In this issue of Molecular Cell, Sanvisens et al. report a new mechanism for regulation of yeast ribonucleotide reductase activity that occurs during iron deprivation.
DNA replication and repair are essential processes in which the limiting step is the availability of deoxynucleotides. The size of deoxynucleotide pools is controlled by the activity of the ribonucleotide reductase (RNR) that catalyses the conversion of all four nucleotides to the corresponding deoxynucleotides and provides the monomeric precursors for DNA synthetic processes. RNR is an important target for cell growth control and is therefore tightly regulated through the cell cycle and by DNA damage. RNR is regulated at both transcriptional and post-translational levels. During DNA damage or S phase, the activity of RNR is also regulated by selective movement of RNR subunits from nucleus to cytosol. In this issue of Molecular Cell, Sanvisens et al. demonstrate a new mechanism of RNR regulation based on selective movement of RNR subunits in response to iron limitation.
RNRs are multimeric enzymes for which metals are essential co-factors for enzymatic activity. While many organisms like Escherichia coli have multiple RNRs, eukaryotes only possess one type termed class Ia. Class Ia RNR is a tetramer comprised of one large homodimer (subunit R1) containing the catalytic and regulatory sites and a smaller R2 subunit, which in yeast is a heterodimer of the iron containing Rnr2 and Rnr4. Rnr4 is required to stabilize iron on Rnr2 (Cotruvo and Stubbe, 2011). In mammalian cells, iron deprivation results in decreased RNR activity leading to a cell growth arrest (Nyholm et al., 1993). In contrast, iron scarcity in yeast does not lead to a dramatic inhibition of RNR activity. To optimize iron utilization during iron limitation Saccharomyces cerevisiae undergoes a metabolic reprogramming in which there is increased utilization of non-iron containing enzymes as well as decreased utilization of iron-containing metabolic pathways such as mitochondrial respiration (Shakoury-Elizeh et al., 2010). Since RNR activity is essential and requires iron as a co-factor, the question was posed how does RNR activity persist under iron-limited conditions in yeast? Sanvisens et al.(2011) investigated RNR activity under iron-replete and iron-limited conditions by measuring dATP and dCTP pools in yeast. In spite of a low quantity of metal co-factors for catalysis, pools of dATPs and dCTPs surprisingly increased twofold, suggesting that the function of RNR during iron deficiency was preserved.
RNR activity takes place in the cytosol where the R1 subunit resides. Most of the R2 subunit, however, resides in the nucleus. During S phase or in response to DNA damage, R2 transits from the nucleus to the cytosol forming the holoenzyme. Under normal conditions nuclear retention of the Rnr2-Rnr4 complex is due to two proteins: Dif1, a cytosolic protein that promotes nuclear import (Lee et al., 2008; Wu and Huang, 2008) and the nuclear protein Wtm1, which binds to R2 tethering it to the nuclear membrane and limiting export from the nucleus (Lee and Elledge, 2006; Zhang et al., 2006). Sansivisens et al. showed that iron deprivation resulted in the movement of R2 from nucleus to cytosol. The movement of R2 in response to DNA damage checkpoint is mediated by the kinases Mec1, Rad53 and Dun1. Sansivisens et al. ruled out the involvement of the DNA damage checkpoint during iron deficiency showing that in the absence of Mec1 or Rad53 the R2 heterodimer can exit from the nucleus at the same rate as in a wild type strain. These results suggest the existence of a new regulatory mechanism that is independent of the cell cycle checkpoint.
Previous studies have shown that iron deficiency results in the expression of Cth1 and Cth2 and that these proteins play a major role in iron-dependent remodeling of metabolism (Puig et al., 2005). These proteins bind to mRNAs, which contain AU-rich elements (AREs) in the untranslated region, and destabilize those mRNAs. The RNAs they bind to encode proteins that can be dispensed during iron limitation remodeling (such as respiratory enzymes). The authors then investigated whether Cth1 and Cth2 could be involved in the redistribution of R2 upon iron starvation. When cells lacking both Cth1 and Cth2 were incubated in iron-depleted media, Rnr2 and Rnr4 were retained in the nucleus in contrast to wild type cells. The authors then showed that Cth1 and Cth2 controlled Rnr2-Rnr4 subcellular localization by targeting WTM1 mRNA for degradation and that the presence of AU-rich elements (ARE) in the 3’ untranslated region of WTM1 mRNA were critical for that regulation. Reduced levels of Wmt1 result in decreased nuclear tethering of R2 and increased R2 in the cytosol (Figure 1).
Figure 1. Regulation of RNR activity during iron limitation.
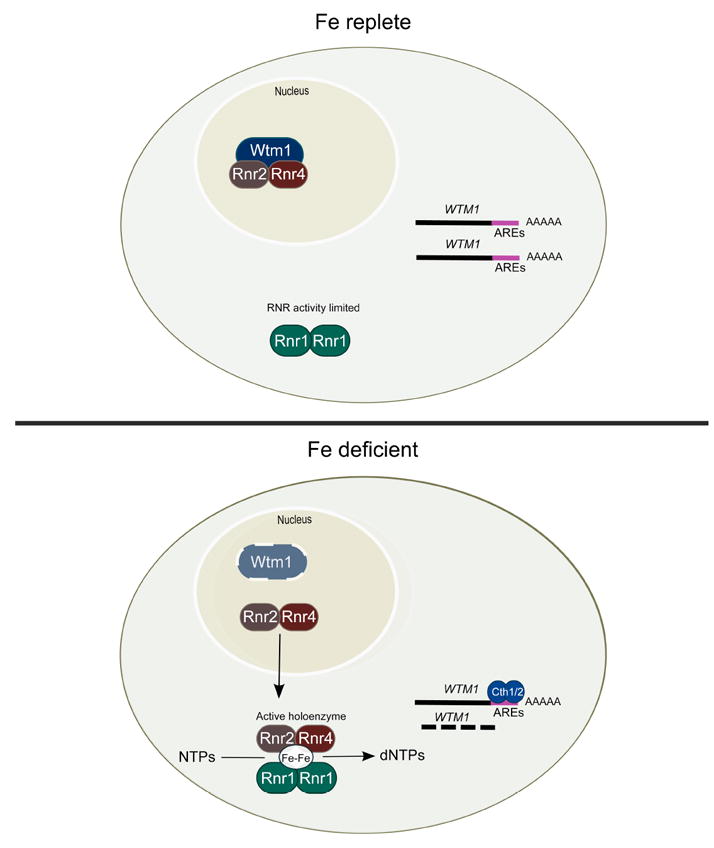
RNR activity in iron-replete conditions is limited by the physical separation of the two subunits of the RNR enzyme. A homodimer of Rnr1 (R1) containing the catalytic domain resides in the cytosol while the iron-containing heterodimer Rnr2-Rnr4 (R2) is tethered in the nucleus through binding to Wtm1. Upon iron limitation there is increased transcription of the mRNA binding proteins Cth1 and Cth2. These proteins bind to specific sequences in the 3’region of WTM1 mRNA, which targets that mRNA for degradation leading to decreased levels of Wtm1. The loss of Wtm1 releases R2 from the nucleus permitting it to translocate to the cytosol, bind to R1 forming an active enzyme.
This work demonstrates a new mechanism of RNR regulation that is independent of the DNA damage/S phase checkpoint. In addition, it enforces the idea that cell metabolism is reorganized to retain essential iron utilizing pathways during iron limitation. The relationship between iron and RNR activity, however, is complex and further investigations could yield more insights into this process. The authors also reported that RNR2 and RNR4 mRNAs have AREs in their 3’untranslated regions leading to a decrease in the level of R2, yet in iron-limitation nucleotide pools were increased two-fold over iron-sufficient cells. It would be of interest to determine if expression of RNR2 and RNR4 lacking AREs had effects on nucleotide pools. The implication is that under iron limitation, while most nuclear R2 is now cytosolic, the activity of RNR is limited by reduced amounts of R2. In DNA checkpoint control of RNR activity, an inhibitor of Sml1 that binds to R1 is degraded (Zhao et al., 2001). It is unclear what happens to Sml1 during iron limitation.
A study from Zhang et al. (Zhang et al., 2011), showing that glutaredoxins and iron-sulfur cluster proteins are involved in the iron loading of Rnr2, demonstrates that iron homeostasis has a critical role in regulating RNR activity and function. Based on this and other work, analysis of the effects of iron deprivation may provide a rich trove that when mined will yield insights into new mechanisms of regulation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Cotruvo JA, Stubbe J. Annual Review Biochem. 2011;80:733–767. doi: 10.1146/annurev-biochem-061408-095817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YD, Elledge SJ. Genes Dev. 2006;20:334–344. doi: 10.1101/gad.1380506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YD, Wang J, Stubbe J, Elledge SJ. Mol Cell. 2008;32:70–80. doi: 10.1016/j.molcel.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholm S, Mann GJ, Johansson AG, Bergeron RJ, Graslund A, Thelander L. J Biol Chem. 1993;268:26200–26205. [PubMed] [Google Scholar]
- Savisens N, Bano MC, Huang M, Puig S. Mol Cell. 2011 doi: 10.1016/j.molcel.2011.09.021. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig S, Askeland E, Thiele DJ. Cell. 2005;120:99–110. doi: 10.1016/j.cell.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Shakoury-Elizeh M, Protchenko O, Berger A, Cox J, Gable K, Dunn TM, Prinz WA, Bard M, Philpott CC. J Biol Chem. 2010;285:14823–14833. doi: 10.1074/jbc.M109.091710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Huang M. Mol Cell Biol. 2008;28:7156–7167. doi: 10.1128/MCB.01388-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu L, Wu X, An X, Stubbe J, Huang M. J Biol Chem. 2011 Sep 20; doi: 10.1074/jbc.M111.294074. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, An X, Yang K, Perlstein DL, Hicks L, Kelleher N, Stubbe J, Huang M. Proc Natl Acad Sci (USA) 2006;103:1422–1427. doi: 10.1073/pnas.0510516103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Chabes A, Domkin V, Thelander L, Rothstein R. EMBO J l. 2001;20:3544–3553. doi: 10.1093/emboj/20.13.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]


