Abstract
Background
The aim of this study was to evaluate the quality of red blood cell concentrates obtained from donated whole blood, selected for transfusion therapy of thalassaemic patients, by measuring the following parameters: haemoglobin, haematocrit, percentage haemolysis, residual leucocyte count and residual protein content.
Materials and methods
Overall 345 red cell concentrates were evaluated, of which 205 had been filtered in-line pre-storage and washed and 140 were buffy coat-depleted and used within 2 days of collection. Of the buffy coat-depleted concentrates, 62 were leucodepleted and 78 washed and leucodepleted post-storage all within 2 days of collection. The off-line filters used for the leucodepletion were gamma-irradiated polyester with a pore size of 200 μm. The washing procedure was automated (Haemonetics ACP 215, Braintree, MA, USA). The haematological parameters were evaluated by a blood cell counter (Coulter, Ramsey, IL, USA) and the white blood cell count by cytofluorimetry (FACScan).
Results
Ninety-five percent (194/205) of the red cell concentrates that had been filtered pre-storage and washed, 92% (57/62) of the red cell concentrates that had been leucodepleted post-storage and 94% (73/78) of the those subjected to both treatments had normal values of haemoglobin (>40 g/unit), haematocrit (between 50–70%), percentage haemolysis (<0.8/unit), white cell count (<1×106) and residual protein content (<0.5 g/L). Five percent (11/205) of the red cell concentrates that had been filtered pre-storage and washed, 8% (5/62) of those leucodepleted post-storage after 2 days and 6% (5/78) of those that underwent both procedures had a haemoglobin content <40 g/unit and a haematocrit <50%.
Conclusions
The preparation procedures had been carried out satisfactorily; nevertheless, transfusion therapy with some “low dose” normal units could be less effective and might, therefore, result in greater transfusion requirements in patients receiving such units.
Keywords: post-storage leucodepletion, washed red blood cells, haemoglobin
Introduction
The transfusion standards of the Italian Society of Immunohaematology and Transfusion Medicine (SIMTI)1 define as “second level” a series of blood components derived from treatments such as post-storage leucodepletion in the laboratory, cycles of washing, irradiation, pathogen inactivation, assembly of other blood components from single products and fractionation of single units into subunits. In the laboratories of the Unit of Collection, Processing and Validation of Blood Components, of San Camillo Forlanini Hospital in Rome, red cell concentrates are normally washed and filtered within 4 days of collection for a population of 14 patients with β-thalassaemia who have attended our Thalassaemia Day Hospital for transfusions from the age of 3 years old up to the present. Some of these patients have now reached the age of 47 years old. These multiply transfused patients who have had previous transfusion reactions and are immunised (antibodies to plasma proteins) cannot receive transfusion therapy with untreated blood components2 (i.e. units that have not been washed and/or filtered pre- or post-storage). On the basis of the thalassaemic patient’s history and immunohaematological studies, the transfusion doctor defines and personalises the type of treatment that the chosen red blood cell concentrates for a given patient must undergo.
Leucodepletion and washing of red cell concentrates, widely recommended for multiply transfused patients3,4 with problems of immunisation5–7, must be carried out exploiting the technological8 and performance improvements of the operative systems. Removal of leucocytes reduces the risk of development of anti-HLA and anti-HPA and, therefore, of refractoriness to platelet transfusions and Graft-versus-Host disease9–11, and lowers the risk of transmission of viruses such as cytomegalovirus, human immunodeficiency virus, HTLV1/2, Epstein-Barr virus, human herpes viruses 6 and 8, and parvovirus B19 if performed before storage; furthermore, it reduces the formation of microaggregates and the release of cytokines12–14, responsible for adverse effects of transfusion such as febrile, non-haemolytic transfusion reactions2,15, and leads to a lower concentration of potassium in the supernatant16–18.
Given that the concentration of cytokines such as interleukin-6, but above all interleukin-8, increases in patients with beta-thalassaemia because the chronic haemolysis in these patients induces macrophage hyperactivity19, leucodepletion plays a very important role in transfusion therapy. It traps residual leucocytes in the filters, preventing the release of cytokines if performed within 4 days of the donation, because even if the small amounts of cytokines infused within every transfusion remain in the circulation for only a short time20, it should not be overlooked that interleukin-8 binds to the Duffy antigen21 of red blood cells or accumulates in polymorphonuclear leucocytes, thus escaping detection by usual serum assays, but is subsequently released22.
Of the main techniques in use for leucodepletion, off-line filtration in the laboratory is the one that allows the best control of the process, in particularly regarding flow velocity, duration of the filtration and storage temperature12,23 and a precise evaluation of the blood component at the time of filtration, with consequent better standardisation compared to the bedside method24. In both cases the filter used should be chosen carefully in order that it guarantees a residual leucocyte content of 0.5×106/unit or less (SIMTI transfusion standards). With regards to washing procedures, it is important to use a sufficient amount of isotonic solution, at 20–24 °C, so that with one or more washes, residual plasma, leucocytes and platelet present are removed from the red cell concentrate leaving the supernatant with a residual protein content of less than 0.3 g/unit, thus decreasing the risk of immune reactions25.
For the purposes of effective transfusion therapy, the calculated haematocrit of the final red cell concentrate can range from 65% to 75%, depending on the clinical needs; each unit should contain at least 40 g of haemoglobin and the percentage of haemolysis of the red cell mass should be less than 0.8%26.
The equipment used for the production of washed red cell concentrates should be serviced regularly in order to control the correct calibration of the system’s fixed parameters. This is important for the good outcome of the chosen treatment procedures and of the quality controls. Furthermore, in accordance with the SIMTI transfusion standards, it is considered appropriate to use sterile connectors to guarantee a closed system, which not only reduces the possible risk of contamination of the product, but also allows a longer shelf-life compared to that of products prepared using an open circuit system under a sterile hood.
The operating procedures conforming with the standards defined in the most recent edition of the recommendations of the Council of Europe R(95)15 and validated by documented and objective proof, supplied by the companies distributing the automated or non-automated kits and systems, define the reference quality standards, the operative methods, the control criteria during production and the recording methods, in order to obtain qualified products. These products are evaluated by quality controls set out in regulations, and the blood components that do not fulfil the established requisites are segregated and eliminated27,28.
Materials and methods
Three hundred and forty-five second level red cell concentrates which had undergone treatment in our Unit between July 2007 and July 2008 were examined. These units of red cell concentrates, previously selected and assigned by the transfusion doctor to thalassaemic patients undergoing transfusion therapy in the Thalassaemia Day Hospital of San Camillo Forlanini Hospital, underwent quality controls aimed at determining whether the tested units were suitable for efficient transfusion therapy.
These controls, in compliance with regulations and the European Recommendations R(95) 2008, 16th edition, and in accordance with the transfusion standards of SIMTI were carried out on every unit after treatment. The controls included an evaluation of the unit’s haematocrit (accepted values: between 50 and 70%), haemoglobin (accepted values: total haemoglobin >40 g/unit), percentage haemolysis (<0.8%/unit), sterility of the unit and protein concentration of the supernatant (<0.5 g/unit). Although not required by the regulations for washed red cells, an evaluation of residual white blood cells was nevertheless carried out using cytofluorimetry (FACscan, Becton Dickinson, Franklin Lakes, NJ, USA) on all units undergoing post-storage off-line filtration, while sterility controls were conducted on the post-treatment washing fluids.
The choice of the type of treatment to use on the red cells, selected by the transfusion doctor on the basis of blood group, phenotype and compatibility, was made by the doctor of the Thalassaemia Day Hospital in the light of any transfusion reactions of the polytransfused patient. In the absence of particular contraindications (the presence of antibodies to plasma proteins, severe post-transfusion reactions), the treatment could be one or two manual washes in a centrifuge at 2100 rpm for 10 minutes with 300 mL of 0.9% physiological saline solution and resuspension of the red cell concentrate in the same saline solution to reconstitute the initial volume of the unit treated (manual washing is currently automated).
In the case in which the patient has antibodies to plasma proteins and previous post-transfusion reactions, automated washing, divided into four steps, is used. The units are washed with 980 to 1,840 mL of 0.9% physiological saline solution in proportion to the weight of the unit to be washed, restoring the concentrated product to its initial weight.
As far as concerns red blood cells subjected to post-storage leucodepletion with off-line filtration, the filters used must meet certain characteristics regarding the membrane porosity (asymmetrical membrane filters are ideal, since the calibre of the pores decreases progressively in the direction of flow, thus maintaining continuous removal of leucocytes together with continuous blood flow), type of material (for example, polyurethane has an efficacy of about 99.99%, is highly biocompatible, does not release bradykinin, and does not cause activation of complement or contact activation; polyester filters are equally valid) and safety valves for the reflux of air, guaranteeing sterility and a product with a residual cell content less than 0.5–1×109 as requested by current regulations29,30.
Results
Three hundred and forty-five red cell concentrates assigned to patients with β-thalassaemia attending the Thalassaemia Day Hospital of San Camillo Forlanini Hospital were examined. The advised treatment for the units for these multiply transfused patients with a high possibility of developing antibodies to plasma proteins is filtration with one or more washes.
The above described quality controls were carried out on all the treated units to verify their transfusion efficacy7,26,27.
Eighteen percent (62/345) of the units were leucodepleted after storage (2 days after donation) with off-line filters, 22% (78/345) were leucodepleted post-storage and washed and the remaining 60% (205/345) were only washed because the red cell concentrates had been filtered pre-storage.
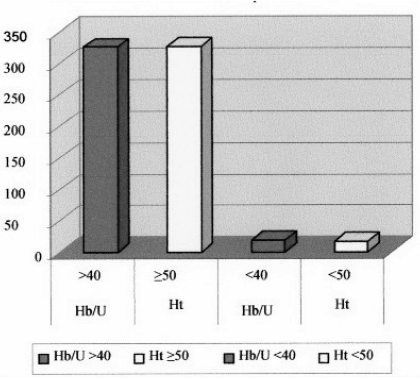
Considering the results of the quality controls carried out on all the units, it was found that 94.5% (326/345) had a haemoglobin content greater than 40 g/U (range, 40–58 g/U), while 5.5% (19/345) had a content below 40 g/U (range, 35–39 g/U).
As far as concerns the haematocrit, 95.4% (327/345) of the units had a haematocrit of 60% or more (range, 60–70%), while 4.6 % (16/345) had a value below 60% (range, 50–59%) (Figure 1).
Figure 1.
Distribution of the values of haemoglobin and haematocrit per unit in the 345 units of red cell concentrates after treatment.
The residual protein content and residual white blood cells in all samples were below 0.3 g/L/U (range, 0.2–0.3 g/L/U) and below 0.1×106 (range, 0.001–0.01×106), respectively. The level of haemolysis was below 0.8% in all the units (range, 0.1–0.4%).
In more detail, 92% (57/62) of the 62 units that underwent only post-storage leucodepletion by off-line filtration 2 days after donation had a haemoglobin content greater than 40 g/unit and a haematocrit greater than 60%; consequently, 8% (5/62) had a haemoglobin content below 40 g/U with values ranging between 35 and 39 g/unit and a haematocrit greater than 50%.
Ninety-four percent (73/78) of the 78 units that underwent post-storage leucodepletion 2 days after donation and were subsequently washed met the normal reference values for the quality controls, while 6% (5/78) had a low total haemoglobin content.
Of the remaining 205 units filtered pre-storage which were subsequently only washed, 95% (194/205) had both haemoglobin content and haematocrit within the norm, while 5% (11/205) of the units had a total haemoglobin content and haematocrit below the normal reference values (Table I).
Table I.
Treatments performed on the red cell concentrates and results of quality control of the units.
| Units of RCC (total=345) | Treatment process | N. of units (%) | Hb g/unit | Hct % | RPC/unit | R-WBC/unit | Haemolysis |
|---|---|---|---|---|---|---|---|
| 205 | Filtered pre-storage washing | 194/205 (95%) | 40–58 g/U M=53 SD=4.949 |
60–70% M=62 SD=2.577 |
0.1–0.3 g/L M=0.2 SD=0.035 |
0.001–0.01×106 M=0.001 SD=0.002 |
No |
| 11/205 (5%) | 35–39 g/U M=38 SD=0.828 |
50–59% M=52 SD=2.806 |
0.1–0.3 g/L M=0.2 SD=0.070 |
0.001–0.01×106 M=0.001 SD=0.002 |
No | ||
| 78 | Post-storage leucodepletion and washing | 73/78 (94%) |
40–58 g/U M=53 SD=4.949 |
60–70% M=60 SD=2.522 |
0.1–0.3 g/L M=0.19 SD=0.026 |
0.001–0.01×106 M=0.001 SD=0.002 |
No |
| 5/78 (6%) | 35–39 g/U M=36 SD=1.303 |
50–59% M=51 SD=3.535 |
0.1–0.3 g/L M=0.2 SD=0.070 |
0.001–0.01×106 M=0.002 SD=0.004 |
No | ||
| 62 | Post-storage leucodepletion | 57/62 (92%) | 40–58 g/U M=52 SD=5.566 |
60–70% M=63 SD=2.751 |
0.1–0.3 g/L M=0.2 SD=0.070 |
0.001–0.01×106 M=0.001 SD=0.006 |
No |
| 5/62 (8%) | 35–39 g/U M=36 SD=1.414 |
50–59% M=50 SD=0.447 |
0.1–0.3 g/L M=0.2 SD=0.070 |
0.001–0.01×106 M=0.002 SD=0.004 |
No |
Legend: RCC: red cell concentrate; Hb g/unit: haemoglobin (standard value: >40 g/unit); Hct of unit (%): haematocrit of the unit (standard value: 50–70%); RPC/unit: residual protein content/unit (standard value: <0.5 g/L); R-WBC/unit: residual white blood cell count/unit (standard: <1×106); M: mean value; SD: standard deviation.
All the units underwent post-filtration quality controls to determine residual white blood cell count: these counts were below 0.1×106 WBC/unit (0.001–0.01×106) and post-washing controls to determine the residual protein content, which was less than 0.3 g/L/U (range, 0.1–0.2 g/L/U).
Discussion and conclusions
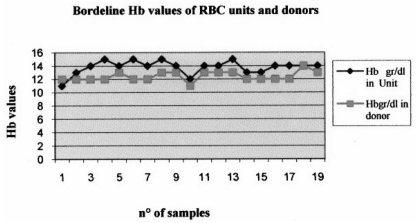
The results of this study show that for each type of treatment about 5% of the units have values outside the normal reference ranges. A detailed analysis revealed that the haemoglobin and haematocrit values of the corresponding donors were, at the time of the donation, at the minimum of the range allowed for by law (12.5 g/dL) to be able to give blood (Figure 2).
Figure 2.
Relation between low haemoglobin and haematocrit values in units of treated red cell concentrates and in the respective donors.
It was, therefore, demonstrated that if the red cell units had not undergone the treatments described, during which the loss of a small amount of haemoglobin is inevitable, their values would have met the standards. Overall, the results of the quality controls on normal red cell concentrates showed good performance of the preparation procedures; nevertheless, transfusion therapy in which some normal, low-dose units are used could be less effective, leading to an increased risk of infection, immunological complications and iron overload31. For this reason, when units are being selected for thalassaemic recipients, whose mean value of haemoglobin in 9.5 g/dL, it would be useful to choose units based not only on compatibility, but also on the full blood count of the donor and on the haemoglobin content of the unit prior to treatment, but essentially, it would be useful to enrol donors for the apheretic collection of red cell units.
Acknowledgments
The authors thank Dr. Emilio Mannella for his scientific advice.
References
- 1.Bonomo P, Alfano G, Gandini G, et al. Standard di Medicina Trasfusionale. 2nd ed. Milano: Edizioni SIMTI; 2010. [Google Scholar]
- 2.Matteocci A, Scocchera R, Cianciulli P, et al. Reazioni trasfusionali febbrili non emolitiche e citochine in pazienti beta-talassemici. La Trasfus del Sangue. 2002;47:470–7. [Google Scholar]
- 3.Matteocci A, Albano M, Damico C, et al. Immunizzazioni ed eventi avversi alla trasfusione di sangue in pazienti talassemici: esperienza del gruppo di studio cooperativo laziale della talassemia. Il Servizio Trasfusionale. 2006;5:38–9. [Google Scholar]
- 4.Baroti Tòth C, Kramer J, Pinter J, et al. IgA content of washed red blood cell concentrates. Vox Sang. 1998;74:13–4. doi: 10.1046/j.1423-0410.1998.7410013.x. [DOI] [PubMed] [Google Scholar]
- 5.Weisbach V, Riego W, Strasser E, et al. The in vitro quality of washed, prestorage leucocyte-depleted red blood cell concentrates. Vox Sang. 2004;87:19–26. doi: 10.1111/j.1423-0410.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 6.Noor Haslina MN, Ariffin N, Illuni Hayati I, Rosline H. Red cell immunization in multiply transfused Malay thalassemic patients. Southeast Asian J Trop Med Public Health. 2006;37:1015–20. [PubMed] [Google Scholar]
- 7.Voulgari PV, Chaidos A, Tzouvara E, et al. Antierythropoietin antibodies in thalassemia patients. Ann Hematol. 2004;83:22–7. doi: 10.1007/s00277-003-0777-z. [DOI] [PubMed] [Google Scholar]
- 8.Butch SH. Applying quality improvement tools in the transfusion service. Clin Lab Sci. 2007;20:113–21. [PubMed] [Google Scholar]
- 9.Mirmomen S, Alavian SM, Hajarizadeh B, et al. Epidemiology of hepatitis B, hepatitis C, and human immunodeficiency virus infections in patients with beta-thalassemia in Iran: a multicenter study. Arch Iran Med. 2006;9:319–23. [PubMed] [Google Scholar]
- 10.Chiou SS, Chang TT, Tsai SP, et al. Lipid peroxidation and antioxidative status in beta-thalassemia major patients with or without hepatitis C virus infection. Clin Chem Lab Med. 2006;44:1226–33. doi: 10.1515/CCLM.2006.219. [DOI] [PubMed] [Google Scholar]
- 11.Cardo LJ, Hmel P, Wilder D. Stored packed red blood cells contain a procoagulant phospholipid reducible by leukodepletion filters and washing. Transfus Apher Sci. 2008;38:141–7. doi: 10.1016/j.transci.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 12.Bordin LO, Heddle NM, Blajchman MA. Biologic effects of leukocytes present in transfused cellular blood products. Blood. 1994;84:1703–21. [PubMed] [Google Scholar]
- 13.Di Monte D, Cassato L, Poliseno G, et al. Leucodeplezione “prestorage”. Influenza sui livelli di citochine. La Trasf del Sangue. 2000;45(Suppl):S47. [Google Scholar]
- 14.Kavalleron L, Frogaton S, Polihs C. Serum concentrations of IL-2,IL-4,IL-6 e IL-8 cytokines in thalassemic patients before and after transfusion with pre-storage leucodepleted red cells. Vox Sang. 2000;78(Suppl):258. [Google Scholar]
- 15.Lin JS, Tzeng CH, Hao TC, et al. Cytokine release in febrile non haemolytic red cell transfusion reaction. Vox Sang. 2002;82:156. doi: 10.1046/j.1423-0410.2002.00159.x. [DOI] [PubMed] [Google Scholar]
- 16.Bansal I, Calhoun BW, Joseph C, et al. A comparative study of reducing the extracellular potassium concentration in red blood cells by washing and by reduction of additive solution. Transfusion. 2007;47:248–50. doi: 10.1111/j.1537-2995.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- 17.Mehdizadeh M, Zamani G, Tabatabaee S. Zinc status in patients with major beta-thalassemia. Pediatr Hematol Oncol. 2008;25:49–54. doi: 10.1080/08880010701773738. [DOI] [PubMed] [Google Scholar]
- 18.Avall A, Hyllner M, Bengtson JP, et al. Greater increase in cytokine concentration after salvage with filtered whole blood than with washed red cells, but no difference in postoperative hemoglobin recovery. Transfusion. 1999;39:271–6. doi: 10.1046/j.1537-2995.1999.39399219283.x. [DOI] [PubMed] [Google Scholar]
- 19.Heddle NM, Klama LN, Griffith L, et al. A prospective study to identify the risk factors associated with acute reactions to platelet and red cell transfusions. Transfusion. 1993;33:794. doi: 10.1046/j.1537-2995.1993.331094054613.x. [DOI] [PubMed] [Google Scholar]
- 20.Borzini P. Le citochine in medicina trasfusionale. La Trasf del Sangue. 1998;43:131. [Google Scholar]
- 21.Jilma-Stohlawetz P, Homoncik M, Drucker C, et al. Fy phenotype and gender determine plasma levels of monocyte chemotactic protein. Transfusion. 2001;41:378. doi: 10.1046/j.1537-2995.2001.41030378.x. [DOI] [PubMed] [Google Scholar]
- 22.Horuk R, Chitnis CE, Darbonne WC, et al. A receptor for the malarial parasite Plasmodium vivax: the erythrocyte chemokine receptor. Science. 1993;261:1182. doi: 10.1126/science.7689250. [DOI] [PubMed] [Google Scholar]
- 23.Alcorta I, Pereira A, Sanz C, et al. Influence of the red cell preparation method on the efficacy of a leukocyte reduction filter. Vox Sang. 1996;71:78–83. doi: 10.1046/j.1423-0410.1996.7120078.x. [DOI] [PubMed] [Google Scholar]
- 24.Ledent E, Berlin G. Inadequate white cell reduction by bed-side filtration of red cell concentrates. Transfusion. 1994;34:765–8. doi: 10.1046/j.1537-2995.1994.34994378276.x. [DOI] [PubMed] [Google Scholar]
- 25.Ledent E, Berlin G. Factors influencing white cell removal from red cell concentrates by filtration. Transfusion. 1996;36:714–8. doi: 10.1046/j.1537-2995.1996.36896374375.x. [DOI] [PubMed] [Google Scholar]
- 26.Valeri CR, Dennis RC, Ragno G, et al. Survival, function, and hemolysis of shed red blood cells processed as nonwashed blood and washed red blood cells. Ann Thorac Surg. 2001;72:1598–602. doi: 10.1016/s0003-4975(01)03097-1. [DOI] [PubMed] [Google Scholar]
- 27.Robinson EA. The European Union Blood Safety Directive and its implications for blood services. Vox Sang. 2007;93:122–30. doi: 10.1111/j.1423-0410.2007.00942.x. [DOI] [PubMed] [Google Scholar]
- 28.Liu WG, Wang WJ, Wang PP, et al. Application of comparison method in internal quality control of hematology analyzer by using fresh blood. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2008;37:88–92. doi: 10.3785/j.issn.1008-9292.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Slopecki A, Smith K, Moore S. The value of Good Manufacturing Practice to a Blood Service in managing the delivery of quality. Vox Sang. 2007;92:187–96. doi: 10.1111/j.1423-0410.2006.00878.x. [DOI] [PubMed] [Google Scholar]
- 30.Reali G. La leucodeplezione totale II. Il Servizio Trasfusionale. 2000;3 [Google Scholar]
- 31.Tsironi M, Polonifi K, Deftereos S, et al. Transfusional hemosiderosis and combined chelation therapy in sickle thalassemia. Eur J Haematol. 2005;75:355–8. doi: 10.1111/j.1600-0609.2005.00528.x. [DOI] [PubMed] [Google Scholar]