Abstract
Di (2-ethylhexyl) phthalate (DEHP) is a plasticizer that has been shown to inhibit growth of mouse antral follicles, however, little is known about the mechanisms by which DEHP does so. Oxidative stress has been linked to follicle growth inhibition as well as phthalate-induced toxicity in non-ovarian tissues. Thus, we hypothesized that DEHP causes oxidative stress and that this leads to inhibition of the growth of antral follicles. To test this hypothesis, antral follicles isolated from CD-1 mice (age 32–35 days) were cultured with vehicle control (dimethylsulfoxide [DMSO]) or DEHP (1–100μg/ml) ± N-acetyl cysteine (NAC, an antioxidant at 0.25–1mM). During culture, follicles were measured daily. At the end of culture, follicles were collected and processed for in vitro reactive oxygen species (ROS) assays to measure the presence of free radicals or for measurement of the expression and activity of various key antioxidant enzymes: Cu/Zn superoxide dismutase (SOD1), glutathione peroxidase (GPX) and catalase (CAT). The results indicate that DEHP inhibits the growth of follicles compared to DMSO control and that NAC (0.25–1mM) blocks the ability of DEHP to inhibit follicle growth. Furthermore, DEHP (10μg/ml) significantly increases ROS levels and reduces the expression and activity of SOD1 compared to DMSO controls, whereas NAC (0.5mM) rescues the effects of DEHP on ROS levels and SOD1. However, the expression and activity of GPX and CAT were not affected by DEHP treatment. Collectively, these data suggest that DEHP inhibits follicle growth by inducing production of ROS and by decreasing the expression and activity of SOD1.
Keywords: di (2-ethylhexyl) phthalate, antral follicles, oxidative stress, ovary
Introduction
Phthalates or phthalate esters (PE) are synthetic plasticizers that impart flexibility to polyvinylchloride products. They are present in a wide variety of products, including building materials, food packaging, toys, cosmetics, clothing and medical devices. More than 18 billion pounds of phthalates are used worldwide each year (Crinnion, 2010). Humans are exposed to phthalates through inhalation, ingestion and dermal absorption on a daily basis (Heudorf et al., 2007; Halden, 2010). Di (2-ethylhexyl) phthalate (DEHP) is one of the most commonly used phthalate plasticizers. Since DEHP is not covalently bound to plastic matrix, it can leach out of products and contaminate the external environment. DEHP exposure is predominantly via food and appears to be close to the tolerable daily intake of 2mg/day in the general population (Lyche et al., 2009). However, individuals undergoing certain medical procedures may be exposed to even higher levels of DEHP via plastic medical devices (Kamrin, 2009).
DEHP is considered to be an endocrine disrupting chemical (EDC) based on its adverse effects on reproductive organs. Animal studies have shown that postnatal exposure to DEHP causes testicular atrophy and that in utero exposure to DEHP causes a number of abnormalities in male reproductive tract development in rodents (Clark and Cochrum, 2007; Martino-Andrade and Chahoud, 2009; Christiansen et al., 2010). In female rats, high dose acute exposure to DEHP reduces estradiol production and causes anovulation (Lovekamp-Swan and Davis, 2003; Lyche et al., 2009; Martino-Andrade and Chahoud, 2009). Numerous human epidemiologic studies have reported associations between chronic exposure to DEHP and various adverse reproductive outcomes, including altered male reproductive development and function (Main et al., 2006; Swan, 2008; Halden, 2010), endometriosis (Cobellis et al., 2003; Kim et al., 2010a), and increased risk of premature birth and various pregnancy complications (Latini et al., 2006; Martino-Andrade and Chahoud, 2009; Whyatt et al., 2009). However, the mechanisms by which DEHP affects the reproductive system are not fully elucidated.
One potential mechanism by which DEHP causes reproductive abnormalities may be via oxidative stress. Oxidative stress is caused by an imbalance between the production and elimination of reactive oxygen species (ROS) in the system, leading to damage to DNA, lipid peroxidation of membranes and oxidative modification of proteins (Agarwal et al., 2005). ROS are a two-edged sword: they serve as key signal molecules in various physiological processes, but also play roles in pathological processes in female reproductive organs (Agarwal et al., 2005). Studies have shown that ROS modulate multiple physiological processes from oocyte maturation to fertilization, embryo development and pregnancy (Al-Gubory et al., 2010; Dennery, 2010; Zhang et al., 2011). However, extra accumulation of ROS leads to ovarian apoptosis, the process by which follicles undergo atresia and corpora lutea undergo regression (Zhang et al., 2006; Agarwal et al., 2008; Lim and Luderer, 2010). Excess ROS also affect angiogenesis, which is critical for follicular growth and corpus luteum formation (Agarwal et al., 2005). Epidemiology studies also have shown that oxidative stress is associated with the age-related decline in fertility, endometriosis, pre-term labor, and unexplained infertility (Agarwal et al., 2005; Ruder et al., 2009; Al-Gubory et al., 2010).
Although studies have shown that DEHP is hydrolyzed into an active metabolite mono(2-ethylhexyl) phthalate (MEHP) by lipases and esterases in the intestine (Frederiksen et al., 2007), we elected to focus on revealing the effect of DEHP on mouse antral follicles for the following reasons. First, after DEHP exposure, detectable amounts of DEHP remain in the plasma and peritoneal fluid and thus, can affect the functions of reproductive organs (Cobellis et al., 2003). Second, high plasma levels of DEHP have been correlated with various female reproductive disorders. For example, several studies have reported an association between high plasma levels of DEHP and endometriosis, indicating a possible role of DEHP in the pathogenesis of endometriosis (Cobellis et al., 2003; Reddy et al., 2006a; Reddy et al., 2006b; Kim et al., 2010a). A study by Durmaz et al. also reported that high plasma levels of DEHP are correlated with pubertal gynecomastia (Durmaz et al., 2010). Third, DEHP has been used in different in vitro systems and has been shown to exert toxic effects on endometrial cells, granulocytes and oocytes (Mlynarcikova et al., 2009; Palleschi et al., 2009; Kim et al., 2010b).
While there is limited information on whether DEHP causes oxidative stress in the female reproductive system, oxidative stress was previously suggested to represent a common mechanism in endocrine disruptor-mediated dysfunction in reproduction (Latchoumycandane and Mathur, 2002; Gupta et al., 2006a; Gupta et al., 2006b). Recent animal studies have shown that DEHP causes oxidative stress in male reproductive tissues, specifically in Leydig cells, Sertoli cells and germ cells by disrupting antioxidant defenses and increasing ROS (Liu et al., 2005; Botelho et al., 2009; Erkekoglu et al., 2010). Previous studies have also shown that DEHP inhibits follicle growth of antral follicles in the ovary (Gupta et al., 2010). However, the mechanisms by which DEHP inhibits growth are not fully understood. Thus, this study tested the hypothesis that DEHP inhibits antral follicle growth through an oxidative stress pathway. To test this hypothesis, we determined whether DEHP reduces ROS in the ovary and tested whether N-acetyl-cysteine (NAC, an antioxidant) rescues follicles from the toxic effects of DEHP on antral follicles. Furthermore, we investigated the mechanism by which DEHP induces oxidative stress by examining the effect of DEHP on key antioxidant enzymes: Cu/Zn superoxide dismutase (SOD1), glutathione peroxidase (GPX) and catalase (CAT).
Materials and Methods
Chemicals
DEHP was purchased from Sigma (St. Louis, MO). Stock solutions of DEHP were prepared using dimethylsulfoxide (DMSO) (Sigma, St. Louis, MO) as the solvent in various concentrations (133, 13.3, and 1.33mg/ml) that allowed an equal volume to be added to culture wells for each treatment group to control for solvent concentration. Final concentrations in culture were 1, 10, and 100μg/ml of DEHP, which are equivalent to approximately 2.56, 25.6, and 256μM. We chose these doses based on previous studies on the effects of DEHP on cultured cells or follicles and based on their clinical relevance (Gillum et al., 2009, Lenie and Smitz, 2009). We also chose these doses based on our preliminary dose–response experiment. N-acetyll-cysteine (NAC) was purchased from Sigma (St. Louis, MO). We chose NAC as opposed to other antioxidants as it is a known antioxidant, free-radical scavenger, glutathione precursor, and increases the activity of SOD and GPX enzymes (Tilly and Tilly, 1995). NAC has also been shown to protect from oxidative stress in other tissues and cell types (Hecht et al., 2002; Gupta et al., 2006a). For culture, a 100mM stock solution of NAC was prepared using α-MEM and the final concentrations of NAC in each well of the culture were 0.25, 0.5, 1 and 2mM.
Animals
CD-1 mice that were 31–35 days old were used for all experiments. The mice were housed at the University of Illinois at Urbana-Champaign, Veterinary Medicine Animal Facility under a 12:12 dark: light cycle. Food and water were provided ad libitum. All animal procedures were approved by the University of Illinois Institutional Animal Care and Use Committee.
Follicle culture
Female CD-1 mice were euthanized and ovaries were removed. Based on relative size (250–350μm) and appearance, antral follicles were isolated mechanically from the ovaries and interstitial tissue was removed using fine watchmaker forceps (Gupta et al., 2006a). About 3–4 mice were used per experiment, providing approximately 25–35 follicles per mouse. The isolated follicles were randomly divided into different treatment groups (10–16 follicles per group). Doses of vehicle control (DMSO), DEHP (1, 10, 100μg/ml), DEHP (10μg/ml)+NAC (0.5, 1, 2mM) were individually prepared in supplemented α-MEM as described previously (Gupta et al., 2006a). The final solvent concentration was 0.075%. Antral follicles were cultured for 96 h at 37°C in 95% air and 5% CO2. Non-treated controls (supplemented media only) were used in each experiment as a control for culture conditions. At the end of culture, follicles were collected, snap frozen, and stored at −80°C for later use.
Analysis of follicle growth
Follicle size was assessed at 24 h intervals by measuring follicle diameter on perpendicular axes using an inverted microscope equipped with a calibrated ocular micrometer. Follicle diameter measurements were averaged among treatment groups and plotted to compare the effects of chemical treatments on growth over time. Data were presented as percent change over time. The statistical analyses were performed based on percentage change. At least three separate experiments were performed for each treatment group.
Gene expression analysis
Total RNA was extracted from follicles using the RNeasy Micro Kit (Qiagen, Inc., Valencia, CA) according to the manufacturer’s protocol. To remove any possible genomic DNA contamination, RNA was further treated with DNAse. Messenger RNA (mRNA; 200ng) was reverse transcribed to cDNA using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) following the manufacturer’s instructions. The cDNA was diluted 1:4 with nuclease free water. Quantitative real-time (qPCR) was conducted using a CFX96 Real-Time PCR Detection System (Bio-Rad Inc.) and accompanying software (CFX Manager Software) according to the manufacturer’s instructions. Specific qPCR primers for the genes of interest are listed in Table 1. An initial incubation of 95°C for 10 min was followed by 40–50 cycles of 94°C for 10 s (denaturation step), 55–60°C for 10 s (annealing step), and 72°C for 10 s (extension step), along with final extension at 72°C for 10 min. At the end of the each reaction, a melting curve was generated at 55–90°C to monitor the generation of a single product. β-actin was used as reference gene for each sample. Relative fold changes were calculated as the ratio to DMSO group level, which was set as 1.0. All samples were measured in triplicate from at least three separate experiments.
Table 1.
Sequences of primer sets used for gene expression analysis
| Gene name | Abbreviation | Forward | Reverse |
|---|---|---|---|
| Superoxide dismutase 1 | Sod1 | 5′-AAGGCCGTGTGCGTGCTGAA-3′ | 5′-CAGGTCTCCAACATGCCTCT-3′ |
| Glutathione peroxidase | Gpx | 5′-CCTCAAGTACGTCCGACCTG-3′ | 5′-CAATGTCGTTGCGGCACACC-3′ |
| Catalase | Cat | 5′-GCAGATACCTGTGAACTGTC-3′ | 5′-GTAGAATGTCCGCACCTGAG-3′ |
| Actin, beta | Actb | 5′-GGGCACAGTGTGGGTGAC-3′ | 5′-CTGGCACCACACCTTCTAC-3′ |
Enzyme activity assays
Follicles were collected after 24–96 h of follicle culture and subjected to various enzyme activity assays according to the manufacturer’s instructions to measure the enzyme activities of superoxide dismutase 1 (SOD1), glutathione peroxidase (GPX) and catalase (CAT). Enzyme activity kits and reagents were all purchased from Cayman Chemical Company (Ann Arbor, MI). For each sample, enzyme activity was normalized to its own protein concentration (measured by BCA Protein Assay Kit, Thermo Scientific, Rockford, IL). All samples were run in duplicate from at least three separate experiments.
In Vitro ROS/RNS Assay
Follicles were collected at selected time points (24–96h) and subjected to in vitro ROS/RNS assays for measurement of total free radicals, including ROS and reactive nitrogen species (RNS) using an OxiSelect In Vitro ROS/RNS Assay Kit (Cell Biolabs, Inc., San Diego, CA) according to the manufacturer’s instructions. Data were first normalized to protein level (measured by BCA Protein Assay Kit, Thermo Scientific, Rockford, IL), then the relative fold changes were determined after setting that of DMSO at 1.0. All samples were run in duplicate from at least three separate experiments.
Statistical analysis
All data were analyzed using SPSS statistical software (SPSS, Inc., Chicago, IL). Data were expressed as means ± SEM from at least three separate experiments. Multiple comparisons between treatment groups were made using ANOVA followed by Tukey’s post hoc comparison. Comparison between two groups was done using Student’s t-test. Statistical significance was assigned at p≤0.05.
Results
Effect of DEHP on follicle growth
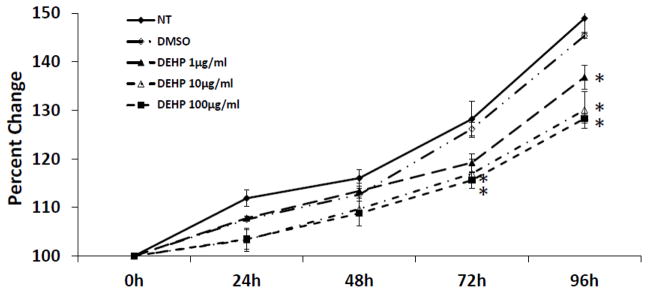
To determine whether DEHP affects antral follicle growth, antral follicles were treated with vehicle or DEHP and follicle diameter was measured every 24 h. Follicles treated with DMSO (vehicle control) showed normal growth compared to non-treated controls (Fig. 1). Exposure to DEHP (10 and 100μg/ml) significantly decreased antral follicle growth compared to DMSO controls beginning at 72 h, and this effect on follicle growth remained throughout the 96 h culture (Fig. 1). By 96 h, even the lowest dose of DEHP (1μg/ml) inhibited growth compared with DMSO controls.
Fig. 1. Effect of DEHP exposure on antral follicle growth.
Antral follicles were cultured in the presence of DMSO or DEHP (1–100μg/ml) for 96h. Growth of follicles was monitored during culture and reported as percent change over time. The graph represents means ± SEMs from at least three separate experiments. Lines with asterisks (*) are significantly different from DMSO controls at 72h and 96h time points (n=10–16 follicles per treatment per experiment; p≤ 0.05).
Effect of NAC supplement on DEHP-induced follicle growth inhibition
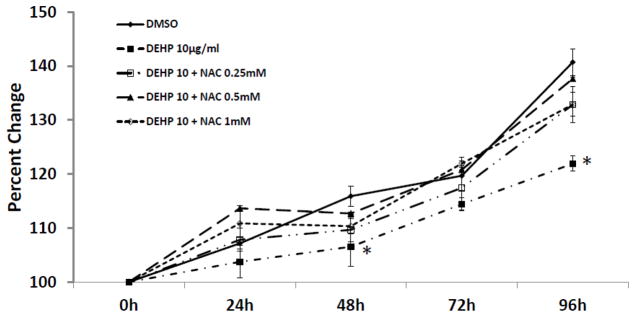
To determine whether N-acetyl cysteine (NAC), an antioxidant, protects antral follicles from DEHP-induced growth inhibition, we conducted preliminary experiments to select a nontoxic level of NAC for the studies. Using the in vitro follicle culture system, the effect of NAC on follicle growth was evaluated for 96 h. No significant follicle growth differences were observed in the NT, DMSO and NAC (0.25–2mM) groups. However, follicles treated with NAC (5–10mM) did not grow (data not shown). Thus, NAC at 0.25–1mM was used in all subsequent experiments.
Inhibition of follicle growth was observed with DEHP (10μg/ml) compared to DMSO controls (Fig. 2). In contrast, NAC (0.25–1mM) blocked the effect of DEHP-induced growth inhibition. Specifically, follicles co-treated with DEHP (10μg/ml) and NAC (0.25–1mM) had similar growth over time to DMSO controls (Fig. 2).
Fig. 2. Effect of DEHP and NAC co-treatment on antral follicle growth.
Antral follicles were cultured in the presence of DMSO or DEHP (10μg/ml) ± NAC (0.25–1mM) for 96h. Growth of follicles was monitored during culture and reported as percent change over time. The graph represents means ± SEMs from at least three separate experiments. The line with asterisks (*) is significantly different from DMSO controls at 48h and 96h time points (n=10–16 follicles per treatment per experiment; p≤ 0.05).
Effect of DEHP on oxidative stress levels in antral follicles in vitro
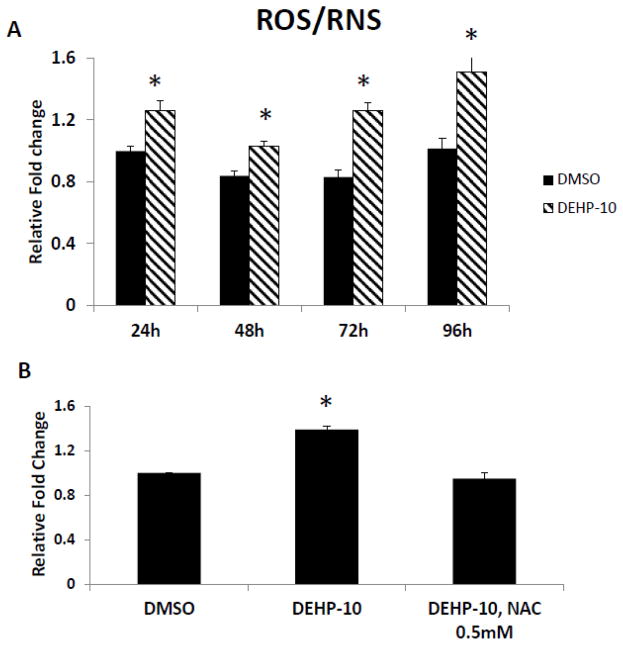
We observed that the DEHP-induced follicle growth inhibition starts as early as 48h (Fig. 2) and 72h (Fig. 1), which suggests that DEHP might induce oxidative stress even before 48h or 72h. To address this question, we compared the levels of ROS/RNS in cultured follicles treated with vehicle or DEHP (10μg/ml) for 24, 48, 72 and 96h. The results show that DEHP (10μg/ml) significantly increased the level of ROS/RNS in follicles compared to DMSO controls at each time point (Fig. 3A).
Fig. 3. Effect of DEHP and NAC on ROS/RNS levels in antral follicles.
A. Antral follicles were exposed in vitro to DMSO or DEHP (10 μg/ml) for 24, 48, 72 and 96h and subjected to ROS/RNS assays to measure ROS/RNS levels. The level of ROS/RNS was normalized to protein level in each sample and reported as relative fold change compared to 24h DMSO controls. Asterisk (*) indicates a significant p value from DMSO controls at the same time point via ANOVA, post hoc Tukey’s HSD (p≤ 0.05). B. After exposure of antral follicles to DMSO or DEHP (10 μg/ml) ± NAC (0.5mM) for 96h, follicles were collected and subjected to in vitro ROS/RNS assay to measure the ROS/RNS levels. The level of ROS/RNS was normalized by protein level in each sample and reported as relative fold change compared to DMSO controls. Asterisk (*) indicates significant difference (p≤0.05) from DMSO controls via ANOVA, followed by post hoc Tukey’s HSD. All data represent mean ± SEM from 3 independent experiments (35 follicles per treatment per experiment).
The results above show that DEHP inhibits antral follicle growth and co-treatment with NAC protects the follicles from the DEHP-induced growth inhibition, suggesting that DEHP induces oxidative stress and therefore inhibits the growth of antral follicles. To directly test this possibility, we examined the level of total free radicals (including ROS and RNS) in cultured follicles in presence of vehicle or DEHP ± NAC at 96h. Accumulation of free radicals is a direct indicator of oxidative stress. DEHP (10μg/ml) significantly increased the level of ROS/RNS in follicles compared to DMSO controls (Fig. 3B). NAC (0.5mM) co-treatment with DEHP (10μg/ml) reduced the levels of ROS/RNS back to control levels.
Effect of DEHP on gene expression of antioxidant enzymes
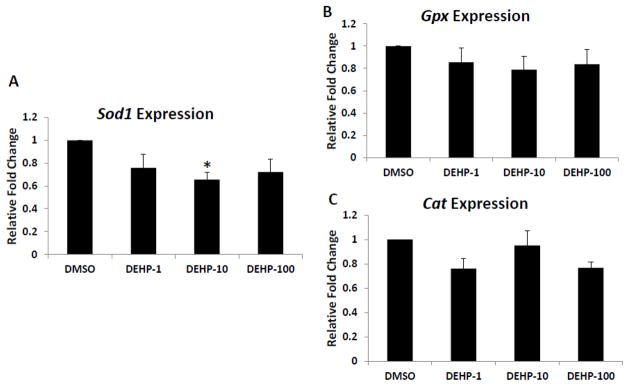
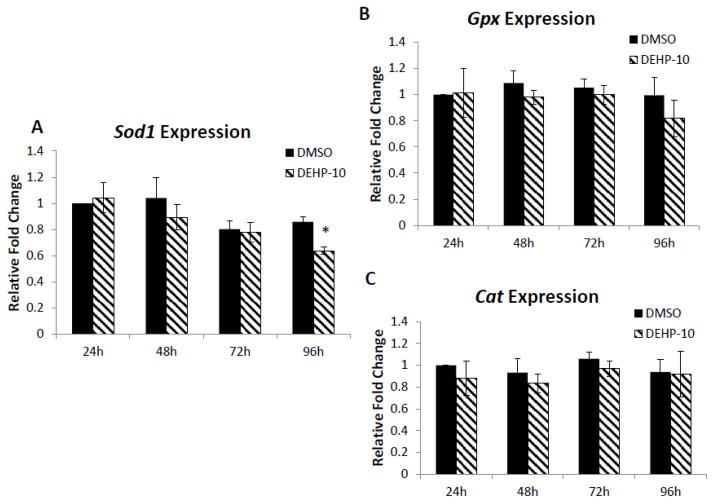
Because DEHP induced oxidative stress in cultured antral follicles, studies were conducted to determine if it did so by altering the expression of antioxidant enzymes required to detoxify the ROS/RNS in the system. Specifically, the expression levels of endogenous antioxidant enzymes Sod1, Gpx and Cat were compared in DMSO control DEHP-treated follicles. At 96h, only DEHP (10μg/ml) significantly decreased the expression of SOD1 compared to DMSO controls. Other DEHP doses (1 and 100μg/ml) did not significantly affect SOD1 expression compared to controls (Fig. 4A). DEHP (1–100μg/ml) did not affect Gpx and Cat expression compared to DMSO controls (Fig. 4B–C).
Fig. 4. Effect of DEHP exposure on Sod1, Gpx and Cat mRNA expression levels.
After exposure of antral follicles to DMSO or DEHP (1–100μg/ml) for 96h in vitro, the follicles were collected and subjected to real-time PCR analysis for Sod1, Gpx and Cat mRNA expression levels. All values were normalized to β-actin as a loading control and reported as relative fold change compared to DMSO controls. Graph represents means ± SEMs from at least three separate experiments. Asterisk (*) indicates a significant difference from the DMSO control via ANOVA followed by Tukey’s HSD post hoc test (n=10–16 follicles per treatment per experiment; p≤ 0.05).
To further elucidate whether DEHP affects gene expression at early time points during the culture, the expression profiles of Sod1, Gpx and Cat were compared in DMSO and DEHP (10μg/ml)-treated follicles at 24h, 48h, 72h and 96h. All the antioxidant enzymes (SOD1, GPX and CAT) were consistently expressed throughout the culture (Fig. 5A–C). Only DEHP (10μg/ml) reduced enzyme expression of SOD1 at 96h (Fig. 5A). No significant changes were observed in any of the selected enzymes at 0–72h compared to DMSO controls (Fig. 5A–C).
Fig. 5. Effect of DEHP exposure on expression of Sod1, Gpx and Cat in antral follicles over time.
Antral follicles were exposed in vitro to DMSO or DEHP (10μg/ml) for 24, 48, 72 and 96h and subjected to real-time PCR for analysis of Sod1, Gpx and Cat mRNA levels. All values were normalized to β-actin as a loading control and reported as relative fold change compared to DMSO controls at 24h. Graph represents means ± SEMs from at least three separate experiments. Asterisk (*) indicates a significant difference from DMSO controls at 24h via ANOVA followed by Tukey’s HSD post hoc test (n=10–16 follicles per treatment per experiment; p≤ 0.05).
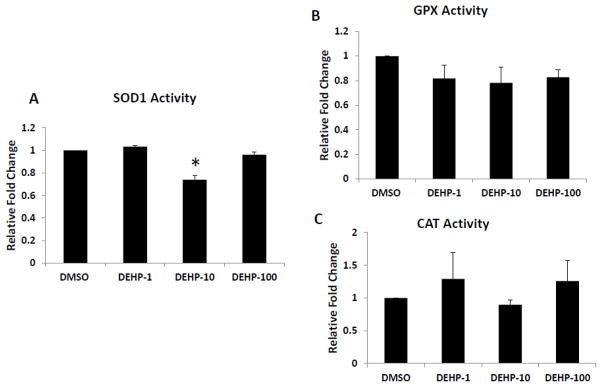
Effect of DEHP on activities of antioxidant enzymes
Since DEHP only inhibited expression of Sod1 and not Gpx or Cat expression, we then determined whether DEHP affects the activities of SOD1, GPX and CAT. Compared to DMSO controls, only DEHP (10μg/ml) significantly inhibited the activity of SOD1, the key antioxidant enzyme that catalyzes the dismutation of superoxide into oxygen and hydrogen peroxide (Fig. 6A). DEHP treatments (1–100μg/ml) did not significantly affect the activities of GPX and CAT, the enzymes that catalyze the decomposition of hydrogen peroxide (Fig. 6B–C).
Fig. 6. Effect of DEHP exposure on SOD1, GPX and CAT activities in antral follicles.
Antral follicles were exposed in vitro to DMSO or DEHP (1–100μg/ml) for 96h and then collected and subjected to specific assays to measure the enzyme activities of SOD1, GPX and CAT. All values were normalized to protein level as a loading control and reported as relative fold change compared to DMSO controls. Graph represents means ± SEMs from at least three separate experiments. Asterisk (*) indicates a significant difference from DMSO controls via ANOVA followed by Tukey’s HSD post hoc test (n=24 follicles per treatment per experiment; p≤ 0.05).
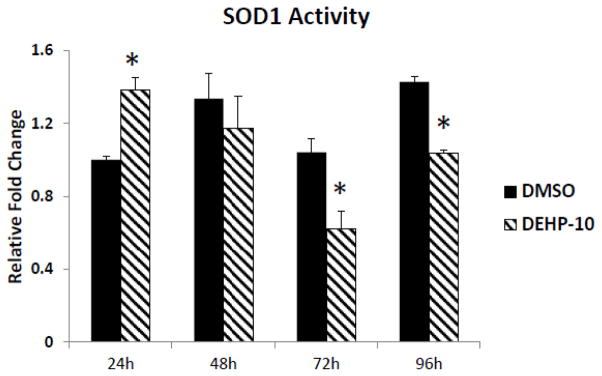
To further elucidate whether DEHP affects SOD1 activity at early time points during the culture, the activity profiles of SOD1 were compared in DMSO and DEHP (10μg/ml)-treated follicles at 24h, 48h, 72h and 96h. The results show that DEHP (10μg/ml) exposure significantly increases SOD1 activity at 24h, but it significantly decreases SOD activity at 72 and 96h (Fig. 7).
Fig. 7. Effect of DEHP exposure on SOD1 activity profile in antral follicles.
Antral follicles were exposed in vitro to DMSO or DEHP (10 μg/ml) for 24, 48, 72 and 96h and subjected to SOD1 activity assays for analysis of Sod1 activity over time. All values were normalized to protein level as a loading control and shown as relative fold change compared to 24h DMSO controls. Graph represents means ± SEMs from at least 3 separate experiments. Asterisk (*) indicates a significant p value from the DMSO controls at same time point via ANOVA, post hoc Tukey’s HSD (n=16 follicles per treatment per experiment; p≤ 0.05).
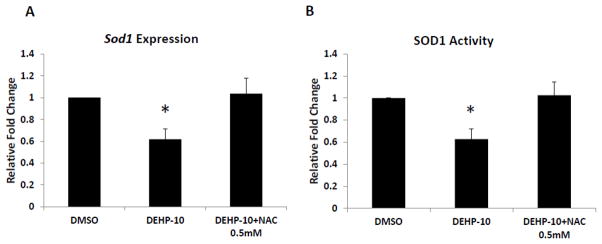
Effect of NAC co-treatment on SOD1 expression and activity
DEHP-induced oxidative stress and inhibition of follicle growth could be the result of decreased SOD1 expression and activity in the follicles since SOD1 is the key antioxidant enzyme required for elimination of accumulated ROS/RNS in the system. Because addition of NAC protects follicle growth from the DEHP-induced inhibition by reducing the oxidative stress in the system, studies were conducted to investigate whether NAC co-treatment protects the follicle from the DEHP-induced oxidative stress by rescuing the expression and activity of SOD1. Compared to DMSO controls, DEHP (10μg/ml) significantly reduced the expression and inhibited the activity of SOD1 (Fig. 8A–B). Addition of NAC (0.5mM) to the supplemented media restored the expression and activity of SOD1 to DMSO control level (Fig. 8A–B).
Fig. 8. Effect of DEHP and NAC co-treatment on SOD1 expression and activity in antral follicles.
Antral follicles were cultured in the presence of DMSO or DEHP (10μg/ml) ± NAC (0.25–1mM) for 96h. After culture, the follicles were collected and subjected to real-time PCR analysis for mRNA expression of Sod1 (A) and activity assays for SOD1 activity (B). The expression of Sod1 was normalized to β-actin as a loading control and the activity of SOD1 was normalized to protein level as a loading control. All values were reported as relative fold change compared to DMSO controls. Graph represents means ± SEMs from at least three separate experiments. Asterisk (*) indicates a significant p value from the DMSO controls via ANOVA followed by Tukey’s HSD post hoc test (n=16–24 follicles per treatment per experiment; p≤ 0.05).
Discussion
Numerous studies have shown that EDCs induce oxidative stress in biological systems. In a study by Jin et al. (2008), TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) induced oxidative stress by decreasing the activity of antioxidant enzymes (SOD, GPX, CAT and glutathione reductase) in mouse testes. Aly et al. (2011) reported a similar effect of TCDD in rat Sertoli cells. In a study by Gupta et al. (2006), the organochlorine pesticide methoxycholor (MXC) induced oxidative stress and caused follicular atresia by decreasing the expression of Sod1, Gpx and Cat in mouse antral follicles. Numerous studies also indicate that DEHP affects antioxidant enzymes in non-ovarian tissues. For example, DEHP has been shown to alter the expression and activity of antioxidant enzymes and to induce oxidative stress in male reproductive tissues (Liu et al., 2005; Botelho et al., 2009; Erkekoglu et al., 2010) and the liver (Rusyn et al., 2006). Thus, this study was designed to evaluate whether DEHP induces oxidative stress in antral follicles.
The present studies have shown that DEHP inhibits growth of mouse antral follicles, induces ROS levels, and decreases the expression and activity of SOD1 in vitro. In addition, we found that co-treatment with NAC, a known antioxidant, rescues antral follicles from DEHP-induced growth inhibition and restores the expression and activity of SOD1 in DEHP-treated follicles to control levels. To our knowledge, this is the first study to show that DEHP induces oxidative stress by disrupting the expression and activity of a key antioxidant enzyme, SOD1, in the mouse ovary.
Oxidative stress is caused by an imbalance between pro-oxidant (ROS) generation and neutralization by antioxidants, which results in damage to cellular components including proteins, lipids and DNA (Valko et al., 2007). ROS have both physiological and pathological roles during folliculogenesis, oocyte maturation, luteal regression and fertilization (Agarwal et al., 2008). A growing number of studies have shown that oxidative stress is directly associated with ovarian follicle aging (Tatone et al., 2008), endometriosis (Agarwal et al., 2005) unexplained female infertility and low success rates in assistant reproductive techniques (Matos et al., 2009). Our study shows that one of most commonly used endocrine disrupting chemicals, DEHP, causes oxidative stress in the ovary. Thus, it is possible that DEHP exposure could lead to infertility and other adverse reproductive outcomes.
Cells have defense systems to prevent injury caused by ROS, such as anti-oxidant enzymes like SOD, GPX and CAT. SOD is responsible for dismutation of superoxide to H2O2. Further, CAT and GPX in the GSH cycle can convert H2O2 to H2O and O2. In humans and rodents, copper/zinc superoxide dismutase (SOD1) and manganese superoxide dismutase (SOD2) are the two major intracellular enzymes in the ovary. SOD1 is present in both cytoplasmic and nuclear compartments, whereas SOD2 is localized to mitochondria. Transgenic animal studies have shown that, compared to SOD2, SOD1 plays important role in ovarian function (Tilly and Tilly, 1995; Matzuk et al., 1998). The expression and activity of SOD is directly related to oocyte quality and now is considered to be a biomarker in assisted reproductive techniques (Matos et al., 2009). Our study shows that DEHP decreases the expression and activity of SOD1, but it does not affect the expression and activity of GPX and CAT in vitro. These results indicate that exposure to DEHP causes accumulation of superoxide in the system, which further induces oxidative stress and inhibits follicle growth.
We observed that the effects of DEHP on SOD1 are not dose dependent. Although the growth curves for DEHP at 10 and 100μg/ml are almost superimposable, only DEHP 10μg/ml causes a significant decrease in SOD1 expression and activity. To address this question, we measured the ROS/RNS levels in follicles when treated with DMSO or DEHP (10 and 100μg/ml) for 24 and 48h. Only DEHP-10 significantly increased the ROS/RNS levels compared to DMSO controls (Fig. 3A). DEHP-100 increased ROS/RNS levels around 20%; however, this increase was not statistically different from controls (data not shown). This suggests that low and high doses of DEHP may work through different mechanisms. Our previous study showed that DEHP inhibits follicle growth and reduces estradiol levels of antral follicles in vitro (Gupta et al., 2010). In this previous study, DEHP (10 and 100μg/ml) significantly reduced estradiol levels compared to DMSO controls in a dose-dependent manner (DEHP 10 μg/ml reduces estradiol levels by 30%, whereas DEHP 100 μg/ml reduces estradiol levels by 80%). Furthermore, DEHP 100μg/ml inhibits the expression of key cell cycle regulatory genes (cyclin-D2, Ccnd2 and cyclin-depended-kinase 4, cdk4) and aromatase. Further, estradiol co-treatment blocked the effect of DEHP-100 induced growth inhibition and restores the expression of the key cell cycle genes and aromatase. These two sets of data suggest that different doses of DEHP might work through different mechanisms to affect the growth of antral follicles. Low doses of DEHP (10μg/ml) may affect the growth of antral follicles mainly through an oxidative stress pathway, while high doses of DEHP (100μg/ml) may inhibit follicle growth via reducing the estradiol level in antral follicles.
The pathway by which DEHP decreases the expression and activity of SOD1 in the ovary is not known. Researchers have identified an antioxidant response element (ARE) in some genes, which responds to oxidative stimuli, and have investigated the mechanism by which ROS affect signal transduction pathways that regulate gene transcription through this element (Knock and Ward, 2010). These studies indicate that ROS can regulate signaling pathways involving extracellular signal-regulated kinases (ERK) and p38 mitogen-activated protein kinases (MAPK) (Knock and Ward, 2010), tyrosine kinase (El-Deeb et al., 2010), phosphotidylinositol-3-kinase (PI3K)/Akt (Rojo et al., 2004), and protein kinase C (PKC) (Ogasawara et al., 2009). Thus, activating/inhibiting the ARE in the promoter region of antioxidant genes results in gene modulation. It is known that two classes of transcription factors, nuclear factor κB (NF-κB) and activator protein 1 (AP-1) are involved in the oxidative stress response in mammalian systems (Scandalios, 2005). Previous studies indicate that both AREs and motifs for NF-κB and AP-1 are present in the promoter regions of most of the antioxidant enzymes, including SOD1, SOD2, CAT and GPX (Banning et al., 2005; Scandalios, 2005). Interestingly, numerous studies have shown that DEHP induces oxidative stress and causes apoptosis in hepatocytes by activating ERK/MAPK and p38/MAPK, therefore activating several transcription factors, including NF-κB, AP-1 c-jun and c-fos (Ghosh et al., 2010; Lee and Lim, 2011a). In mouse allergic effector cells, DEHP increases AP-1 transcriptional activation via p38 MAPK phosphorylation (Oh and Lim, 2010). Further, mono- (2-ethylhexyl) phthalate (MEHP), the active metabolite of DEHP, also activates NF-κB signaling in the rat testis and induces germ cell apoptosis (Rasoulpour and Boekelheide, 2005; Rogers et al., 2008). Thus, it is quite possible that DEHP alters the expression of Sod1 through any of these pathways.
NAC is a thiol antioxidant and has beneficial clinical implications in HIV infection and cancer, as well as in heart, kidney and liver disease (Kelly, 1998). It acts as a free radical scavenger and glutathione precursor in reproductive and non-reproductive tissues and protects them from oxidative stress induced apoptosis (Erkkila et al., 1998; Otala et al., 2002; Priya et al., 2011). Numerous studies have shown that NAC inhibits apoptosis in cultured porcine ovarian primordial germ cells (Lee et al., 2010), rat ovarian follicles (Tilly and Tilly, 1995), bovine corpora luteal cells (Lohrke et al.) and in cultured human ovarian cortex (Otala et al., 2002). NAC was able to restore the fertility of mice lacking γ glutamyl transpeptidase, an enzyme important for glutathione synthesis (Kumar et al., 2000). Our previous studies also show that NAC protects the mouse antral follicles from MXC-induced growth inhibition and atresia (Gupta et al., 2010). Thus, NAC seems to be a survival factor in reproductive organs via its effects of redox control. Our current study supports and expands these findings.
The mechanism by which NAC acts to prevent DEHP–induced toxicity is unknown. Recent studies show that DEHP induces oxidative stress via the MAPK- NF-κB pathway ( Lee and Lim, 2011a; Lee and Lim, 2011b) and that NAC decreases the phosphorylation of various kinases (p38 MAPK and ERK) and inhibits the activation of NF-κ (Lee and Lim, 2011b; Sigala et al., 2011). Further, Rojo et al. (2004) reported that motifs for NF-κB are located in the promoter regions of SOD. We have shown that DEHP treatment decreases the expression and activity of SOD1 and that this effect of DEHP is blocked by NAC. Thus, it is possible that DEHP-induced ROS alters the NF-κB pathway to further regulate the antioxidant expression causing oxidative stress and that NAC prevents these processes. In addition, a recent study shows that NAC pre-treatment upregulates SOD2 and CAT in cadmium-induced oxidative stress (Doi et al., 2010). Thus, it is possible that NAC co-treatment induces SOD2; therefore, decreasing the ROS level and restoring the expression and activity of SOD1.
In conclusion, these studies show that DEHP causes oxidative stress in antral follicles, which leads to growth inhibition of these follicles. Future studies should examine if DEHP-induced oxidative stress leads to infertility in animal models and human and if so, whether NAC can protect from DEHP-induced infertility.
Highlights.
DEHP inhibits growth and increases reactive oxygen species in ovarian antral follicles in vitro.
NAC rescues the effects of DEHP on the growth and reactive oxygen species levels in follicles.
DEHP decreases the expression and activity of Cu/Zn superoxide dismutase, which can be rescued by NAC, in antral follicles.
Acknowledgments
The authors thank Liying Gao for her outstanding technical help. This work was supported by National Institutes of Health (NIH) R01ES019178 (JAF), a Billie Field Fellowship in Reproductive Biology (WW and ZRC) and an Environmental Toxicology Scholarship (MSB).
Footnotes
Conflict of interest statement
The authors have no conflicts to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Wei Wang, Email: weiwang2@illinois.edu.
Zelieann R. Craig, Email: zelieann@illinois.edu.
Mallikarjuna S. Basavarajappa, Email: mbasava2@illinois.edu.
Rupesh Gupta, Email: drrupesh@yahoo.com.
Jodi A. Flaws, Email: jflaws@illinois.edu.
References
- Agarwal A, Gupta S, Sekhon L, Shah R. Redox considerations in female reproductive function and assisted reproduction: from molecular mechanisms to health implications. Antioxid Redox Signal. 2008;10:1375–1403. doi: 10.1089/ars.2007.1964. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Gupta S, Sharma RK. Role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2005;3:28. doi: 10.1186/1477-7827-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010;42:1634–1650. doi: 10.1016/j.biocel.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Banning A, Deubel S, Kluth D, Zhou Z, Brigelius-Flohe R. The GI-GPx gene is a target for Nrf2. Mol Cell Biol. 2005;25:4914–4923. doi: 10.1128/MCB.25.12.4914-4923.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botelho GG, Bufalo AC, Boareto AC, Muller JC, Morais RN, Martino-Andrade AJ, Lemos KR, Dalsenter PR. Vitamin C and resveratrol supplementation to rat dams treated with di(2-ethylhexyl)phthalate: impact on reproductive and oxidative stress end points in male offspring. Arch Environ Contam Toxicol. 2009;57:785–793. doi: 10.1007/s00244-009-9385-9. [DOI] [PubMed] [Google Scholar]
- Christiansen S, Boberg J, Axelstad M, Dalgaard M, Vinggaard AM, Metzdorff SB, Hass U. Low-dose perinatal exposure to di(2-ethylhexyl) phthalate induces anti-androgenic effects in male rats. Reprod Toxicol. 2010;30:313–321. doi: 10.1016/j.reprotox.2010.04.005. [DOI] [PubMed] [Google Scholar]
- Clark BJ, Cochrum RK. The steroidogenic acute regulatory protein as a target of endocrine disruption in male reproduction. Drug Metab Rev. 2007;39:353–370. doi: 10.1080/03602530701519151. [DOI] [PubMed] [Google Scholar]
- Cobellis L, Latini G, De Felice C, Razzi S, Paris I, Ruggieri F, Mazzeo P, Petraglia F. High plasma concentrations of di-(2-ethylhexyl)-phthalate in women with endometriosis. Hum Reprod. 2003;18:1512–1515. doi: 10.1093/humrep/deg254. [DOI] [PubMed] [Google Scholar]
- Crinnion WJ. Toxic effects of the easily avoidable phthalates and parabens. Altern Med Rev. 2010;15:190–196. [PubMed] [Google Scholar]
- Dennery PA. Oxidative stress in development: nature or nurture? Free Radic Biol Med. 2010;49:1147–1151. doi: 10.1016/j.freeradbiomed.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Doi T, Puri P, Bannigan J, Thompson J. Pre-treatment with N-acetylcysteine upregulates superoxide dismutase 2 and catalase genes in cadmium-induced oxidative stress in the chick omphalocele model. Pediatr Surg Int. 2010;27:131–136. doi: 10.1007/s00383-010-2794-z. [DOI] [PubMed] [Google Scholar]
- El-Deeb IM, Yoo KH, Lee SH. ROS receptor tyrosine kinase: a new potential target for anticancer drugs. Med Res Rev. 2010 doi: 10.1002/med.20206. [DOI] [PubMed] [Google Scholar]
- Erkekoglu P, Rachidi W, Yuzugullu OG, Giray B, Favier A, Ozturk M, Hincal F. Evaluation of cytotoxicity and oxidative DNA damaging effects of di(2-ethylhexyl)-phthalate (DEHP) and mono(2-ethylhexyl)-phthalate (MEHP) on MA-10 Leydig cells and protection by selenium. Toxicol Appl Pharmacol. 2010;248:52–62. doi: 10.1016/j.taap.2010.07.016. [DOI] [PubMed] [Google Scholar]
- Erkkila K, Hirvonen V, Wuokko E, Parvinen M, Dunkel L. N-acetyl-L-cysteine inhibits apoptosis in human male germ cells in vitro. J Clin Endocrinol Metab. 1998;83:2523–2531. doi: 10.1210/jcem.83.7.4949. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Skakkebaek NE, Andersson AM. Metabolism of phthalates in humans. Mol Nutr Food Res. 2007;51:899–911. doi: 10.1002/mnfr.200600243. [DOI] [PubMed] [Google Scholar]
- Ghosh J, Das J, Manna P, Sil PC. Hepatotoxicity of di-(2-ethylhexyl)phthalate is attributed to calcium aggravation, ROS-mediated mitochondrial depolarization, and ERK/NF-kappaB pathway activation. Free Radic Biol Med. 2010;49:1779–1791. doi: 10.1016/j.freeradbiomed.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Miller KP, Babus JK, Flaws JA. Methoxychlor inhibits growth and induces atresia of antral follicles through an oxidative stress pathway. Toxicol Sci. 2006a;93:382–389. doi: 10.1093/toxsci/kfl052. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Schuh RA, Fiskum G, Flaws JA. Methoxychlor causes mitochondrial dysfunction and oxidative damage in the mouse ovary. Toxicol Appl Pharmacol. 2006b;216:436–445. doi: 10.1016/j.taap.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Singh JM, Leslie TC, Meachum S, Flaws JA, Yao HH. Di-(2-ethylhexyl) phthalate and mono-(2-ethylhexyl) phthalate inhibit growth and reduce estradiol levels of antral follicles in vitro. Toxicol Appl Pharmacol. 2010;242:224–230. doi: 10.1016/j.taap.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halden RU. Plastics and health risks. Annu Rev Public Health. 2010;31:179–194. doi: 10.1146/annurev.publhealth.012809.103714. [DOI] [PubMed] [Google Scholar]
- Hecht SS, Upadhyaya P, Wang M, Bliss RL, McIntee EJ, Kenney PM. Inhibition of lung tumorigenesis in A/J mice by N-acetyl-S-(N-2-phenethylthiocarbamoyl)-L-cysteine and myo-inositol, individually and in combination. Carcinogenesis. 2002;23:1455–1461. doi: 10.1093/carcin/23.9.1455. [DOI] [PubMed] [Google Scholar]
- Heudorf U, Mersch-Sundermann V, Angerer J. Phthalates: toxicology and exposure. Int J Hyg Environ Health. 2007;210:623–634. doi: 10.1016/j.ijheh.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Kamrin MA. Phthalate risks, phthalate regulation, and public health: a review. J Toxicol Environ Health B Crit Rev. 2009;12:157–174. doi: 10.1080/10937400902729226. [DOI] [PubMed] [Google Scholar]
- Kelly GS. Clinical applications of N-acetylcysteine. Altern Med Rev. 1998;3:114–127. [PubMed] [Google Scholar]
- Kim SH, Chun S, Jang JY, Chae HD, Kim CH, Kang BM. Increased plasma levels of phthalate esters in women with advanced-stage endometriosis: a prospective case-control study. Fertil Steril. 2010a;95:357–359. doi: 10.1016/j.fertnstert.2010.07.1059. [DOI] [PubMed] [Google Scholar]
- Kim YH, Kim SH, Lee HW, Chae HD, Kim CH, Kang BM. Increased viability of endometrial cells by in vitro treatment with di-(2-ethylhexyl) phthalate. Fertil Steril. 2010b;94:2413–2416. doi: 10.1016/j.fertnstert.2010.04.027. [DOI] [PubMed] [Google Scholar]
- Knock GA, Ward JP. Redox Regulation of Protein Kinases as a Modulator of Vascular Function. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3614. [DOI] [PubMed] [Google Scholar]
- Kumar TR, Wiseman AL, Kala G, Kala SV, Matzuk MM, Lieberman MW. Reproductive defects in gamma-glutamyl transpeptidase-deficient mice. Endocrinology. 2000;141:4270–4277. doi: 10.1210/endo.141.11.7760. [DOI] [PubMed] [Google Scholar]
- Latchoumycandane C, Mathur PP. Induction of oxidative stress in the rat testis after short-term exposure to the organochlorine pesticide methoxychlor. Arch Toxicol. 2002;76:692–698. doi: 10.1007/s00204-002-0388-9. [DOI] [PubMed] [Google Scholar]
- Latini G, Del Vecchio A, Massaro M, Verrotti A, CDEF In utero exposure to phthalates and fetal development. Curr Med Chem. 2006;13:2527–2534. doi: 10.2174/092986706778201666. [DOI] [PubMed] [Google Scholar]
- Lee J, Lim KT. Plant-originated glycoprotein (24 kDa) has an inhibitory effect on proliferation of BNL CL.2 cells in response to di(2-ethylhexyl)phthalate. Cell Biochem Funct. 2011a doi: 10.1002/cbf.1777. [DOI] [PubMed] [Google Scholar]
- Lee J, Lim KT. Plant-originated glycoprotein (24 kDa) has an inhibitory effect on proliferation of BNL CL.2 cells in response to di(2-ethylhexyl)phthalate. Cell Biochem Funct. 2011b;29:496–505. doi: 10.1002/cbf.1777. [DOI] [PubMed] [Google Scholar]
- Lee YJ, Lee DM, Lee CH, Heo SH, Won SY, Im JH, Cho MK, Nam HS, Lee SH. Suppression of human prostate cancer PC-3 cell growth by N-acetylcysteine involves over-expression of Cyr61. Toxicol In Vitro. 2010;25:199–205. doi: 10.1016/j.tiv.2010.10.020. [DOI] [PubMed] [Google Scholar]
- Lim J, Luderer U. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol Reprod. 2010;84:775–782. doi: 10.1095/biolreprod.110.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Lehmann KP, Sar M, Young SS, Gaido KW. Gene expression profiling following in utero exposure to phthalate esters reveals new gene targets in the etiology of testicular dysgenesis. Biol Reprod. 2005;73:180–192. doi: 10.1095/biolreprod.104.039404. [DOI] [PubMed] [Google Scholar]
- Lohrke B, Xu J, Weitzel JM, Kruger B, Goldammer T, Viergutz T. N-acetylcysteine impairs survival of luteal cells through mitochondrial dysfunction. Cytometry A. 77:310–320. doi: 10.1002/cyto.a.20873. [DOI] [PubMed] [Google Scholar]
- Lovekamp-Swan T, Davis BJ. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ Health Perspect. 2003;111:139–145. doi: 10.1289/ehp.5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyche JL, Gutleb AC, Bergman A, Eriksen GS, Murk AJ, Ropstad E, Saunders M, Skaare JU. Reproductive and developmental toxicity of phthalates. J Toxicol Environ Health B Crit Rev. 2009;12:225–249. doi: 10.1080/10937400903094091. [DOI] [PubMed] [Google Scholar]
- Main KM, Mortensen GK, Kaleva MM, Boisen KA, Damgaard IN, Chellakooty M, Schmidt IM, Suomi AM, Virtanen HE, Petersen DV, Andersson AM, Toppari J, Skakkebaek NE. Human breast milk contamination with phthalates and alterations of endogenous reproductive hormones in infants three months of age. Environ Health Perspect. 2006;114:270–276. doi: 10.1289/ehp.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino-Andrade AJ, Chahoud I. Reproductive toxicity of phthalate esters. Mol Nutr Food Res. 2009;54:148–157. doi: 10.1002/mnfr.200800312. [DOI] [PubMed] [Google Scholar]
- Matos L, Stevenson D, Gomes F, Silva-Carvalho JL, Almeida H. Superoxide dismutase expression in human cumulus oophorus cells. Mol Hum Reprod. 2009;15:411–419. doi: 10.1093/molehr/gap034. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology. 1998;139:4008–4011. doi: 10.1210/endo.139.9.6289. [DOI] [PubMed] [Google Scholar]
- Mlynarcikova A, Nagyova E, Fickova M, Scsukova S. Effects of selected endocrine disruptors on meiotic maturation, cumulus expansion, synthesis of hyaluronan and progesterone by porcine oocyte-cumulus complexes. Toxicol In Vitro. 2009;23:371–377. doi: 10.1016/j.tiv.2008.12.017. [DOI] [PubMed] [Google Scholar]
- Ogasawara N, Oguro T, Sakabe T, Matsushima M, Takikawa O, Isobe K, Nagase F. Hemoglobin induces the expression of indoleamine 2,3-dioxygenase in dendritic cells through the activation of PI3K, PKC, and NF-kappaB and the generation of reactive oxygen species. J Cell Biochem. 2009;108:716–725. doi: 10.1002/jcb.22308. [DOI] [PubMed] [Google Scholar]
- Oh PS, Lim KT. IgE, COX-2, and IL-4 are Expressed by DEHP through p38 MAPK and Suppressed by Plant Glycoprotein (75 kDa) in ICR Mice. Inflammation. 2010 doi: 10.1007/s10753-010-9238-8. [DOI] [PubMed] [Google Scholar]
- Otala M, Erkkila K, Tuuri T, Sjoberg J, Suomalainen L, Suikkari AM, Pentikainen V, Dunkel L. Cell death and its suppression in human ovarian tissue culture. Mol Hum Reprod. 2002;8:228–236. doi: 10.1093/molehr/8.3.228. [DOI] [PubMed] [Google Scholar]
- Palleschi S, Rossi B, Diana L, Silvestroni L. Di(2-ethylhexyl)phthalate stimulates Ca(2+) entry, chemotaxis and ROS production in human granulocytes. Toxicol Lett. 2009;187:52–57. doi: 10.1016/j.toxlet.2009.01.031. [DOI] [PubMed] [Google Scholar]
- Priya S, Vijayalakshmi P, Vivekanandan P, Karthikeyan S. Influence of N-acetylcysteine against dimethylnitrosamine induced hepatotoxicity in rats. Toxicol Ind Health. 2011 doi: 10.1177/0748233711399323. [DOI] [PubMed] [Google Scholar]
- Rasoulpour RJ, Boekelheide K. NF-kappaB is activated in the rat testis following exposure to mono-(2-ethylhexyl) phthalate. Biol Reprod. 2005;72:479–486. doi: 10.1095/biolreprod.104.034363. [DOI] [PubMed] [Google Scholar]
- Reddy BS, Rozati R, Reddy BV, Raman NV. Association of phthalate esters with endometriosis in Indian women. BJOG. 2006a;113:515–520. doi: 10.1111/j.1471-0528.2006.00925.x. [DOI] [PubMed] [Google Scholar]
- Reddy BS, Rozati R, Reddy S, Kodampur S, Reddy P, Reddy R. High plasma concentrations of polychlorinated biphenyls and phthalate esters in women with endometriosis: a prospective case control study. Fertil Steril. 2006b;85:775–779. doi: 10.1016/j.fertnstert.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Rogers R, Ouellet G, Brown C, Moyer B, Rasoulpour T, Hixon M. Cross-talk between the Akt and NF-kappaB signaling pathways inhibits MEHP-induced germ cell apoptosis. Toxicol Sci. 2008;106:497–508. doi: 10.1093/toxsci/kfn186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo AI, Salinas M, Martin D, Perona R, Cuadrado A. Regulation of Cu/Zn-superoxide dismutase expression via the phosphatidylinositol 3 kinase/Akt pathway and nuclear factor-kappaB. J Neurosci. 2004;24:7324–7334. doi: 10.1523/JNEUROSCI.2111-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruder EH, Hartman TJ, Goldman MB. Impact of oxidative stress on female fertility. Curr Opin Obstet Gynecol. 2009;21:219–222. doi: 10.1097/gco.0b013e32832924ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusyn I, Peters JM, Cunningham ML. Modes of action and species-specific effects of di-(2-ethylhexyl)phthalate in the liver. Crit Rev Toxicol. 2006;36:459–479. doi: 10.1080/10408440600779065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res. 2005;38:995–1014. doi: 10.1590/s0100-879x2005000700003. [DOI] [PubMed] [Google Scholar]
- Sigala I, Zacharatos P, Toumpanakis D, Michailidou T, Noussia O, Theocharis S, Roussos C, Papapetropoulos A, Vassilakopoulos T. MAPKs and NF-kappaB differentially regulate cytokine expression in the diaphragm in response to resistive breathing: the role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1152–1162. doi: 10.1152/ajpregu.00376.2010. [DOI] [PubMed] [Google Scholar]
- Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108:177–184. doi: 10.1016/j.envres.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, Artini PG, Piomboni P, Focarelli R. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update. 2008;14:131–142. doi: 10.1093/humupd/dmm048. [DOI] [PubMed] [Google Scholar]
- Tilly JL, Tilly KI. Inhibitors of oxidative stress mimic the ability of follicle-stimulating hormone to suppress apoptosis in cultured rat ovarian follicles. Endocrinology. 1995;136:242–252. doi: 10.1210/endo.136.1.7828537. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Adibi JJ, Calafat AM, Camann DE, Rauh V, Bhat HK, Perera FP, Andrews H, Just AC, Hoepner L, Tang D, Hauser R. Prenatal di(2-ethylhexyl)phthalate exposure and length of gestation among an inner-city cohort. Pediatrics. 2009;124:e1213–1220. doi: 10.1542/peds.2009-0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Liu Y, An Z, Huang D, Qi Y, Zhang Y. Mediating effect of ROS on mtDNA damage and low ATP content induced by arsenic trioxide in mouse oocytes. Toxicol In Vitro. 2011;25:979–984. doi: 10.1016/j.tiv.2011.03.009. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li XH, Ma X, Wang ZH, Lu S, Guo YL. Redox-induced apoptosis of human oocytes in resting follicles in vitro. J Soc Gynecol Investig. 2006;13:451–458. doi: 10.1016/j.jsgi.2006.05.005. [DOI] [PubMed] [Google Scholar]