Abstract
Aedes aegypti is an important vector of the viruses that cause dengue fever, dengue hemorrhagic fever, and yellow fever. Reverse genetic approaches to the study of gene function in this mosquito have been limited by the lack of a robust inducible promoter to allow precise temporal control over a protein-encoding or hairpin RNA transgene. Likewise, investigations into the molecular and biochemical basis of vector competence would benefit from the ability to activate an anti-pathogen molecule at specific times during infection. We have characterized the ability of genomic sequences derived from two Ae. aegypti hsp70 genes to drive heat-inducible expression of a reporter in both transient and germline transformation contexts. AaHsp70-luciferase transcripts accumulated specifically after heat shock, and displayed a pattern of rapid induction and decay similar to endogenous AaHsp70 genes. Luciferase expression in transgenic Ae. aegypti increased by ∼25-50 fold in whole adults by four hours after heat-shock, with significant activity (∼20 fold) remaining at 24 hr. Heat-induced expression was even more dramatic in midgut tissues, with one strain showing a ∼2500-fold increase in luciferase activity. The AaHsp70 promoters described could be valuable for gene function studies as well as for the precise timing of the expression of anti-pathogen molecules.
Keywords: HSP70, promoter, Aedes aegypti, transgenic, mosquito, heat shock, stress
Introduction
Aedes aegypti is a significant vector of disease agents, and is primarily responsible for transmission of the viruses that cause yellow fever, dengue fever, and dengue hemorrhagic fever (Tatem et al. 2006). Current efforts to control Ae. aegypti populations such as insecticides and habitat removal have not yet been sufficient to interrupt transmission of these disease agents. Genetic control strategies are being developed as an additional alternative to aid in reducing disease transmission, with a number of recent reports describing the development of novel female-killing or pathogen-resistant transgenic mosquito strains (Fu et al. 2010; Isaacs et al. 2011; Kokoza et al. 2010; Meredith et al. 2011; Wise de Valdez et al. 2011).
The generation of pathogen-resistant, genetically sterile or female-killing transgenic mosquitoes depends upon the ability to drive the expression of one or more foreign genes in an appropriate temporal and spatial manner. Likewise, basic research studies addressing questions of gene function using ectopic expression or gene knockdown must often be precisely timed in order to avoid developmentally disruptive phenotypes. A wide range of promoter elements have been characterized and utilized to drive transgene expression or gene knockdown in Aedes aegypti, including the salivary gland specific apyrase (Coates et al. 1999) and 30K (Mathur et al. 2010) promoters, the midgut-specific carboxypeptidase (CP) promoter (Moreira et al. 2000), the fat-body specific vitellogenin (Vg) promoter (Kokoza et al. 2001), ovary-specific vitellogenin receptor and nanos promoters (Adelman et al. 2007; Cho et al. 2006), the testes-specific β2 tubulin promoter (Smith et al. 2007) and the full body polyubiquitin (PUb) promoter (Anderson et al. 2010). While these promoter elements provide a range of spatial controls, the only temporal controls (Vg, CP) are linked to bloodfeeding. Uncoupling transgene expression from the acquisition of a bloodmeal would substantially broaden the types of experiments which could be performed in this species. For example, expressing a foreign gene or establishing a gene knockdown phenotype early after adult emergence would allow investigations into host seeking behavior. Similarly, restricting expression until long after a bloodmeal is taken would allow investigations into host-pathogen interactions, where gene products could be expressed at a specific point in a pathogens' life cycle.
In Drosophila, such precise temporal control is achieved using the hsp70 promoter, where both ectopic expression and gene knockdown experiments are common (Aigaki et al. 1991; Lam and Thummel 2000; Read et al. 1992). However, significant basal expression in Aedes aegypti has limited the utility of this promoter in Ae. aegypti (Morris et al. 1991). Ultimately, no rigorous analysis of any heat shock promoter has been reported for this mosquito.
Recently we characterized the gene structure and expression patterns of six members of the Hsp70 family in Aedes aegypti (Gross et al. 2009). In an effort to identify an Ae. aegypti promoter capable of providing strict temporal control over a transgene, we placed the firefly luciferase ORF under the control of sequences derived from the genomic region upstream of the AaHsp70Aa or AaHsp70Bb genes. The ability of these AaHsp70 sequences to drive luciferase expression was examined in transient cell and embryo assays, as well as in the context of Ae. aegypti chromosomal insertions via the transposon Mos1. Both promoter constructs were found to drive heat-inducible expression throughout adult female mosquitoes.
Results
Bioinformatic analysis of potential heat shock factor binding sites
Hsp70 genes require specific enhancers in order to respond to heat stress, termed heat shock elements (HSE) (reviewed in (Sakurai and Enoki 2010). The consensus HSE consists of the basic pattern nGAAn (where n is any nucleotide) arranged in a cluster of three or more head-tail repeats. We analyzed the genomic intervals between three AaHsp70 inverted gene pairs (Gross et al. 2009) for the presence and abundance of HSEs. Only a single sequence corresponding to a canonical nTTCn-nGAAn-nTTCn trimeric HSE was identified, and was located about midway between genes AaHsp70Aa and AaHsp70Ab. The heat shock transcription factor (HSF) binds to HSE as a trimer, and is known to allow one of its three subunits to wobble (reviewed in (Sakurai and Enoki 2010). Therefore, we also searched for potential trimeric HSE sites where one of the three nGAAn units was allowed to be randomized (Table 1). While tail-tail and head-head arrangements of the nGAAn units have been shown to bind HSF equally well, we observed a preference for the head-head arrangement in Ae. aegypti Hsp70 intergenic sequences (Table 1). However, the most common HSE-like element was the step/gap type, which was significantly enriched in all three intergenic regions (Table 1). This suggests that in Ae. aegypti, the middle subunit may contain the greatest tolerance for deviation from consensus. Alignment of all 28 step/gap type HSE sequences revealed a preference for the nucleotides GA separating the first and second units, with a strong preference for an A/T in the first position of the third unit (Fig. 1). This consensus may help to identify other Ae. aegypti genes that are transcriptionally activated under heat stress.
Table 1. AaHsp70 intergenic regions are enriched for HSEs.
| Intergenic region | Length (bp) | # HSE | Expected (sd)a | z score (P value) |
|---|---|---|---|---|
| 2P tail-tail (nTTCn-nGAAn-nnnnn) | ||||
| AaHsp70Aa-Ab | 2628 | 2 | 0.8 (+/- 0.8) | 1.6 (0.057) |
| AaHsp70Ba-Bb | 3071 | 2 | 1.3 (+/-1.0) | 0.7 (0.242) |
| AaHsp70Ca-Cb | 1077 | 2 | 0.4 (+/-0.7) | 2.3 (0.010) |
| 2P head-head (nGAAn-nTTCn-nnnnn) | ||||
| AaHsp70Aa-Ab | 2628 | 7 | 1.1 (+/- 1.1) | 5.5 (<0.0001) |
| AaHsp70Ba-Bb | 3071 | 6 | 1.0 (+/-0.9) | 5.3 (<0.0001) |
| AaHsp70Ca-Cb | 1077 | 3 | 0.4 (+/-0.6) | 4.7 (<0.0001) |
| Step/Gap Type (nTTCn-nnnnn-nTTCn) | ||||
| AaHsp70Aa-Ab | 2628 | 15 | 1.8 (+/- 1.3) | 9.9 (<0.0001) |
| AaHsp70Ba-Bb | 3071 | 8 | 2.0 (+/- 1.2) | 4.8 (<0.0001) |
| AaHsp70Ca-Cb | 1077 | 5 | 0.9 (+/- 1.1) | 3.7 (<0.0001) |
Mean number of HSE elements as expected by chance based on 30 shuffled versions of the parent sequence (Stothard 2000); sd indicates one standard deviation.
Figure 1. Alignment of step/gap type HSEs found in AaHsp70 intergenic regions.

Consensus bases are highlighted in black, similar bases are highlighted in gray. Asterisk (*) indicates the only perfect trimeric site. In the consensus, bold/underlined bases are predicted to bind HSF (Perisic et al. 1989).
Heat-induced gene expression has been shown to be proportional to the number of consecutive nGAAn subunits present upstream of the transcriptional start site (Fernandes et al. 1995). To determine the size and location of any HSE clusters upstream of AaHsp70 genes, the genomic positions of HSEs identified in Table 1 were compared. With the exception of AaHsp70Cb, all genes contained a pattern immediately upstream (-50 to -90 nt for AaHsp70Aa) of the start of transcription consisting of 4 consecutive subunits, a 6 bp spacer, and an additional 3 subunits. Functionally, this may be similar to an 8-subunit cluster. Both AaHsp70Aa (-672 to -702 nt) and AaHsp70Bb (-936 to -966 nt) contained a second cluster of 6 subunits, while AaHsp70Aa alone contained a third large cluster of 5 subunits (-1264 to -1284). The relative positions of HSE clusters in the AaHsp70Aa-Ab and AaHsp70Ba-Bb intergenic regions are shown in Fig. 2.
Figure 2. Activity of AaHsp70 putative promoter constructs in Aedes aegypti cells and embryos.
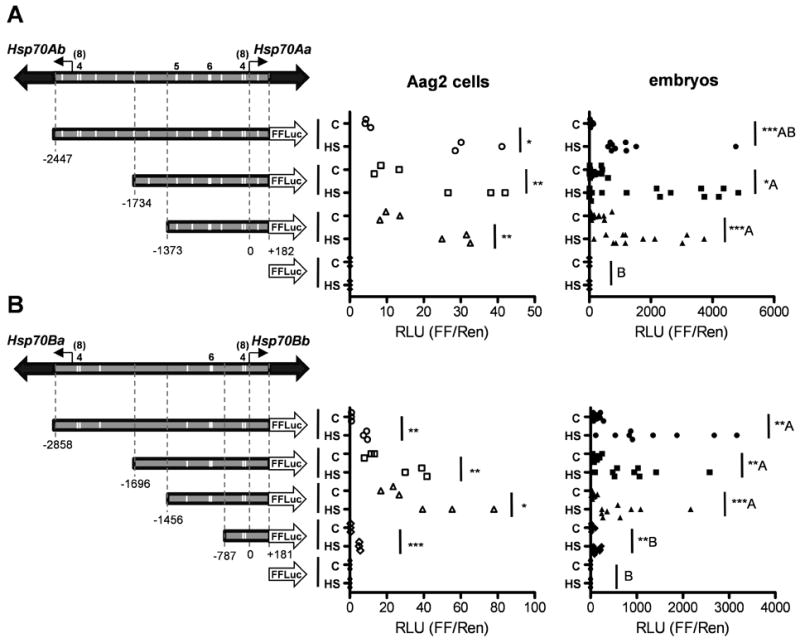
The parent structure of AaHsp70 inverted gene pairs are shown above each of the test constructs, with solid block arrows indicating each hsp70 ORF and open block arrows indicating the FFluc ORF. Line arrows indicate the start of transcription (0), with the number of nucleotides upstream (-) or downstream (+) indicated below each vertical dotted line. White vertical bars indicate the approximate locations of HSE clusters, with numerals above displaying the size of HSE clusters when different from 3. For both AaHsp70Aa (A) and AaHsp70Bb (B), x-axis indicates the ratio of FFluc to Rluc. Heat shocked (HS) and control (C) groups are indicated for each putative promoter construct. For cells, each data point represents a single well of a 12-well plate. Statistical significance between control and heat shock values was determined using an unpaired t-test; Welch's correction was used where variances were found to be unequal. For embryos, each data point represents a group of 80-120 injected individuals; data were rank-transformed and significance was determined using the Mann-Whitney test. Significance at p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***) is indicated. For comparisons between groups, Tukey's Honestly Significant Difference (HSD) test was used based on heat-shock values only; values not connected by the same letter are statistically independent groups. We note that the magnitude of the ratio of FFLuc to RLuc is not directly comparable between cell and embryo data due to the different temperatures used for heat shock as well as different plasmid ratios (see Experimental Procedures).
AaHsp70 promoter testing in cells and embryo assays
Upstream sequences derived from all six AaHsp70 genes (Gross et al. 2009) were tested for core promoter activity in cultured mosquito cells; with regions derived from AaHsp70Aa and AaHsp70Bb as the only ones able to drive the robust expression of a reporter (not shown). In order to test whether all the elements required for steady-state repression and heat activation were present upstream of these genes, genomic segments derived from AaHsp70Aa or AaHsp70Bb containing variable numbers of HSE clusters were placed upstream of the reporter gene, firefly luciferase (FFluc) (Fig. 2). AaHsp70Aa-FFluc and AaHsp70Bb-FFluc constructs were transfected into Ae. aegypti Aag2 cells or injected directly into pre-blastoderm Aedes aegypti embryos. A second plasmid which controlled the expression of Renilla luciferase (Rluc) in a heat shock-independent manner served as an internal normalization control. Following heat shock, the ratio of FFluc to Rluc was determined (Fig. 2). Luciferase activity driven from all AaHsp70 constructs was found to be significantly upregulated in both cells and embryos in response to heat shock. For AaHsp70Aa constructs, the level of luciferase activity following heat shock was similar between all three test constructs, indicating that all of the information needed to respond to heat shock was present between -1373 and +182 relative to the start of transcription, which included the main 8-member cluster as well as the 6- and 5-member clusters (Fig. 2A). While all AaHsp70Bb-based constructs demonstrated significant differences between control and heat shock expression, significantly less heat-induced expression was observed for the AaHsp70Bb-787 construct as compared with AaHsp70Bb-1456, AaHsp70Bb-1696, and AaHsp70Bb-2858. This indicates that for this promoter, critical information (potentially the 6-member HSE cluster) for heat shock-based transcriptional activation is located in the region between -787 and -1456. In both cases the 8-member HSE cluster for the opposite gene (AaHsp70Ab and AaHsp70Ba) was expendable, suggesting little cross-talk between these promoters. Based on these data, promoters AaHsp70Aa-1373 and AaHsp70Bb-1456 were chosen as the best candidates for further experimentation in transformed Ae. aegypti.
Generation of transgenic Ae. aegypti
In order to determine the ability of AaHsp70Aa-1373 and AaHsp70Bb-1456 promoter fragments to provide for the strict temporal control of a transgene in the context of the mosquito chromosome, Mos1 transformation constructs were designed (Fig. S1, A & C) to include each promoter-reporter cassette, and were introduced into Ae. aegypti embryos. G1 progeny obtained from surviving individuals were screened as larvae for DsRed expression under the control of the PUb promoter (Anderson et al. 2010), with putative transgenic individuals identified in three AaHsp70Aa-1373 pools and four AaHsp70Bb-1456 pools (Table S1). Genomic integrations were confirmed using Southern analysis (Fig. S1, B & D), and all transgene cassettes were stably inherited to the G2 generation (Table S1).
Validation of AaHsp70 promoters in adult female Ae. aegypti
In order to determine if the AaHsp70Aa-1373 and AaHsp70Bb-1456 promoters contained all the information required to respond to a heat shock with a burst of transcription in the context of the mosquito chromosome, we measured the total firefly luciferase activity in adult transformed individuals from all seven transgenic strains. Consistent with the data from cell and embryo assays, a significant increase in FFluc activity was observed in all transgenic strains as early as 1 hr post heat shock for both promoters (Fig. 3). FFluc activity was consistently about 10-13 times higher in heat shocked individuals than the corresponding untreated siblings at this time point. A clear difference in the absolute expression levels of the two promoters was also observed, with the AaHsp70Aa-1373 fragment driving the expression of ∼10 times more FFluc before and after heat shock (Fig. 3). Statistical analysis of FFluc levels among all seven control groups using Tukey's HSD test confirmed that the basal expression levels of the AaHsp70Aa-1373 lines was uniformly higher than those of AaHsp70Bb-1456 lines. A similar comparison between all seven sets of heat-shock values revealed a similar, but less dramatic trend, with Aa#P10 > Aa#P5 > Aa#P2 = Bb#P1 = Bb#P6 > Bb#P7 > Bb#P1. These results confirm that both the AaHsp70Aa-1373 and AaHsp70Bb-1456 promoters are capable of driving the heat-induced expression of a foreign gene in transgenic Ae. aegypti, albeit at different overall levels, potentially due to the extra 5-member HSE cluster present in the AaHsp70Aa-1373 fragment.
Figure 3. AaHsp70 promoter activity in adult female transgenic Aedes aegypti.
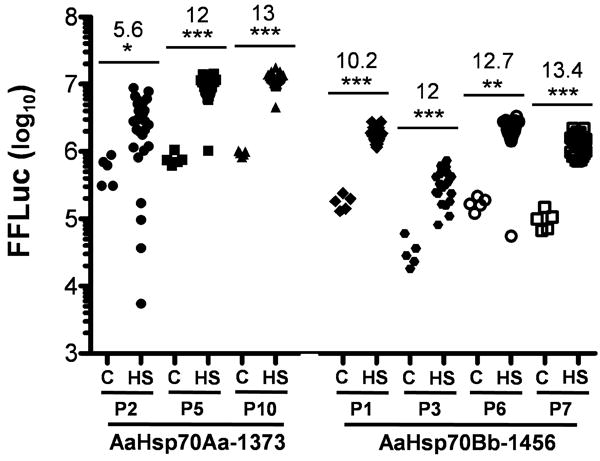
AaHsp70Aa-1373-FFluc and AaHsp70Bb-1456-FFluc transformed adult females were heat shocked (HS) and FFluc values were determined after a 1 hr recovery period and compared with untreated control (C) siblings. Y-axis indicates the log10-transformed value of the total number of light units, with each data point representing a single adult. The Mann-Whitney test was used on rank-transformed data, with significance at p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***) indicated above each bar. Numbers indicate the fold induction as determined by dividing the mean heat-shock value by the mean control value.
Endogenous hsp70 transcripts accumulate rapidly following heat shock, and are subject to degradation following the successful refolding/recycling of damaged proteins (Petersen and Lindquist 1989). In order to determine if FFluc transcripts under the control of AaHsp70 fragments mirrored this pattern, transcript abundance was measured by Northern analysis at various times following heat shock. Analysis of RNA derived from heat-shocked AaHsp70Aa-1373 line #P10 individuals revealed a pattern of luciferase mRNA levels similar to native AaHsp70 genes described previously (Gross et al. 2009), with abundant transcripts immediately following heat shock and a subsequent strong decrease by 24 hours (Fig. 4A). Phosphorimager-based quantitation revealed that FFluc transcripts were 20, 18, 13, and 6 percent of maximal AaHsp70 expression at 0, 1, 2, and 4 hours, respectively. Considering that the probe used to detect AaHsp70 transcripts was capable of hybridizing to all 12 AaHsp70 gene transcripts, while the luciferase probe would recognize only those transcripts resulting from AaHsp70Aa-1373-FFluc expression, this indicates that the AaHsp70Aa-1373 promoter is capable of driving transcription at a rate comparable to its endogenous counterpart.
Figure 4. Transcript abundance and luciferase activity over time in transgenic Aedes aegypti following heat shock.
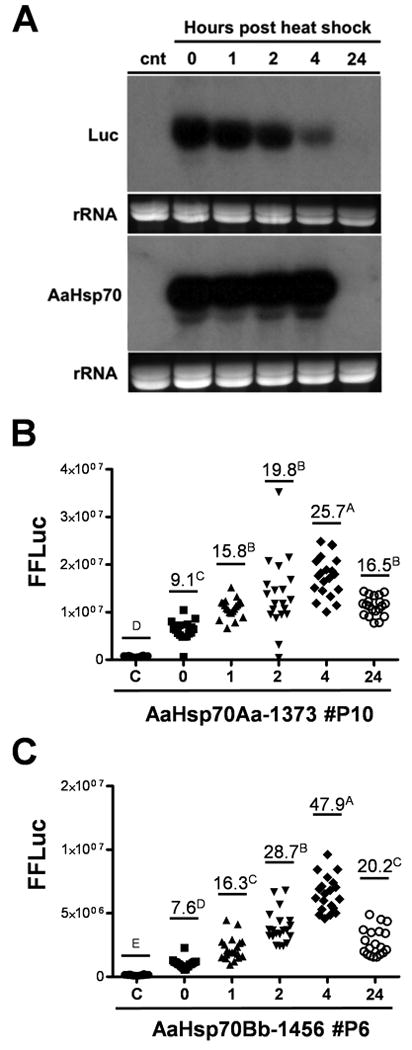
(A) Northern analysis of FFluc or hsp70 transcripts following heat shock of transgenic strain AaHsp70Aa-FFluc #P10. Exposures were standardized based on the specific activity of each probe; ethidium bromide stained rRNA loading controls are shown below each blot. (B-C) Luciferase activity was measured over time for AaHsp70Aa-1373-FFLuc line #P10 (B) or AaHsp70Bb-1456-FFLuc line #P6 (C) adult females following heat shock for 1 hour at 39°C. Fold-changes in luciferase activity compared to the non-heat shocked control (c) are labeled and values not connected by the same letter are significantly different according to Tukey's HSD.
Similarly, we measured the accumulation of FFluc over time in AaHsp70Aa-1373 line #P10 and in AaHsp70Bb-1456 line #P6. Luciferase assays confirmed a pattern of protein abundance similar to that of transcript abundance, though there was a delay in the time needed to reach peak levels (Fig. 4, B & C). FFluc levels were highest at 4 hours following heat shock for both promoters, reaching levels 25-50 times that of untreated females. Substantial luciferase activity remained (16-20 fold above control levels) at 24 hrs post heat shock even though mRNA transcripts could no longer be detected, indicating that FFluc is fairly stable under the conditions used herein. Luciferase activity in AaHsp70Bb-1456-FFluc transformed females followed a similar pattern as AaHsp70Aa-1373, but again with lower overall expression levels (Fig. 4C).
Heat induced expression of native Hsp70 has been previously confirmed in D. melangaster in all tissues assayed, including brain, salivary glands, midgut and female gonadal disks (Krebs and Feder 1997). Similarly, we have shown that AaHsp70 gene transcription is activated in similar tissues following heat shock in Ae. aegypti (Gross et al. 2009). In order to determine the pattern of AaHsp70Aa-1373 and AaHsp70Bb-1456-driven expression in specific tissues, we performed luciferase assays on dissected heads, midguts (sugar-fed), salivary glands and ovaries (sugar-fed) in each transformed line (Fig. 5). Head tissue is enriched for neurons, a common site for arbovirus amplification (Linthicum et al. 1996), and also contains significant amounts of fat body, the main immune organ of the mosquito. All three transgenic lines derived from the AaHsp70Aa-1373-FFluc construct displayed a ∼10 fold increase in luciferase activity in heads at 2-4 hrs following heat shock (Fig. 5A). Heat shock-induced expression was much more dramatic in AaHsp70Bb-1456-derived lines, due to significantly lower basal levels of expression in this tissue, as determined by Tukey's HSD test (Fig. 5A). In midguts, heat-induced luciferase levels were significantly increased in all lines, though there was much more variation between transgenic strains as compared to heads. No clear differences between the two promoters was observed, with the AaHsp70Bb-1456 promoter yielding transgenic strains with both the least and the greatest level of induction as measured by fold change. In particular, AaHsp70Bb-1456 line #P3 showed the greatest change between control and heat shock, with ∼2600-fold induction of FFluc. Differences in fold change were again largely due to differences in basal levels of expression, which spanned three orders of magnitude (Fig. 5B). Heat-induced activation of FFluc was also observed in salivary glands (Fig. 5C) and ovaries (Fig. 5D). These data confirm that AaHsp70 promoters are capable of driving the temporally-controlled expression of a transgene in a wide variety of mosquito tissues.
Figure 5. AaHsp70 promoter activity in transgenic Ae. aegypti tissues.

Firefly luciferase activity was measured at 1-4 h post heat shock for heads (A), midguts (B), salivary glands (C) and ovaries (D) dissected from transformed individuals. Y-axis indicates the log10-transformed value of the total number of light units, with each data point representing a pool of 10. The Mann-Whitney test was used on rank-transformed data, with significance at p < 0.05 (*), p < 0.01 (**) and p < 0.001 (***) indicated above each bar. Numbers indicate the fold induction as determined by dividing the mean heat-shock value by the mean control value.
Discussion
Overall, we found that genomic sequences derived from regions upstream of two AaHsp70 genes are capable of driving the heat-inducible expression of a marker gene both transiently in Aedes aegypti cells and embryos and through the stable integration of a transgene. Both the AaHsp70Aa-1373 and AaHsp70Bb-1456 promoters were found to be capable of transcriptional activation in response to a 1 hr treatment at 39°C in whole adults as well as individual tissues.
We found that the intergenic regions separating all three pairs of AaHsp70 genes we examined were highly enriched for nGAAn-nTTCn and nTTCn-nnnnn-nTTCn HSE motifs, with the latter forming a consensus nTTCg-aGNRn-wTTCn. In flies, multiple trimeric HSEs arranged in clusters show much greater cooperative binding of HSF than the same number of sites spread at a distance; this increase in HSF binding was associated with increased gene activation at lower temperatures (Fernandes et al. 1995). Our data agree with this, as the AaHsp70Aa gene promoter had both the greatest number of large HSE clusters and the strongest basal expression levels. Though FFluc expression was correlated with a higher number of HSE clusters, the large HSE clusters located at the start of transcription of the distal Hsp70 gene were expendable. It is possible that the distance (>2.5 kb) of these clusters from the transcriptional initiation site was too great to exert a significant influence on transcriptional activation (Fernandes et al. 1995). Ultimately, further investigations will be necessary to clarify the precise role of individual HSE clusters in the heat-induced activation of AaHsp70 genes.
The appropriateness of which of these two promoters to use in a given experiment will likely depend on the trait that is more important to the investigator: the ability to express as much gene product as possible, or the ability to strongly suppress transcription prior to heat activation. We emphasize, however, that even though the AaHsp70Aa-1373 promoter exhibited basal expression levels ∼10× higher than the AaHsp70Bb-1456 promoter, we were still unable to detect AaHsp70Aa-FFluc transcripts in the absence of heat shock. Thus, even this “higher” level of expression should be considered extremely low compared with most transcripts. It is likely that the sensitivity of the firefly luciferase reporter, combined with its relatively long half-life, played an important role in our ability to detect expression in the absence of heat shock. While the half-life of firefly luciferase was reported to be ∼3 hours at 37°C (Thompson et al. 1991), our data indicate that this is substantially lengthened at 28°C to between 8-16 hours (Fig. 4). This issue might be avoided with experiments where no protein is produced, such as the expression of hairpin RNAs for gene knockdown, or where protein products can be intentionally destabilized.
In contrast to our inability to detect FFluc transcripts in the absence of heat shock, these transcripts became very abundant immediately after the one hour treatment at 39°C, mirroring the pattern exhibited by the endogenous AaHsp70 genes (Gross et al. 2009). Surprisingly, luciferase transcripts decreased much more quickly than the corresponding AaHsp70 transcripts. This is notable because our transformation constructs did not include the native AaHsp70 3′ untranslated region (UTR). In flies, the 3′UTR is important for heat shock transcript regulation, as it targets messages for de-adenylation and degradation once stress conditions have passed (Dellavalle et al. 1994; Feder et al. 1992; Petersen and Lindquist 1989). Utilizing the 3′UTR sequence from SV40 in place of the native AaHsp70 3′UTRs in our transgenic constructs, the stability of luciferase transcripts was expected to increase. These results may indicate that the stability of AaHsp70 transcripts is regulated differently than in flies, with additional signals present in AsHsp70 transcripts which identify them as targets for deadenylation during recovery from heat stress.
Luciferase expression levels between the three AaHsp70Aa-1373 transgenic lines was fairly consistent in whole adults, heads, ovaries and salivary glands, indicating that the chromosomal position of each Mos1 insertion did not exert a strong influence on the expression or activation of this promoter. Likewise, with the exception of line #P3, luciferase expression in AaHsp70Bb-1456 transgenic strains was similar in whole adults and heads alone. In contrast, for both promoter constructs basal expression levels in the midguts were much more variable; indicating that expression in this tissue is much more susceptible to chromosomal position effects. We note that both transformation constructs included an attP recombination site, allowing the re-use of any of these integration sites using PhiC31-mediated recombination (Nimmo et al. 2006).
In conclusion, we have shown that two independent AaHsp70 promoter regions can activate robust transcription in response to heat shock in Ae. aegypti. Such precise temporal control of a transgene will be vital for gene knockdown or ectopic expression studies of genes whose loss/gain would adversely affect the successful progression through development. Uncoupling temporal control of transgene expression from a bloodmeal will allow investigations into events which occur before a mosquito obtains her first meal, such as host-seeking or mating, as well as events which happen late in bloodmeal digestion or oogenesis. Additionally, the precisely timed expression of a transgene can be used to dissect host-pathogen interactions, by allowing the expression of a foreign gene at a specific point in a pathogens life cycle. Finally, the transgenic strains we have developed here can be used directly as sensors to detect activation of the stress response [as reviewed in (Mukhopadhyay et al. 2003)]. While the stress response of D. melanogaster has been studied extensively (as reviewed in Craig 1985; Lindquist 1986), there is only limited understanding of conditions such as environmental contaminants, insecticides, infectious agents or biological processes which may induce the stress response in Aedes aegypti (Benoit et al. 2011; Muturi et al. 2011).
Experimental Procedures
Cell culture and transient embryo assays
Aedes aegypti Aag2 cells were maintained at 28°C and 5% CO2 in Schneider's Drosophila medium supplemented with 10% fetal bovine serum, 1% L-glutamine, and 1% penicillin/streptomycin. Twelve-well plates were seeded with to be transfected with luciferase promoter plasmid constructs. A total of 0.5 μg of DNA was transfected with 12.5 μl of Effectene into Aag2 cells at approximately 80% confluency with a 1:1 ratio of experimental plasmid and the pRL Renilla luciferase control plasmid (Promega). Aedes aegypti embryos were collected from bloodfed females and injected 30 to 90 minutes after being laid with 300 ng/μl experimental plasmid and 15 ng/μl pRL in 1X injection buffer (Jasinskiene et al. 1998). Twenty-four hours following transfection/injection, cells/embryos were heat shocked at 37°C/39°C, respectively, for one hour and were allowed to recover for 1 hr at 28°C prior to harvest. Dual luciferase reporter assays (Promega) were performed on cell lysates after active cell lysis using rubber policemen according to the manufacturer's protocol for 12-well plates. Embryos were crushed in a total of 100 μl of passive lysis buffer (Promega) and luciferase assays were performed as for cell lysates. Luciferase activity was measured using a Hidex Chameleon plate reader (Turku, Finland).
Mosquito assays
The Liverpool strain of Aedes aegypti was reared at 28°C and approximately 60% relative humidity with a 14:10 daylight cycle. Larvae were fed pulverized fish food and reared ≈ 400 per pan in 4 liters of reverse osmosis purified water until pupation. Adult mosquitoes were maintained on sucrose and were bloodfed using artificial membrane feeders and defibrinated sheep blood (Colorado Serum Company, Denver, CO). Mosquitoes were heat shocked at 3-6 days post eclosion for one hour at 39°C as described previously (Gross et al. 2009). Adult females were frozen individually for luciferase assays, or in groups of 6 for RNA extraction. Tissues were dissected 1-4 hours post heat shock and snap frozen in pools of 10. Adults (100 μl) and tissues (50 μl) were homogenized in 1X passive lysis buffer and luciferase assays were performed according to the manufacturer's protocol (Promega, Dual Luciferase Assay, firefly reading only).
Construction of AaHsp70 promoter-reporter plasmids
Sequences corresponding to genomic position 380841-383453 and position 404011-406759 on supercontig 1.680 were amplified from Aedes aegypti Liverpool genomic DNA using Platinum Pfx polymerase chain reaction (PCR; Invitrogen, Carlsbad, CA) (2× Pfx amplification buffer, 0.3 mM dNTPs, 1 mM MgSO4, 0.3 μM primers, 1 U Platinum Pfx DNA polymerase). Prior to amplification, mosquito genomic DNA was digested with either SacII or BglII to separate inverted hsp70 gene pairs. Several amplification products were generated and assembled to reconstitute the entire intergenic region between the AaHsp70Aa-AaHsp70Ab and AaHsp70Ba-AaHsp70Bb gene pairs. Each genomic interval was cloned into pGL3-Basic upstream of the firefly luciferase ORF, yielding plasmids AaHsp70Aa-2447-FFluc and AaHsp70Bb-2858-FFluc.
To produce AaHsp70Aa-1734-FFluc, AaHsp70Aa-2447-FFluc was digested with NcoI and HindIII, and the resulting 1916 base pair fragment was ligated into the NcoI and HindIII of pGL3Basic. To produce AaHsp70Aa-1383-FFluc, AaHsp70Aa-2447-FFluc was linearized with MluI, and partially digested with AseI to remove 1064bp of sequence. The remaining AaHsp70Aa-FFluc/pGL3 backbone was treated with Klenow to produce blunt ends, followed by self ligation to restore circularity. To produce both AaHsp70Bb-1696-FFluc and AaHsp70Bb-767-FFluc, AaHsp70Bb was linearized with MluI and partial digests were performed using EcoRI. The two shortened AaHsp70Bb-FFluc/pGL3-Basic containing fragments resulting from these digests were treated with Klenow and self-ligated. AaHsp70Bb-1456-FFluc was obtained by digesting AaHsp70Bb-1696-FFluc with PvuI, followed by self-ligation of the remaining plasmid sequence. Final AaHsp70Aa-1373 and AaHsp70Bb-1456 promoters sequences have been deposited to GenBank under accession numbers JN393894 and JN393895, respectively.
To build Mos1 donor constructs, the AaHsp70Aa-1373-FFluc gene cassette was excised from pGL3-Basic using MluI and BamHI and ligated into the AscI/BglII sites of pMos-PUbDsRed-5HE-MCS-5HE (Anderson et al. 2010). Similarly, the AaHsp70Bb-1456-FFluc gene cassette was excised from pGL3-Basic with PvuI/BamHI and was ligated into the PacI and BglII sites of pMos-PUbDsRed-5HE-MCS-5HE.
Mos1-mediated transformation
Transgenic Aedes aegypti were produced by injecting Liverpool strain embryos with 0.5 μg/μl donor plasmid and 0.3 μg/μl helper plasmid (pGL3-PUb-Mos1) in 1X injection buffer [see above]. All microinjections were performed using a Leica micromanipulator and a FemtoJet microinjector (Eppendorf, Westbury, NY). Needles were produced using a Sutter Instruments (Novato, CA) Model P-2000 needle puller (Heat = 270, Fil = 3, Vel = 37, Del = 250, Pul = 140). Surviving G0 females were pooled into groups of 20-25 individuals and mated to Liverpool males. G0 males were mated individually to 5 Liverpool females and then pooled into groups of 24 males and 120 females, as previously described (Anderson et al. 2010). G1 progeny were screened for DsRed+ using a Leica MZ-16FL microscope.
Northern/Southern analysis
Total RNA was extracted from pools of 6 female mosquitoes using TRIzol (Invitrogen) according to the manufacturer's protocol. RNA (5 μg) from each experimental group was electrophoresed in a 1.2 percent agarose, 1X MOPS, 2% formaldehyde gel and blotted onto a positively charged nylon membrane (Immobilon-NY+, Milllipore, Concord, MA). Genomic DNA was extracted from pools of six females as described previously (Anderson et al. 2010) and was digested with the indicated restriction endonuclease, followed by gel electrophoresis and capillary transfer to a nylon membrane.
For Northern analysis, a probe specific to the luciferase open reading frame was generated by digesting pGL3Basic (Promega) with XbaI and NcoI, resulting in an approximately 1.6 kb fragment (Moreira et al. 2000). Probe sequence common to all AaHsp70 open reading frames was as described previously (Gross et al. 2009). For Southern analysis, probes were produced corresponding to HindIII restriction fragments derived from the parent Mos1 construct, as described previously (Anderson et al. 2010). Random primed probes were labeled with [α-32P]dATP, using the Amersham Megaprime DNA Labeling System (GE Healthcare, Buckinghamshire, UK). Probes were purified using illustra NICK columns (GE Healthcare) and added to pre-warmed Church's buffer to hybridize overnight at 65°C. Following washes, blots were exposed to Kodak BioMax maximum sensitivity film at -80°C. Hybridization signals were also quantified using a Storm 820 phosphoimager and ImageQuantTL software (GE Healthcare).
Supplementary Material
Figure S1. Southern analysis of AaHsp70A-1373 and AaHsp70B-1456 in Mos1 transformed Ae. aegypti. (A, C) Hypothetical representation of AaHsp70Aa-1373-FFluc and AaHsp70Bb-1456-FFluc transgene insertions. Block arrows represent right (R) and left (L) arms of the Mos1 transposon. Dashed lines indicate Aedes aegypti genomic DNA. Predicted restriction sites for EcoRI (E), HindIII (H), and NdeI (N) are indicated. Bracketed bars indicate the size of the entire insertion as well as the expected size of one of the bands resulting from HindIII digestion. (B, D) Genomic DNA from wild type (wt) or transgenic mosquitoes was hybridized with a probe corresponding to the Mos1 right arm and DsRed cassette. Molecular weight markers are indicated to the left of each image. For (B), black arrows indicate the internal HindIII fragment which should be common to all insertions, white arrows indicate junction fragments with mosquito genomic DNA. For (C), white arrows again indicate junction fragments with mosquito genomic DNA. All transgenic lines appear to contain only a single insertion, with the exception of line #P3. In this case, the EcoRI digest produced two strong hybridization fragments, while the NdeI digest only produced one. Thus, it is not clear if there are one or two insertions in this line.
Table S1. Mos1-mediated transformation of Aedes aegypti with AaHsp70-FFluc constructs
Acknowledgments
We thank members of the Adelman and Myles laboratories for technical assistance. This work was supported by the National Institute of Allergy and Infectious Diseases grants AI071208 and AI085091 as well as the Fralin Life Science Institute and Department of Entomology at Virginia Tech.
References
- Adelman ZN, Jasinskiene N, Onal S, Juhn J, Ashikyan A, Salampessy M, MacCauley T, James AA. nanos gene control DNA mediates developmentally regulated transposition in the yellow fever mosquito Aedes aegypti. Proc Natl Acad Sci U S A. 2007;104:9970–5. doi: 10.1073/pnas.0701515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aigaki T, Fleischmann I, Chen PS, Kubli E. Ectopic expression of sex peptide alters reproductive behavior of female D. melanogaster. Neuron. 1991;7:557–63. doi: 10.1016/0896-6273(91)90368-a. [DOI] [PubMed] [Google Scholar]
- Anderson MA, Gross TL, Myles KM, Adelman ZN. Validation of novel promoter sequences derived from two endogenous ubiquitin genes in transgenic Aedes aegypti. Insect Mol Biol. 2010;19:441–9. doi: 10.1111/j.1365-2583.2010.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit JB, Lopez-Martinez G, Patrick KR, Phillips ZP, Krause TB, Denlinger DL. Drinking a hot blood meal elicits a protective heat shock response in mosquitoes. Proc Natl Acad Sci U S A. 2011;108:8026–9. doi: 10.1073/pnas.1105195108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho KH, Cheon HM, Kokoza V, Raikhel AS. Regulatory region of the vitellogenin receptor gene sufficient for high-level, germ line cell-specific ovarian expression in transgenic Aedes aegypti mosquitoes. Insect Biochem Mol Biol. 2006;36:273–81. doi: 10.1016/j.ibmb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Coates CJ, Jasinskiene N, Pott GB, James AA. Promoter-directed expression of recombinant fire-fly luciferase in the salivary glands of Hermes-transformed Aedes aegypti. Gene. 1999;226:317–25. doi: 10.1016/s0378-1119(98)00557-5. [DOI] [PubMed] [Google Scholar]
- Craig EA. The heat shock response. CRC Crit Rev Biochem. 1985;18:239–80. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Dellavalle RP, Petersen R, Lindquist S. Preferential deadenylation of Hsp70 mRNA plays a key role in regulating Hsp70 expression in Drosophila melanogaster. Mol Cell Biol. 1994;14:3646–59. doi: 10.1128/mcb.14.6.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder JH, Rossi JM, Solomon J, Solomon N, Lindquist S. The Consequences of Expressing Hsp70 in Drosophila Cells at Normal Temperatures. Genes & Development. 1992;6:1402–1413. doi: 10.1101/gad.6.8.1402. [DOI] [PubMed] [Google Scholar]
- Fernandes M, Xiao H, Lis JT. Binding of heat shock factor to and transcriptional activation of heat shock genes in Drosophila. Nucleic Acids Res. 1995;23:4799–804. doi: 10.1093/nar/23.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu G, Lees RS, Nimmo D, Aw D, Jin L, Gray P, Berendonk TU, White-Cooper H, Scaife S, Kim Phuc H, Marinotti O, Jasinskiene N, James AA, Alphey L. Female-specific flightless phenotype for mosquito control. Proc Natl Acad Sci U S A. 2010;107:4550–4. doi: 10.1073/pnas.1000251107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross TL, Myles KM, Adelman ZN. Identification and characterization of heat shock 70 genes in Aedes aegypti (Diptera: Culicidae) J Med Entomol. 2009;46:496–504. doi: 10.1603/033.046.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacs AT, Li F, Jasinskiene N, Chen X, Nirmala X, Marinotti O, Vinetz JM, James AA. Engineered Resistance to Plasmodium falciparum Development in Transgenic Anopheles stephensi. PLoS Pathog. 2011;7:e1002017. doi: 10.1371/journal.ppat.1002017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasinskiene N, Coates CJ, Benedict MQ, Cornel AJ, Rafferty CS, James AA, Collins FH. Stable transformation of the yellow fever mosquito, Aedes aegypti, with the Hermes element from the housefly. Proc Natl Acad Sci U S A. 1998;95:3743–7. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoza V, Ahmed A, Wimmer EA, Raikhel AS. Efficient transformation of the yellow fever mosquito Aedes aegypti using the piggyBac transposable element vector pBac[3×P3-EGFP afm] Insect Biochem Mol Biol. 2001;31:1137–43. doi: 10.1016/s0965-1748(01)00120-5. [DOI] [PubMed] [Google Scholar]
- Kokoza V, Ahmed A, Woon Shin S, Okafor N, Zou Z, Raikhel AS. Blocking of Plasmodium transmission by cooperative action of Cecropin A and Defensin A in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A. 2010;107:8111–6. doi: 10.1073/pnas.1003056107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs RA, Feder ME. Tissue-specific variation in Hsp70 expression and thermal damage in Drosophila melanogaster larvae. J Exp Biol. 1997;200:2007–15. doi: 10.1242/jeb.200.14.2007. [DOI] [PubMed] [Google Scholar]
- Lam G, Thummel CS. Inducible expression of double-stranded RNA directs specific genetic interference in Drosophila. Curr Biol. 2000;10:957–63. doi: 10.1016/s0960-9822(00)00631-x. [DOI] [PubMed] [Google Scholar]
- Lindquist S. The heat-shock response. Annu Rev Biochem. 1986;55:1151–91. doi: 10.1146/annurev.bi.55.070186.005443. [DOI] [PubMed] [Google Scholar]
- Linthicum KJ, Platt K, Myint KS, Lerdthusnee K, Innis BL, Vaughn DW. Dengue 3 virus distribution in the mosquito Aedes aegypti: an immunocytochemical study. Med Vet Entomol. 1996;10:87–92. doi: 10.1111/j.1365-2915.1996.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Mathur G, Sanchez-Vargas I, Alvarez D, Olson KE, Marinotti O, James AA. Transgene-mediated suppression of dengue viruses in the salivary glands of the yellow fever mosquito, Aedes aegypti. Insect Mol Biol. 2010;19:753–63. doi: 10.1111/j.1365-2583.2010.01032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith JM, Basu S, Nimmo DD, Larget-Thiery I, Warr EL, Underhill A, McArthur CC, Carter V, Hurd H, Bourgouin C, Eggleston P. Site-specific integration and expression of an anti-malarial gene in transgenic Anopheles gambiae significantly reduces Plasmodium infections. PLoS ONE. 2011;6:e14587. doi: 10.1371/journal.pone.0014587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira LA, Edwards MJ, Adhami F, Jasinskiene N, James AA, Jacobs-Lorena M. Robust gut-specific gene expression in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A. 2000;97:10895–8. doi: 10.1073/pnas.97.20.10895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AC, Schaub TL, James AA. FLP-mediated recombination in the vector mosquito, Aedes aegypti. Nucleic Acids Res. 1991;19:5895–900. doi: 10.1093/nar/19.21.5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay I, Nazir A, Saxena DK, Chowdhuri DK. Heat shock response: hsp70 in environmental monitoring. J Biochem Mol Toxicol. 2003;17:249–54. doi: 10.1002/jbt.10086. [DOI] [PubMed] [Google Scholar]
- Muturi EJ, Kim CH, Alto BW, Berenbaum MR, Schuler MA. Larval environmental stress alters Aedes aegypti competence for Sindbis virus. Trop Med Int Health. 2011;16:955–964. doi: 10.1111/j.1365-3156.2011.02796.x. [DOI] [PubMed] [Google Scholar]
- Nimmo DD, Alphey L, Meredith JM, Eggleston P. High efficiency site-specific genetic engineering of the mosquito genome. Insect Mol Biol. 2006;15:129–36. doi: 10.1111/j.1365-2583.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisic O, Xiao H, Lis JT. Stable binding of Drosophila heat shock factor to head-to-head and tail-to-tail repeats of a conserved 5 bp recognition unit. Cell. 1989;59:797–806. doi: 10.1016/0092-8674(89)90603-x. [DOI] [PubMed] [Google Scholar]
- Petersen RB, Lindquist S. Regulation of Hsp70 Synthesis by Messenger-Rna Degradation. Cell Regulation. 1989;1:135–149. doi: 10.1091/mbc.1.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read D, Levine M, Manley JL. Ectopic expression of the Drosophila tramtrack gene results in multiple embryonic defects, including repression of even-skipped and fushi tarazu. Mech Dev. 1992;38:183–95. doi: 10.1016/0925-4773(92)90052-l. [DOI] [PubMed] [Google Scholar]
- Sakurai H, Enoki Y. Novel aspects of heat shock factors: DNA recognition, chromatin modulation and gene expression. FEBS J. 2010;277:4140–9. doi: 10.1111/j.1742-4658.2010.07829.x. [DOI] [PubMed] [Google Scholar]
- Smith RC, Walter MF, Hice RH, O'Brochta DA, Atkinson PW. Testis-specific expression of the beta2 tubulin promoter of Aedes aegypti and its application as a genetic sex-separation marker. Insect Mol Biol. 2007;16:61–71. doi: 10.1111/j.1365-2583.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- Stothard P. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques 28. 2000;1102:1104. doi: 10.2144/00286ir01. [DOI] [PubMed] [Google Scholar]
- Tatem AJ, Hay SI, Rogers DJ. Global traffic and disease vector dispersal. Proc Natl Acad Sci U S A. 2006;103:6242–7. doi: 10.1073/pnas.0508391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JF, Hayes LS, Lloyd DB. Modulation of firefly luciferase stability and impact on studies of gene regulation. Gene. 1991;103:171–7. doi: 10.1016/0378-1119(91)90270-l. [DOI] [PubMed] [Google Scholar]
- Wise de Valdez MR, Nimmo D, Betz J, Gong HF, James AA, Alphey L, Black WCt. Genetic elimination of dengue vector mosquitoes. Proc Natl Acad Sci U S A. 2011;108:4772–5. doi: 10.1073/pnas.1019295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Southern analysis of AaHsp70A-1373 and AaHsp70B-1456 in Mos1 transformed Ae. aegypti. (A, C) Hypothetical representation of AaHsp70Aa-1373-FFluc and AaHsp70Bb-1456-FFluc transgene insertions. Block arrows represent right (R) and left (L) arms of the Mos1 transposon. Dashed lines indicate Aedes aegypti genomic DNA. Predicted restriction sites for EcoRI (E), HindIII (H), and NdeI (N) are indicated. Bracketed bars indicate the size of the entire insertion as well as the expected size of one of the bands resulting from HindIII digestion. (B, D) Genomic DNA from wild type (wt) or transgenic mosquitoes was hybridized with a probe corresponding to the Mos1 right arm and DsRed cassette. Molecular weight markers are indicated to the left of each image. For (B), black arrows indicate the internal HindIII fragment which should be common to all insertions, white arrows indicate junction fragments with mosquito genomic DNA. For (C), white arrows again indicate junction fragments with mosquito genomic DNA. All transgenic lines appear to contain only a single insertion, with the exception of line #P3. In this case, the EcoRI digest produced two strong hybridization fragments, while the NdeI digest only produced one. Thus, it is not clear if there are one or two insertions in this line.
Table S1. Mos1-mediated transformation of Aedes aegypti with AaHsp70-FFluc constructs


