Abstract
Background
Storage of red blood cells at 4 °C is associated with deleterious metabolic and biochemical changes, collectively referred to as “storage lesions”. Lipid peroxidation of the red cell membrane leading to lysis contributes to these storage lesions. The aim of the present study was to investigate oxidative injury to red cells during storage for 28 days and its correlation with markers of red cell membrane damage.
Materials and methods
Samples from 30 units of red blood cells stored at 4 °C for 28 days were withdrawn aseptically on day 0, day 14 and day 28 of storage. Markers of membrane damage including plasma haemoglobin, plasma potassium and lactate dehydrogenase (LDH) concentrations and markers of oxidative injury such as malondialdehyde (MDA) levels, haemoglobin oxidation and osmotic fragility were studied in all samples.
Results
Statistically significant (p<0.001) increases in the mean values of plasma haemoglobin, plasma potassium, LDH and markers of oxidative injury such as MDA and haemoglobin oxidation were observed over the storage period of 28 days. Direct correlations of MDA and haemoglobin oxidation with membrane damage, as reflected by plasma haemoglobin concentration, were observed.
Conclusion
Oxidative injury to red blood cells during storage leads to membrane damage and lysis. The role of antioxidants in the prevention of this deleterious effect of storage warrants investigation.
Keywords: red blood cells, storage lesions, oxidative injury
Introduction
Storage of red blood cells in preservative medium is associated with deleterious metabolic, biochemical and molecular changes to erythrocytes: these changes are collectively referred to as “storage lesions”1.
Oxidative damage to red cells during storage contributes to the storage lesions considerably. Identified sites of oxidative injury in stored red cells include cytoskeleton proteins and membrane phospholipids2. The oxidative damage renders red cells more susceptible to stress as shown by increased osmotic fragility during storage and consequent release of haemoglobin (Hb), potassium ions (K+) and intracellular enzymes such as lactate dehydrogenase (LDH) into the supernatant plasma. The aim of the present study was to investigate oxidative injury to red cells during storage for 28 days by measuring malondialdehyde (MDA) levels, Hb oxidation and osmotic fragility and the correlation of the oxidative injury with plasma Hb levels.
Material and methods
A total of 30 buffy coat depleted red cell blood units preserved in SAGM were included in the study. All red cell units were stored at 4 °C for 28 days and samples were withdrawn aseptically on day 0, day 14 and day 28 of storage. Markers of membrane damage including plasma Hb, plasma K+, LDH and markers of oxidative injury such as MDA levels, Hb oxidation and osmotic fragility were studied in all samples.
Red cell indices such as Hb, haematocrit, mean corpuscular volume, mean corpuscular haemoglobin concentration and red cell distribution width were measured on automated cell counter (Micros 60, ABX Diagnostica, Biomeriux, France).
Measurement of markers of membrane damage
Plasma Hb concentration was measured using a commercially available HemoCue Plasma/Low Hb system (HemoCue®, Ängelholm, Sweden) while the plasma K+ concentration was determined by an automated electrolyte analyser (Easylyte, Medica Bedford, Massachusetts, USA). LDH was assayed using a commercially available kit (Bio Systems S.A., Barcelona, Spain).
Measurement of markers of oxidative injury to red cells
Oxidative injury to red cells was studied by measuring the level of MDA using a previously described method3. Briefly, a suspension of 200 μL red cells and 0.1 M NADPH was prepared and incubated at 37 °C for 60 minutes. To this suspension, 6% trichloroacetic acid and 0.6% thiobarbituric acid were added and boiled at 100 °C for 15 minutes. The supernatant was obtained after centrifugation and was read at 532 nm. Hb oxidation and osmotic fragility were measured using standard techniques4.
Statistical analysis
Mean values and standard deviations were calculated for all parameters. Pearson’s correlation was used to correlate the markers of oxidative injury (MDA levels and conversion of methaemoglobin) and markers of membrane damage (plasma Hb). Comparisons between days of sampling were done using a t-test. SPSS software (IBM® SPSS® software version 13) was used for all statistical evaluations.
Results
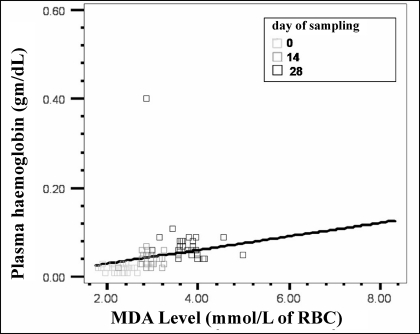
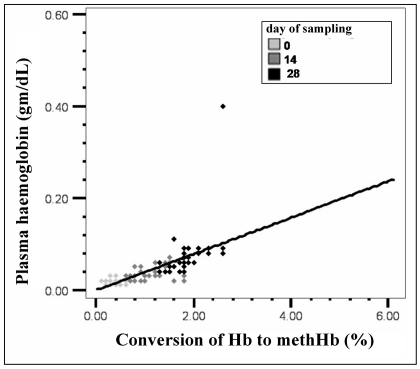
The mean values of markers of membrane damage and oxidative injury are presented in Table I. There were statistically significant (p<0.001) progressive increases in the mean levels of plasma Hb, plasma K+ and LDH over the storage period of 28 days. Mean values of markers of oxidative injury such as MDA and Hb oxidation also increased progressively during storage. A significant increase in osmotic lysis during storage was seen: the mean osmotic fragility on day 0 was 1.07% and increased to 16.2% on day 28 (p<0.005). A direct relationship between lipid peroxidation and plasma Hb, a marker of membrane damage, was noted on day 14 (r=0.578, p<0.001) and day 28 (r=0.513, p<0.005), while it was not statistically significant on day 0 (Figure 1). Similarly, as the conversion of Hb to methaemoglobin increased during storage, plasma Hb also increased on all three days of sampling: day 0 (r=0.392, p<0.005), day 14 (r=0.587, p<0.001) and day 28 (r=0.552, p<0.001) (Figure 2).
Table I.
Mean (SD) levels of various markers of membrane damage and oxidative injury during storage of red cells.
| Parameters | Day 0 | Day 14 | Day 28 | P value |
|---|---|---|---|---|
| Plasma Hb (g/dL) | 0.017±0.01 | 0.037±0.01 | 0.077±0.06 | <0.005 |
| K+ levels (mmol/l) | 5.16±1.2 | 15.7±3.0 | 35.1±4.6 | <0.005 |
| LDH levels (IU/L) | 52.9±7.2 | 204.3±41 | 366.9±67.8 | <0.005 |
| MDA levels (mmol/L) | 2.25±0.21 | 2.9±0.22 | 3.76±0.41 | <0.005 |
| Hb oxidation (%) | 0.366±0.16 | 1.16±0.33 | 1.87±0.34 | <0.005 |
SD: standard deviation; LDH: lactate dehydrogenase; MDA: malondialdehyde
Figure 1.
Correlation between plasma Hb and lipid peroxidation of red cells (r =0.532, p<0.005). As the lipid peroxidation increases (MDA levels), plasma Hb increases simultaneously.
Figure 2.
Correlation between plasma Hb and Hb oxidation (r=0.567, p<0.001). As the percentage conversion of Hb to methaemoglobin (Hb oxidation) increase, plasma Hb increases simultaneously.
Discussion
Lipid peroxidation, the oxidative deterioration of polyunsaturated fatty acids, is a common mechanism of cell injury and death. Although, earlier studies failed to detect MDA, a marker of lipid peroxidation in stored red cells5–6, more recent investigations using sensitive techniques have found increased levels of this marker during red cell storage. We observed a progressive and significant increase in mean MDA levels over 28 days of storage. The mean MDA on day 0 was 2.25 mmol/mg of protein and increased to 3.76 mmol/mg of protein on day 28 (p<0.005). Our results are in agreement with earlier published data7–10.
The relative importance of lipid peroxidation during red cell storage is still unclear. We observed a direct relationship between lipid peroxidation and plasma Hb, a marker of membrane damage (Figure 1, r=0.532). It is, therefore, possible that addition of antioxidants to units of blood could decrease not only lipid peroxidation but also reduce leakage of K+, LDH and Hb. However, no relationship was noted between lipid peroxidation and other markers of membrane damage (plasma K+ and LDH).
Normally, about 3% of Hb mass is converted to methaemoglobin each day11. This methaemoglobin is rapidly converted back to oxyhaemoglobin by methaemoglobin reductase in the presence of NADH. During storage of red blood cells, the activity of methaemoglobin reductase is diminished resulting in increased levels of methaemoglobin which is not converted back to oxyhaemoglobin. Our results are in agreement with earlier published findings8,10.
In the present study, we observed that Hb oxidation started soon after storage and increased progressively, as shown by the significant difference between the mean percentages on day 0 (0.36%) and day 14 (1.16%) (p<0.005). This is in contrast to changes observed in lipid peroxidation, which were more pronounced after the second week of storage.
The increase in Hb oxidation during storage of red blood cells may be due to a decrease in their antioxidant capacity, resulting in oxidation and deterioration of membrane lipids and proteins which can ultimately lead to irreversible damage to the membrane. We observed a direct correlation between increasing oxidation of Hb and a marker of red cell membrane damage, plasma Hb.
The loss of phospholipids from red cells during storage may contribute to increased osmotic fragility and the formation of echinocytes and spheroechinocytes. The majority of red cells in stored blood have normal osmotic fragility but a minor population with increased susceptibility to osmotic lysis can be identified. This latter population is believed to represent those cells removed within 24 hours of transfusion. Our results are in accordance with those of previously published studies9,12.
During storage, spontaneous lysis of a small fraction of red cells takes place and vesicles containing both lipids and Hb from intact red cells are shed into the supernatant plasma. As a result of this lysis, not only is the red cell Hb released into plasma but also intracellular enzymes, such as LDH and cations (K+), are released. The levels of these parameters during red cell storage are considered to be markers of red cell lysis during storage. We observed significant and progressive increases in levels of these markers during storage (Table I).
Red cell lysis due to oxidative injury during storage is now a well established fact, further confirmed by the findings of the present study. The potential effects of adding antioxidants to blood bags are, therefore, of interest. Sharifi et al. studied the effects of antioxidants on the lipid peroxidation of red cells after gamma irradiation9. It was noted that normal human plasma was very effective at protecting against oxidative damage of irradiated red cells. Furthermore, the synthetic antioxidant trilazad mesylate was very effective at protecting red cells against oxidative damage as this antioxidant can scavenge reactive oxygen species that are found within the membrane and block the process of lipid peroxidation. Further studies are required to investigate the role of antioxidants, either given to the donor before donation or added to the blood bag after red cell separation, in preventing oxidative damage to red cells during storage.
Acknowledgments
This work is part of the research supported by an intramural grant from the Sanjay Gandhi Postgraduate Institute of Medical Sciences to the first author. All authors are grateful to the Institute for this intramural grant.
References
- 1.Chin-Yee I, Arya N, d’Almeida MS. The red cell storage lesion and its implication for transfusion. Transfusion Science. 1997;18:447–58. doi: 10.1016/S0955-3886(97)00043-X. [DOI] [PubMed] [Google Scholar]
- 2.Halliwell B. Oxygen radicals: a commonsense look at their nature and medical importance. Med Biol. 1984;62:71–7. [PubMed] [Google Scholar]
- 3.Wright JR, Colb HD, Miles PR. Cytosolic factors which affect microsomal lipid peroxidation in lung and liver. Arch Biochem Biophys. 1981;206:296–304. doi: 10.1016/0003-9861(81)90095-3. [DOI] [PubMed] [Google Scholar]
- 4.Parpart AK, Lorenz PB, Parpart ER, et al. The osmotic resistance (fragility) of human red cells. J Clin Invest. 1947;26:636–40. [PubMed] [Google Scholar]
- 5.Wolfe LC. The membrane and the lesions of storage in preserved red cells. Transfusion. 1985;25:185–203. doi: 10.1046/j.1537-2995.1985.25385219897.x. [DOI] [PubMed] [Google Scholar]
- 6.Bhargava AB, Pavri RS, Bhatia HM. Susceptibility of red cell to the oxidative injury during preservation. Indian J Med Res. 1988;87:202–5. [PubMed] [Google Scholar]
- 7.Knight JA, Voorhees RP, Martin L, Anstall H. Lipid peroxidation in stored red cells. Transfusion. 1992;32:354–7. doi: 10.1046/j.1537-2995.1992.32492263451.x. [DOI] [PubMed] [Google Scholar]
- 8.Anand AJ, Dzik WH, Imam A, Sadrzadeh SMH. Radiation induced red cell damage: role of reactive oxygen species. Transfusion. 1997;37:160–5. doi: 10.1046/j.1537-2995.1997.37297203518.x. [DOI] [PubMed] [Google Scholar]
- 9.Sharifi S, Dzik WH, Sadrzadeh SMH. Human plasma and tirilazad mesylate protects stored human erythrocytes against the oxidative damage of gamma irradiation. Transfus Med. 2000;10:125–9. doi: 10.1046/j.1365-3148.2000.00242.x. [DOI] [PubMed] [Google Scholar]
- 10.Cicha I, Suzuki Y, Tateishi N, et al. Gamma ray irradiated red blood cells stored in mannitol adenine phosphate medium: rheological evaluation and susceptibility to oxidative stress. Vox Sang. 2000;79:75–82. doi: 10.1159/000031216. [DOI] [PubMed] [Google Scholar]
- 11.Carrell RW, Winterbourn CC, Rachmilewitz EA. Activated oxygen and hemolysis. Br J Haematol. 1975;30:259–64. doi: 10.1111/j.1365-2141.1975.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 12.Beutler E, Kuhl W, West C. The osmotic fragility of erythrocytes after prolonged liquid storage and after reinfusion. Blood. 1982;59:1141–7. [PubMed] [Google Scholar]