Abstract
Background
There are no reported studies on whether a helicopter flight affects the quality and shelf-life of red blood cells stored in mannitol-adenine-phosphate.
Materials and methods
Seven days after donation, five aliquots of red blood cells from five donors were packed into an SS-BOX-110 container which can maintain the temperature inside the container between 2 °C and 6 °C with two frozen coolants. The temperature of an included dummy blood bag was monitored. After the box had been transported in a helicopter for 4 hours, the red blood cells were stored again and their quality evaluated at day 7 (just after the flight), 14, 21 and 42 after donation. Red blood cell quality was evaluated by measuring adenosine triphosphate, 2,3-diphosphoglycerate, and supernatant potassium, as well as haematocrit, intracellular pH, glucose, supernatant haemoglobin, and haemolysis rate at the various time points.
Results
During the experiment the recorded temperature remained between 2 and 6 °C. All data from the red blood cells that had undergone helicopter transportation were the same as those from a control group of red blood cell samples 7 (just after the flight), 14, 21, and 42 days after the donation. Only supernatant Hb and haemolysis rate 42 days after the donation were slightly increased in the helicopter-transported group of red blood cell samples. All other parameters at 42 days after donation were the same in the two groups of red blood cells.
Discussion
These results suggest that red blood cells stored in mannitol-adenine-phosphate are not significantly affected by helicopter transportation. The differences in haemolysis by the end of storage were small and probably not of clinical significance.
Keywords: red blood cells, storage, quality, shelf-life, helicopter transportation
Introduction
The storage time of red blood cells (RBC) is determined primarily based on in vitro data on adenosine triphosphate (ATP) levels and haemolysis1 and on in vivo 24-hour survival radiolabelling studies2. RBC in Europe are generally stored in citrate-phosphate-dextrose (CPD)-adenine solution or saline-adenine-glucose-mannitol; in these preservative solutions their shelf-life is 42 days3. In the UK, Canada and USA the storage time is also 42 days, with common additive solutions being the Adsol, Nutricel and Optisol preservative solutions4–6. In Japan, it had been a long-standing practice to store RBC in CPD/mannitol-adenine-phosphate (MAP) solution, again with a 42-day shelf-life. In April 1995 the Japanese Red Cross Society, which makes all blood components in Japan, declared that the storage time should be reduced to 21 days based on concerns about bacterial growth7.
The decision to perform a blood transfusion is usually made by a doctor or a nurse8,9. When a helicopter or airplane carries a doctor to the site of a road accident, often in a mountainous or inaccessible area, the doctor is able to begin the diagnostic process and the necessary treatment immediately10–14. For hypovolaemic shock due to bleeding from trauma, low-molecular weight dextran solution is effective, as is a saline infusion. However, in cases of very severe hypovolaemic shock or if amputation surgery is needed, RBC transfusion is the most effective management8,15.
RBC components for transfusion have been carried by aircraft since World War II16–18. However, there are no reports on whether a helicopter flight affects the storage of RBC. We, therefore, investigated the quality of RBC samples that had undergone a helicopter flight, comparing the values for these samples with those of control RBC that remained in a laboratory refrigerator.
Materials and methods
Study design
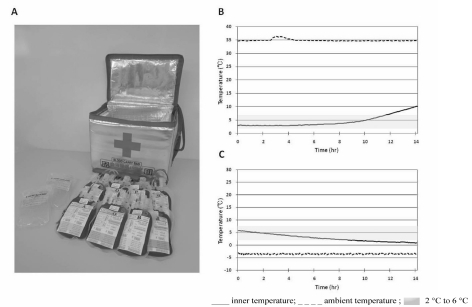
In this experiment, 7 days after donation, five aliquots of RBC from five donors were packed into a newly designed compact container (Figure 1A) whose internal temperature can be maintained between 2 and 6 °C for at least 8 hours (Figure 1B,C). The container also included two frozen coolants and a dummy blood bag to monitor the temperature. After the container had been transported for 4 h in a helicopter, the blood samples were analysed immediately after the flight, corresponding to 7 days after the donation, and then 14, 21 and 42 days after the donation. The quality of the RBC was investigated by measuring ATP, 2,3-diphosphoglycerate (2,3-DPG), and supernatant potassium which are most important to RBC viability. Other parameters measured were haematocrit (Hct), intracellular pH, glucose, supernatant haemoglobin (Hb) and haemolysis rate.
Figure 1.
A. A SS-BOX-110 container is displayed. This container holds a maximum of eight red blood cell units and two coolants (frozen in use). The weight of the box is 1.7 kg (3.7 lb). B,C. The preliminary data for the internal temperature of the box containing five red blood cell units and two frozen coolants at the ambient temperature of 35 °C (B) and −5 °C (C). The internal temperature of the box can be retained 2 °C and 6 °C for 11 hours, 20 min when the external, ambient temperature is 35 °C (B) and for 8 hours, 15 minutes in an ambient temperature of −5 °C (C).
Blood collection and most assays were done at Hokkaido Red Cross Blood Centre in Sapporo. Some measurements were done at the Red Cross Hospital in Asahikawa. The helicopter flight originated from Asahikawa.
The shipping container
A compact container, SS-BOX-110, was newly designed by the Hokkaido Red Cross Blood Centre (Figure 1A)19. Its outer dimensions (length×width×height) were 34.0×25.0×28.5 cm (13.4×9.8×11.2 in) while its internal dimensions were 25.7×15.7×18.8 cm (10.1×6.2×7.5 in); the container weighed 1.7 kg (3.7 lb). The material was polyurethane (polyisocyanurate) with an aluminium sheet attached to the inner surface of the box. The container can hold a maximum of eight RBC bags with space for the two frozen coolants (Figure 1A). The coolant was 250 mL of a gel-based, non-toxic, water-absorbing polymer (Coolplanets, HRCBC RZXA-025S, Planets, Nagoya, Japan). They were wrapped with customary bubble wrap and placed at the bottom of the container and on top of the RBC.
The internal temperature of the box was measured at external, ambient temperatures of 35 °C (Figure 1B) and −5 °C (Figure 1C). This container with two frozen coolants can maintain the internal temperature between 2 and 6 °C for 11 h, 20 min when the ambient temperature is 35 °C (Figure 1B), and for 8 h, 15 min when it is −5 °C (Figure 1C). The temperature stability of this small box is as long as that of other larger boxes20,21.
Helicopter
A McDonnell Douglas Helicopter Explorer MD902 was used to carry the container. This helicopter is powered by two Pratt and Whitney Canada PW207E turboshafts of 530 kW each. Anti-torque control is provided by the NOTAR system. The turboshaft engine rotates at 50,000/min at maximum, main rotor 392/min, and NOTAR 5,412/min. Its cruising speed is 160 mph (258 km/h) while its maximal speed is 161 mph (259 km/h).
Blood collection and preparation of the red blood cell aliquots
In accordance with a standard Japanese blood bank method for making red cell components, whole blood (400 mL) was collected from five healthy donors after informed consent had been obtained and each sample was put into a quadruple bag (Imuflex CPD-MAP, Terumo Co., Tokyo, Japan) designed for 400 mL containing 56 mL of CPD.
The net weight of the whole blood was 481.7±2.4 g, and the estimated volume was 454.3±2.4 mL. The whole-blood units were filtered through an integrally attached filter (Spacell RZ-2000, Asahi Medical Co., Tokyo, Japan) to remove white blood cells. The net weight after filtration was 440.5±1.9 g, and the estimated volume was 415.6±1.7 mL. The units were then centrifuged in a large capacity refrigerated centrifuge (Kubota 9900, Kubota Co., Tokyo, Japan) at 4780×g for 6 min at 22 °C. RBC and plasma were separated and transferred into satellite bags designed for 400 mL with an automated blood component separator (KL-121, Kawasumi Laboratories Inc., Tokyo, Japan). MAP solution (90 mL) was added automatically after the separation22.
Two days after the blood collection, each of the five units of leucocyte-reduced RBC were divided into two aliquots of equal volume in separate bags (BB-T030DJ, Terumo Co., Tokyo, Japan) designed for 300 mL using a sterile connection device (TSCD202, Terumo Co., Tokyo, Japan).
Finally, the ten aliquots from five donors were prepared, and kept at 5±1 °C in a refrigerator.
Preparation of the container and the helicopter flight
Initially, the SS-BOX-11 was equipped with three probes to monitor temperature. Two thermocouple resin-coated probes (TR-0106, T&D Co., Nagoya, Japan) were used to measure the air temperature. One probe was attached to the upper inside of the side wall to record the internal temperature of the box (Figure 2B), and the other was attached to the outside of the box to record the ambient temperature (Figure 2C). Both sensors were connected to a data-logging system (Thermo Recorder TR-71U, T&D Co., Nagoya, Japan) for recording temperatures both inside and outside the container (Figure 2B, C). Another adaptable probe (Pt100, T&D Co., Nagoya, Japan) was used to monitor the blood temperature of the dummy blood bag in the container (Figure 2A) and was connected to a data-logging system (Thermo Recorder TR-81, T&D Co., Nagoya, Japan) (Figure 2A, B). Five aliquots of RBC were taken, in the container, for a flight in the helicopter (flight group) (Figure 2A); the other five aliquots formed the control group. For the flight group, the five aliquots of RBC and one temperature-monitoring dummy blood bag were laid flat between the two frozen coolants with bubble-sheet wrapping (Figure 2A, B).
Figure 2.
A . The SS-BOX-110, equipped with an internal thermocouple probe to record the internal temperature and an outer probe to measure the ambient temperature during transport. The five units of red blood cell components and a dummy blood bag for recording the liquid temperature are also displayed.
B. Five aliquots of red blood cells were put one by one in opposite directions after a bubble-sheet-wrapped frozen coolant had been placed on the bottom. Finally, the other wrapped frozen coolant was put on the top of the red blood cell bags, and a foam plug was used as the ceiling to ensure a tight fit.
C. The container containing five bags of red blood cells, a dummy blood bag and two frozen coolants is shown. The outer thermocouple probe for measuring the ambient temperature and the data logger can be seen.
A foam plug was used as the ceiling for tight packing. These procedures were performed as quickly as possible in order not to warm the inside of the container which had been cooled with two coolants for 1 hour beforehand (Figure 3).
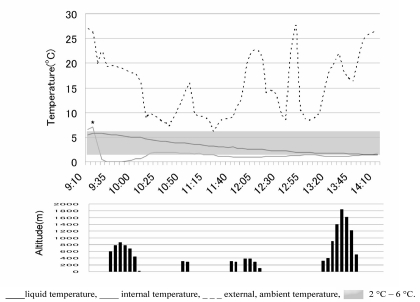
Figure 3.
Top: the temperature of a dummy blood bag and the inside and the outside of the container.
Bottom: the altitude of the helicopter after leaving the Red Cross Hospital.
The liquid temperature (___) was kept between 2 °C and 6 °C during the flight (
 )
)
The box had been cooled with two frozen coolants 1 hour before the red blood cell units were put into it. The rapid increase (*) and decrease of the internal temperature was due to the opening and closing of the box to put the five red blood cell units and the dummy bag into the container.
The container was then immediately carried by hand to the helicopter.
The explorer MD902 carrying the container launched from the roof of Asahikawa Red Cross Hospital at 9:28 am. It stopped at Yubetsu, Saroma, Kitami, and Memanbetsu airports returning to the Red Cross Hospital at 1:47 pm. The time from the start to the return was 4 h, 19 min including time on the ground, with an actual flying time of 115 minutes.
The flight distance covered was 247.9 km (154.1 miles). The highest flight altitude was 1,957 m (6,421 ft) above sea level, when the helicopter flew over the Byobu-dake mountain (Figure 3).
For the control group, the corresponding five aliquots were stored in a refrigerator kept at a temperature of 5±1 °C.
Time points for quality assessment
Blood samples of 15 mL were taken on day 7 (just after the flight), 14, 21, and 42 after donation. Blood sampling was performed using a sterile connection device (TSCD202, Terumo Co., Tokyo, Japan) to avoid contamination. After sterile sampling, the five aliquots of RBC were quickly put back into a refrigerator.
Assays
In vitro measurements of RBC were done as described below. ATP level represents the morphological stability of red cells. 2,3-DPG level represents the amount of oxygen that can be delivered to tissues by the haemoglobin molecule. Supernatant potassium concentration increases when the lipoprotein membrane of red cells is damaged. These parameters are, therefore, considered to be important markers of the quality of RBC23–25. In addition, Hct value, intracellular pH, glucose level, and supernatant Hb concentration were measured, and the haemolysis rate was calculated20.
Hct value and total Hb concentration were determined with an automated blood analyser (ADVIA 210i, Siemens Healthcare Diagnostics Manufacturing Ltd., Dublin, Ireland) immediately after sampling. Intracellular pH was measured at 37 °C with a blood gas analyser (ABL 800Flex, Radiometer Medical ApS Co., Copenhagen, Denmark) immediately after sampling according to the method of Meryman and Hornblower26.
ATP levels were measured from frozen supernatants of deproteinised RBC. Cell aliquots were mixed with 0.6 N KClO4 to deproteinise blood proteins. After 10 min on ice, perchlorate precipitates were removed by centrifugation and the extracts were neutralised with 2.5 M K2CO3 and centrifuged to remove the precipitate. The supernatants were kept frozen at −80 °C until tested. After being thawed, ATP was assayed enzymatically with a bioluminescence ATP Determination Kit (Lucifel 250 Plus, Kikkoman Co., Tokyo, Japan)27. Luminescence was detected using a luminometer (Gene Light55, Microtech Nichion Co., Tokyo, Japan).
Levels of 2,3-DPG were measured from frozen supernatants of deproteinised RBC. Cell aliquots were mixed with 0.6 N KClO4 to deproteinise the blood proteins. After 10 min on ice, deproteinised samples were kept frozen at −80 °C until tested. After being thawed, samples were centrifuged to remove perchlorate precipitates and the supernatant was neutralised with 2.5 M K2CO3 and centrifuged to remove the precipitate. 2,3-DPG was assayed enzymatically with a commercially available test kit (2,3-DPG, Roche, Mannheim, Gemany)28. Levels of 2,3-DPG were indirectly determined by spectrometric measurement of the decrease of NADH to NAD+ at a wavelength of 340 nm using a spectrophotometer (U-2000, Hitachi Co., Tokyo, Japan).
Glucose levels were measured from the frozen supernatant at −20 °C. After being thawed, the levels were determined by a red glucose-glucose oxidase assay kit (Glucose CII-test WAKO, Wako Junyaku Inc., Tokyo, Japan) using a spectrophotometer (U-2000, Hitachi Co., Tokyo, Japan).
Supernatant potassium concentration was measured from the frozen supernatant at −20 °C. After being thawed, the concentration was measured on a programmable chemical analyser (644 Na+/K+/Cl− analyzer, Ciba Corning Diagnostics Ltd., Cambridge, MA, USA).
Supernatant Hb concentration was measured from the frozen supernatant at −20 °C. After being thawed, the concentration was measured with three-wavelength direct spectrophotometry, the modified Neo analysis29,30, using a spectrophotometer (U-2000, Hitachi Co., Tokyo, Japan).
The rate of haemolysis was calculated from the haematological parameters using the following formula20:
Statistics analysis
The results are given as means and standard deviations (SD). Difference between the flight group and control group were compared with paired t-tests. Analyses of variances (F tests) were performed and comparisons for which a p value was less than 0.05 were considered statistically significant. These statistical calculations were performed with computer software (Microsoft Excel spreadsheets, Microsoft Co., Redmond, WA, USA).
Results
The temperature of the dummy blood bag during the flight
During the flight the temperature of the dummy blood bag remained between 2 and 6 °C although the ambient temperature ranged from 6.1 to 27.8 °C (Figure 3). The altitude of Asahikawa is 113 m (371 ft) above sea level; the flight attitude ranged from 113 m (371 ft) to 1,957 m (6,421 ft) (Figure 3). The highest air pressure calculated was 755 mmHg (1,006 mbar) on the top of the Red Cross Hospital, and the lowest was 351 mmHg (468 mbar) over the Byobu-dake mountain.
Quality control parameters after the flight
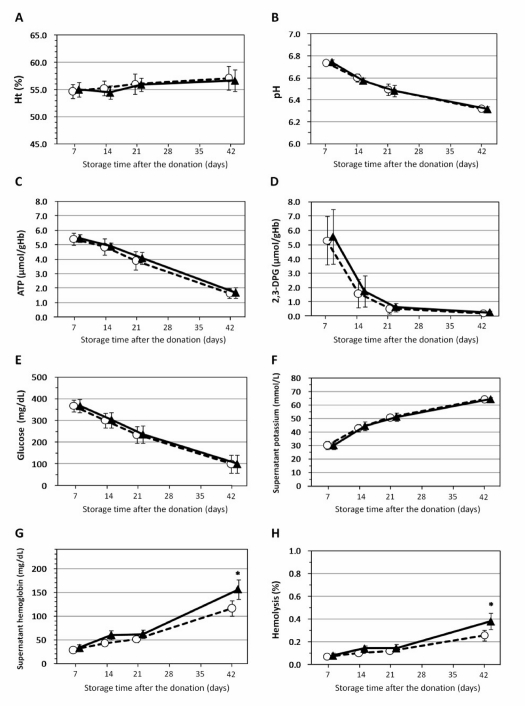
In both the control group and flight group, there were very slight progressive increases in Hct values (Figure 4A). In contrast the intracellular pH gradually declined in both groups of RBC samples (Figure 4B). There was also a slow decline in ATP levels in both groups (Figure 4C). In both groups the 2,3-DPG levels declined rapidly 7 and 14 days after the flight, and thereafter declined slowly (Figure 4D).
Figure 4.
The effects of the helicopter flight on red blood cell parameters. (A) Haematocrit (Ht), (B) intracellular pH, (C) ATP levels, (D) 2,3-diphosphoglycerate (2,3-DPG) levels, (E) glucose levels, (F) supernatant K+, (G) supernatant Hb, and (H) rate of haemolysis. The flight had no effect on any parameters at day 7 (immediately after the flight), day 14 and day 21 after the donation. However, 42 days after the donation the supernatant Hb and rate of haemolysis were statistically increased in the units of red blood cells that had undergone the flight. (G,H). Control group (○: n=5) Flight group (▴: n=5). Error bars represent standards error of distributions (SD); *Significant differences (p<0.05) between the control group and the flight group.
Glucose levels decreased slowly in both groups (Figure 4E), while there were slight progressive increases in supernatant potassium concentrations in both groups (Figure 4F).
Hct values, intracellular pH, ATP levels, 2,3-DPG levels, glucose levels and supernatant potassium concentrations in the flight group were not statistically different from those of the control group at any time after the donation, i.e. day 7 (just after the flight), 14, 21, or 42 after the donation (Figure 4F).
Supernatant haemoglobin and haemolysis rate after the flight
In the control group, there were slight progressive increases in supernatant Hb concentrations and haemolysis rates (Figure 4G, H).
The supernatant Hb concentrations and haemolysis rates also increased in the flight group, without there being statistical differences from the control group at day 7 (just after the flight), 14 and 21 after the donation (Figure 4G, H). However, 42 days after the donation, supernatant haemoglobin concentration and haemolysis rate were statistically higher in the flight group than in the control group (Figure 4G, H, Table I).
Table I.
RCC qualities after the flight.
| Control group (n=5) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Days after flight | Days after donation | Ht [%] | pH | ATP [μmol/gHb] | 2,3-DPG [μmol/gHb] | Glucose [mg/dL] | Sup* K [mmol/L] | Sup* Hb [mg/dL] | Hemolysis [%] |
| 0# | 7 | 54.7±1.3 | 6.74±0.03 | 5.39±0.42 | 5.27±1.69 | 367±26.4 | 30.1±3.0 | 28.9±2.2 | 0.068±0.01 |
| 7 | 14 | 55.3±1.3 | 6.60±0.04 | 4.84±0.56 | 1.58±0.99 | 299±32.8 | 42.8±2.6 | 43.0±5.7 | 0.101±0.01 |
| 14 | 21 | 56.1±1.9 | 6.49±0.05 | 3.90±0.62 | 0.52±0.35 | 235±37.4 | 50.6±2.1 | 51.4±7.4 | 0.119±0.02 |
| 35 | 42 | 57.1±2.2 | 6.32±0.03 | 1.65±0.35 | 0.19±0.12 | 100±41.2 | 64.1±1.9 | 116.5±16.9* | 0.256±0.04* |
| Flight group (n=5) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Days after flight | Days after donation | Ht [%] | pH | ATP [μmol/gHb] | 2,3-DPG [μmol/gHb] | Glucose [mg/dL] | Sup* K [mmol/L] | Sup* Hb [mg/dL] | Hemolysis [%] |
| 0# | 7 | 55.0±1.3 | 6.74±0.03 | 5.44±0.25 | 5.56±1.92 | 366±31.0 | 29.8±2.9 | 33.1±7.4 | 0.079±0.02 |
| 7 | 14 | 54.5±1.2 | 6.57±0.03 | 4.88±0.24 | 1.74±1.07 | 303±35.1 | 44.2±3.3 | 60.2±9.7 | 0.141±0.03 |
| 14 | 21 | 55.9±1.2 | 6.48±0.05 | 4.08±0.43 | 0.62±0.29 | 236±40.7 | 51.0±2.9 | 62.1±9.4 | 0.144±0.03 |
| 35 | 42 | 56.7±2.0 | 6.32±0.02 | 1.70±0.36 | 0.25±0.11 | 101±41.9 | 64.1±1.3 | 156.0±20.9* | 0.342±0.07* |
0# just after the flight;
Significant differences(p<0.05) between Control and Flight group
Discussion
A doctor carried by a helicopter or air plane to a road accident can make a diagnosis on the spot and start treatment8–14. Although blood transfusion is the most effective treatment for hypovolaemic shock due to massive bleeding, sometimes a blood transfusion may be cancelled due to “wrong” information or a different decision of the flight doctor. If the RBC quality is not affected by helicopter transport, unopened RBC could be used until their storage time elapses.
To the best of our knowledge, only one French study has investigated the effects of helicopter transport on Hct, haemolysis, pH, and supernatant potassium concentration of RBC just after the flight, and after 30 days31. In that study haemolysis was increased at both time points. The study did not monitor the temperature in the box or that of a fluid dummy bag. The immediate increase of the haemolysis rate just after the flight suggests that the temperature of the RBC was not maintained between 2 and 6 °C. This hypothesis is supported by data from an in vitro experiment by Klose et al.20 who found that low air pressure did not affect the haemolysis rate of “temperature-controlled” RBC just after a flight. Furthermore, our temperature-controlled RBC showed no increase of haemolysis just after the flight (Figure 4H, Table I) again suggesting that the findings in the French study may have been due to a problem with temperature control of the RBC during the flight.
In our experiment, the temperatures of the dummy bag contained in the SS-BOX-110 remained between 2 and 6 °C (Figure 3), and all control group data were comparable to those previously reported23–25. The results suggest that Hct values, intracellular pH, ATP levels, 2,3-DPG levels, glucose levels, and supernatant potassium concentrations of RBC stored in MAP are not significantly affected by helicopter transportation.
Although supernatant Hb concentration and haemolysis rate were not statistically different between the flight group and the control group up to 21 days after the donation, at 42 days after the donation they were statistically higher in the flight group. These data are comparable to those in the in vitro experiment by Klose et al20. The rate of haemolysis did, however, remain at all times below the level (0.8%) recommended by European and UK guidelines4.
Possible causes of the increased haemolysis at 42 days after donation are air pressure and vibration. In a previous study it was found that air pressure decreases were associated with large increases in volume in both surrogates and RBC, but that all materials met maximum requirements for 1 h and returned to their normal appearance without showing any defects20. With regards to the effect of different atmospheric air pressures (1,000, 600, and 200 mbar) on the degree of haemolysis, it was reported that at 600 mbar and 200 mbar the degree of haemolysis, 28, 35 and 42 days after blood donation, increased compared with that occurring at 1,000 mbar (similar to ground pressure)20. The lowest air pressure that the RBC experienced in the flight in our study was 486 mbar over the Byobu-dake mountain. Our data on increased haemolysis rate 42 days after donation are compatible with the data from Klose et al.20 (Figure 3).
As to the second possibility of an effect of vibration, a high prevalence of back pain has been reported in helicopter pilots32–34. However, although a vibration effect from a rotor engine cannot be denied32,33, to our knowledge there are no in vitro or in vivo data showing a relationship between vibration and haemolysis of RBC.
In conclusion, although the haemolysis rate of RBC in our study increased slightly 42 days after the donation, a level of 0.342±0.07%, as observed in the flight group, is not harmful to humans35 and the blood can be transfused.
These results suggest that RBC in MAP are not significantly affected by helicopter transportation. By the end of the shelf-life of transported RBC, the changes in haemolysis are small and probably have no clinical implications.
Acknowledgments
The authors thank Mr. Katsuya Morishita and Mr. Seiji Chiba in Hokkaido Asahikawa Red Cross Blood Centre for their many efforts to ensure that this collaboration would be a success, Mr. Daisuke Tsugoshi for his skilful helicopter flight technique, and Mr. Tatsuya Goto for the management of the flight.
References
- 1.Högman CF, Hedlund K. Storage of red cells in a CPD/SAGM system using Teruflex® PVC. Vox Sang. 1985;49:177–80. doi: 10.1111/j.1423-0410.1985.tb00790.x. [DOI] [PubMed] [Google Scholar]
- 2.Högman CF, Akerblom O, Hedlund K, et al. Red cell suspensions in SAGM medium- Further experience of in vivo survival of red cells, clinical usefulness and plasma-saving effects. Vox Sang. 1983;45:217–23. doi: 10.1111/j.1423-0410.1983.tb01907.x. [DOI] [PubMed] [Google Scholar]
- 3.Cedex: EDQM; 2008. Guide to the Preparation, Use and Quality Assurance of Blood Components. [Google Scholar]
- 4.Guidelines for the Blood Transfusion Services in the United Kingdom. 7th ed. London: TSO; 2005. [Google Scholar]
- 5.Clinical Guideline to Transfusion. Toronto: Canadian Blood Service; 2006. [Google Scholar]
- 6.Roback JD, Combs MR, Grossman BJ, Hillyer CD. Technical Manual. 16th ed. Bethesda, MD: AABB; 2008. [Google Scholar]
- 7.Transfusion information from the Japanese Red Cross Society 9504-14. Tokyo, JRCS, 1995.
- 8.Berns KS, Zietlow SP. Blood usage in rotor-wing transport. Air Med J. 1998;17:105–8. doi: 10.1016/s1067-991x(98)90104-3. [DOI] [PubMed] [Google Scholar]
- 9.Parris E. Deciding when to use universal blood. Emerg Nurse. 2010;18:18–9. doi: 10.7748/en2010.09.18.5.18.c7972. [DOI] [PubMed] [Google Scholar]
- 10.Thomas SH. Helicopter emergency medical services transport outcomes literature: annotated review of articles published 2000–2003. Prehosp Emerg Care. 2004;8:322–33. doi: 10.1016/j.prehos.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 11.Mashiko K. Trauma systems in Japan: history, present status and future perspectives. J Nippon Med Sch. 2005;72:194–202. doi: 10.1272/jnms.72.194. [DOI] [PubMed] [Google Scholar]
- 12.Exadaktylos AK, Haffejee F, Wood D, Erasmus P. South Africa Red Cross flying doctors service quality and safety in the rural and remote South African environment. Aust J Rural Health. 2005;13:106–10. doi: 10.1111/j.1440-1854.2005.00663.x. [DOI] [PubMed] [Google Scholar]
- 13.Garne DL, Perkins DA, Boreland FT, Lyle DM. Frequent users of the royal flying doctor service primary clinic and aeromedical services in remote New South Wales: a quality study. Med J Aust. 2009;191:602–4. doi: 10.5694/j.1326-5377.2009.tb03344.x. [DOI] [PubMed] [Google Scholar]
- 14.Warne G. The flying doctor. Natl Med J India. 2009;22:151–2. [PubMed] [Google Scholar]
- 15.Dries DJ. Blood transfusion and acute intravascular volume loss. Air Med J. 1998;17:108–10. doi: 10.1016/s1067-991x(98)90105-5. [DOI] [PubMed] [Google Scholar]
- 16.Rous P, Turner JR. The preservation of living red cells in vitro. J Exper Med. 1916;23:219–37. doi: 10.1084/jem.23.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oberman HA. The history of transfusion medicine. In: Petz LD, Swisher SN, Kleinman S, et al., editors. Clinical Practice of Transfusion Medicine. 3rd edition. New York: Churchill Livingstone; 1996. pp. 11–32. [Google Scholar]
- 18.Janatpour KA, Holland PV. A brief history of blood transfusion. In: Hillyer CD, Silverstein LE, Ness PM, et al., editors. Blood Banking and Transfusion Medicine. 2nd edition. Philadelphia: Churchill Livingstone; 2007. pp. 3–11. [Google Scholar]
- 19.Kikuchi H, Sato N, Suzuki K, et al. [Development and evaluation of a packing procedure for red cells concentrates using transportation container] [abstract] (Japanese) J Jpn B Prgm. 2007;30(Suppl 1):322. [Google Scholar]
- 20.Klose T, Borchert HH, Pruβ A, et al. Current concepts for quality assured long-distance transport of temperature-sensitive red blood cell concentrates. Vox Sang. 2010;99:44–53. doi: 10.1111/j.1423-0410.2009.01302.x. [DOI] [PubMed] [Google Scholar]
- 21.van der Meer PF, Pietersz RN. Overnight storage of whole blood: a comparison of two designs of butane-1,4-diol cooling plates. Transfusion. 2007;47:2038–43. doi: 10.1111/j.1537-2995.2007.01427.x. [DOI] [PubMed] [Google Scholar]
- 22.Fujihara M, Akino M, Sato M, et al. Prestorage leucofiltration prevents the accumulation of matrix metalloproteinase-9 in red cell concentrates stored in mannitol-adenine-phosphate medium. Vox Sang. 2005;89:114–5. doi: 10.1111/j.1423-0410.2005.00667.x. [DOI] [PubMed] [Google Scholar]
- 23.Hess JR, Hill HR, Oliver CK, et al. Twelve-week RBC storage. Transfusion. 2003;43:867–72. doi: 10.1046/j.1537-2995.2003.00442.x. [DOI] [PubMed] [Google Scholar]
- 24.Weisbach V, Riego W, Strasser E, et al. The in vitro quality of washed, prestorage leucocyte-depleted red blood cell concentrates. Vox Sang. 2004;87:19–26. doi: 10.1111/j.1423-0410.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 25.de Korte D, Kleine M, Korsten HG, Verhoevev AJ. Prolonged maintenance of 2,3-diphosphoglycerate acid and adenosine triphosphate in red blood cells during storage. Transfusion. 2008;48:1081–9. doi: 10.1111/j.1537-2995.2008.01689.x. [DOI] [PubMed] [Google Scholar]
- 26.Meryman HT, Hornblower M. Manipulating red cell intra- and extracellular pH by washing. Vox Sang. 1991;60:99–104. doi: 10.1111/j.1423-0410.1991.tb00881.x. [DOI] [PubMed] [Google Scholar]
- 27.Nakao M, Motegi T, Nakao T, et al. A direct relationship between adenosine triphosphate-level and in vivo viability of erythrocytes. Nature. 1962;194:877–8. doi: 10.1038/194877a0. [DOI] [PubMed] [Google Scholar]
- 28.Ericson A, de Verdier CH. A modified method for the determination of 2,3-diphosphoglycerate in erythrocytes. Scand J Clin Lab Invest. 1972;29:84–90. [PubMed] [Google Scholar]
- 29.Neo AD, Weedn V, Bell RW. Direct spectrophotometry of serum hemoglobin: an Allen correction compared with a three-wavelength polychromatic analysis. Clin Chem. 1984;30:627–30. [PubMed] [Google Scholar]
- 30.Yamada T, Yoshinaga Y, Hirayama K, et al. The determination of plasma hemoglobin by use of direct spectrophotometry with three wavelength (in Japanese with English abstract) Jap J Med Tech. 1991;40:146–52. [Google Scholar]
- 31.Almanza L, Lataillade JJ, Almanza BA, et al. Concentrés érythrocytaires et transport aérien: evaluation de la qualité. TCB. 1995;5:343–7. doi: 10.1016/s1246-7820(05)80077-1. [DOI] [PubMed] [Google Scholar]
- 32.Bongers PM, Hulshof CTJ, Dijkstra L, et al. Back pain and exposure to whole body vibration in helicopter pilots. Ergonomics. 1990;33:1007–26. doi: 10.1080/00140139008925309. [DOI] [PubMed] [Google Scholar]
- 33.Thomae MK, Porteous JE, Brock JR, et al. Back pain in Australian military helicopter pilots: a preliminary study. Aviat Space Environ Med. 1998;69:468–73. [PubMed] [Google Scholar]
- 34.Froom P, Froom J, Van Dyk D, et al. Lytic spondylolithesis in helicopter pilots. Aviat Space Environ Med. 1984;55:556–7. [PubMed] [Google Scholar]
- 35.Valeri CR, Bomd JC. Observations on the preservation of autologous human erythrocytes using glycerol, slow-freeze technique and agglomeration. Transfusion. 1965;6:254–62. [Google Scholar]