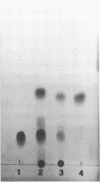
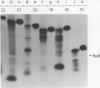
Abstract
The paper describes an improved method for the synthesis of oligodeoxyribonucleotides using phosphoramidite chemistry. Our procedure relies on novel phosphoramidite intermediates, the deoxyribonucleoside-3'-morpholine-methoxyphosphins. These compounds are extremely stable and can be purified readily. Condensation reactions during solid-phase synthesis can thus be performed with high efficiency and result in a high yield synthesis of long chain oligodeoxyribonucleotides.
Full text
PDF

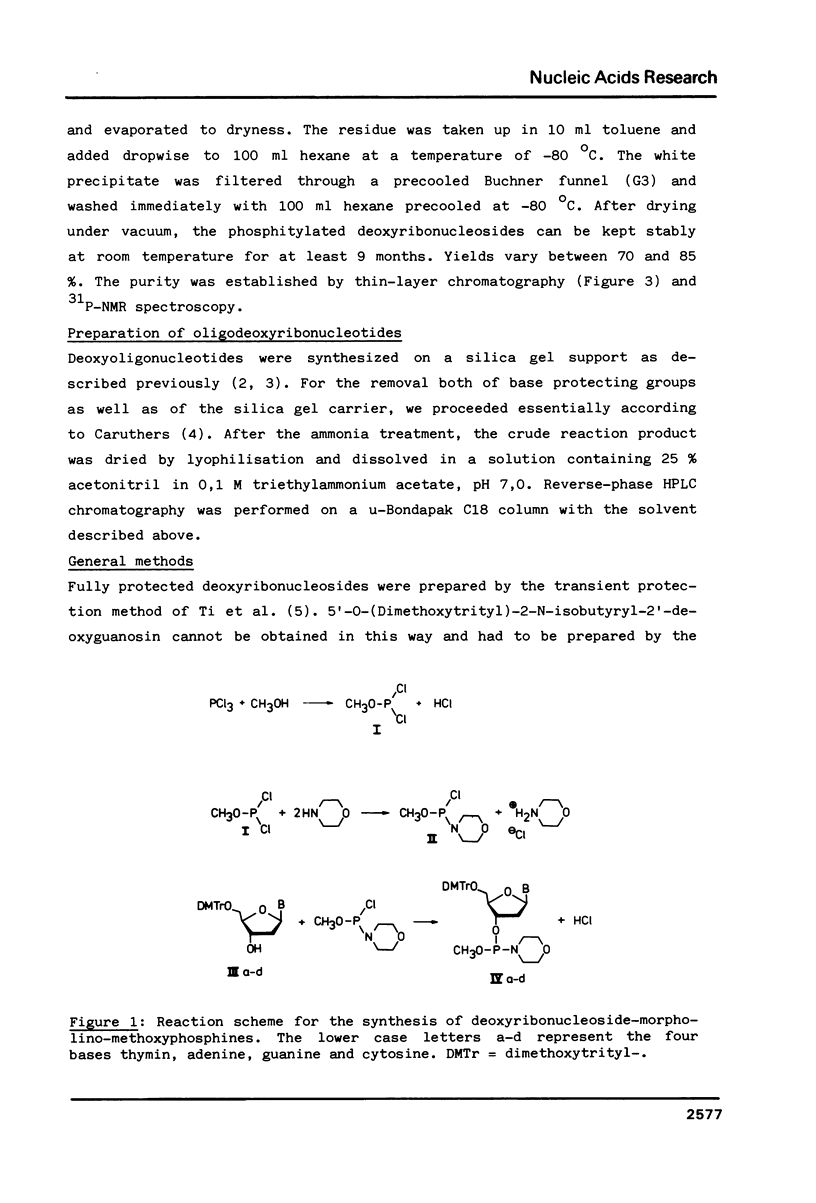
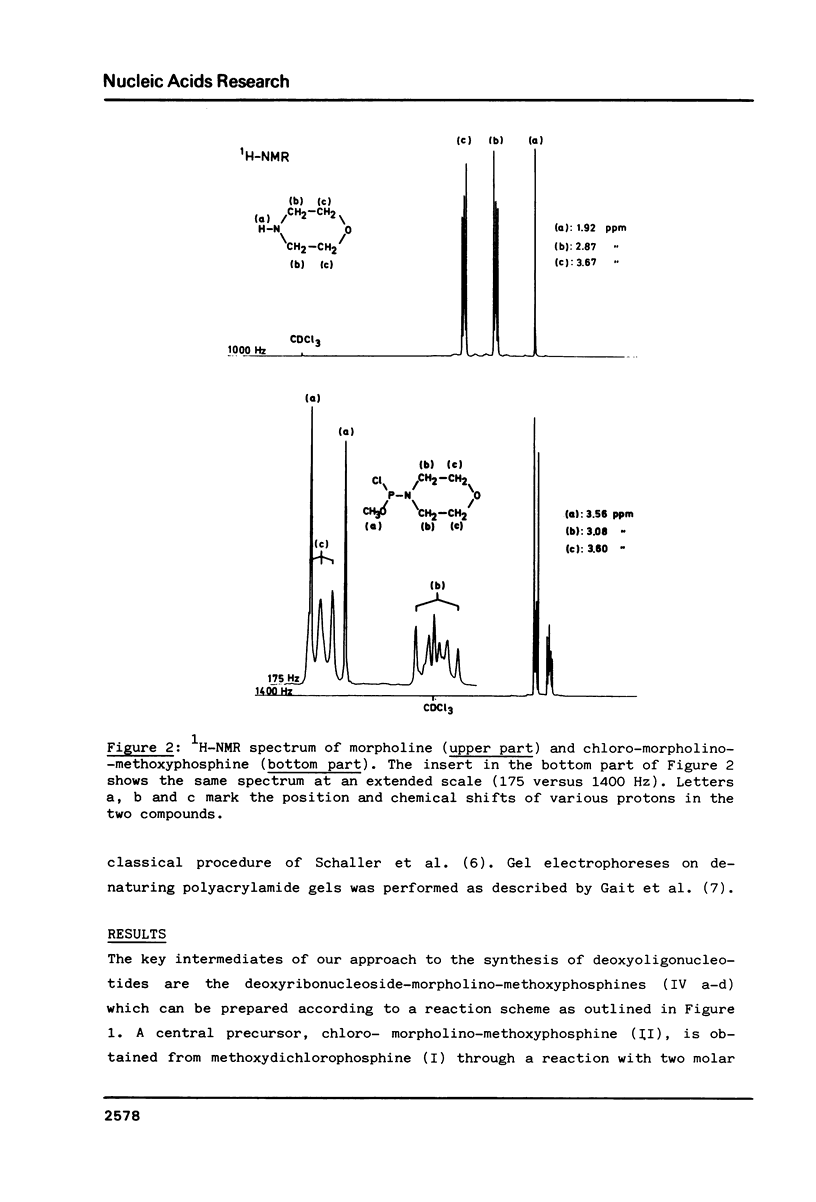
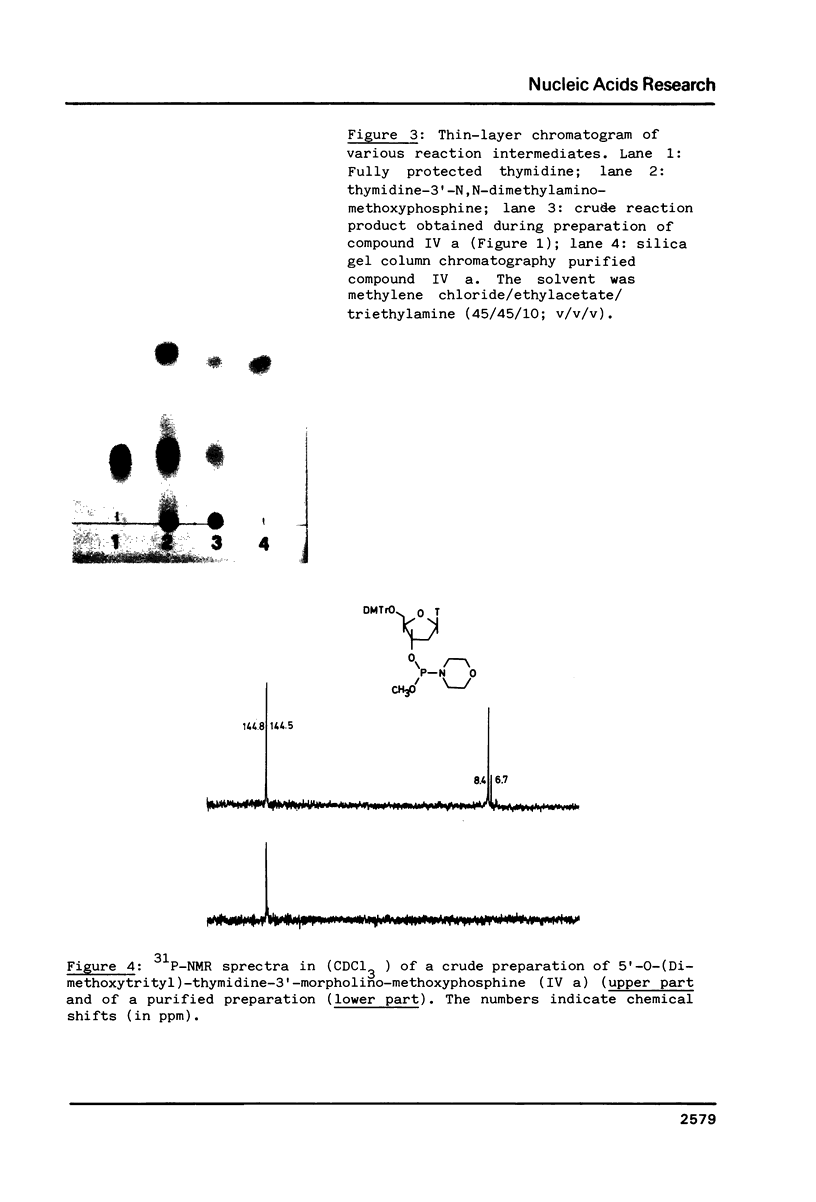
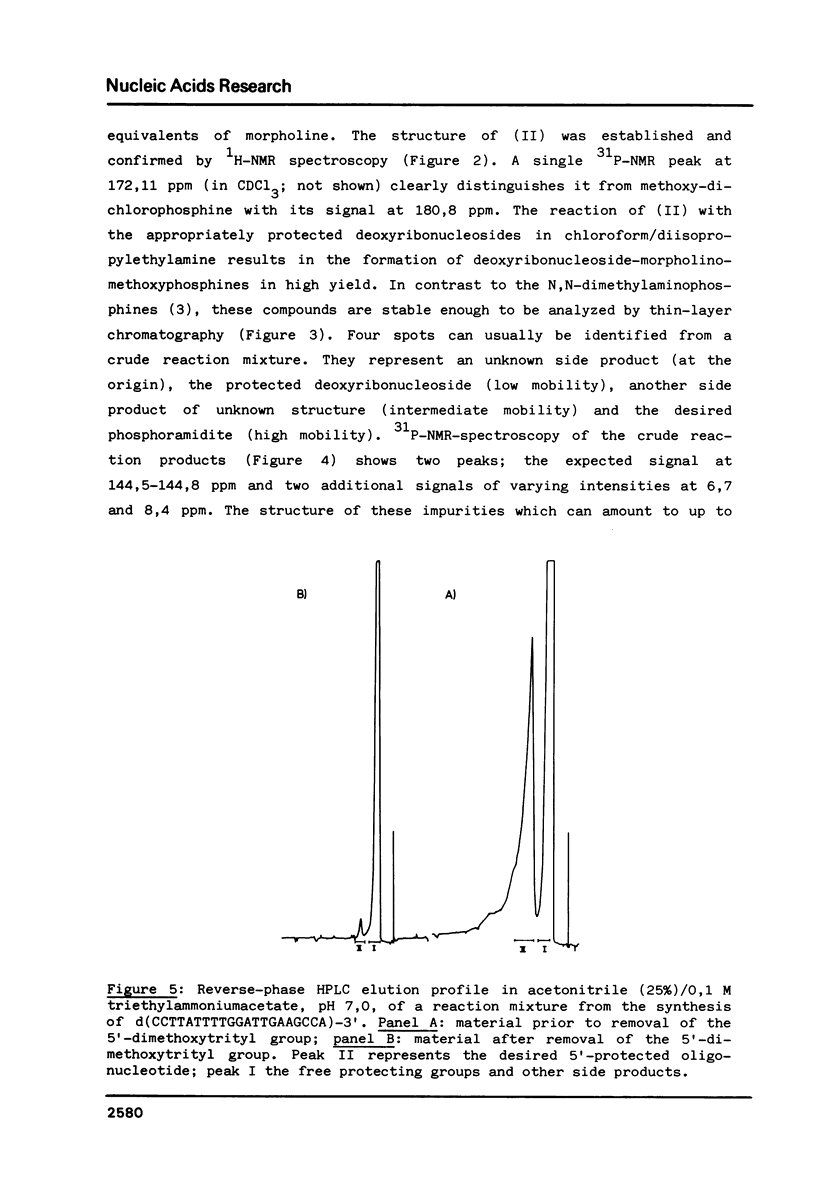




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daub G. W., van Tamelen E. E. Synthesis of oligoribonucleotides based on the facile cleavage of methyl phosphotriester intermediates. J Am Chem Soc. 1977 May 11;99(10):3526–3528. doi: 10.1021/ja00452a069. [DOI] [PubMed] [Google Scholar]
- Jay E., Seth A. K., Rommens J., Sood A., Jay G. Gene expression: chemical synthesis of E. coli ribosome binding sites and their use in directing the expression of mammalian proteins in bacteria. Nucleic Acids Res. 1982 Oct 25;10(20):6319–6329. doi: 10.1093/nar/10.20.6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letsinger R. L., Lunsford W. B. Synthesis of thymidine oligonucleotides by phosphite triester intermediates. J Am Chem Soc. 1976 Jun 9;98(12):3655–3661. doi: 10.1021/ja00428a045. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]