Abstract
Background
Neuropathic pain is a chronic pain due to disorder in the peripheral or central nervous system with different pathophysiological mechanisms. Current treatments are not effective. Analgesic drugs combined can reduce pain intensity and side effects. Here, we studied the analgesic effect of nimesulide, nefopam, and morphine with different mechanisms of action alone and in combination with other drugs in chronic constriction injury (CCI) model of neuropathic pain.
Methods
Male Wistar rats (n = 8) weighing 150-200 g were divided into 3 different groups: 1- Saline-treated CCI group, 2- Saline-treated sham group, and 3- Drug-treated CCI groups. Nimesulide (1.25, 2.5, and 5 mg/kg), nefopam (10, 20, and 30 mg/kg), and morphine (1, 3, and 5 mg/kg) were injected 30 minutes before surgery and continued daily to day 14 post-ligation. In the combination strategy, a nonanalgesic dose of drugs was used in combination such as nefopam + morphine, nefopam + nimesulide, and nimesulide + morphine. Von Frey filaments for mechanical allodynia and acetone test for cold allodynia were, respectively, used as pain behavioral tests. Experiments were performed on day 0 (before surgery) and days 1, 3, 5, 7, 10, and 14 post injury.
Results
Nefopam (30 mg/kg) and nimesulide (5 mg/kg) blocked mechanical and thermal allodynia; the analgesic effects of morphine (5 mg/kg) lasted for 7 days. Allodynia was completely inhibited in combination with nonanalgesic doses of nefopam (10 mg/kg), nimesulide (1.25 mg/kg), and morphine (3 mg/kg).
Conclusions
It seems that analgesic drugs used in combination, could effectively reduce pain behavior with reduced adverse effects.
Keywords: combination therapy, morphine, nefopam, neuropathy, nimesulide
INTRODUCTION
Neuropathic pain can arise as a result of damage to the peripheral or central nervous system and includes a variety of conditions that differ in etiology as well as location. Sensory abnormalities which manifest as allodynia (pain evoked by normally non-noxious stimuli), and hyperalgesia (an increased response to a noxious stimuli) are routinely observed in human neuropathic pain conditions as well as in relevant animal models [1-3]. Many drugs have tried to reduce neuropathic pain but the underlying mechanisms of neuropathic pain are multiple and complex; therefore, treatment and management of this distressing condition are suggesting the use of more than one type of medication [4].
Among analgesics, morphine is a widely used drug in the treatment of moderate to severe pain. There is considerable controversy for opioid analgesics to treat chronic pain [3]. For example, opioids were reported to be ineffective in some patients with neuropathic pain [5], whereas other observations suggest that opioids are effective in attenuating neuropathic pain [6,7]. However, respiratory depression, sedation, constipation and tolerance are the most important side effects of opioids which limit their clinical application in neuropathic pain [8].
Non Steroidal Anti Inflammatory Drugs (NSAIDs) are used in the management of chronic, inflammatory and postoperative pain [9]. Nimesulide, a highly selective COX-2 inhibitor, has induced analgesia compared to other NSAIDs such as celecoxib, indomethacin, and naproxen in painful osteoarthritis and some other conditions [10-12]. The efficacy of nimesulide in neuropathic pain is not well established. However the most common side effects of NSAIDs are gastrointestinal troubles, hepatotoxicity, and nephrotoxicity [13].
Nefopam, a nonopioid drug with a mechanism different from other analgesics is a centrally acting agent [14]. The antinociceptive effect of nefopam has been shown in animal models of acute and chronic pain and in human. Nefopam reduced pain in some behavioral tests (the hot plate [15], formalin [16]; carrageenan and incision induced thermal hyperalgesia tests [17]). Moreover, many clinical studies have evaluated the analgesic efficacy of nefopam in postoperative pain [18,19]. Nefopam has shown a protective analgesic effect when used in single doses in the CCI model of chronic neuropathic pain [20]. However, as for other analgesic drugs, some unpleasant adverse effects of nefopam including dizziness, headache, nausea, vomiting, and sweating are consistent with its central mechanism of action [14,21].
Multidrug therapy (MDT) has been widely accepted in the pharmacological management of a wide variety of complex chronic medical disorders including hypertension, HIV, cancer, and pain conditions like postoperative pain and neuropathic pain. The use of drug combinations from different pharmacological classes is expected to improve analgesia and to decrease the incidence and/or severity of the side effects of each drug [22].
From this perspective, our study was designed to evaluate the antiallodynic effects of different pharmacological classes of analgesic drugs. In this regards, we used an opioid drug (morphine), a highly selective COX-2 inhibitor (nimesulide), and a nonopioid drug (nefopam) in a dose-dependent manner alone, and in combination in chronic constriction injury (CCI) model of neuropathic pain in rats.
MATERIALS AND METHODS
1. Animals
Experiments were carried out on male Wistar rats (150-200 g) that were housed one rat per cage and placed under a 12 hour light/dark cycle in a temperature-controlled room (22 ± 1℃). Animals had free access to food and water. Rats were divided randomly into several experimental groups, each made-up of 8 animals. All experiments followed the IASP guidelines on ethical standards for investigation of experimental pain in animals [23]. The animals were allowed to habituate to the housing facilities for one week before the experiments began. Behavioral studies were performed in a quiet room between 9:00 to 11:00 AM. Efforts were made to limit distress and to use the minimum number of animals necessary to achieve statistical significance.
2. Surgery
We used the CCI model of neuropathic pain [24]. The surgical procedure was performed under ketamine (60 mg/kg) and xylazine (10 mg/kg) anaesthesia. The left sciatic nerve was exposed and 4 loose chromic gut ligatures were placed around the nerve proximal to the trifurcation. The distance between the two adjacent ligatures was 1 mm. The wound was irrigated with saline (0.9%) and closed in two layers with 4-0 silk (facial plane) and surgical skin staples. In the saline-treated sham group, rats underwent the same surgical procedure except for the ligation.
3. Drug preparation
Nimesulide (Sigma, USA) was prepared as suspension in saline 0.9%. Nefopam (Biocodex Laboratories, France) and morphine (Sigma, USA) were dissolved in saline 0.9%. Ketamine hydrochloride (Sigma, USA) and Xylazine hydrochloride (Sigma, USA) were used for the anesthesia. All drugs were injected by the intra-peritoneal (IP) route.
4. Drug administration
Animals were randomly divided into three experimental groups: 1- Saline-treated CCI group, 2- Saline-treated sham group, and 3- Drug-treated CCI groups. In one study, animals received morphine (1, 3, and 5 mg/kg) [25], nefopam (10, 20, and 30 mg/kg) [20], and nimesulide (1.25, 2.5, and 5 mg/kg) [10]. In another study, a nonanalgesic dose of each drug was selected and used in combination as follows: "nefopam-nimesulide", "nefopam-morphine", and "morphine-nimesulide". Drugs were injected 30 minutes before surgery and continued daily to day 14 post-ligation. All behavioral tests were recorded on day 0 (control day) before the surgery and on days 1, 3, 5, 7, 10, and 14 post-nerve injury. The order of pain testing was mechanical and cold allodynia, respectively (the interval between each test was 30 minutes).
5. Behavioral tests and experimental design
The sciatic nerve territory (mid-plantar hind paw) was tested for sensitivity to noxious and innocuous stimuli using standard behavioural assays done sequentially at several intervals up to 14 days following surgery. Animals were acclimated to the testing chambers for 30 min prior to testing. Mechanical and cold allodynia were evaluated in the animals.
1) Mechanical allodynia
Mechanical sensitivity to nonnoxious stimuli was measured with a set of calibrated nylon monofilaments (Stoelting, USA). The von Frey methodology was used to assess the sensitivity of the skin to tactile stimulation. Von Frey filaments are calibrated to have a characteristic bending force when pressure is applied. Each rat was placed under a transparent Plexiglas cage on an elevated metal screen surface with 1 cm mesh openings. Increasing strengths of von Frey filaments were applied sequentially to the plantar surface of the left hind paw of each animal. The minimum paw withdrawal threshold (PWT), defined as the minimum gram strength eliciting two sequential responses with 3 min intervals between them (withdrawal from pressure), was recorded for the left paw. The intensity of the mechanical stimulation increased from 2 to 60 g in a graded manner using successively greater diameter filaments until the hind paw was withdrawn. For successive tests, the placement of these stimuli was varied slightly from one trial to the next to avoid sensitization of the hind paw [26] .
2) Cold allodynia
The acetone test [27] was used to determine the reactivity to an acetone stimulus. Rats were placed under a transparent Plexiglas cage, as described previously, and an acetone bubble was formed at the end of a piece of small polyethylene tubing that was connected to a syringe; then, the bubble was lightly touched to the heel. The acetone was applied 5 times with an interval of 1 min between application, and the number of paw lifts from surface was the response measured. The response was calculated as the percent of paw withdrawal frequency (%PWF) using the following equation:
(Number of paw withdrawals / 5 trials) × 100.
6. Statistical analysis
Parametric data were analyzed for significance with analysis of variance (ANOVA) followed by a post-hoc Tukey's test. Non-parametric data were analyzed with 2 related samples followed by the Wilcoxon test. In all cases, data were shown as the mean ± SEM (standard error) and P < 0.05 was considered significant.
RESULTS
1. Dose-dependent effects of nefopam, nimesulide, and morphine
1) Response to mechanical allodynia (von Frey Filament test)
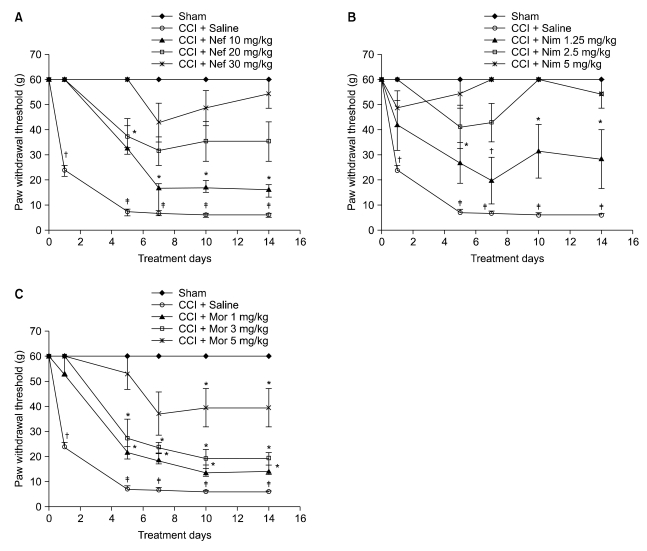
Fig. 1: All saline-treated CCI groups were strongly allodynic at the fifth day post-ligation (P < 0.001) compared to the control day; this effect was sustained until the end of the study. On the contrary, the saline-treated sham group did not show any mechanical allodynia for the duration of the experimental period as compared to the pre-surgery day. During the period of the study, in the drug-treated CCI groups, nefopam reduced mechanical allodynia for 20 and 30 mg/kg doses, but not for a 10 mg/kg dose (P < 0.05) (Fig. 1A); anti-allodynic effect of nimesulide was seen for 2.5 and 5 mg/kg doses, but not for a 1.25 mg/kg dose (P < 0.05) (Fig. 1B); morphine (5 mg/kg) decreased the tactile allodynia until day 7, but not for 1 and 3 mg/kg doses (P < 0.05) (Fig. 1C).
Fig. 1.
Paw withdrawal threshold in response to von Frey filaments before and at several time points after surgery in the saline-treated CCI group, saline-treated sham group, and drug-treated CCI group. Nefopam (10, 20, and 30 mg/kg (A)), Nimesulide (1.25, 2.5, and 5 mg/kg (B)), morphine (1, 3, and 5 mg/kg (C)) were injected i.p. Results are expressed as the mean ± SEM of 8 animals per group. Nef: Nefopam, Nim: Nimesulide, Mor: Morphine. Asterisks indicate a significant difference between post surgery days compared with day 0 (*P < 0.05, †P < 0.01, ‡P < 0.001).
2) Response to cold allodynia (Acetone test)
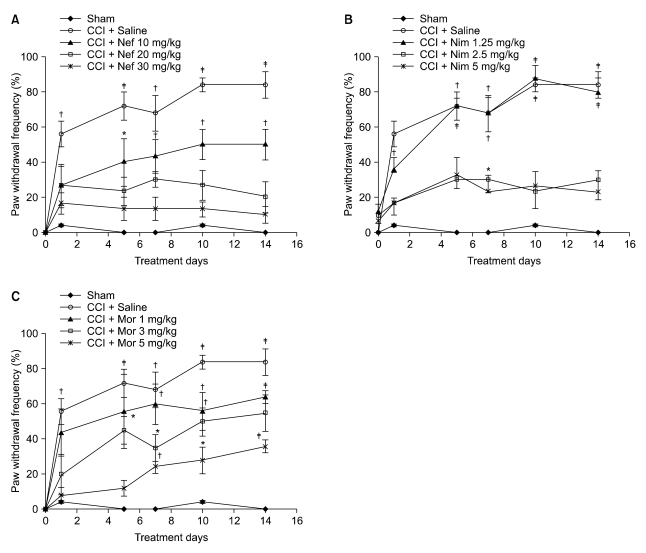
Fig. 2: In the saline-treated CCI group, a significant difference in pain behavior (P < 0.001) was seen on the fifth day postinjury compared to day 0; this effect continued until the end of the study. However, cold allodynia was not observed in the saline-treated sham group. In the drug-treated CCI group, nefopam reduced cold allodynia for 20 and 30 mg/kg doses, but not for a 10 mg/kg dose (P < 0.05) (Fig. 2A); nimesulide produced an antiallodynic effect for 2.5 and 5 mg/kg doses but not for a 1.25 mg/kg dose (P < 0.01) (Fig. 2B); the antiallodynic effect of morphine (5 mg/kg) lasted for 5 days, but not for the 1 and 3mg/kg doses (P < 0.01) (Fig. 2C).
Fig. 2.
The frequency of paw withdrawal in response to acetone before and at several time points after surgery in the saline-treated CCI group, saline-treated sham group, and drug-treated CCI group. Nefopam (10, 20, and 30 mg/kg (A)), Nimesulide (1.25, 2.5, and 5 mg/kg (B)), morphine (1, 3, and 5 mg/kg (C)) were injected i.p. Results are expressed as the mean ± SEM of 8 animals per group. Nef: Nefopam, Nim: Nimesulide, Mor: Morphine. Asterisks indicate a significant difference between post surgery days compared with day 0 (*P < 0.05, †P < 0.01, ‡P < 0.001).
2. The effect of combination therapy
1) Response to mechanical allodynia (von Frey Filament)
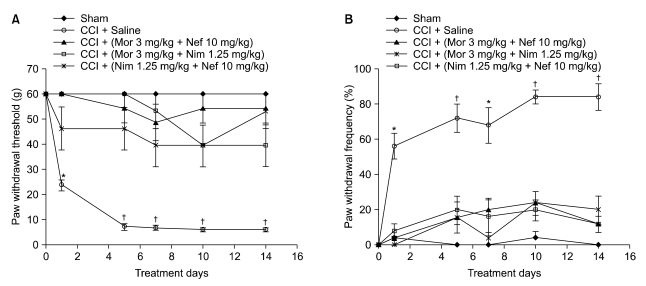
Fig. 3A: Pain behavior was not seen in all drug-treated CCI groups when used in combination [(morphine 3 mg/kg + nefopam 10 mg/kg), (morphine 3 mg/kg + nimesulide 1.25 mg/kg), (nimesulide 1.25 mg/kg + nefopam 10 mg/kg)] during the experimental period compared to day 0 presurgery.
Fig. 3.
Paw withdrawal threshold in response to von Frey filaments (A) and the frequency of paw withdrawal in response to acetone (B) before and at several time points after surgery in saline-treated CCI group, saline-treated sham group and drug-treated CCI groups in combination: (morphine 3 mg/kg + nefopam 10 mg/kg, morphine 3 mg/kg + nimesulide 1.25 mg/kg, nimesulide 1.25 mg/kg + nefopam 10 mg/kg) were injected i.p. Results are expressed as the mean ± SEM of 8 animals per group. Nef: Nefopam, Nim: Nimesulide, Mor: Morphine. Asterisks indicate a significant difference between post surgery days compared with day 0 (*P < 0.01, †P < 0.001).
2) Response to cold allodynia (Acetone test)
Fig. 3B: All drug-treated CCI groups [(morphine 3 mg/kg + nefopam 10 mg/kg), (morphine 3 mg/kg + nimesulide 1.25 mg/kg), (nimesulide 1.25 mg/kg + nefopam 10 mg/kg) did not produce pain behavior compared to day 0 presurgery when used in combination.
DISCUSSION
In this study, the analgesic effects of drugs with different mechanisms of action (nimesulide, nefopam, and morphine) were investigated alone and in combination, in a rat model of neuropathic pain. We used the CCI model of nerve injury because it has an inflammatory and a nerve injury components which are reported to mimic types of neuropathic pain found in humans [24]. The management of neuropathic pain remains a major clinical challenge. This is in part due to an inadequate understanding of the mechanisms involved in the disease etiology, but also a result of the relative absence of clinically effective treatments [28].
Opioids are the drugs of choice for the treatment of moderate to severe pain, however, a large number of clinical studies have reported that opioids, particularly morphine, had weak analgesic efficacy in neuropathic pain in humans [29]. In our research, lower doses of morphine did not reduce mechanical and cold allodynia during the experimental period. The antiallodynic effect of morphine used at a high dose lasted for a few days. However, it seems that the reduced analgesic effect of morphine may be due to the tolerance of its analgesic effects. There are some controversies about the relative efficacy of opiate analgesics against neuropathic pain in clinical and experimental researches [30].
Pre-emptive analgesia can provide an effective treatment which prevents the establishment of altered central processing, amplifying post-operative pain. Evidence showed that pre-emptive injection of nefopam and nimesulide had produced effective analgesia [20,31], while morphine produced a slight antiallodynic effect in the CCI model of neuropathic pain [29].
Nefopam, a nonopioid analgesic drug, can produce analgesia in animals and humans. It was reported that in CCI model of neuropathic pain, a single dose of nefopam, significantly reduced pain behavior. Moreover, it was shown that nefopam has preventive analgesic effects [20]. Acute administration of nefopam (intraperitoneal, subcutaneous, and oral) exhibited a dose-dependent attenuation of pain behavior in hot plate and plantar tests [15]. In our experiment, high doses of nefopam showed pain reducing effects, which is consistent with previous studies [15,20]. Although a single dose of nefopam (30 mg/kg) reduced pain behavior in the CCI model of neuropathic pain [20], we observed that daily administration of nefopam (30 & 20 mg/kg) showed analgesic effects. It seems that chronic administration of nefopam could probably attenuate pain behavior in a dose-dependent manner. However, we noticed a hyperexcitability state, which was seen when a high dose of nefopam (30 mg/kg) was injected into the rats. This effect was transient and disappeared after 30 minutes of drug administration.
Nimesulide, a highly selective COX-2 inhibitor, inhibits PGE2 and nitric oxide production and modulates neural transmission at the level of the dorsal horn [31]. Among numerous properties of nimesulide, decreased tumor necrosis factor α (TNF-α) and neutrophil accumulation in the inflammatory exudates were reported [9]. Nimesulide has a relatively rapid onset of antihyperalgesic effects in humans when compared with well-established NSAIDs such as diclofenac, celecoxib, and rofecoxib. It was shown that nimesulide can properly reduce pain and inflammation in formalin and other inflammatory tests [10]. Our results show that moderate and high doses of nimesulide reduced mechanical and cold allodynia, which are consistent with the results of previous works [9,10,31]
The use of multiple drug therapy is common in clinical and experimental conditions, including neuropathic pain. The rationale behind drug combination is that there are multiple mechanisms involved in generating chronic pain [22]. In one study, patients with diabetic neuropathy and post herpetic neuralgia were treated with a combination of gabapentin and morphine, compared with either drug alone or with a placebo. The combination of gabapentin and morphine provided better pain relief at smaller doses for each drug [32]. It seems that it is unlikely that a single drug can effectively reduce pain and treat neuropathic pain.
According to our results, nonanalgesic doses of nefopam, nimesulide, and morphine were selected. Co-administration of these drugs exhibited antiallodynic effects.
Few experimental studies have investigated the effects of the co-administration of nefopam with morphine or opioid agonists. In one study, carrageenan-induced tactile allodynia test, co-administration of weak analgesic doses of nefopam with moderate analgesic doses of morphine significantly reduced allodynia. Moreover, co-administration of nonanalgesic doses of nefopam with a nonanalgesic doses of morphine completely inhibited carrageenan- or incision-induced thermal hyperalgesia [17]. In clinical studies concerning abdominal, urogenital, orthopedic surgery, and hepatic resection, nefopam has been shown to reduce morphine consumption [18,19]. Enhancement of morphine antinociception by other monoamine reuptake inhibitors has been shown with clomipramine, amitriptyline, and fluoxetine in animals [33,34]. We can conclude that, by inhibiting monoamine reuptake, these drugs as well as nefopam can induce an increase in noradrenaline, serotonin, and/or dopamine levels in the central nervous system; therefore, the enhancement of morphine-associated antinociception can thus be rationalized.
In a recent study, the antinociceptive characteristics of the combination of nefopam and paracetamol were evaluated using four different animal models of pain. Data analysis revealed that, drug combination significantly blocked allodynia in the carrageenan test, in the inflammatory phase in the formalin test, in thermal hyperalgesia in the incision model of postoperative pain, and in abdominal writhes in the acetic acid test [35]. Evidence showed an interaction in co-administration of nefopam and ketoprofen in postoperative pain, which increases their analgesic efficacy and reduces their side effects [36]. In our research, we found that the combination of nefopam and nimesulide can reduce pain behavior, which is consistent with the above mentioned studies [35,36]. As stated before, NSAIDs may exert their antinociceptive activity by inhibition of prostaglandins biosynthesis and through other complex mechanisms. These include modulation by endogenous opioids and cholinergic, serotonergic, and noradrenergic mechanisms [37,38]. Experimental studies in animal models have demonstrated that nimesulide inhibits COX-2 and nitric oxide synthetase (NOS) in the spinal cord confirming the central action of the drug. Therefore, the antinociceptive activity of nimesulide partly involves the inhibition of inflammation, a mechanism of action different from that of the other analgesic drugs acting centrally like nefopam [31,39].
Several combinations of opioids and anti-inflammatory drugs have been examined in various models of pain and inflammation. For example, in one study, the combination of morphine and diclofenac has been shown to be effective in a model of acute inflammatory pain [40]. A synergistic antiallodynic effect of spinal morphine administered with ketorolac in nerve injured rats has also been reported [41]. Recently, it has been shown that the antinociceptive activity of morphine combined with different NSAIDs (including nimesulide, meloxicam, diclofenac, naproxen, piroxicam, parecoxib, and ketoprofen) induced a synergistic interaction in the mice model of visceral acute pain [42].
Considering these data, in our study, we found that a combination of nonanalgesic doses of nefopam, morphine, and nimesulide could reduce effectively mechanical and cold allodynia. Our data are consistent with previous works on combination therapy in an attempt to reduce pain behavior [40-42].
In conclusion, our results show that nefopam, morphine, and nimesulide used pre-emptively reduced pain in a dose-dependent manner throughout the experiments. Although the analgesic effect of morphine was not comparable to nefopam and nimesulide, we found an effective response for combination therapy in reducing pain behaviors. Further evaluations are needed to establish the type of interaction between these drugs in combination therapy in the CCI model of neuropathic pain.
ACKNOWLEDGEMENTS
This research was supported by a grant from the Neuroscience Research Center of Shahid Beheshti University of Medical Sciences. The authors would like to thank the staff of the Department of Pharmacology for their kind collaboration.
References
- 1.Blackburn-Munro G. Pain-like behaviours in animals - how human are they? Trends Pharmacol Sci. 2004;25:299–305. doi: 10.1016/j.tips.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18:350–354. doi: 10.1097/00002508-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Moulin DE, Clark AJ, Gilron I, Ware MA, Watson CP, Sessle BJ, et al. Pharmacological management of chronic neuropathic pain - consensus statement and guidelines from the Canadian Pain Society. Pain Res Manag. 2007;12:13–21. doi: 10.1155/2007/730785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suzuki R, Dickenson AH. Differential pharmacological modulation of the spontaneous stimulus-independent activity in the rat spinal cord following peripheral nerve injury. Exp Neurol. 2006;198:72–80. doi: 10.1016/j.expneurol.2005.10.032. [DOI] [PubMed] [Google Scholar]
- 5.Arnér S, Meyerson BA. Lack of analgesic effect of opioids on neuropathic and idiopathic forms of pain. Pain. 1988;33:11–23. doi: 10.1016/0304-3959(88)90198-4. [DOI] [PubMed] [Google Scholar]
- 6.Raja SN, Haythornthwaite JA, Pappagallo M, Clark MR, Travison TG, Sabeen S, et al. Opioids versus antidepressants in postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology. 2002;59:1015–1021. doi: 10.1212/wnl.59.7.1015. [DOI] [PubMed] [Google Scholar]
- 7.Wu CL, Tella P, Staats PS, Vaslav R, Kazim DA, Wesselmann U, et al. Analgesic effects of intravenous lidocaine and morphine on postamputation pain: a randomized doubleblind, active placebo-controlled, crossover trial. Anesthesiology. 2002;96:841–848. doi: 10.1097/00000542-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Dobecki DA, Schocket SM, Wallace MS. Update on pharmacotherapy guidelines for the treatment of neuropathic pain. Curr Pain Headache Rep. 2006;10:185–190. doi: 10.1007/s11916-006-0044-9. [DOI] [PubMed] [Google Scholar]
- 9.Rainsford KD. Current status of the therapeutic uses and actions of the preferential cyclo-oxygenase-2 NSAID, nimesulide. Inflammopharmacology. 2006;14:120–137. doi: 10.1007/s10787-006-1505-9. [DOI] [PubMed] [Google Scholar]
- 10.Bianchi M, Broggini M. Anti-hyperalgesic effects of nimesulide: studies in rats and humans. Int J Clin Pract Suppl. 2002;(128):11–19. [PubMed] [Google Scholar]
- 11.Binning A. Nimesulide in the treatment of postoperative pain: a double-blind, comparative study in patients undergoing arthroscopic knee surgery. Clin J Pain. 2007;23:565–570. doi: 10.1097/AJP.0b013e3180e00dff. [DOI] [PubMed] [Google Scholar]
- 12.Bianchi M, Broggini M, Balzarini P, Franchi S, Sacerdote P. Effects of nimesulide on pain and on synovial fluid concentrations of substance P, interleukin-6 and interleukin-8 in patients with knee osteoarthritis: comparison with celecoxib. Int J Clin Pract. 2007;61:1270–1277. doi: 10.1111/j.1742-1241.2007.01453.x. [DOI] [PubMed] [Google Scholar]
- 13.Bennett A, Villa G. Nimesulide: an NSAID that preferentially inhibits COX-2, and has various unique pharmacological activities. Expert Opin Pharmacother. 2000;1:277–286. doi: 10.1517/14656566.1.2.277. [DOI] [PubMed] [Google Scholar]
- 14.Durrieu G, Olivier P, Bagheri H, Montastruc JL; Overview of adverse reactions to nefopam: an analysis of the French Pharmacovigilance database. Fundam Clin Pharmacol. 2007;21:555–558. doi: 10.1111/j.1472-8206.2007.00499.x. [DOI] [PubMed] [Google Scholar]
- 15.Girard P, Pansart Y, Coppe MC, Gillardin JM. Nefopam reduces thermal hypersensitivity in acute and postoperative pain models in the rat. Pharmacol Res. 2001;44:541–545. doi: 10.1006/phrs.2001.0886. [DOI] [PubMed] [Google Scholar]
- 16.Girard P, Coppé MC, Verniers D, Pansart Y, Gillardin JM. Role of catecholamines and serotonin receptor subtypes in nefopam-induced antinociception. Pharmacol Res. 2006;54:195–202. doi: 10.1016/j.phrs.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Girard P, Pansart Y, Gillardin JM. Nefopam potentiates morphine antinociception in allodynia and hyperalgesia in the rat. Pharmacol Biochem Behav. 2004;77:695–703. doi: 10.1016/j.pbb.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 18.Beloeil H, Delage N, Nègre I, Mazoit JX, Benhamou D. The median effective dose of nefopam and morphine administered intravenously for postoperative pain after minor surgery: a prospective randomized double-blinded isobolographic study of their analgesic action. Anesth Analg. 2004;98:395–400. doi: 10.1213/01.ANE.0000093780.67532.95. [DOI] [PubMed] [Google Scholar]
- 19.Kapfer B, Alfonsi P, Guignard B, Sessler DI, Chauvin M. Nefopam and ketamine comparably enhance postoperative analgesia. Anesth Analg. 2005;100:169–174. doi: 10.1213/01.ANE.0000138037.19757.ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biella GE, Groppetti A, Novelli A, Fernández-Sánchez MT, Manfredi B, Sotgiu ML. Neuronal sensitization and its behavioral correlates in a rat model of neuropathy are prevented by a cyclic analog of orphenadrine. J Neurotrauma. 2003;20:593–601. doi: 10.1089/089771503767168519. [DOI] [PubMed] [Google Scholar]
- 21.Novelli A, Groppetti A, Rossoni G, Manfredi B, Ferrero-Gutiérrez A, Pérez-Gómez A, et al. Nefopam is more potent than carbamazepine for neuroprotection against veratridine in vitro and has anticonvulsant properties against both electrical and chemical stimulation. Amino Acids. 2007;32:323–332. doi: 10.1007/s00726-006-0419-6. [DOI] [PubMed] [Google Scholar]
- 22.Backonja MM, Irving G, Argoff C. Rational multidrug therapy in the treatment of neuropathic pain. Curr Pain Headache Rep. 2006;10:34–38. doi: 10.1007/s11916-006-0007-1. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- 24.Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. doi: 10.1016/0304-3959(88)90209-6. [DOI] [PubMed] [Google Scholar]
- 25.Zhao C, Tall JM, Meyer RA, Raja SN. Antiallodynic effects of systemic and intrathecal morphine in the spared nerve injury model of neuropathic pain in rats. Anesthesiology. 2004;100:905–911. doi: 10.1097/00000542-200404000-00021. [DOI] [PubMed] [Google Scholar]
- 26.Stuesse SL, Crisp T, McBurney DL, Schechter JB, Lovell JA, Cruce WL. Neuropathic pain in aged rats: behavioral responses and astrocytic activation. Exp Brain Res. 2001;137:219–227. doi: 10.1007/s002210000630. [DOI] [PubMed] [Google Scholar]
- 27.Choi Y, Yoon YW, Na HS, Kim SH, Chung JM. Behavioral signs of ongoing pain and cold allodynia in a rat model of neuropathic pain. Pain. 1994;59:369–376. doi: 10.1016/0304-3959(94)90023-X. [DOI] [PubMed] [Google Scholar]
- 28.Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–1534. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 29.Hamidi GA, Manaheji H, Janahmadi M, Noorbakhsh SM, Salami M. Co-administration of MK-801 and morphine attenuates neuropathic pain in rat. Physiol Behav. 2006;88:628–635. doi: 10.1016/j.physbeh.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 30.Erichsen HK, Hao JX, Xu XJ, Blackburn-Munro G. Comparative actions of the opioid analgesics morphine, methadone and codeine in rat models of peripheral and central neuropathic pain. Pain. 2005;116:347–358. doi: 10.1016/j.pain.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Tassorelli C, Greco R, Sandrini G, Nappi G. Central components of the analgesic/antihyperalgesic effect of nimesulide: studies in animal models of pain and hyperalgesia. Drugs. 2003;63(Suppl 1):9–22. doi: 10.2165/00003495-200363001-00003. [DOI] [PubMed] [Google Scholar]
- 32.Baillie JK, Power I. Morphine, gabapentin, or their combination for neuropathic pain. N Engl J Med. 2005;352:2650–2651. doi: 10.1056/NEJM200506233522520. [DOI] [PubMed] [Google Scholar]
- 33.Nayebi AR, Hassanpour M, Rezazadeh H. Effect of chronic and acute administration of fluoxetine and its additive effect with morphine on the behavioural response in the formalin test in rats. J Pharm Pharmacol. 2001;53:219–225. doi: 10.1211/0022357011775235. [DOI] [PubMed] [Google Scholar]
- 34.Ho KY, Tay W, Yeo MC, Liu H, Yeo SJ, Chia SL, et al. Duloxetine reduces morphine requirements after knee replacement surgery. Br J Anaesth. 2010;105:371–376. doi: 10.1093/bja/aeq158. [DOI] [PubMed] [Google Scholar]
- 35.Girard P, Niedergang B, Pansart Y, Coppé MC, Verleye M. Systematic evaluation of the nefopam-paracetamol combination in rodent models of antinociception. Clin Exp Pharmacol Physiol. 2011 doi: 10.1111/j.1440-1681.2011.05477.x. [in press] [DOI] [PubMed] [Google Scholar]
- 36.Girard P, Verniers D, Coppé MC, Pansart Y, Gillardin JM. Nefopam and ketoprofen synergy in rodent models of antinociception. Eur J Pharmacol. 2008;584:263–271. doi: 10.1016/j.ejphar.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 37.Miranda HF, Lemus I, Pinardi G. Effect of the inhibition of serotonin biosynthesis on the antinociception induced by nonsteroidal anti-inflammatory drugs. Brain Res Bull. 2003;61:417–425. doi: 10.1016/s0361-9230(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 38.Miranda HF, Sierralta F, Pinardi G. An isobolographic analysis of the adrenergic modulation of diclofenac antinociception. Anesth Analg. 2001;93:430–435. doi: 10.1097/00000539-200108000-00039. [DOI] [PubMed] [Google Scholar]
- 39.Sandrini G, Tassorelli C, Cecchini AP, Alfonsi E, Nappi G. Effects of nimesulide on nitric oxide-induced hyperalgesia in humans--a neurophysiological study. Eur J Pharmacol. 2002;450:259–262. doi: 10.1016/s0014-2999(02)02188-x. [DOI] [PubMed] [Google Scholar]
- 40.Fletcher D, Benoist JM, Gautron M, Guilbaud G. Isobolographic analysis of interactions between intravenous morphine, propacetamol, and diclofenac in carrageenin-injected rats. Anesthesiology. 1997;87:317–326. doi: 10.1097/00000542-199708000-00019. [DOI] [PubMed] [Google Scholar]
- 41.Lashbrook JM, Ossipov MH, Hunter JC, Raffa RB, Tallarida RJ, Porreca F. Synergistic antiallodynic effects of spinal morphine with ketorolac and selective COX1- and COX2-inhibitors in nerve-injured rats. Pain. 1999;82:65–72. doi: 10.1016/S0304-3959(99)00031-7. [DOI] [PubMed] [Google Scholar]
- 42.Miranda HF, Prieto JC, Puig MM, Pinardi G. Isobolographic analysis of multimodal analgesia in an animal model of visceral acute pain. Pharmacol Biochem Behav. 2008;88:481–486. doi: 10.1016/j.pbb.2007.10.005. [DOI] [PubMed] [Google Scholar]