Abstract
Background
Umbilical cord blood (UCB) is a source of hematopoietic precursor cells for transplantation. The creation of UCB banks in 1992 led to the possibility of storing units of UCB for unrelated transplants. The distribution of cell contents in historical inventories is not homogenous and many units are not, therefore, suitable for adults. The aim of this study was to analyse our UCB bank inventory, evaluate the units released for transplantation and calculate the cost of the current process per unit of UCB stored.
Methods
Three study periods were defined. In the first period, from January 1996 to January 2006, the total nucleated cell (TNC) count acceptable for processing was 4–6×108 and a manual processing system was used. In the second period, from October 2006 to July 2010, processing was automated and the acceptable TNC count varied from 8–10×108. In the third period, from January 2009 to June 2010, an automated Sepax-BioArchive procedure was used and the accepted initial TNC count was >10×108. Within each period the units were categorised according to various ranges of cryopreserved TNC counts in the units: A, >16.2×108; B1, from 12.5–16.1×108; B2, from 5.2–12.4×108; and C, <5.1×108.
Results
The third period is best representative of current practices, with homogenous TNC acceptance criteria and automated processing. In this period 15.7% of the units were category A and 25.5% were category B. Overall, the mean TNC count of units released for transplantation was 14×108 (range, 4.6×108 to 36.5×108). The cost of the processed UCB in 2009 was 720.41 euros per unit.
Conclusion
An UCB bank should store units of high-quality, in terms of the TNC count of units issued for transplantation, have a training programme to optimise the selection of donors prior to delivery, use similar volume reduction systems and homogenous recovery indices, express its indicators in the same units, use validated analytical techniques, and bear in mind ethnic minorities.
Keywords: umbilical cord blood, cord blood bank, quality, cost
Introduction
The Andalusian Umbilical Cord Blood Bank started its activity in 1996, when it stored umbilical cord blood (UCB) collected from two nearby maternity units (within 2 km) that had received authorisation for donation within this distance. The bank now receives UCB from 61 maternity units, all within 600 km.
Initially, whole volume units were processed manually with programmed freezing and storage in conventional liquid nitrogen tanks. In 1999 the freezing area was optimised and the manually processed units were volume reduced by hydroxyethyl starch with double centrifugation, followed by programmed freezing and storage in liquid nitrogen. In 2006 a volume reduction system with hydroxyethyl starch and automated separation (Sepax®) was introduced; the volume reduced units then underwent programmed freezing and automated storage (BioArchive®).
The total nucleated cell (TNC) counts considered acceptable for storage were increased following physicians’ demands: in 1996 a TNC count >4×108 was considered acceptable, in 2006 a TNC count >8×108 (assuming 40% discarded units) and from November 2008 a TNC count >10×108 was required (a criterion of the Spanish National Umbilical Cord Plan). The percentage of discarded units has risen to 60%1.
The aim of this study was to analyse the UCB units in our inventory, evaluate the units sent for transplantaion and calculate the cost per unit of stored UCB prepared using our current process.
Materials and methods
A total of 20,762 units processed between 1996 and 2010 were studied. Three periods were defined for the purpose of this analysis. In the first period, from January 23, 1996 to January 9, 2006, in which UCB was provided from two maternity units, there were no acceptance criteria concerning TNC count and a manual processing system was used. In the second period, from October 1, 2006 to July 30, 2010, coinciding with the inclusion of most of the other maternity units, automated processing was used and the acceptable TNC count varied. The third period, from January 1, 2009 to June 30, 2010, is best representative of the current situation in which we now use an automated Sepax-BioArchive procedure and the acceptable TNC count is >10×108. The overlapping of the second and third periods was in order to examine the incidence of variables in the selection criteria.
Within each period, the cryopreserved units were categorised according to various ranges of TNC count:2 category A (TNC >16.2×108), category B1 (TNC from 12.5 to 16.1×108), category B2 (TNC from 5.2 to 12.4×108) and category C (TNC <5.1×108). As we considered the range of counts in category B2 to be excessively wide, for this analysis we divided this category into three subcategories: B2.1 (TNC from 10.0 to 12.4×108), B2.2 (TNC from 8.0 to 9.9×108) and B2.3 (TNC from 5.2 to 7.9×108).
The units issued for transplantation were analysed according to the defined periods and TNC category, considering also the distribution. An analysis was made of both inventory and disposable material, together with the cost of personnel working in the process. Information provided by external services (blood cultures and haemoglobin studies) and transport was not included.
Statistical analysis
A descriptive analysis was made with mean values and ranges. The data are presented as absolute values, percentages and standard deviations.
Results
Overall, 21,206 units of UCB were analysed: 3,567 from the first period, 12,388 from the second and 5,251 from the third. For the whole study period, 93.4% of the donations were stored in a single bag and 6.6% in a double bag. The TNC counts considered acceptable for processing were 4–6×108 for the first period, 8–10×108 for the second and >10×108 for the third (Table I).
Table I.
Initial TNC accepted and number of units processed in the different study periods.
| Periods | I | II | III |
|---|---|---|---|
| Initial TNC accepted (×108) | 4–6 | 8–10 | >10 |
| UCB units processed (n.) | 3,567 | 12,388 | 5,251 |
| UCB units processed in 1 bag, n. and % | 3,527 93.1% |
11,924 96.3% |
4,807 90.8% |
| UCB units processed in 2 bags, n. and % | 40 6.9% |
464 3.7% |
444 9.2% |
Of the 3,567 units in the first period, 248 (7%) were category A (i.e. TNC >16.2×108) and 434 (12.35%) were category B1 (Table II). The volumes indicated in the Table correspond to those that were frozen and are high because both total volume units and reduced units were stored in this period (Table II).
Table II.
Category of units in period I (January 23, 1996 to January 1, 2006).
| Category TNC (×108) | A >16.2 | B1 12.5–16.1 | B2.1. 10.0–12.4 | B2.2. 8.0–9.9 | B2.3. 5.2–7.9 | C <5.2 | Total I |
|---|---|---|---|---|---|---|---|
| UCB units, n. (%) | 248 (7) | 434 (12.3) | 597 (16.9) | 625 (17.1) | 1,185 (33.5) | 478 (13.5) | 3567 (100) |
| 1 bag | 241 | 418 | 589 | 618 | 1,183 | 478 | 3527 |
| 2 bags | 7 | 16 | 8 | 7 | 2 | 0 | 40 |
| Mean volume±SD* | 128±41.01 | 115±39.68 | 102±39.55 | 86±36.25 | 71±29.17 | 67±22.06 | 82±38.33 |
Frozen volume mL
Of the 12,388 units in the second period, 1,302 (10.5%) were category A and 2,417 (19.5%) were category B1. The initial volumes reported correspond to those of the donation (Table III).
Table III.
Category of units in period II (October 1, 2006 to July 30, 2010).
| Category TNC (×108) | A >16.2 | B1 12.5–16.1 | B2.1. 10.0–12.4 | B2.2. 8.0–9.9 | B2.3. 5.2–7.9 | C <5.2 | Total II |
|---|---|---|---|---|---|---|---|
| UCB units, n. (%) | 1,302 (10.5) | 2,417 (19.5) | 3,034 (24.4) | 2,884 (23.2) | 2,536 (20.4) | 215 (1.7) | 12,388 (100) |
| 1 bag | 1,023 | 2,288 | 2,994 | 2,868 | 2,536 | 215 | 11,924 |
| 2 bags | 279 | 129 | 40 | 16 | 0 | 0 | 464 |
| Mean volume*±SD | 127±20.48 | 111±18.69 | 99±17.33 | 89±15.58 | 78±14.11 | 68±13.83 | 95±20.96 |
Initial volume mL
The third period is best representative of current practices in our UCB bank as the TNC acceptance criteria were homogenous and the processing automated. Of the 5,251 units in this third period, 825 (15.7%) were category A and 1,341 (25.5%) were category B1 (Table IV).
Table IV.
Category of units in period III (January 1, 2009 to June 30, 2010).
| UCB units, n. (%) | 825 (15.7) | 1,341 (25.5) | 1,626 (30.9) | 1,207 (22.9) | 81 (1.5) | 5 (0.0) | 5.251 (100) |
| 1 bag | 564 | 1,214 | 1,589 | 1,194 | 81 | 5 | 4,807 |
| 2 bags | 261 | 127 | 37 | 13 | 0 | 0 | 444 |
| Mean volume*±SD | 126±20.67 | 110±18.66 | 98±16.43 | 89±15.43 | 83±21.84 | 87±23.02 | 102±22–03 |
Initial volume mL
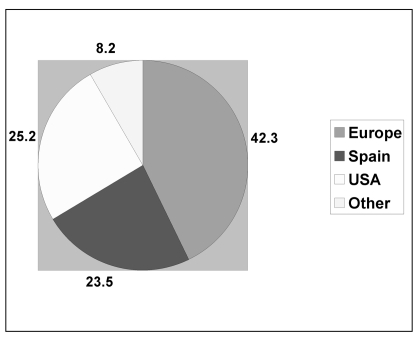
The number of units issued for transplantation increased in proportion to the number of units stored and the increase in TNC content. The units issued were transplanted in Spain (23.7%), the rest of Europe (42.7%), the USA (25.4%) and elsewhere (8.2%) (Figure 1). The mean TNC of the units sent for transplantation was 14×108 (range, 4.6×108 to 36.5×108) (Table V).
Figure 1.
Geographical destination of the units issued for transplantation.
Table V.
Number of units issued for transplantation in each year, with mean and range of their TNC content.
| Year | N. of transplants | TNC (×108) | Range TNC (×108) |
|---|---|---|---|
| 1998 | 2 | 7.8 | 5.8–9.9 |
| 1999 | 3 | 16.0 | 13.3–20.1 |
| 2000 | 7 | 18.3 | 8.8–28.1 |
| 2001 | 4 | 14.1 | 12.0–14.9 |
| 2002 | 4 | 11.2 | 7.2–13.7 |
| 2003 | 3 | 12.6 | 9.0–17.8 |
| 2004 | 5 | 18.1 | 8.5–24.2 |
| 2005 | 5 | 14.3 | 7.1–22.8 |
| 2006 | 12 | 17.2 | 4.6–28.2 |
| 2007 | 18 | 13.3 | 7.1–26.2 |
| 2008 | 23 | 15.7 | 6.2–36.5 |
| 2009 | 34 | 17.7 | 6.6–31.1 |
| 2010 | 52 | 18.7 | 5.5–29.5 |
The cost of the UCB units processed in 2009 was 720.41 euros per unit. Of this, the collection cost was 17.1 euros, which included a Macopharma® collection bag, tubes, containers, forms and various collection material. The processing cost was 310.15 euros and included validation, separation by Sepax Biosafe®, cryopreservation and storage using BioArchive®, as well as administrative expenses. This heading also included sample storage (foetal red blood cells, foetal and maternal plasma bank, and tissue fragments). The quality controls included HLA typing, flow cytometry (CD34+ count), viability, blood cultures, ABO and Rh grouping, and testing for transmissible diseases (hepatitis B and C viruses, syphilis, human immunodeficiency virus, chagas, malaria).
The cost analysis for the personnel considered each person involved throughout the process: midwife, laboratory technician, administrative staff, and professional staff. The analysis did not include units that were not processed (Table VI).
Table VI.
Cost of processing 3243 umbilical cord units in 2009.
| Process Stages | €/Unit | N=3243 |
|---|---|---|
| Collection | 17.10 | x 3,243=5,540.4 |
| Processing | 310.15 | x 3,243=75,366.45 |
| Quality control | 180.86 | x 3,243=586,528.98 |
| Human resources | 163.84 | x 3,243=531,333.12 |
| External preventive Maintenance | 35.2 | x 3,243=114,153.8 |
| External corrective maintenance | 13.26 | x 3,243=43,002.18 |
| Total | 720.41€ | 2,336,289.63€ |
Discussion and conclusions
The introduction of quality systems, their legislation and the use of practices based on scientific evidence have all contributed to the continual improvement in the quality of stored UCB units. Previously used only for children weighing less than 20 kg, UCB transplants are now indicated as one more source of hematopoietic progenitors, alongside bone marrow and peripheral blood, for all patients3. The relationships between TNC count, CD34+ cell count and transplant outcome have now been defined. The higher the dose of infused cells the greater the likelihood of engraftment in both children and adults4–6 and the transplanting physician can choose the most suitable unit for a patient in relation to higher values of TNC count and CD34+ cells/Kg and adequate HLA compatibility, as well as other factors, such as the recipient’s diagnosis and conditioning regimen.
Nevertheless, the various procedures used by different laboratories could confuse the physician concerning the final decision regarding the actual number of infused CD34+ cells and TNC content, leading to mistakes of interpretation. There is a lack of uniform criteria concerning the values each UCB bank includes in the search registries, with the values varying according to whether the TNC count relates to the cells collected, cryopreserved or infused. The mean recovery of cryopreserved cells in infused units is 76.4%6 with respective means of 3.9 and 5.1×107/kg. The mean TNC count in those studies in which it was reported was markedly lower at 4.1×107/kg7. It is, therefore, important to know both the cryopreserved TNC count and the percentage recovery after the thawing process in order that the physician can make an informed decision. The Spanish Hematopoietic Transplant Group (GETH), in its protocol for transplantation of UCB from an unrelated donor for patients with haematological cancers, recommends a TNC count of >15×108, which for a patient weighing 70 kg is equivalent to >2×107 TNC/kg8.
The CD34+ cell count can also predict the success of a transplant. Measurement of this parameter is not a standardized technique and the values given by the various laboratories are not comparable, so the information could lead to mistakes. The recommended transplant dose is >70×105 CD34+ cells, which for a patient of 70 kg is equivalent to 1×107 CD34+ cells/kg8. Spanish transplant centres currently select UCB units for transplantation according to this protocol. The Spanish National Umbilical Cord Plan recommends that the dose of CD34+/kg of recipient body weight in cryopreserved units should be >0.6×105 and that of TNC >2×107. These figures coincide with those of other groups, with small differences9. It should be possible to update guidelines in accordance with recommendations from the scientific societies representing transplant centres, with clear and agreed criteria.
A more homogenous analysis of units transplanted would be useful in order to determine the minimum TNC count and CD34+ dose necessary to guarantee a successful transplant. These data are crucial for UCB banks as they provide information concerning the expectations for current deposits, compel the banks to audit stockpiled units and establish new ranges of cellularity and minimum requirements when processing the units received. Just storing units in categories A and B1 would result in up to 58.8% of the units in the other categories being discarded. The analysis of our third period, in which the initial TNC count considered acceptable for processing was >10×108, indicates that the acceptance ranges should be 12.5–16.1 (category B) and >16.5 (category A). If the recovery index of the initial TNC count is 85%, the initial TNC count acceptable for processing the unit should be >14.7×108.
UCB is scarce and expensive, and the protocols for obtaining it should be optimised and involve careful selection of donors in order to reduce the number of units discarded. Various epidemiological, obstetric and neonatal factors have been described to influence the different quality parameters. The role of these variables has been studied and defined in numerous articles10–14. Careful pre-selection of the mother during pregnancy, considering parameters such as gestational age, foetal weight, expected type of delivery, sex of the newborn and multiparity, could reduce the number of units discarded, as all these factors influence the final volume of the donation and the initial TNC count. Another point to consider is the cell recovery yield using the various volume reduction systems (automated or manual) to optimise storage space15. With the Sepax® automated system, high volume, cell-rich units processed in one step and stored in a single bag have lower recovery yields of both TNC and mononuclear cells. Each bank should optimise its resource use by establishing the optimal volume to process in one or two bags. Our bank has validated the process for bags weighing <170 g, equivalent to a volume of about 150 mL, above which the sample is separated into two bags but with no loss of traceability and with a single identifier. This process implies increased costs in terms of disposable materials, time and human resources. Optimisation of the cell separators by installing new software would modify the process and require validation. It may be necessary to raise the processing cut-off point for separation into two units, or even suppress it altogether, in order to reduce costs and storage space.
The main aim of an UCB bank is to provide ideal units for transplantation. In our case, we saw a progressive increase in requests for our units, which was directly proportional to the number of units stored. The mean TNC threshold of these units was 14×108, which shows the need to increase cellularity in the units stored.
The importance of the HLA system and its antigen variability should not be overlooked, as it may be possible to store units with the same type. In this case, use of the units with greater cellularity will result in the others not being used. Likewise, consideration should be given to ethnic minorities: in this case, UCB banks should not restrict the levels of acceptance of initial TNC. Our bank contains a very important antigen diversity as a result of the history of this region and the cross-mix in civilizations. Its important ethnic mix has led to the availability of broad HLA diversity. Accordingly, any historical review of the inventory should be made with caution, first analysing the HLA type before discarding units with low cell volumes. A TNC count >4×108 could guarantee a supply of transplantable units for these patients.
Units that are discarded could be used for research purposes or as a cell factory for other study groups. This would help to repay the cost of the units collected but not processed. An UCB bank should aim to store high-quality units, defined as those with TNC values >14×108. The banks should also have a training programme for obstetric personnel in order to optimise donor selection prior to delivery. The banks should use similar volume reduction systems, with homogenous recovery indices and use the same criteria to express the values of cryopreserved TNC and CD34+ cells, using validated techniques.
References
- 1.Querol S, Rubinstein P, Marsh S, et al. Cord blood banking: “providing cord blood banking for a nation”. Br J Haematol. 2009;147:227–35. doi: 10.1111/j.1365-2141.2009.07818.x. [DOI] [PubMed] [Google Scholar]
- 2.Querol S, Gomez SG, Pagliuca A, et al. Quality rather than quantity: the cord blood bank dilemma. Bone Marrow Transplant. 2010;45:970–8. doi: 10.1038/bmt.2010.7. [DOI] [PubMed] [Google Scholar]
- 3.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Center for International Blood and Marrow Transplant Research; Acute Leukemia Working Party Eurocord (the European Group for Blood Marrow Transplantation). National Cord Blood Program of the New York Blood Center. Lancet Oncol. 2010;11:653–60. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peffault de Latour R, Purtill D, Ruggeri A, et al. Influence of nucleated cell dose on overall survival of unrelated cord blood transplant for patients with severe acquired aplastic anemia: a study by EUROCORD and the Aplastic Anemia Working Party of the EBMT. Biol Blood Marrow Transplant. 2010;17:78–85. doi: 10.1016/j.bbmt.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues CA, Sanz G, Brunstein CG, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27:256–63. doi: 10.1200/JCO.2007.15.8865. [DOI] [PubMed] [Google Scholar]
- 6.Kurtzberg J, Prasad VK, Carter SL, et al. COBLT Steering Committee. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318–27. doi: 10.1182/blood-2007-06-098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herr AL, Kabbara N, Bonfim CM, et al. Long-term follow-up and factors influencing outcomes after related HLA-identical cord blood transplantation for patients with malignancies: an analysis on behalf of Eurocord-EBMT. Blood. 2010;116:1849–56. doi: 10.1182/blood-2010-02-271692. [DOI] [PubMed] [Google Scholar]
- 8.Protocolo de TSCU Grupo Español de Trasplante Hematopoyético (GETH 2009) 2009 Junio; Trasplante de Sangre de Cordón Umbilical de Donante no emparentado en paciente con neoplasias hematológicas tras acondicionamiento con tiotepa, busulfan intravenoso en dosis única diaria, fludarabina y timoglobulina. [Google Scholar]
- 9.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–70. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ballen KK, Wilson M, Wuu J, et al. Bigger is better: maternal and neonatal predictors of hematopoietic potential of umbilical cord blood units. Bone Marrow Transplant. 2001;27:7–14. doi: 10.1038/sj.bmt.1702729. [DOI] [PubMed] [Google Scholar]
- 11.Nakagawa R, Watanabe T, Kawano Y, et al. Analysis of maternal and neonatal factors that influence the nucleated and CD34 cell yield for cord blood banking. Transfusion. 2004;44:262–7. doi: 10.1111/j.1537-2995.2004.00645.x. [DOI] [PubMed] [Google Scholar]
- 12.Redzko S, Przepiesc J, Zak J, et al. Influence of perinatal factors on hematological variables in umbilical cord blood. J. Perinat Med. 2005;33:42–5. doi: 10.1515/JPM.2005.007. [DOI] [PubMed] [Google Scholar]
- 13.Mancinelli F, Tamburini A, Spagnoli A, et al. Optimizing umbilical cord blood collection: impact of obstetric factors versus quality of cord blood units. Transplant Proc. 2006;38:1174–6. doi: 10.1016/j.transproceed.2006.03.052. [DOI] [PubMed] [Google Scholar]
- 14.Solves P, Perales A, Mirabet V, et al. Donors selection and retrieval of units in an umbilical cord blood bank. Med Clin (Barc) 2007;129:561–5. doi: 10.1157/13111706. [DOI] [PubMed] [Google Scholar]
- 15.Lapierre V, Pellegrini N, Bardey I, et al. Cord blood volume reduction using an automated system (Sepax) vs. a semi-automated system (Optipress II) and a manual method (hydroxyethyl starch sedimentation) for routine cord blood banking: a comparative study. Cytotherapy. 2007;9:165–9. doi: 10.1080/14653240701196811. [DOI] [PubMed] [Google Scholar]