Abstract
Feces from 142 animals were collected on 15 farms in the region of Brittany, France. Each sample was directly collected from the rectum of the animal and identified with the ear tag number. Animals were sampled three times, at 5, 15 and 22 weeks of age. After DNA extraction from stool samples, nested PCR was performed to amplify partial 18S-rDNA and 60 kDa glycoprotein genes of Cryptosporidium. The parasite was detected on all farms. One hundred out of 142 calves (70.4%) were found to be parasitized by Cryptosporidium. Amplified fragments were sequenced for Cryptosporidium species identification and revealed the presence of C. parvum (43.8%), C. ryanae (28.5%), and C. bovis (27%). One animal was infected with Cryptosporidium ubiquitum. The prevalence of these species was related to the age of the animal. C. parvum caused 86.7% of Cryptosporidium infections in 5-week-old calves but only 1.7% in 15-week-old animals. The analysis of the results showed that animals could be infected successively by C. parvum, C. ryanae, and C. bovis for the study period. C. parvum gp60 genotyping identifies 6 IIa subtypes of which 74.5% were represented by IIaA15G2R1. This work confirms previous studies in other countries showing that zoonotic C. parvum is the dominant species seen in young calves.
Introduction
Cryptosporidium is a genus of protozoan parasites infecting a wide range of hosts [1]. All groups of vertebrates are susceptible to Cryptosporidium infection worldwide. This parasite is the etiological agent of cryptosporidiosis, which is mainly characterized by diarrhea in humans and livestock. Massive outbreaks of enteritis in people such as in Milwaukee, Wisconsin (USA) have increased public awareness of this parasite [2]. In humans, the prevalence and severity of infection increase in immunodeficient individuals such as AIDS patients. In immunocompetent patients, the disease is self-limited [3]. No drug therapy is yet available and the high resistance of oocysts to environmental conditions and chemical treatment make cryptosporidiosis difficult to control [4]. Cattle have been considered to be a primary reservoir for Cryptosporidium oocysts for zoonotic C. parvum [5]. These animals could be a risk factor via environmental contamination from their manure being spread on farmland or their grazing on watersheds [6]. On farms, transmission of Cryptosporidium spp. can result from ingestion of contaminated food or water, by direct transmission from host to host, or through insect vectors [7]. In cattle, infection by Cryptosporidium spp. was first reported in 1971 [8]. Since vaccines have become commercially available against Escherichia coli K99, rotavirus, and coronavirus, Cryptosporidium has emerged as the main neonatal diarrheic agent in calves [9]. In farm animals, the economic impact is related to morbidity, mortality and growth retardation [10]. Among the 24 species previously described (if the three fish species are accepted without complete genetic characterization) [1,11-13], C. parvum, C. bovis, C. ryanae and C. andersoni usually infect cattle. C. parvum has zoonotic potential and is a frequent cause of human cryptosporidiosis [14]. C. bovis and C. ryanae have not been found in humans and there is only one description of C. andersoni in a patient [15]. Recent reports have described an age-related distribution of these aforementioned species in dairy cattle on the east coast of the United States [16-18], India, China, Georgia [19], Malaysia [20], and Denmark [21]. The most prevalent species were C. parvum in preweaned calves, C. ryanae and C. bovis in postweaned calves and C. andersoni in adult cows [16,17].
In France, previous studies on the prevalence of Cryptosporidium in cattle were based on microscopic determination [22] or coproantigen detection using ELISA [23]. These studies on dairy calves reported a within herd prevalence of Cryptosporidium without identifying species or the relation to the host's age. The present study was conducted in 15 farms in Brittany, France to determine the prevalence of Cryptosporidium in veal calves. We used genotyping and subtyping for the molecular study of Cryptosporidium isolates. Follow-up of the same animal allowed us to determine the outcome of the infection and the age distribution of Cryptosporidium species.
Material and methods
Specimen sources and collection
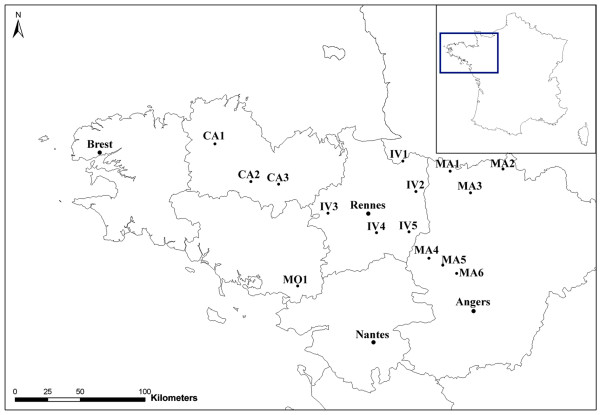
Fifteen fattening units in Brittany (France) were included in this work. They belonged to three industrial veal producers representative of integrators in France and did not present any known history of Cryptosporidium infection. These farms were located in four administrative regions (Figure 1): Côtes d'Armor (CA1-CA3), Morbihan (MO1), Ile-et-Vilaine (IV1-IV5), and Mayenne (MA1-MA6). During the summer and autumn of 2007, all farms were visited three times and fecal samples were taken from 142 animals exhibiting diarrhea at the age of 5 weeks old. Calves arrived in fattening units at the age of 2 weeks old and were confined in small groups from four to six animals per pen. Because of a concomitant welfare study [24], calves had to stay 2 to 3 weeks without any external stress despite the farmer's presence. At the age of 22 weeks old, calves were finally sent to the slaughterhouse. Consequently, sampling was done at the ages of 5 weeks, 15 weeks, and 22 weeks (Table 1). These points of sampling corresponded to the beginning, the middle and the end of the fattening period. Fecal samples were collected and shipped by a veterinarian. Collectors respected the following criteria: use of a single pair of latex gloves per animal, a single plastic sterile cup per animal, and collection of at least 5 g of feces per sample. Feces were collected directly from the rectum of each animal and stored at 4 °C in potassium dichromate (2.5% wt/vol) until processed. Cups were capped, labeled with the animal's ear tag number, and accompanied by a form recording the date of sampling, the animal's sex, breed, identification number, and the mean age of the herd.
Figure 1.
Map of administrative regions in Brittany showing the location of farms included in the study: Côtes d'Armor (CA), Ile-et-Vilaine (IV), Mayenne (MA), and Morbihan (MO) in France.
Table 1.
Cryptosporidium prevalence in veal herds found in Brittany farms according to animal age.
| Animal age | ||||
|---|---|---|---|---|
| Farm | 5 weeks No. positive/No. sample (%) |
15 weeks No. positive/No. sample (%) |
22 weeks No. positive/No. sample (%) |
Total number of positive animals* (%) |
| CA1 | 1/6 (16.6%) | 2/6 (33.3%) | 1/6 (16.6%) | 4/6 (66.6%) |
| CA2 | 3/10 (30%) | 4/10 (40%) | 3/10 (30%) | 8/10 (80%) |
| CA3 | 4/10 (40%) | 6/10 (60%) | 0/10 (0%) | 6/10 (60%) |
| MO1 | 0/10 (0%) | 0/10 (0%) | 1/10 (10%) | 1/10 (10%) |
| IV1 | 6/10 (60%) | 7/10 (70%) | 1/10 (10%) | 10/10 (100%) |
| IV2 | 4/10 (40%) | 4/10 (40%) | 3/10 (30%) | 6/10 (60%) |
| IV3 | 2/8 (25%) | 7/8 (87.5%) | 3/8 (37.5%) | 8/8 (100%) |
| IV4 | 3/10 (30%) | 4/10 (40%) | 0/10 (0%) | 6/10 (60%) |
| IV5 | 4/10 (40%) | 2/10 (20%) | 3/10 (30%) | 5/10 (50%) |
| MA1 | 8/10 (80%) | 3/10 (30%) | 1/10 (10%) | 9/10 (90%) |
| MA2 | 7/10 (70%) | 3/10 (30%) | 1/10 (10%) | 7/10 (70%) |
| MA3 | 6/10 (60%) | 6/9** (66.6%) | 0/9** (0%) | 8/10 (80%) |
| MA4 | 6/10 (60%) | 4/9** (44.4%) | 1/9** (11.1%) | 6/10 (60%) |
| MA5 | 7/8 (87.5%) | 6/8 (75%) | 0/8 (0%) | 8/8 (100%) |
| MA6 | 7/10 (70%) | 3/10 (30%) | 2/10 (20%) | 8/10 (80%) |
| Total | 68/142 (47.9%) |
59/140 (42.1%) |
20/140 (14.3%) |
100/142 (70.4%) |
* A calf is considered to be positive if at least one out of the three samples is positive.
**The number of animals is 9 because one calf died between the age of 5 and 15 weeks.
Cryptosporidium detection
After washing steps in water to eliminate potassium dichromate from the samples, DNA was extracted according to the method previously described [25] without the Cetyl TrimethylAmmonium Bromide (CTAB) and PolyVinylPyrrolidone (PVP) treatment steps. An 18S RNA gene fragment was amplified by nested PCR according to Xiao et al. [26]. The partial gp60 gene was amplified according to Gatei et al., [27]. PCR products were analyzed on 2% agarose gel and visualized by ethidium bromide staining. To ensure purity and limit the presence of PCR inhibitors, all PCR-negative samples were reprocessed. Samples were treated for oocyst purification by immunomagnetic separation (Dynabeads ®anti-Cryptosporidium, Invitrogen ™, Norway) according to the manufacturer's instructions. These samples were finally processed as previously for DNA extraction and PCR amplification.
Cryptosporidium species identification
PCR products were purified on an ultracel YM50 membrane (Microcon, Millipore, Bedford, MA, USA) according to the manufacturer's instructions. DNA sequencing reactions were performed using internal primers of the nested PCR with the ABI Prism Big Dye Terminator cycle sequencing kit (Applied Biosystem, Foster City, CA, USA). Capillary electrophoresis was performed by Genoscreen (Lille, France). Sequences were analyzed using BLAST at NCBI [28].
Results
Cryptosporidium prevalence
The prevalence of Cryptosporidium infection on 15 farms from four administrative regions in Brittany (France) was studied (Figure 1). All Cryptosporidium-positive specimens generated the expected SSU-RNA products in nested PCR and revealed that no farm was free of Cryptosporidium. The molecular analysis of 422 fecal samples revealed that 147 (34.8%) were positive for Cryptosporidium. As shown in Table 1, the overall prevalence of infected animals was 70.4% (100/142) and ranged from 10% on a farm in Morbihan (MO1) to 100% on farms in Ile-et-Vilaine (IV1, IV3) and in Mayenne (MA5). Amongst the specimens sampled from 5-week-old and 15-week-old animals, Cryptosporidium prevalence was 47.9% and 42.1%, respectively (range, 0%-87.5%). In 22-week-old calves, the prevalence decreased to 14.3% (range, 0%-37.5%). The prevalence of infection decreased as the age of the calves increased.
Cryptosporidium species identification by 18S rDNA sequencing
For species identification, the 147 positive nested PCR products were sequenced. Sequence analysis from 137 readable electrophoregrams revealed the presence of C. parvum, C. bovis, and C. ryanae. One additional Cryptosporidium genotype showing 99% identity with Cryptosporidium ubiquitum (EU827413) (previously identified as Cryptosporidium cervine genotype [13]) was detected in one calf. This sequence was deposited in GenBank under the accession number GU124629. Sixty (43.8%) samples were identified as C. parvum as follows: forty-six sequences had 100% identity with the GenBank AF093490 nucleotide sequence, 11 had 100% identity with the AF308600 nucleotide sequence and three had 99% identity compared to both references. These sequences were deposited in GenBank under the accession numbers GU124615 to GU124617. For the other positive specimens, 39 (28.5%) were identified as C. ryanae (previously described as Cryptosporidium deer-like genotype). Thirty-one of these had 100% identity with the AY587166 sequence [17] and eight were 99% identical to this reference. These nucleotide sequences were deposited in GenBank under the accession numbers GU124621 to GU124628. For the last positive samples, 37 (27%) had an identical nucleotide sequence with C. bovis (GenBank accession number, AY120911) formerly known as the Cryptosporidium Bovine B genotype. Within these sequences, 34 had 100% identity to the reference deposited in GenBank, three sequences had 99% identity. These last sequences were deposited in Genbank under the accession numbers GU124618 to GU124620.
Prevalence of C. parvum, C. ryanae, and C. bovis in relation to calf age
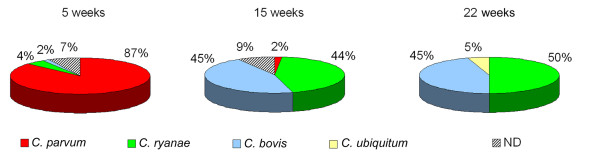
The distribution of Cryptosporidium species identified in animals at the age of 5, 15, and 22 weeks is shown in Figure 2. The prevalence of each species changed with the age of the calves. C. parvum prevalence was 86.7% in the 5-week-old calves and decreased to 1.7% in 15-week-old animals. This species was not identified in 22-week-old calves. C. ryanae and C. bovis were identified in 5-week-old calves in 4.4% and 1.5% of the specimens, respectively. The prevalence of these species in 15-week-old animals increased to 44.1% and 45.7%, respectively. This prevalence evolved to 50% and 45% in 22-week-old animals.
Figure 2.
Prevalence of Cryptosporidium species/genotype: C. parvum, C. ryanae, C. bovis, C. C.ubiquitum and not determined species because of unreadable sequences (ND) in calves from 5 weeks to 22 weeks of age.
Sequential infection profile
The presence of one, two, or three species of Cryptosporidium was determined in each animal (n = 91) for which the sequences were readable in all positive samples. Three calves positive for C. parvum at the age of 5 weeks were excluded because Cryptosporidium species could not be identified in all of the following samples collected in these animals. As shown in Table 2, Cryptosporidium species determination over time showed that only one species was identified in 63.7% (58/91) of the animals analyzed. Thus, 35.1% (32/91) had excreted only C. parvum, 15.4% (14/91) shed only C. ryanae, and 12.1% (11/91) only C. bovis. The C. ubiquitum identified in one sample accounted for 1.1%. In the time lapse of this study, 34% of the animals (31/91) were found to excrete two different species of Cryptosporidium successively. Indeed, 13.2% (12/91) produced C. parvum and C. ryanae, 12.1% (11/91) excreted C. parvum and C. bovis, and 8.8% (8/91) excreted C. ryanae and C. bovis. Finally, 2.2% (2/91) of the animals studied were detected to produce C. parvum, C. ryanae, and C. bovis.
Table 2.
Number of Cryptosporidium species identified in animals and sequential infection.
|
No. Cryptosporidium species/genotype per animal |
5 weeks | 15 weeks | 22 weeks | n |
|---|---|---|---|---|
| C. parvum | 31 | |||
| C. ryanae | 2 | |||
| C. ryanae | 10 | |||
| C. bovis | 7 | |||
| C. parvum | 1 | |||
| C. bovis | 2 | |||
| 1 | C. ryanae | 1 | ||
| C. ubiquitum | 1 | |||
| C. bovis | C. bovis | 2 | ||
| C. ryanae | C. ryanae | 1 | ||
| C. parvum | C. ryanae | 10 | ||
| C. parvum | C. bovis | 10 | ||
| C. ryanae | C. bovis | 1 | ||
| C. bovis | C. ryanae | 4 | ||
| 2 | C. ryanae | C. bovis | 2 | |
| C. parvum | C. ryanae | 1 | ||
| C. parvum | C. ryanae | C. ryanae | 1 | |
| C. parvum | C. bovis | C. bovis | 1 | |
| C. bovis | C. ryanae | C. ryanae | 1 | |
| 3 | C. parvum | C. ryanae | C. bovis | 1 |
| C. parvum | C. bovis | C. ryanae | 1 | |
| ND* | C. parvum | ND | ND | 3 |
ND: not determined due to unreadable sequence.
Cryptosporidium parvum subtyping by gp60 sequence analysis
The subtyping analysis was performed on C. parvum positive specimens. From 60 targeted samples, 51 could be used for sequence analysis. As shown in Table 3, all alleles identified belong to the IIa family. The most common subtype IIaA15G2R1 (100% identity with reference strain AB 514090) was found in 38 out of 51 samples (74.51%). Six samples (11.76%) were typed as subtype IIaA17G1R1 (100% identity with reference strain GQ983359), three samples (5.89%) as subtype IIaA16G3R1 (100% identity with reference strain DQ192506) and two samples (3.92%) as subtype IIaA16G2R1 (100% identity with reference strain DQ192505). Finally one sample (1.96%) was subtyped as IIaA16G1R1 (100% identity with reference strain DQ192504) and another one (1.96%) as subtype IIaA13G1R1 (100% identity with reference strain DQ192502).
Table 3.
gp60 gene subtypes of C. parvum positive samples.
| Sub-genotype | No/No tot samples (%) |
% identity with reference |
Reference sequence in GenBank |
|---|---|---|---|
| IIaA15G2R1 | 38/51 (74.51%) | 100 | AB514090 |
| IIaA17G1R1 | 6/51 (11.76%) | 100 | GQ983359 |
| IIaA16G3R1 | 3/51 (5.89%) | 100 | DQ192506 |
| IIaA16G2R1 | 2/51 (3.92%) | 100 | DQ192505 |
| IIaA16G1R1 | 1/51 (1.96%) | 100 | DQ192504 |
| IIaA13G1R1 | 1/51 (1.96%) | 100 | DQ192502 |
*Total number of samples (No tot samples) = 51 because 9 C. parvum positive samples gave no readable sequence for the gp60 gene marker.
Discussion
Calves under 1 month of age are frequently infected with Cryptosporidium sp [29] which results in economic loss [10]. In France, up to date, the prevalence of Cryptosporidium in diarrheic calves has been studied only by Elisa and microscopic strategies [22,23,30]. No data are available on a molecular basis to study Cryptosporidium species in calf herds in that country. The present study based on 18S rDNA and gp60 gene analysis is the first in France to include molecular characterization to describe the prevalence and the host age related susceptibility to different Cryptosporidium species after a follow up of the same animal.
Our results showed that all fifteen farms were contaminated with Cryptosporidium. The parasite prevalence on farms ranged from 10% to 100% of the sampled animals. This observation was in accordance with results in Michigan (USA) where this parameter ranged from 0% to 100% [31]. The prevalence of 70.4% obtained in this work tended toward the upper end of the scale compared to other investigations done in France which ranged from 15.6% in beef herds [30] to 95% in suckling calves [23] and in other European countries where prevalence ranged from 3.4% to 96% [32,33]. However, the sampling program did not allow the study of animals under 5 weeks of age. Indeed, the animals arrived in these structures at the age of 2 to 3 weeks and farmers did not allow sampling before two complete resting weeks for each animal. Therefore, our results could underestimate the real prevalence as Huetink et al. showed that the percentage of parasite excreting animal declines after the third week of age [34] and that the first peak of prevalence is at the age of 15 days [17].
In our study, the higher prevalence of cryptosporidiosis was observed in calves 5 weeks old (47.9%) and the lowest (14.3%) in the 22-week-old animals. This observation shows that prevalence of Cryptosporidium infection decreases with increasing age of the cattle in France as in many other countries [17,19,33-38].
Additionally, our data confirmed the presence in France of a host age-related susceptibility to the infection with different Cryptosporidium species. C. parvum was predominantly detected in 5-week-old calves (86.7%) compared to C. ryanae or C. bovis detected in 4.4% and 1.5% of the positive samples respectively. It is noteworthy that these results are very similar to data obtained in Ireland on calves under 30 days of age with 95%, 3.6%, and 1.3% of prevalence of the same species, respectively [39] and in the UK on animals over 3 weeks old with 93% C. parvum, 6% C. bovis, and 2% C. ryanae [40]. In contrast to previous studies [17,41], C. ryanae and C. bovis were found with similar prevalence predominantly in 15 week and 22 week old calves. This association between the age of the cattle and the Cryptosporidium species identification has been supported by several studies [17,19,21,38,40] but different reports suggest that Cryptosporidium species repartition regarding the age of the host could be due to a technical artifact. Despite the fact that the methodological strategy based on PCR using genus specific primers and partial direct sequencing of the 18S rDNA is commonly used to identify Cryptosporidium species [42], this molecular tool is limited in the case of mixed infections. Feng et al., [19] suggested that the important shedding of C. parvum in preweaned calves had probably masked the concurrent infection of these animals by C. bovis or C. ryanae. Furthermore, previous reports suggested that a dominant Cryptosporidium species in a sample can be preferentially amplified by PCR [43,44]. It is noteworthy that this situation of mixed Cryptosporidium species infection in farm animals would be more prevalent than originally believed [45-47]. Mixed Cryptosporidium species could also explain sequencing difficulties encountered in this work. The simultaneous presence of several species in the same sample could lead to amplification and sequencing of different genetic fragments leading to unreadable superimposition of electrophoregrams.
Consequently, in our work based on the utilization of Cryptosporidium generic primers, the amplification of a single fragment with a single sequence is not conclusive evidence that the sample contains only a single species. However, based on our results, it is possible to confirm the predominance of different species of Cryptosporidium by group of age among the calves.
Particularly, our data showed that animals can be sequentially infected with C. parvum, C. ryanae and C. bovis as well as C. parvum, C. bovis and C. ryanae. This observation provides evidence that a previous infection with C. parvum did not protect calves against an infection with other Cryptosporidium species. Fayer et al. suggested that the peak of cryptosporidiosis prevalence in young calves could reflect the immaturity of the immune status [48]. It was also suggested that the low excretion of C. parvum oocysts in older calves might be related to the development of immunity that also protected the animal against a secondary challenge [49]. It has been reported that immunity arises in the first two weeks after infection [50]. Interestingly, Fayer et al. [51] described that calves previously challenged with C. parvum were able to excrete oocysts after a second challenge with C. bovis but not with C. parvum. The authors concluded that immunity to C. parvum was not extended to C. bovis. Consistently, in our study, the presence in the same animal during sequential sampling of C. parvum, C. bovis and C. ryanae suggests that immunity against C. parvum and against C. bovis did not extend to C. ryanae. Furthermore, the observation that one animal excreted sequentially C. parvum, C. ryanae and C. bovis suggests that immunity against C. ryanae did not extend to C. bovis as well.
Finally, the risk to human health posed by Cryptosporidium infected cattle in France was assessed. The detection of C. ubiquitum (a rare infectious agent detected in humans [52]), C. ryanae and C. bovis (which are mainly specific for cattle) led to consider that the 22-week-old calves are not likely a public health concern. However, the major detection of C. parvum, a prevalent zoonotic species, in 5-week-old calves was in agreement with the report of Atwill et al., who considered that the contribution of cattle to human cryptosporidiosis is limited to calves under 2 months of age [53].
To determine C. parvum subtypes, the sequence analysis of a fragment of the gp60 gene was done. Our results show that in the region of Brittany, all identified C. parvum gp60 subtypes belonged to the IIa family which was previously found in both animals and humans [42]. Particularly, human infections with the IIa subtype are commonly seen in areas with intensive animal production [54]. Among the 48 gp60 subtypes formerly described in cattle [55], only six were identified in this work, being IIaA15G2R1 the most commonly found. This subtype has been widely reported in calves and humans in different countries such as in Portugal [54], Slovenia [56] and The Netherlands [57]. This observation confirms previous works and suggests a zoonotic transmission of the parasite also in this region.
It is noteworthy that the three predominant subtypes (IIaA15G2R1, IIaA17G1R1, and IIaA16G3R1) found in this work were also described in cattle with an equivalent distribution in The Netherlands [57] and England [40]. Thus, the subtype IIaA15G2R1 was found in 74.5% of the samples in this work, 68.9% in The Netherlands and 68.6% in England. The IIaA17G1R1 was identified in 11.7% of the samples in this report, 10.8% in The Netherlands and 13.8% in England. The IIaA16G3R1 determined in 5.9% of our samples, was characterized in 4.65% in The Netherlands and 5.8% in England. It is remarkable that subtypes, IIaA16G2R1, IIaA16G1R1 and IIaA13G2R1 were equivalently underrepresented in these three countries. This observation could suggest that the proportion of a gp60 subtype would not be randomly represented in a population.
Finally, the zoonotic transmission assessment of C. parvum in France would require a comparative investigation of variable genetic loci both in human and animal samples.
This is the first report on the molecular identification of Cryptosporidium species or genotypes in veal calves in France. According to data reported previously in many countries, a sequential distribution of species is observed in cattle according to age. C. parvum was mainly observed in the youngest calves, while C. ryanae and C. bovis became predominant in stool specimens collected in older animals. In some cases, several Cryptosporidium species were successively detected in the same calf, suggesting that the immune defense against C. parvum is not efficient against C. ryanae or C. bovis. Finally, the major identification of the IIaA15G2R1 subtype in France suggests that 5-week old calves could be a reservoir for zoonotic parasites transmissible to humans.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JF and KG participated in the conception and design of the study, carried out the experiments and drafted the manuscript. HL designed the sampling strategy and collected samples on farms. JF, KG and AFD designed the protocol for molecular assay and participated in the analysis result. OHG carried out molecular assays. EDC, GC and PH participated in the coordination of the study and helped draft the manuscript. All authors read and approved the final manuscript.
Contributor Information
Jérôme Follet, Email: jerome.follet@isa-lille.fr.
Karine Guyot, Email: karine.guyot@pasteur-lille.fr.
Hélène Leruste, Email: helene.leruste@isa-lille.fr.
Anne Follet-Dumoulin, Email: annef@icl-lille.fr.
Ourida Hammouma-Ghelboun, Email: ourida.hammouma@isa-lille.fr.
Gabriela Certad, Email: gabriela.certad@pasteur-lille.fr.
Eduardo Dei-Cas, Email: eduardo.dei-cas@pasteur-lille.fr.
Patrice Halama, Email: patrice.halama@isa-lille.fr.
Acknowledgements
This study was supported by the Catholic University of Lille through the "Projet Grande Campagne Ensemble Innovons" genotyping program. We would like to thank the veal unit managers who participated in this study.
References
- Fayer R. Taxonomy and species delimitation in Cryptosporidium. Exp Parasitol. 2009;124:90–97. doi: 10.1016/j.exppara.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Mac Kenzie WR, Hoxie NJ, Proctor ME, Gradus MS, Blair KA, Peterson DE, Kazmierczak JJ, Addiss DG, Fox KR, Rose JB, Davis JP. A massive outbreak in Milwaukee of cryptosporidium infection transmitted through the public water supply. N Engl J Med. 1994;331:161–167. doi: 10.1056/NEJM199407213310304. [DOI] [PubMed] [Google Scholar]
- O'Hara SP, Chen XM. The cell biology of cryptosporidium infection. Microbes Infect. 2011;13:721–730. doi: 10.1016/j.micinf.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccio SM, Pozio E. Advances in the epidemiology, diagnosis and treatment of cryptosporidiosis. Expert Rev Anti Infect Ther. 2006;4:429–443. doi: 10.1586/14787210.4.3.429. [DOI] [PubMed] [Google Scholar]
- Chalmers RM, Giles M. Zoonotic cryptosporidiosis in the UK - challenges for control. J Appl Microbiol. 2010;109:1487–1497. doi: 10.1111/j.1365-2672.2010.04764.x. [DOI] [PubMed] [Google Scholar]
- Monis PT, Thompson RC. Cryptosporidium and Giardia-zoonoses: fact or fiction? Infect Genet Evol. 2003;3:233–244. doi: 10.1016/j.meegid.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Follet-Dumoulin A, Guyot K, Duchatelle S, Bourel B, Guilbert F, Dei-Cas E, Gosset D, Cailliez JC. Involvement of insects in the dissemination of Cryptosporidium in the environment. J Eukaryot Microbiol. 2001;Suppl:36S. doi: 10.1111/j.1550-7408.2001.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Panciera RJ, Thomassen RW, Garner FM. Cryptosporidial infection in a calf. Vet Pathol. 1971;8:479–484. [Google Scholar]
- Moore DA, Zeman DH. Cryptosporidiosis in neonatal calves: 277 cases (1986-1987) J Am Vet Med Assoc. 1991;198:1969–1971. [PubMed] [Google Scholar]
- de Graaf DC, Vanopdenbosch E, Ortega-Mora LM, Abbassi H, Peeters JE. A review of the importance of cryptosporidiosis in farm animals. Int J Parasitol. 1999;29:1269–1287. doi: 10.1016/S0020-7519(99)00076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R, Santin M. Cryptosporidium xiaoi n. sp. (Apicomplexa: Cryptosporidiidae) in sheep (Ovis aries) Vet Parasitol. 2009;164:192–200. doi: 10.1016/j.vetpar.2009.05.011. [DOI] [PubMed] [Google Scholar]
- Robinson G, Wright S, Elwin K, Hadfield SJ, Katzer F, Bartley PM, Hunter PR, Nath M, Innes EA, Chalmers RM. Re-description of Cryptosporidium cuniculus Inman and Takeuchi, 1979 (Apicomplexa: Cryptosporidiidae): morphology, biology and phylogeny. Int J Parasitol. 2010;40:1539–1548. doi: 10.1016/j.ijpara.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Fayer R, Santin M, Macarisin D. Cryptosporidium ubiquitum n. sp. in animals and humans. Vet Parasitol. 2010;172:23–32. doi: 10.1016/j.vetpar.2010.04.028. [DOI] [PubMed] [Google Scholar]
- Chalmers RM, Smith R, Elwin K, Clifton-Hadley FA, Giles M. Epidemiology of anthroponotic and zoonotic human cryptosporidiosis in England and Wales, 2004-2006. Epidemiol Infect. 2011;139:700–712. doi: 10.1017/S0950268810001688. [DOI] [PubMed] [Google Scholar]
- Leoni F, Amar C, Nichols G, Pedraza-Diaz S, McLauchlin J. Genetic analysis of Cryptosporidium from 2414 humans with diarrhoea in England between 1985 and 2000. J Med Microbiol. 2006;55:703–707. doi: 10.1099/jmm.0.46251-0. [DOI] [PubMed] [Google Scholar]
- Fayer R, Santin M, Trout JM, Greiner E. Prevalence of species and genotypes of Cryptosporidium found in 1-2-year-old dairy cattle in the eastern United States. Vet Parasitol. 2006;135:105–112. doi: 10.1016/j.vetpar.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Santin M, Trout JM, Xiao L, Zhou L, Greiner E, Fayer R. Prevalence and age-related variation of Cryptosporidium species and genotypes in dairy calves. Vet Parasitol. 2004;122:103–117. doi: 10.1016/j.vetpar.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Santin M, Trout JM, Fayer R. A longitudinal study of cryptosporidiosis in dairy cattle from birth to 2 years of age. Vet Parasitol. 2008;155:15–23. doi: 10.1016/j.vetpar.2008.04.018. [DOI] [PubMed] [Google Scholar]
- Feng Y, Ortega Y, He G, Das P, Xu M, Zhang X, Fayer R, Gatei W, Cama V, Xiao L. Wide geographic distribution of Cryptosporidium bovis and the deer-like genotype in bovines. Vet Parasitol. 2007;144:1–9. doi: 10.1016/j.vetpar.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Muhid A, Robertson I, Ng J, Ryan U. Prevalence of and management factors contributing to Cryptosporidium sp. infection in pre-weaned and post-weaned calves in Johor, Malaysia. Exp Parasitol. 2011;127:534–538. doi: 10.1016/j.exppara.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Langkjaer RB, Vigre H, Enemark HL, Maddox-Hyttel C. Molecular and phylogenetic characterization of Cryptosporidium and Giardia from pigs and cattle in Denmark. Parasitology. 2007;134:339–350. doi: 10.1017/S0031182006001533. [DOI] [PubMed] [Google Scholar]
- Lefay D, Naciri M, Poirier P, Chermette R. Prevalence of Cryptosporidium infection in calves in France. Vet Parasitol. 2000;89:1–9. doi: 10.1016/S0304-4017(99)00230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naciri M, Lefay MP, Mancassola R, Poirier P, Chermette R. Role of Cryptosporidium parvum as a pathogen in neonatal diarrhoea complex in suckling and dairy calves in France. Vet Parasitol. 1999;85:245–257. doi: 10.1016/S0304-4017(99)00111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brscic M, Heutinck LF, Wolthuis-Fillerup M, Stockhofe N, Engel B, Visser EK, Gottardo F, Bokkers EA, Lensink BJ, Cozzi G, Van Reenen CG. Prevalence of gastrointestinal disorders recorded at postmortem inspection in white veal calves and associated risk factors. J Dairy Sci. 2011;94:853–863. doi: 10.3168/jds.2010-3480. [DOI] [PubMed] [Google Scholar]
- Guyot K, Follet-Dumoulin A, Lelievre E, Sarfati C, Rabodonirina M, Nevez G, Cailliez JC, Camus D, Dei-Cas E. Molecular characterization of Cryptosporidium isolates obtained from humans in France. J Clin Microbiol. 2001;39:3472–3480. doi: 10.1128/JCM.39.10.3472-3480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Alderisio K, Limor J, Royer M, Lal AA. Identification of species and sources of Cryptosporidium oocysts in storm waters with a small-subunit rRNA-based diagnostic and genotyping tool. Appl Environ Microbiol. 2000;66:5492–5498. doi: 10.1128/AEM.66.12.5492-5498.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatei W, Hart CA, Gilman RH, Das P, Cama V, Xiao L. Development of a multilocus sequence typing tool for Cryptosporidium hominis. J Eukaryot Microbiol. 2006;53(Suppl 1):S43–S48. doi: 10.1111/j.1550-7408.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- Basic Local Alignment Search Tool. http://www.ncbi.nlm.nih.gov/BLAST/
- Quilez J, Sanchez-Acedo C, del Cacho E, Clavel A, Causape AC. Prevalence of Cryptosporidium and Giardia infections in cattle in Aragon (northeastern Spain) Vet Parasitol. 1996;66:139–146. doi: 10.1016/S0304-4017(96)01015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendali F, Bichet H, Schelcher F, Sanaa M. Pattern of diarrhoea in newborn beef calves in south-west France. Vet Res. 1999;30:61–74. [PubMed] [Google Scholar]
- Peng MM, Wilson ML, Holland RE, Meshnick SR, Lal AA, Xiao L. Genetic diversity of Cryptosporidium spp. in cattle in Michigan: implications for understanding the transmission dynamics. Parasitol Res. 2003;90:175–180. doi: 10.1007/s00436-003-0834-5. [DOI] [PubMed] [Google Scholar]
- Duranti A, Caccio SM, Pozio E, Di Egidio A, De Curtis M, Battisti A, Scaramozzino P. Risk factors associated with Cryptosporidium parvum infection in cattle. Zoonoses Public Health. 2009;56:176–182. doi: 10.1111/j.1863-2378.2008.01173.x. [DOI] [PubMed] [Google Scholar]
- Maddox-Hyttel C, Langkjaer RB, Enemark HL, Vigre H. Cryptosporidium and Giardia in different age groups of Danish cattle and pigs--occurrence and management associated risk factors. Vet Parasitol. 2006;141:48–59. doi: 10.1016/j.vetpar.2006.04.032. [DOI] [PubMed] [Google Scholar]
- Huetink RE, van der Giessen JW, Noordhuizen JP, Ploeger HW. Epidemiology of Cryptosporidium spp. and Giardia duodenalis on a dairy farm. Vet Parasitol. 2001;102:53–67. doi: 10.1016/S0304-4017(01)00514-3. [DOI] [PubMed] [Google Scholar]
- Khan SM, Debnath C, Pramanik AK, Xiao L, Nozaki T, Ganguly S. Molecular characterization and assessment of zoonotic transmission of Cryptosporidium from dairy cattle in West Bengal, India. Vet Parasitol. 2001;171:41–47. doi: 10.1016/j.vetpar.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Singh BB, Sharma R, Kumar H, Banga HS, Aulakh RS, Gill JP, Sharma JK. Prevalence of Cryptosporidium parvum infection in Punjab (India) and its association with diarrhea in neonatal dairy calves. Vet Parasitol. 2006;140:162–165. doi: 10.1016/j.vetpar.2006.03.029. [DOI] [PubMed] [Google Scholar]
- Feltus DC, Giddings CW, Khaitsa ML, McEvoy JM. High prevalence of Cryptosporidium bovis and the deer-like genotype in calves compared to mature cows in beef cow-calf operations. Vet Parasitol. 2008;151:191–195. doi: 10.1016/j.vetpar.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Maikai BV, Umoh JU, Kwaga JK, Lawal IA, Maikai VA, Cama V, Xiao L. Molecular characterization of Cryptosporidium spp. in native breeds of cattle in Kaduna State, Nigeria. Vet Parasitol. 2011;178:241–245. doi: 10.1016/j.vetpar.2010.12.048. [DOI] [PubMed] [Google Scholar]
- Thompson HP, Dooley JS, Kenny J, McCoy M, Lowery CJ, Moore JE, Xiao L. Genotypes and subtypes of Cryptosporidium spp. in neonatal calves in Northern Ireland. Parasitol Res. 2007;100:619–624. doi: 10.1007/s00436-006-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook EJ, Anthony Hart C, French NP, Christley RM. Molecular epidemiology of Cryptosporidium subtypes in cattle in England. Vet J. 2009;179:378–382. doi: 10.1016/j.tvjl.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Feng Y, Li N, Duan L, Xiao L. Cryptosporidium genotype and subtype distribution in raw wastewater in Shanghai, China: evidence for possible unique Cryptosporidium hominis transmission. J Clin Microbiol. 2009;47:153–157. doi: 10.1128/JCM.01777-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L. Molecular epidemiology of cryptosporidiosis: an update. Exp Parasitol. 2010;124:80–89. doi: 10.1016/j.exppara.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Reed C, Sturbaum GD, Hoover PJ, Sterling CR. Cryptosporidium parvum mixed genotypes detected by PCR-restriction fragment length polymorphism analysis. Appl Environ Microbiol. 2002;68:427–429. doi: 10.1128/AEM.68.1.427-429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanriverdi S, Arslan MO, Akiyoshi DE, Tzipori S, Widmer G. Identification of genotypically mixed Cryptosporidium parvum populations in humans and calves. Mol Biochem Parasitol. 2003;130:13–22. doi: 10.1016/S0166-6851(03)00138-5. [DOI] [PubMed] [Google Scholar]
- Jenikova M, Nemejc K, Sak B, Kvetonova D, Kvac M. New view on the age-specificity of pig Cryptosporidium by species-specific primers for distinguishing Cryptosporidium suis and Cryptosporidium pig genotype II. Vet Parasitol. 2011;176:120–125. doi: 10.1016/j.vetpar.2010.11.010. [DOI] [PubMed] [Google Scholar]
- Santin M, Zarlenga DS. A multiplex polymerase chain reaction assay to simultaneously distinguish Cryptosporidium species of veterinary and public health concern in cattle. Vet Parasitol. 2009;166:32–37. doi: 10.1016/j.vetpar.2009.07.039. [DOI] [PubMed] [Google Scholar]
- Wang R, Wang H, Sun Y, Zhang L, Jian F, Qi M, Ning C, Xiao L. Characteristics of Cryptosporidium transmission in preweaned dairy cattle in Henan, China. J Clin Microbiol. 2011;49:1077–1082. doi: 10.1128/JCM.02194-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R, Gasbarre L, Pasquali P, Canals A, Almeria S, Zarlenga D. Cryptosporidium parvum infection in bovine neonates: dynamic clinical, parasitic and immunologic patterns. Int J Parasitol. 1998;28:49–56. doi: 10.1016/S0020-7519(97)00170-7. [DOI] [PubMed] [Google Scholar]
- Harp JA, Woodmansee DB, Moon HW. Resistance of calves to Cryptosporidium parvum: effects of age and previous exposure. Infect Immun. 1990;58:2237–2240. doi: 10.1128/iai.58.7.2237-2240.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters JE, Villacorta I, Vanopdenbosch E, Vandergheynst D, Naciri M, Ares-Mazas E, Yvore P. Cryptosporidium parvum in calves: kinetics and immunoblot analysis of specific serum and local antibody responses (immunoglobulin A [IgA], IgG, and IgM) after natural and experimental infections. Infect Immun. 1992;60:2309–2316. doi: 10.1128/iai.60.6.2309-2316.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fayer R, Santin M, Xiao L. Cryptosporidium bovis n. sp. (Apicomplexa: Cryptosporidiidae) in cattle (Bos taurus) J Parasitol. 2005;91:624–629. doi: 10.1645/GE-3435. [DOI] [PubMed] [Google Scholar]
- Trotz-Williams LA, Martin DS, Gatei W, Cama V, Peregrine AS, Martin SW, Nydam DV, Jamieson F, Xiao L. Genotype and subtype analyses of Cryptosporidium isolates from dairy calves and humans in Ontario. Parasitol Res. 2006;99:346–352. doi: 10.1007/s00436-006-0157-4. [DOI] [PubMed] [Google Scholar]
- Atwill ER, Johnson E, Klingborg DJ, Veserat GM, Markegard G, Jensen WA, Pratt DW, Delmas RE, George HA, Forero LC, Philips RL, Barry SJ, McDougald NK, Gildersleeve RR, Frost WE. Age, geographic, and temporal distribution of fecal shedding of Cryptosporidium parvum oocysts in cow-calf herds. Am J Vet Res. 1999;60:420–425. [PubMed] [Google Scholar]
- Alves M, Xiao L, Antunes F, Matos O. Distribution of Cryptosporidium subtypes in humans and domestic and wild ruminants in Portugal. Parasitol Res. 2006;99:287–292. doi: 10.1007/s00436-006-0164-5. [DOI] [PubMed] [Google Scholar]
- Jex AR, Gasser RB. Genetic richness and diversity in Cryptosporidium hominis and C. parvum reveals major knowledge gaps and a need for the application of "next generation" technologies-research review. Biotechnol Adv. 2010;28:17–26. doi: 10.1016/j.biotechadv.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Soba B, Logar J. Genetic classification of Cryptosporidium isolates from humans and calves in Slovenia. Parasitology. 2008;135:1263–1270. doi: 10.1017/S0031182008004800. [DOI] [PubMed] [Google Scholar]
- Wielinga PR, de Vries A, van der Goot TH, Mank T, Mars MH, Kortbeek LM, van der Giessen JW. Molecular epidemiology of Cryptosporidium in humans and cattle in The Netherlands. Int J Parasitol. 2008;38:809–817. doi: 10.1016/j.ijpara.2007.10.014. [DOI] [PubMed] [Google Scholar]