iron therapy in patients scheduled for surgery, parenteral routes of iron administration should be preferred, even though many patients will respond to oral iron. Nowadays, intramuscular administration of iron is not recommended. Thus, intravenous iron has emerged as a safe and effective alternative for the management of IDA. The indication for intravenous iron in surgical patients takes into consideration factors such as:
- intolerance or contraindications to oral iron therapy;
- poor adherence or failure of oral iron therapy;
- malabsorptive status;
- inflammatory status;
- severe IDA or ACD/IDA (Hb <10 g/dL);
- use of erythropoiesis-stimulating agents;
- short time to surgery;
- predicted blood loss;
- active bleeding.
Seven different products are principally used in clinical practice: iron gluconate, iron sucrose, low molecular weight (LMW) iron dextran, ferric carboxymaltose, iron isomaltoside 1000, high molecular weight (HMW) iron dextran, and ferumoxytol (the last two are only available in USA) (Table I). Their efficacy in treating anaemia has been consistently proven in a variety of clinical settings39. All intravenous iron agents are colloids with spheroid iron-carbohydrate nanoparticles. For most compounds, each particle consists of an iron-oxyhydroxide core (Fe [III]) and a carbohydrate shell that stabilises the iron-oxyhydroxide core. However, the structure of iron isomaltoside 1000 is somewhat different as the linear oligosaccharide isomaltoside 1000 allows for the formation of a matrix with interchanging iron and carbohydrate.
Table I.
Some characteristics of the different intravenous iron formulations.
| Iron gluconate | Iron sucrose | Low molecular weight iron dextran | Ferric carboxymaltose | Iron isomaltoside 1000 | High molecular weight iron dextran | Ferumoxytol | |
|---|---|---|---|---|---|---|---|
| Carbohydrate shell | Gluconate (monosaccharide) | Sucrose (disaccharide) | Dextran (branched polysaccharide) | Carboxymaltose (branched polysaccharide | Isomaltoside (linear oligosaccharide) | Dextran (branched polysaccharide) | Polyglucose sorbitol carboxymethylether |
| Complex type | Type III Labile and weak | Type II Semi-robust and moderately strong | Type I Robust and strong | Type I Robust and strong | Type I Robust and strong | Type I Robust and strong | Type I Robust and strong |
| Molecular weight (kD) | 289–440 | 30–60 | 165 | 150 | 150 | 265 | 750 |
| Initial distribution volume (L) | 6 | 3.4 | 3.5 | 3.5 | 3.4 | 3.5 | 3.16 |
| Plasma half-life (h) | 1 | 6 | 20 | 16 | 20 | 60 | 15 |
| Labile iron release | +++ | ± | - | - | - | - | - |
| Direct iron donation to transferrin (% injected dose) | 5–6 | 4–5 | 1–2 | 1–2 | <1 | 1–2 | <1 |
| Test dose required | No | Yes/No (*) | Yes | No | No | Yes | No |
| Iron content (mg/mL) | 12.5 | 20 | 50 | 50 | 100 | 50 | 30 |
| Maximal single dose (mg) | 125 | 200 | 20 mg/kg | 15 mg/kg (max 1,000 mg in one infusion) | 20 mg/kg | 20 mg/kg | 510 |
| Premedication | No | No | TDI only | No | No | TDI only | No |
| Life-threatening ADE (×106 doses) | 0.9 | 0.6 | 3.3 | ?? | ?? | 11.3 | ?? |
Legend: ADE: adverse drug effect; TDI: total dose infusion;
Only in some countries in the European Union.
Complexes can generally be classified as labile or robust (kinetic variability), and as weak or strong (thermodynamic variability), with all possible intermediates. Each iron product is taken up into the reticuloendothelial system, where the carbohydrate is degraded for iron to become bioavailable. Although the efficacy of intravenous iron is directly related to the amount of iron administered, differences in core size and carbohydrate chemistry determine pharmacological and biological differences between the different iron complexes. These include clearance after injection, iron release in vitro, early evidence of iron bioactivity in vivo, and maximum tolerated dose and rate of infusion, as well as effects on oxidative markers, propensity for inducing hypophosphataemia, and propensity to cause transient proteinuria following administration40–45.
Experience/evidence on the use of intravenous iron in orthopaedic patients
Non-elective surgery
In two preliminary studies, including 15 patients with hip fracture, post-operative transfusion requirements were significantly reduced by the administration of intravenous iron dextran plus erythropoietin46,47. Recently, the safety and utility of iron sucrose, an intravenous iron formulation with lesser and milder side effects than iron dextran, for the treatment of acute anaemia in patients with pertrochanteric and subcapital hip fracture was assessed in six studies.
After evaluation of the safety of iron sucrose administration in a small number of patients with pertrochanteric hip fracture, the effectiveness of pre-operative intravenous 200–300 mg iron sucrose on the reduction of transfusion requirements and on post-operative morbidity-mortality in patients with pertrochanteric or subcapital hip fractures was prospectively investigated48–50. No adverse reactions to iron administration were observed, but pre-operative iron sucrose reduced the percentage of transfused patients, compared to a control group. This reduction in allogeneic blood transfusion rate was significant in patients with subcapital hip fracture (15% vs. 36.8%, respectively; p=0.059) or with admission Hb >12 g/dL (26.3% vs. 41.3%, respectively; p <0.05). These results were recently confirmed by a randomised controlled trial of patients receiving 600 g iron sucrose pre-operatively, which was associated with a significant reduction in allogeneic blood transfusion rate in patients with subcapital hip fracture (14.3% vs. 45.7%, respectively; p=0.04) or with admission Hb >12 g/dL (18.3% vs. 30%, respectively; p=0.049)51.
In addition, there were lower post-operative infection and 30-day mortality rates, plus a trend towards shorter hospital stay, in patients receiving iron sucrose49,50 (Table II). On the other hand, data from patients with admission Hb≤12 g/dL suggested that a benefit could be obtained if iron sucrose and rHuEPO were jointly administered.
Table II.
Characteristics of the clinical studies involving hip fracture patients that compared intravenous iron administration with oral iron or no intervention included in this review.
| Study year (ref) | n | Study design | Fracture type | IVI mg (Schedule) | rHuEPO (dose) | ABT rate (%) | Infection rate (%) | 30-day mortality rate (%) |
|---|---|---|---|---|---|---|---|---|
| Cuenca et al. 2004 (48,49)* | 180 | Single centre, prospective, observational; retrospective control | PHF | 100–300 (100 mg/48h) | No | Control: 55.9%* IVI: 42.3% | Control: 33.3% IVI: 19.2% | Control: 16.7% IVI. 11.5%: |
| Cuenca et al. 2005 (50) | 77 | Single centre, Prospective, observational; retrospective control | SHF | 200–300 (100 mg/48h) | No | Control: 36.8% IVI: 15% | Control: 33.3% IVI: 15% | Control: 19.3% IVI: 0% |
| Serrano-Trenas et al. 2010 (51) | 200 | Single centre, randomised, double blind | PHF, SHF | 600 (200 mg/48h) | No | Control: 41.3%** IVI:33.1% | Control: 13% IVI: 16% | Control: 7% IVI: 10% |
| García-Erce et al. 2006 (52) | 124 | Single centre, prospective, observational; parallel control | PHF, SHF | 600 (200 mg/48h) | If Hb <13 g/dL (1×40,000 IU) | Control: 53.7% IVI: 19.3% | Control. 31.4% IVI: 12.5% | Control: 15% IVI:7.3% |
| García-Erce et al. 2009 (53) | 196 | Single centre, prospective, observational; parallel control | Anaemic patients PHF, SHF | 600 (200 mg/48h) | 1×40,000 IU | IVI: 60% IVI+rHuEPO: 42% | IVI: 11% IVI+rHuEPO: 5% | IVI: 5% IVI+rHuEPO: 2% |
Legend: n: number of patients; IVI: intravenous iron; PHF: pertrochanteric hip fracture; SHF: subcapital hip fracture; rHuEPO: recombinant human erythropoietin; ABT: allogeneic blood transfusion.
Combined data from two studies with the same historical control; difference in ABT rate was significant for patients with admission Hb >12 g/dL.
Difference in ABT rate was significant for patients with admission Hb >12 g/dL or with SHF.
The effects of this combined therapy was explored in a prospective study including patients with pertrochanteric or subcapital hip fracture who received 600 mg iron sucrose (plus 40,000 IU rHuEPO subcutaneously if Hb <13 g/dL; the cut-off for rHuEPO use in orthopaedic surgery) and a restrictive transfusion protocol (transfusion trigger: Hb <8 g/dL and/or symptoms of acute anaemia)52. Once again, the treatment resulted in a significant reduction of both the percentage of postoperatively transfused patients (54 vs. 19%, respectively), the number of transfused units (1.7±1.3 vs. 0.6±1.1 units per patient, respectively), and the postoperative infection rate (31 vs. 13%, respectively), when compared to a control group. In addition, there was a trend to lower 30-day mortality, and no adverse reactions to iron administration were found (Table II).
As this protocol was adopted by the authors’ institution as standard of care for hip fracture patients, 2 years later they prospectively audited the adhesion to the protocol for anaemic patients in everyday clinical practice53. Only 81 out of 196 anaemic patients received rHuEPO (41%). Overall, 103 out of 196 patients (52.5%) received at least one unit of allogeneic blood (2.1±1.0 units per patient). However, there were significant differences in peri-operative allogeneic blood transfusion rates between groups (42% vs. 60%, for those with or without rHuEPO, respectively; P=0.013) (Table II). Haemoglobin levels on post-operative days 7 and 30 were higher among those receiving rHuEPO, who also presented with higher Hb levels on post-operative day 30 than on admission (12.7±1.0 g/dL vs. 11.9±0.8 g/dL, respectively; P=0.030). Administration of rHuEPO did not increase post-operative complications or 30-day mortality rate. Only three mild intravenous iron adverse effects were witnessed. Therefore, in anaemic patients with hip fracture managed with peri-operative intravenous iron and a restrictive transfusion protocol, pre-operative administration of rHuEPO is associated with reduced allogeneic blood transfusion requirements. However, appropriate training, education and awareness are needed to avoid protocol violations and to limit further exposure to allogeneic blood transfusion and its related risks.
Finally, it worth noting that the use of a restrictive transfusion trigger (Hb <8 g/dL or Hb <9 g/dL if cardiac disease), which was a corner stone in this protocol, is supported by data from several randomised controlled trials showing that in euvolaemic surgical patients a restrictive transfusion threshold did not result in an increase of mortality, morbidity, or length of hospital stay54–56. The exception is the study by of Foss et al.57, but it was not designed to assess this objective and lacked statistical power to do so.
Elective surgery
In a recent consensus statement on the management of peri-operative anaemia, the panel suggests intravenous iron administration during the peri-operative period for patients undergoing orthopaedic surgery who are expected to develop severe post-operative anaemia13. To this end, in the NATA guidelines on the detection, evaluation, and management of pre-operative anaemia in patients undergoing elective orthopaedic surgery, the panel recommends that elective orthopaedic surgical patients have a Hb level determination 28 days before the scheduled surgical procedure if possible as well as further laboratory testing to evaluate those with anaemia for nutritional deficiencies, chronic renal insufficiency, and/or chronic inflammatory disease14. Finally, both panels recommend that nutritional deficiencies, with or without anaemia, be treated13,14.
Following these recommendations, we evaluated the efficacy of intravenous iron sucrose administration (1,000±400 mg over 3–5 weeks) for the correction of pre-operative anaemia in 84 patients who were scheduled for major elective surgery (30 colon cancer resections, 33 abdominal hysterectomies, 21 lower limb arthroplasties)58. In the orthopaedic patients, administration of intravenous iron caused a significant increase of Hb levels (1.8±1.1 g/dL; p <0.001) and anaemia was resolved in 67% of patients.
No life-threatening adverse effects were seen, and the overall transfusion rate was only 21%. These results were somewhat better than those reported by Theussinger et al.59 who observed a Hb increment of 1.0±0.6 g/dL 2 weeks after the start of intravenous iron treatment, and suggested administration of intravenous iron 2–3 weeks before the scheduled surgical procedure.
From these results, we concluded that because of the low incidence of side effects and the rapid increase of Hb levels, intravenous iron is a safe, effective drug for treating pre-operative anaemia in these populations of patients. However, quite often we do not have such a time frame to investigate anaemia and implement the appropriate treatment. In this regard, we evaluated the effects of perioperative administration of iron sucrose (2×200 mg intravenously, one dose 24 hours before surgery and the other 24 hours after surgery) plus folic acid and vitamin C until discharge, on transfusion requirements in patients undergoing total knee replacement. In addition, patients with a preoperative Hb <13 g/dL received one dose of rHuEPO (40,000 IU subcutaneously, 24 hours before surgery), and allogeneic blood transfusion was given if the post-operative Hb value was lower than 8 g/dL or there were clinical symptoms of acute anaemia (Hb <9 g/dL for patients with active cardiac disease). No adverse effects of iron sucrose or rHuEPO administration were seen and, overall, only 4% of patients required allogeneic blood transfusion60. No major differences in peri-operative blood counts or iron metabolism parameters were observed between sub-groups of patients, but stimulation of erythropoiesis seemed to be more pronounced in those patients receiving rHuEPO. Interestingly, the allogeneic blood transfusion rate in patients with a pre-operative Hb <13 g/dL (9%) was no different from that reported with the pre-operative administration of rHuEPO at the dosage of 4×40,000 IU (10.8%)32.
We also evaluated the effectiveness of this blood-saving protocol on recovery from post-operative anaemia in another series of 148 patients undergoing total knee replacement.
Mean Hb loss at post-operative day 7 was 3.6 g/dL, but only seven patients were transfused (5%).
Pre-operatively, 66 (45%) patients did not have enough stored iron to compensate for this Hb loss (ferritin <100 ng/mL).
On post-operative day 30, only 15% were anaemic: 71% of Hb loss and 92% of pre-operative Hb had been recovered, and iron stores were increased, although erythropoietic response was higher in patients receiving erythropoietin (p<0.05)61.
This protocol does, therefore, seem to reduce allogeneic blood transfusion requirements and may hasten the recovery from post-operative anaemia in patients undergoing total knee replacement, although further studies are needed to ascertain which patients may benefit from extended intravenous iron and/or rHuEPO administration.
As stated above, the results of several randomised controlled trials suggested that the administration of oral iron was not effective in correcting anaemia after orthopaedic surgery33–38. Similarly, one interrupted randomised controlled trial in cardiac surgery patients and a small group of orthopaedic patients showed that post-operative administration of intravenous iron, alone or in combination with rHuEPO, was not associated with a greater increase in Hb concentration than placebo62.
However, iron sucrose (3 mg/kg/day) was shown to be a more effective oral iron for restoring postoperative Hb levels after spinal surgery in children63.
More recently, the effect of post-operative administration of 300 mg of intravenous iron sucrose on allogeneic blood transfusion requirements in patients undergoing total hip replacement was evaluated64.
Compared with patients in a previous series not given iron therapy, the group given iron showed a lower transfusion index (1.68 vs. 0.96 units per patient, respectively), and a trend to a lower transfusion rate (73% vs. 46%, respectively). This reduction could not be related to changes in transfusion practice, since there were no differences in pre-transfusion Hb levels between the two groups.
The percentage of transfused patients in this study was higher than that reported in other studies65, most probably due to the high prevalence of pre-operative anaemia (37%) in this series. However, it is worth noting that admission Hb levels for non-transfused patients were lower in the iron group than in the control group (12.7±0.9 g/dL vs. 14.0±1.2 g/dL, respectively; p=0.017), suggesting an erythropoietic effect of iron. Additionally, since 65% of transfusions were given on the third or fourth post-operative day or later, an extended post-operative iron administration schedule or a higher dose might be useful for further reducing late post-operative allogeneic blood transfusion.
This possibility was examined in a subsequent series of patients undergoing total hip or knee replacement who were given 600 mg intravenous iron post-operatively; the haematological and transfusion data of these patients were compared to those from patients receiving 300 mg66.
All patients were operated on by the same surgeon, using the same implants, and received the same antibiotic and anti-thrombotic prophylaxis.
There were no differences between groups regarding age, gender distribution or co-morbidity, but there were a higher proportion of hip procedures and slightly lower preoperative Hb in the 300 mg intravenous iron group (but not post-operatively).
Compared to patients receiving 300 mg intravenous iron (n=53), those receiving 600 mg (n=169) had a lower allogeneic blood transfusion rate (12.4% vs. 26.4%, respectively; p=0.028) and index (0.55±1.0 unit per patient vs. 0.25±0.7 units per patient, respectively; p=0.015), without differences in pre-transfusion levels (7.8±0.7 g/dL vs. 7.9±0.8 g/dL, respectively; p=0.658), spent a shorter time in hospital (8.9±3.4 days vs. 8.0±2.2 days, respectively; p=0.018), and had a trend to a lower post-operative infection rate (7.5% vs. 1.6%, respectively; p=0.058). No adverse reactions to iron administration were seen.
Post-operative administration of 600 mg of intravenous iron does, therefore, seem to be safe and more effective than that of 300 mg for reducing allogeneic blood transfusion requirements in patients undergoing total hip or knee replacement.
A large, randomised controlled trial to confirm these results is warranted.
Lower or higher intravenous iron doses in orthopaedic patients?
Although iron sucrose is the most frequently used intravenous iron compound in the reviewed studies, the main disadvantage of this compound is the need for multiple infusions as the maximum weekly dose should not exceed 600 mg (200 mg intravenously, 1–3 times per week). The availability of stable parenteral iron compounds allowing for higher dose infusion (ferric carboxymaltose [≤15 mg/kg body weight, up to 1000 mg intravenously], LMW iron dextran or iron isomaltoside 1000 [≤20 mg/kg body weight intravenously]) may greatly facilitate iron replacement therapy in orthopaedic patients. The use of these stable compounds has benefits for both the patient (less disruption of life, less time away from home/work, fewer injections, fewer side effects, etc.) and for hospital/health service (fewer visits, reduced physician and nurse time, improved out-patient management, better cost-effectiveness, etc.).
Are there any other advantages from the use of higher doses of intravenous iron? The benefits of high doses (total dose infusion) have been highlighted in several studies. We compared data from a study by Gisbert et al67 who used iron sucrose (2×200 mg/week) in 22 anaemic patients with inflammatory bowel disease (Hb <10 g/dL), and those from a study by Kulnigg et al.68 who used ferric carboxymaltose (up to 2000 mg, 1–2 infusions) in a similar group of 137 patients with inflammatory bowel disease. A Hb increase ≥2 g/dL or normalisation of Hb level was observed in 77% of patients in both groups, without differences in the incidence of side effects (0% vs. 1.5%). However, the duration of the study was 26 week for patients receiving iron sucrose and only 12 weeks for those given ferric carboxymaltose.
In another study, García-Erce et al.69 evaluated the impact of administering multiple doses of intravenous iron sucrose (300 mg/session) compared with high doses of LMW iron dextran or ferric carboxymaltose (500 mg or more/session) in 47 patients with IDA caused by a variety of conditions. It was found that patients receiving the higher dose administration achieved similar post-treatment Hb levels (increase in Hb of 2 g/dL or Hb ≥12 g/L) but much more rapidly (21 vs. 39 days). Moreover, the higher dose was also associated with higher ferritin levels (271 ng/mL vs. 116 ng/mL), which may be important to delay the recurrence of IDA70.
More recently, Evstatiev et al. also found that higher doses of intravenous iron in patients with inflammatory bowel disease were superior, in a comparative clinical trial of the efficacy and safety of standardised ferric carboxymaltose doses (n=240) with respect to individually calculated iron sucrose doses (n=235)71. At week 12 after the initiation of iron therapy, the response rate (defined as an increase in Hb of >2 g/dL) (66.1% vs. 54.1%), the proportion of non-anaemic patients (72.8% vs. 61.8%), and full adherence to the treatment regimen (92.5% vs. 79.1%) were all significantly greater in the ferric carboxymaltose group than in the iron sucrose group. In addition, there was no significant difference in treatment-related adverse events between the two groups (13.9% vs. 11.3%).
These results endorse the use of high dose infusion regimens and may explain the benefit of administering high doses of intravenous iron to patients with iron deficiency and inflammation (faster and more complete response). What, however, is the underlying mechanism? It has been postulated that while the bone marrow requires 20–30 mg iron/day for erythropoiesis, only a small proportion of the infused iron is delivered in a ferric form into the plasma and taken up by transferrin. According to data from in vitro studies, approximately 45 mg of iron can be sustained in the plasma after the administration of 1,000 mg of iron administered intravenously. Meanwhile, most of the administered iron dose is taken up by the macrophages. The iron overload of the macrophages in the reticulo-endothelial system may induce over-expression of ferroportin (thereby by-passing the hepcidin block) allowing iron to enter the bone marrow, transported by transferrin, to sustain the erythropoiesis20. In addition, in autoimmune diseases, iron loading of macrophages may inhibit pro-inflammatory immune effector pathways, thus reducing disease activity72.
What are the true risks of intravenous iron therapy?
Although almost no severe adverse drug effects have been reported, the numbers of patients included in the studies evaluating the role of intravenous iron for treating anaemia in orthopaedic surgical patients are not big enough to draw definite conclusions regarding the safety of intravenous iron compounds in this clinical setting. However, according to an analysis of data from the Food and Drug Administration (FDA) (2001–2003; 30×106 doses), the incidence of life-threatening adverse drug effects (2.2 per million doses), including deaths (0.4 per million doses), associated with the use of four intravenous iron preparations (iron gluconate, iron sucrose, HMW iron dextran, and LMW iron dextran), is much lower than that associated with the use of allogeneic blood transfusion (10 and 4 per million units, respectively)73,74.
It must be borne in mind that all intravenous preparations have been reported to cause anaphylactoid reactions characterised by nausea, hypotension, tachycardia, chest pain, dyspnoea (lung oedema), and bilateral oedema of the hands and feet, and should not be misinterpreted as anaphylaxis40,44. However, iron dextran complexes may cause well-known dextran-induced anaphylactic reactions, which are significantly more frequent with HMW iron dextran than with LMW iron dextran. Although the exact mechanism of the anaphylactic reaction to iron dextran has not yet been clarified, it seems to be related to the antibody-mediated release of mediators by mast cells. HMW iron dextran is not commercially available in Europe, the National Comprehensive Cancer Network recommends against the use of HMW iron dextran and the FDA has altered the labelling of HMW iron dextran to warn that it is not clinically interchangeable with LMW iron dextran75,76.
Regarding the newer iron formulations, ferric carboxymaltose has not yet been approved by the FDA because of a mortality safety signal and imbalance in serious adverse events and occurrence of hypophosphataemia (http://www.fda.gov/ohrms/dockets/ac/08/briefing/2008-4337b1-01-fda.pdf). The FDA is continuing to evaluate ferumoxytol due to reports of serious cardiac disorders, to determine the need for any regulatory action (http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Surveillance/AdverseDrugEffects/ucm223734.htm). In addition, ferumoxytol interferes with magnetic resonance imaging which is frequently used for the diagnosis and follow-up of orthopaedic pathologies, and consequently does not seem to be an appropriate intravenous iron compound for orthopaedic patients. The iron isomaltoside 1000 regulatory application includes references to general intravenous iron documentation, including evidence from iron dextran treatment. The regulatory authorities have therefore placed iron isomaltoside 1000 in the B03AC06 (iron trivalent, parenteral preparations, ferric oxide dextran complex) pharmacotherapeutic group of the Anatomical Therapeutic Chemical (ATC) classification system. Nevertheless, they recognise the unique properties of iron isomaltoside 1000, the unique generic name iron isomaltoside 1000 (a non-anaphylactic carbohydrate), and no requirement for a test dose application which is always the case with iron dextran.
Overall, with the exception of HMW iron dextran (increased rates of severe side effects and deaths), it seems that the acute safety differences among intravenous iron products are small when the products are given at the recommended doses, although comparative trials are needed to be certain.
Current information on the relationship between intravenous iron and infection, and between intravenous iron and oxidative stress deserves special consideration. Elemental iron is an essential growth factor for bacteria with many species expressing iron transport proteins that compete with transferrin, and it has long been suggested that patients with iron overload are at increased risk of infection77. However, in a population of patients undergoing peritoneal dialysis, the risk of peritonitis did not differ between patients who did or did not receive intravenous iron78. In addition, a meta-analysis of six observational studies (807 patients) revealed that the administration of intravenous iron to patients undergoing major orthopaedic surgery led to a significant decrease in both transfusion rates (RR: 0.60; 95% confidence interval [CI]: 0.50–0.72; p <0.001) and infection rates (RR: 0.45; 95%CI: 0.32–0.63; p <0.001)79. However, despite the absence of definitive clinical data, it seems sensible to avoid intravenous iron administration in the setting of acute infection, and to withhold intravenous iron in patients with pre-treatment ferritin values >500 ng/mL13. On the other hand, the available evidence relating intravenous iron administration to atherogenesis is indirect, and there is little evidence that intravenous iron adversely affects survival in patients with dialysis-dependent chronic kidney disease.
In addition, intravenous iron therapy has not been associated with an increase in tumour incidence80. Nevertheless, the evidence argues for caution, not complacency, in prescribing intravenous iron40.
Key learning points
- Iron supplementation should be initiated as soon as iron deficiency, with or without anaemia, is diagnosed.
- Pre-operative oral or intravenous iron may correct anaemia and reduce the number of transfused units and the percentage of transfused patients.
- Peri-operative intravenous iron reduces the frequency and volume of allogeneic blood transfusion in orthopaedic and trauma surgery, and may hasten the recovery from post-operative anaemia, while preserving iron stores. These effects seem to be increased by the addition of a single dose of recombinant human erythropoietin.
- Intravenous iron seems to decrease infection rate or mortality. Nevertheless, despite the absence of definitive clinical data, intravenous iron should not be given to patients with ongoing bacteraemia or iron overload.
- With the exception of high molecular weight iron dextran (increased rates of severe side effects and deaths), it seems that the acute safety differences among intravenous iron products are small when they given at the recommended doses, and smaller that those associated with red blood cell transfusions.
References
- 1.Spahn DR. Anemia and patient blood management in hip and knee surgery. A systematic review of the literature. Anesthesiology. 2010;113:482–95. doi: 10.1097/ALN.0b013e3181e08e97. [DOI] [PubMed] [Google Scholar]
- 2.Wu WC, Schifftner TL, Henderson WG, et al. Pre-operative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007;297:2481–8. doi: 10.1001/jama.297.22.2481. [DOI] [PubMed] [Google Scholar]
- 3.Saleh E, McClelland DBL, Hay A, et al. Prevalence of anaemia before major joint arthroplasty and the potential impact of pre-operative investigation and correction on peri-operative blood transfusions. Br J Anaesth. 2007;99:801–8. doi: 10.1093/bja/aem299. [DOI] [PubMed] [Google Scholar]
- 4.Bisbe E, Castillo J, Sáez M, et al. Prevalence of preoperative anemia and hematinic deficiencies in patients scheduled for major orthopedic surgery. Transfus Altern Transfus Med. 2008;4:166–73. [Google Scholar]
- 5.Salido JA, Marín LA, Gómez LA, et al. Pre-operative haemoglobin levels and the need for transfusion after prosthetic hip and knee surgery: analysis of predictive factors. J Bone Joint Surg Am. 2002;84-A:216–20. doi: 10.2106/00004623-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Aderinto J, Brenkel IJ. Pre-operative predictors of the requirement for blood transfusion following total hip replacement. J Bone Joint Surg Br. 2004;86-B:970–3. doi: 10.1302/0301-620x.86b7.14682. [DOI] [PubMed] [Google Scholar]
- 7.García-Erce JA, Solano VM, Cuenca J, Ortega P. La hemoglobina preoperatoria como único factor predictor de las necesidades transfusionales en la artroplastia de rodilla. Rev Esp Anestesiol Reanim. 2002;49:254–60. [PubMed] [Google Scholar]
- 8.Rosencher N, Kerkkamp HE, Macheras G, et al. OSTHEO Investigation: Orthopedic Surgery Transfusion Haemoglobin European Overview (OSTHEO) study: blood management in elective knee and hip arthroplasty in Europe. Transfusion. 2003;43:459–69. doi: 10.1046/j.1537-2995.2003.00348.x. [DOI] [PubMed] [Google Scholar]
- 9.García-Erce JA, Cuenca J, Solano VM. Factores predictores de transfusión en pacientes mayores de 65 años con fractura subcapital de cadera. Med Clin (Barc) 2003;120:161–6. doi: 10.1016/s0025-7753(03)73637-9. [DOI] [PubMed] [Google Scholar]
- 10.Beattie WS, Karkouti K, Wijeysundera DN, Tait G. Risk associated with preoperative anemia in noncardiac surgery: a single-center cohort study. Anesthesiology. 2009;110:574–81. doi: 10.1097/ALN.0b013e31819878d3. [DOI] [PubMed] [Google Scholar]
- 11.Muñoz M, García-Erce JA, Remacha AF. Disorders of iron metabolism. Part II: Iron deficiency and iron overload. J Clin Pathol. 2011;64:287–96. doi: 10.1136/jcp.2010.086991. [DOI] [PubMed] [Google Scholar]
- 12.Mercuriali F, Inghilleri G. Proposal of an algorithm to help the choice of the best transfusion strategy. Curr Res Med Opin. 1996;13:465–78. doi: 10.1185/03007999609115227. [DOI] [PubMed] [Google Scholar]
- 13.Beris P, Muñoz M, García-Erce JA, et al. Perioperative anaemia management: consensus statement on the role of intravenous iron. Br J Anaesth. 2008;100:599–604. doi: 10.1093/bja/aen054. [DOI] [PubMed] [Google Scholar]
- 14.Goodnough LT, Maniatis A, Earnshaw P, et al. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106:13–22. doi: 10.1093/bja/aeq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muñoz M, Romero A, Gómez JF, et al. Utility of point-of-care haemoglobin measurement in the HemoCue-B haemoglobin for the initial diagnosis of anaemia. Clin Lab Haematol. 2005;27:99–104. doi: 10.1111/j.1365-2257.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- 16.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747–50. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cook JD. Diagnosis and management of iron-deficiency anaemia. Best Pract Res Clin Haematol. 2005;18:319–32. doi: 10.1016/j.beha.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 18.Thomas C, Thomas L. Biochemical markers and hematologic indices in the diagnosis of functional iron deficiency. Clin Chem. 2002;48:1066–76. [PubMed] [Google Scholar]
- 19.Basora C, Deulofeu R, Salazar F, et al. Improved preoperative iron status assessment by soluble transferrin receptor in elderly patients undergoing knee and hip replacement. Clin Lab Haem. 2006;28:370–5. doi: 10.1111/j.1365-2257.2006.00821.x. [DOI] [PubMed] [Google Scholar]
- 20.Muñoz M, García-Erce JA, Remacha AF. Disorders of iron metabolism. Part 1: Molecular basis of iron homeostasis. J Clin Pathol. 2011;64:281–6. doi: 10.1136/jcp.2010.079046. [DOI] [PubMed] [Google Scholar]
- 21.Thomas C, Kobold U, Balan S, Roeddiger R, Thomas L. Serum hepcidin-25 may replace the ferritin index in the Thomas plot in assessing iron status in anemic patients. Int J Lab Hematol. 2011;33:187–93. doi: 10.1111/j.1751-553X.2010.01265.x. [DOI] [PubMed] [Google Scholar]
- 22.Thomas L, Franck S, Messinger M, Linssen J, et al. Reticulocyte hemoglobin measurement-comparison of two methods in the diagnosis of iron-restricted erythropoiesis. Clin Chem Lab Med. 2005;43:1193–202. doi: 10.1515/CCLM.2005.207. [DOI] [PubMed] [Google Scholar]
- 23.Brugnara C, Schiller B, Moran J. Reticulocyte hemoglobin equivalent (RetHe) and assessment of iron-deficient states. Clin Lab Haematol. 2006;28:303–8. doi: 10.1111/j.1365-2257.2006.00812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canals C, Remacha AF, Sarda MP, et al. Clinical utility of the new Sysmex XE 2100 parameter - reticulocyte hemoglobin equivalent - in the diagnosis of anemia. Haematologica. 2005;90:1133–4. [PubMed] [Google Scholar]
- 25.Ganzoni AM. Eisen-Dextran intravenös: therapeutische und experimentalle Möglichkeiten. Schweizerische medizinische Wochenschrift. 1970;100:301–3. [PubMed] [Google Scholar]
- 26.Crichton RR, Danielsson BG, Geisser P. Iron therapy with special emphasis on intravenous administration. 2008. UNI-Med Verlag AG, Bremen,
- 27.Gisbert JP, Gomollón F. Common misconceptions in the diagnosis and management of anemia in inflammatory bowel disease. Am J Gastroenterol. 2008;103:1299–307. doi: 10.1111/j.1572-0241.2008.01846.x. [DOI] [PubMed] [Google Scholar]
- 28.Andrews CM, Lane DW, Bradley JG. Iron pre-load for major joint replacement. Transfus Med. 1997;7:281–6. doi: 10.1046/j.1365-3148.1997.d01-42.x. [DOI] [PubMed] [Google Scholar]
- 29.Cuenca J, García-Erce JA, Martínez F, et al. Pre-operative haematinics and transfusion protocol reduce the need for transfusion after total knee replacement. Inter J Surg. 2007;5:89–94. doi: 10.1016/j.ijsu.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Muñoz M, Kühlmorgen B, Ariza D, et al. Which patients are more likely to benefit of postoperative shed blood reinfusion after unilateral total knee replacement? Vox Sang. 2007;92:136–41. doi: 10.1111/j.1423-0410.2006.00868.x. [DOI] [PubMed] [Google Scholar]
- 31.Rogers BA, Cowie A, Alcock C, Rosson JW. Identification and treatment of anaemia in patients awaiting hip replacement. Ann R Coll Surg Engl. 2008;90:504–7. doi: 10.1308/003588408X301163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weber EW, Slappendel R, Hémon Y, et al. Effects of epoetin alfa on blood transfusions and postoperative recovery in orthopaedic surgery: the European Epoetin Alfa Surgery Trial (EEST) Eur J Anaesthesiol. 2005;22:249–57. doi: 10.1017/s0265021505000426. [DOI] [PubMed] [Google Scholar]
- 33.Zauber NP, Zauber AG, Gordon FJ, et al. Iron supplementation after femoral head replacement for patients with normal iron stores. JAMA. 1992;267:525–7. [PubMed] [Google Scholar]
- 34.Sutton PM, Cresswell T, Livesey JP, et al. Treatment of anaemia after joint replacement. J Bone Joint Surg Br. 2004;86-B:31–3. [PubMed] [Google Scholar]
- 35.Wheatheral M, Maling TJ. Role of iron therapy for anaemia after orthopaedic surgery: randomized clinical trial. ANZ J Surg. 2004;74:1049–51. doi: 10.1111/j.1445-1433.2004.03265.x. [DOI] [PubMed] [Google Scholar]
- 36.Mundy GM, Birtwistle SJ, Power RA. The effect of iron supplementation on the level of haemoglobin after lower limb arthroplasty. J Bone Joint Surg Br. 2005;87-B:213–7. doi: 10.1302/0301-620x.87b2.15122. [DOI] [PubMed] [Google Scholar]
- 37.Prasad N, Rajamani V, Hullin D, Murray JM. Postoperative anaemia in femoral neck fracture patients: does it need treatment? A single blinded prospective randomised controlled trial. Injury. 2009;40:1073–6. doi: 10.1016/j.injury.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 38.Parker MJ. Iron supplementation for anemia after hip fracture surgery: a randomized trial of 300 patients. J Bone Joint Surg Am. 2010;92-A:265–9. doi: 10.2106/JBJS.I.00883. [DOI] [PubMed] [Google Scholar]
- 39.Muñoz M, Breymann C, García-Erce JA, et al. Efficacy and safety of intravenous iron therapy as an alternative/adjunct to allogeneic blood transfusion. Vox Sang. 2008;94:172–83. doi: 10.1111/j.1423-0410.2007.01014.x. [DOI] [PubMed] [Google Scholar]
- 40.Aronoff GR. Safety of intravenous iron in clinical practice: implications for anemia management protocols. J Am Soc Nephrol. 2004;15:S99–S106. doi: 10.1097/01.ASN.0000143815.15433.87. [DOI] [PubMed] [Google Scholar]
- 41.Silverstein SB, Rodgers GM. Parenteral iron therapy options. Am J Hematol. 2004;76:74–8. doi: 10.1002/ajh.20056. [DOI] [PubMed] [Google Scholar]
- 42.Fishbane S, Kowalski EA. The comparative safety of intravenous iron dextran, iron saccharate, and sodium ferric gluconate. Semin Dial. 2000;13:381–4. doi: 10.1046/j.1525-139x.2000.00104.x. [DOI] [PubMed] [Google Scholar]
- 43.Danielson BG. Structure, chemistry, and pharmacokinetics of intravenous iron agents. J Am Soc Nephrol. 2004;15:S93–S98. doi: 10.1097/01.ASN.0000143814.49713.C5. [DOI] [PubMed] [Google Scholar]
- 44.Van Wyck DB. Labile iron: manifestations and clinical implications. J Am Soc Nephrol. 2004;15(Suppl 2):S107–S111. doi: 10.1097/01.ASN.0000143816.04446.4C. [DOI] [PubMed] [Google Scholar]
- 45.Jahn MR, Andreasen HB, Fütterer S, et al. A comparative study of the physicochemical properties of iron isomaltoside 1000 (Monofer®), a new intravenous iron preparation and its clinical implications. Eur J Pharm Biopharm. 2011;78:480–91. doi: 10.1016/j.ejpb.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 46.Goodnough LT, Merkel K. Parenteral iron and recombinant human erythropoietin therapy to stimulate erythropoiesis in patients undergoing repair of hip fracture. Hematology. 1996;1:163–6. doi: 10.1080/10245332.1996.11746300. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt AH, Templeman DC, Kyle RF. Blood conservation in hip trauma. Clin Orthop. 1998;357:68–73. doi: 10.1097/00003086-199812000-00010. [DOI] [PubMed] [Google Scholar]
- 48.Cuenca J, García-Erce JA, Martínez AA, et al. Utilidad del hierro intravenoso en el tratamiento de la fractura de cadera en el anciano. Med Clin (Barc) 2004;123:281–5. doi: 10.1016/s0025-7753(04)74493-0. [DOI] [PubMed] [Google Scholar]
- 49.Cuenca J, García-Erce JA, Muñoz M, et al. Patients with pertrochanteric hip fracture may benefit from pre-operative intravenous iron therapy: a pilot study. Transfusion. 2004;44:1447–52. doi: 10.1111/j.1537-2995.2004.04088.x. [DOI] [PubMed] [Google Scholar]
- 50.Cuenca J, García-Erce JA, Martínez AA, et al. Role of parenteral iron in the management of anaemia in the elderly patient undergoing displaced subcapital hip fracture repair: preliminary data. Arch Orthop Trauma Surg. 2005;125:342–7. doi: 10.1007/s00402-005-0809-3. [DOI] [PubMed] [Google Scholar]
- 51.Serrano-Trenas JA, Font-Ugalde P, Muñoz-Cabello L, et al. Role of perioperative intravenous iron therapy in elderly hip fracture patients: a single-center randomized controlled trial. Transfusion. 2011;51:97–104. doi: 10.1111/j.1537-2995.2010.02769.x. [DOI] [PubMed] [Google Scholar]
- 52.García-Erce JA, Cuenca J, Muñoz M, et al. Peri-operative stimulation of erythropoiesis with intravenous iron and erythropoietin reduces transfusion requirements in patients with hip fracture. A prospective observational study. Vox Sang. 2005;88:235–43. doi: 10.1111/j.1423-0410.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- 53.García-Erce JA, Cuenca J, Haman-Alcober S, et al. Efficacy of preoperative recombinant human erythropoietin administration for reducing transfusion requirements in patients undergoing surgery for hip fracture repair. An observational cohort study. Vox Sang. 2009;97:260–7. doi: 10.1111/j.1423-0410.2009.01200.x. [DOI] [PubMed] [Google Scholar]
- 54.Carson JL, Terrin ML, Barton FB, et al. A pilot randomized trial comparing symptomatic vs. hemoglobin-level-driven red blood cell transfusions following hip fracture. Transfusion. 1998;38:522–9. doi: 10.1046/j.1537-2995.1998.38698326331.x. [DOI] [PubMed] [Google Scholar]
- 55.Grover M, Talwalkar S, Casbard A, et al. Silent myocardial ischaemia and haemoglobin concentration: a randomized controlled trial of transfusion strategy in lower limb arthroplasty. Vox Sang. 2006;90:105–12. doi: 10.1111/j.1423-0410.2006.00730.x. [DOI] [PubMed] [Google Scholar]
- 56.Carson JL. Transfusion triggers in orthopedic patients. Transfus Altern Transfus Med. 2011;12:9. [Google Scholar]
- 57.Foss NB, Kristensen MT, Jensen PS, et al. The effects of liberal versus restrictive transfusion thresholds on ambulation after hip fracture surgery. Transfusion. 2009;49:227–34. doi: 10.1111/j.1537-2995.2008.01967.x. [DOI] [PubMed] [Google Scholar]
- 58.Muñoz M, García-Erce JA, Diéz-Lobo AI, et al. Utilidad de la administración de hierro sacarosa intravenoso para la corrección de la anemia preoperatoria en pacientes programados para cirugía mayor. Med Clin (Barc) 2009;132:303–6. doi: 10.1016/j.medcli.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 59.Theusinger OM, Leyvraz PF, Schanz U, et al. Treatment of iron deficiency anemia in orthopedic surgery with intravenous iron: efficacy and limits: A prospective study. Anesthesiology. 2007;107:923–7. doi: 10.1097/01.anes.0000291441.10704.82. [DOI] [PubMed] [Google Scholar]
- 60.Cuenca J, García-Erce JA, Martínez F, et al. Perioperative intravenous iron, with or without erythropoietin, plus restrictive transfusion protocol reduce the need for allogeneic blood after knee replacement surgery. Transfusion. 2006;46:1112–9. doi: 10.1111/j.1537-2995.2006.00859.x. [DOI] [PubMed] [Google Scholar]
- 61.García-Erce JA, Cuenca J, Martínez F, et al. Perioperative intravenous iron preserves iron stores and may hasten the recovery from postoperative anaemia after knee replacement surgery. Transf Med. 2006;16:335–41. doi: 10.1111/j.1365-3148.2006.00682.x. [DOI] [PubMed] [Google Scholar]
- 62.Karkouti K, McCluskey SA, Ghannam M, et al. Intravenous iron and recombinant erythropoietin for the treatment of postoperative anaemia. Can J Anesth. 2006;53:11–19. doi: 10.1007/BF03021522. [DOI] [PubMed] [Google Scholar]
- 63.Bernière J, Dehullu JP, Gall O, Murat I. Intravenous iron in the treatment of postoperative anaemia in surgery of the spine in infants and adolescents. Rev Chir Orthop. 1998;84:319–22. [PubMed] [Google Scholar]
- 64.Muñoz M, Naveira E, Seara J, et al. Role of parenteral iron on transfusion requirements after total hip replacement. A pilot study. Transfus Med. 2006;16:137–42. doi: 10.1111/j.1365-3148.2005.00629.x. [DOI] [PubMed] [Google Scholar]
- 65.Feagan BG, Wong CJ, Kirkley A, et al. Erythropoietin with iron supplementation to prevent allogeneic blood transfusion in total hip joint arthroplasty. Ann Intern Med. 2000;133:845–54. doi: 10.7326/0003-4819-133-11-200012050-00008. [DOI] [PubMed] [Google Scholar]
- 66.Muñoz M, Naveira E, Seara J, et al. Postoperative iron after lower limb arthroplasty: a comparative study of the effects of 300 vs. 600 mg v iron administration on transfusion requirements. Transfus Altern Transfus Med. 2011;12:34–5. [Google Scholar]
- 67.Gisbert JP, Bermejo F, Pajares R, et al. Oral and intravenous iron treatment in inflammatory bowel disease: Hematological response and quality of life improvement. Inflamm Bowel Dis. 2009;15:1485–91. doi: 10.1002/ibd.20925. [DOI] [PubMed] [Google Scholar]
- 68.Kulnigg S, Stoinov S, Simanenkov V, et al. A novel intravenous iron formulation for treatment of anemia in inflammatory bowel disease: the ferric carboxymaltose (FERINJECT) randomized controlled trial. Am J Gastroenterol. 2008;103:1182–92. doi: 10.1111/j.1572-0241.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- 69.García-Erce JA, Soria B, Cuenca J, et al. [Benefits from ambulatory administration of intravenous iron up to one gram per session]. LI Annual Meeting of the Spanish Association of Haematology and Haemotherapy; Barcelona. 2009. [Google Scholar]
- 70.Kulnigg S, Teischinger L, Dejaco C, et al. Rapid recurrence of IBD-associated anemia and iron deficiency after intravenous iron sucrose and erythropoietin treatment. Am J Gastroenterol. 2009;104:1460–7. doi: 10.1038/ajg.2009.114. [DOI] [PubMed] [Google Scholar]
- 71.Evstatiev R, Marteau P, Iqbal T, et al. Efficacy and safety of standardised ferric carboxymaltose doses vs. individually calculated iron sucrose doses for IBD-associated iron deficiency anemia: a multicentre, randomised controlled trial. Presented at the 18th United European Gastroenterology Week; Barcelona. 2010; p. P0420. [Google Scholar]
- 72.Weiss G, Meusburger E, Radacher G, et al. Effect of iron treatment on circulating cytokine levels in ESRD patients receiving recombinant human erythropoietin. Kidney Int. 2003;64:572–8. doi: 10.1046/j.1523-1755.2003.00099.x. [DOI] [PubMed] [Google Scholar]
- 73.Chertow GM, Mason PD, Vaaga-Nilsen O, Ahlmén J. Update on adverse drug events associated with parenteral iron. Nephrol Dial Transplant. 2006;21:378–82. doi: 10.1093/ndt/gfi253. [DOI] [PubMed] [Google Scholar]
- 74.Stainsby D, Jones H, Asher D, et al. SHOT Steering Group Serious hazards of transfusion: a decade of hemovigilance in the UK. Transfus Med Rev. 2006;20:273–82. doi: 10.1016/j.tmrv.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 75.Rodgers GM, Auerbach M, Cella D, et al. High-molecular weight iron dextran: a wolf in sheep’s clothing? J Am Soc Nephrol. 2008;19:833–4. doi: 10.1681/ASN.2008030255. [DOI] [PubMed] [Google Scholar]
- 76.Coyne DW, Auerbach M. Anemia management in chronic kidney disease: intravenous iron steps forward. Am J Hematol. 2010;85:311–2. doi: 10.1002/ajh.21682. [DOI] [PubMed] [Google Scholar]
- 77.Weiss G. Iron and immunity: a double-edged sword. Eur J Clin Invest. 2002;32(Suppl 1):70–8. doi: 10.1046/j.1365-2362.2002.0320s1070.x. [DOI] [PubMed] [Google Scholar]
- 78.Vychytil A, Haag-Weber M. Iron status and iron supplementation in peritoneal dialysis patients. Kidney Int. 1999;55(Suppl):S71–S78. doi: 10.1046/j.1523-1755.1999.055suppl.69071.x. [DOI] [PubMed] [Google Scholar]
- 79.García-Erce JA, Cuenca J, Gómez-Ramírez S, et al. Posibilidades de tratamiento de la anemia perioperatoria en cirugía ortopédica y traumatología. Anemia. 2009;2:17–27. [Google Scholar]
- 80.Huang X. Iron overload and its association with cancer risk in humans: evidence for iron as a carcinogenic metal. Mutat Res. 2003;533:153–71. doi: 10.1016/j.mrfmmm.2003.08.023. [DOI] [PubMed] [Google Scholar]