Abstract
The Vanin genes are a family that encode pantetheinases involved in recycling Coenzyme A, caytalysing the breakdown of intermediate panetheine to vitamin B5 for reuse in CoA biosynthesis. The role of pantetheinase in this most fundamental of cellular processes, was substantially characterised by the 1970s. The next 20 years saw little further interest in pantetheinase until various genetic studies implicated the vanin locus in a range of normal and disease phenotypes, and a consequent interest in the other product of pantetheinase activity, cysteamine. This report seeks to bring together the early biochemical studies with recent biological data implicating cysteamine as a regulator of the oxidative state of a cell. Numerous studies now report a role for Vanin in inflammation, oxidative stress, cell migration and numerous diseases including cardiovascular disease.
Keywords: Vanin, pantetheinase, cysteamine, cardiovascular disease
Introduction
Belying its original description as Vascular Non-Inflammatory Molecule 1, Vanin 1 and related family members Vanin 2 and Vanin 3, which code for pantetheine hydrolase isoforms, have wide-ranging and diverse effects, although some of them are related to inflammation. Pantetheine hydrolase or pantetheinase, catalyses the hydrolysis of pantetheine to pantothenic acid (vitamin B5) and cysteamine, a powerful anti-oxidant. Functional studies indicate a role for Vanin in inflammation, oxidative stress, cell migration and numerous diseases. We discuss the role of the Vanin family in these biological pathways and explore how Vanin and the metabolic products reveal insights into cellular function.
2. Gene and Protein
Genomic organisation of the Vanin gene family
The human genome encodes three Vanin genes lying in the same transcriptional orientation within an 80 kb region on chromosome 6q23-6q24[1,2]. Vanin 3 is upstream of Vanin 1 and downstream of Vanin 2[1]. Orthologues of Vanin 1 and Vanin 3 have been identified as mouse Vanin 1 and Vanin 3 respectively[1,2].
Human and mouse Vanin genes show approximately 40% amino acid sequence similarity to biotinidase (Figure 1), although no biotinidase activity is detected from Vanin 1[1,3,4,5]. Biotinidase is part of the same amidohydrolase family, but with different substrate specificity[3,4]. The human Vanin 1 protein show significant sequence similarity to the mouse form (77.9%) and with human Vanin 2 (63.5%)[2]. Human Vanin 3 was recently classified a pseudogene as the open reading frame is disrupted by a frameshift. Nine splice variants for Vanin 3 have been reported in human neutrophils, however none of these extend the full span of exon 7, suggesting they don't encode the predicted Vanin protein[6].
Figure 1.
Human Vanin family structural predictions. Predicted N glycosylation sites shown as underscored residues in green: N-Signal peptides in bold; predicted active site residues (based on biotinidase) boxed in red including * ; predicted α-helices in yellow (light shading); predicted β-sheets shaded in grey (dark); predicted GPI-anchor site residues in white with blue box; * identical residues; : conservative substitution; . non-conservative substitution; Missense residue in VNN3 shown in strikeout and red has been changed to allow readthrough to the predicted stop codon.
Vanin molecules can be membrane bound
The human Vanin genes are similarly organised into 7 exons [1]. For Vanin 1 and Vanin 2, the 3’ end of exon 7 encodes a 20 amino acid hydrophobic region preceded by an 8-10 residue hydrophilic spacer region containing a glycosylphosphatidylinositol (GPI)-anchored cleavage site (see Figure 1)[3,7]. In addition, each protein contains a 23 amino acid leader peptide at the N-terminus. Both sequences are required for coupling with a GPI anchor and subsequent attachment to the cell membrane. Murine Vanin 3 lacks this GPI-anchoring consensus [1]. GPI-anchored proteins can be released from cell surfaces by phosphatidylinositol-specific phospholipase C mediated cleavage[8,9]. The Vanin 2 protein (originally called GPI-80) exists in both soluble and membrane bound forms[10].
Vanin Molecules are widely expressed
Although several studies have investigated expression of the Vanin genes, a conclusive tissue distribution pattern remains to be determined. At the mRNA level, mouse Vanin 1 expression is primarily in kidney epithelia, intestine and liver[5]. However, expression can be detected at some level by PCR in almost all organs tested[5]. Mouse Vanin 3 mRNA expression can be detected in myeloid cells with lower levels in the spleen and liver[1]. In other animals including pig and cow, pantetheinase activity has been observed in leukocytes, fibroblasts, intestinal cells and plasma[4,11].
In humans, Vanin 1 mRNA expression has been demonstrated in the spleen, thymus, lymph nodes, peripheral blood leukocytes, urethra, kidney, parts of the respiratory tracts, liver and intestinal tract[2,12]. More recently, Vanin 1 was detected at high abundance in CD15+ granulocytes and CD14+ monocytes. Platelets show moderate levels of Vanin 1 mRNA, whilst CD4/8+ T-cells and CD20+ B-cells show relatively low levels[13]. This distribution pattern in leukocytes suggests a role for Vanin 1 in innate immunity.
Human Vanin 2 mRNA expression has been demonstrated in almost all tissues with highest expression in the spleen, kidney and blood, particularly neutrophils[1,2,7,12]. Based on the expression patterns, protein structural similarities and the likelihood that the human Vanin 3 gene is a pseudogene, mouse Vanin 3 functionally corresponds to human Vanin 2. Vanin 3 mRNA has been detected in liver, peripheral blood leukocytes, placenta, urethra and parts of the respiratory tracts[1,12] but the functional significance of this remains unknown.
3. Biochemistry of Vanin
Pantetheinase has absolute specificity
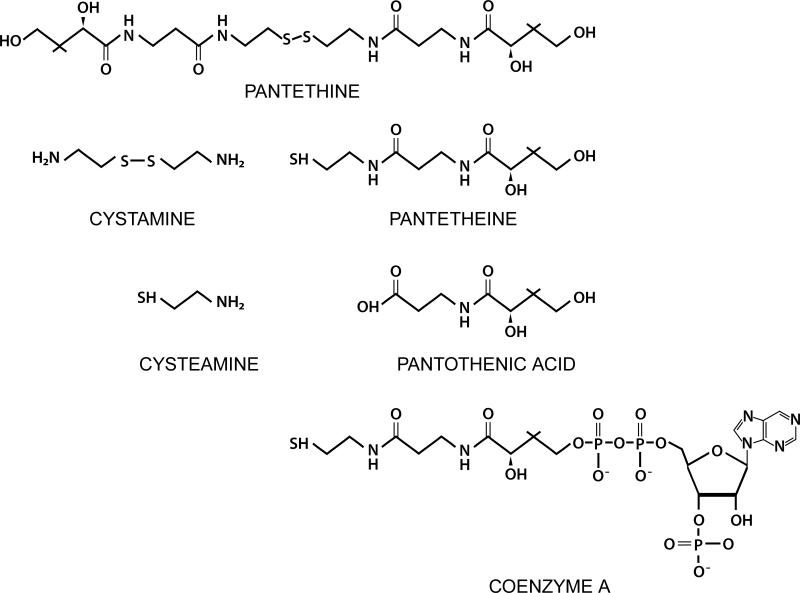
Pantetheinase activity was first ascribed to the Vanin genes in 1996. Pantetheinase (Pantetheine hydrolyase EC 3.5.1.92) hydrolyses one carboamide linkage in D-pantetheine forming D-pantothenate (pantothenic acid or vitamin B5), and cysteamine (2-aminoethanethiol)[4]. The chemical structures are shown in Figure 2.
Figure 2.
Substrates and metabolites of Vanin Activity.
Pantetheinase has absolute substrate specificity acting on the intact pantetheine molecule at one of the amide bonds[14,15]. The presence of a reduced thiol is required for enzyme activity[4,16]. Pantetheinase activity is seen in a pH range from 4-8 with a pH optima of 6.5-7.5 for leukocytes[11,16]. Pantetheinase activity is inhibited by the reduced pantetheine dimer, pantethine (see Figure 1)[11,14,17].
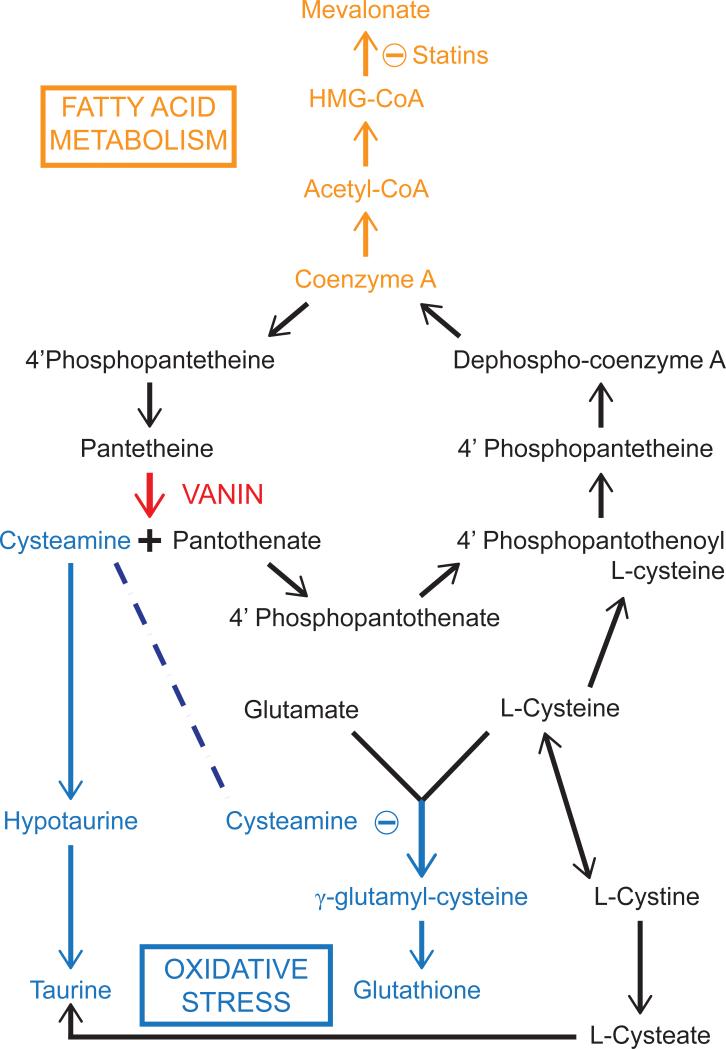
The pantetheinase-mediated hydrolysis of pantetheine to pantothenate and cysteamine impacts on a number of biological processes including fatty acid metabolism and the oxidative stress response (Figure 3). First, pantothenic acid acts as the structural component of Coenzyme A (CoA)[18]. CoA can be recycled via conversion to pantetheine, which is then hydrolysed to pantothenate. This turnover of CoA allows for its controlled and continuous production, which may be mediated by the rate of pantetheine hydrolysis and, in turn, may have significant biological implications in a number of cellular pathways. If this is the case, it is possible that the regulation of Vanin gene expression controls fatty acid metabolism.
Figure 3.
Overview of the main pathways influenced by Vanin Activity.
Second, cysteamine is a decarboxylated cysteine that acts as a nucleophile, donating both bonding electrons to its reaction partner[11,16]. Cysteamine is converted either to taurine through a hypotaurine intermediate, or is oxidised to its disulfide dimer, cystamine[19]. Both pantothenic acid and cysteamine concentrations are in flux with pantetheine/pantethine availability. This may ultimately be determined by the extracellular redox conditions that control the pools of pantetheine and pantethine and hence the activity of pantetheinase.
Cysteamine can avidly attack disulfide groups to yield mixed disulfides and so inactivate proteins. This highly effective process is attributed to the low pKa value of cysteamine, due to the protonated amine[17]. A well-studied example of this phenomenon comes from the autosomal recessive disease Cystinosis which is characterised by a build up of cystine in lysosomes of cells causing widespread cell damage[20]. Oral administration of cysteamine alleviates the accumulation of cystine[11,20]. Administration of pantethine instead of cysteamine produces comparable results with reduced side-effects whilst administration of pantothenic acid alone shows no effect[20]. In a cell culture model using skin fibroblasts obtained from cystinotic patients, cystamine was reduced to cysteamine allowing it to react with the disulfide bond in cystine yielding the mixed disulfide cysteamine-cysteine[20]. This complex is then able to move from the lysosome to the cytoplasm for removal. Cystamine is often used in laboratory and clinical investigations as a more stable source of cysteamine. Although cystamine may have cysteamine-independent functions, it is likely that because it is rapidly reduced, cysteamine is in fact the functional element.
More recent studies show addition of cysteamine, cystamine or pantethine to murine neural cells increased cellular cysteine level following an increase in cysteamine[21]. This was attributed to thiol-disulfide exchange reactions with cystine, which could either release cysteine or increase uptake of cystine[21,22,23]. Free cysteine could interact with free cysteamine, be oxidised to cystine or enter the glutathione pool[20]. In one study, this resulted in the removal of by-products of lipid peroxidation and other metabolic reactions preventing cytotoxicity[21]. Administration of cystamine to cultured cells or mouse brain resulted in an increase in L-cysteine[22,24]. An increase in total glutathione (GSH) was also observed in cell culture models[22,25]. Cysteamine must therefore play a significant role in regulating the redox status of the cell, acting not only as a potent antioxidant itself, but also by regulating cysteine, cystine and GSH levels.
However, cysteamine may also be an inhibitor of gamma glutamylcysteine synthetase (γ-GCS), the rate-limiting enzyme in reduced glutathione (GSH) synthesis. γ-GCS is composed of two subunits that interact via a reversible disulfide bridge[26]. This disulfide bridge can undergo a thiol-disulfide exchange with cysteamine ultimately rendering γ-GCS inactive. Vanin 1 knockout mice (Vanin 1-/-) mice, which lack free cysteamine in tissues, display not only an enhanced resistance to oxidative stress, but also show down-regulated tissue inflammation in response to oxidative stress[15,27,28]. In murine liver for example, γ-GCS protein levels were significantly higher in Vanin 1-/- mice than their wild type counterparts and were associated with a higher level of GSH, a response attributed to the enhanced resistance to oxidative stress[15]. Similar results were seen upon induction of acute or chronic inflammation. Interestingly, these results were not replicated in intestinal tissue.
These antithetical effects of cysteamine could be an artifact of Vanin 1 knockout or may be a consequence of tissue specific effects. Variations in the amount and time-dependant release of cysteamine in different tissues in response to a wide range of stimuli may be a mechanism behind Vanin gene involvement in redox regulation, oxidative stress and disease.
4. Functions of Vanin in Disease
A number of recent studies have implicated the vanin gene family in the development and maintainence of disease (see Table 1). Many of these effects have been attributed directly to the effects of cysteamine, but the other product of vanin activity, pantothenic acid may also play a role.
Table.
| Disease | Cell type | Result | Reference |
|---|---|---|---|
| Inflammation (NSAID- and Schistosoma-induced intestinal inflammation) | Intestine (mouse) | Vanin 1-/- mice ↑ GSH and γ-GCS vs. WT + cysteamine negated effect. | Martin et al. 2004 |
| Inflammation (Colitis) | Gut epithelial cells (mouse) | Vanin 1-/- mice ↑ PPARγ mRNA and protein vs. WT. + cysteamine negated effect. | Berruyer et al. 2006 |
| Inflammation (Psoratic skin) | Differentiated keratinocytes (human) | ↑ Vanin 1/Vanin 3 mRNA after addition of Th1 cytokines. | Jensen et al. 2009 |
| Malaria (Inflammation and Oxidative stress) | Spleen, Liver, Blood (mouse) | Vanin 1-/- Vanin 3-/- mice ↑ susceptibility to malaria. + cystamine: ↓ mortality, ↓ blood paresitemia. | Min-Oo et al. 2007 |
| Carcinogenesis (Inflammation-driven colorectal cancer) | Gut epithelial cells (mouse) | Vanin 1-/- mice: 70% ↓ in tumours and ↓ NF-κβ vs. WT. | Pouyet et al. 2010 |
| Huntington's Disease | Brain (mouse) | + cysteamine: ↓ TGase, ↓abnormal movements and prolonged survival. | Karpuj et al. 2002 |
| Huntington's Disease | Cultured astrocytes | + cysteamine: partial suppression of cell death and ↓ cleavage of caspase-3. | Ientile et al. 2003 |
| Wound Healing | Dermal fibroblasts (human) | + pantothenic acid: ↑ number of fibroblasts, ↑ speed and distance travelled. | Weimann et al. 1999 |
| Type 1 diabetes (Drug-induced and auto immune diabetes) | Pancreatic islet cells and kidney capsules | Pancreas: Vanin 1-/- ↑ incidence diabetes ↑ number of cleaved caspase-3 positive cells ↑ susceptibility to death compared to wt. | Roisin-Bouffay et al. 2008 |
| Lipedemia | Blood | + pantethine or + cysteamine ↓ TG, LDL, ApoB ↑ HDL-C and ApoA + pantothenic acid, no change. |
Wittwer et al. 1987 |
Diverse Cysteamine-mediated Effects
In a mouse model of colitis, Vanin 1-/- mice exhibit a loss of cysteamine-mediated inhibition of peroxisome proliferator-activated receptor gamma (PPARγ) resulting in an increase in anti-inflammatory signals and a diminished inflammatory response. In intestinal and thymic epithelial cells PPARγ was reported to be higher in Vanin 1-/- mice than in wild type controls[28]. Importantly, administration of cystamine abrogated this effect. Suppression of PPARγ resulted in the increased expression of inflammatory cytokines and chemokines, a response that may be mediated by cysteamine[15,28,29,30,31].
Cysteamine is a Transglutaminase (TGase) inhibitor[32,33]. TGase catalyses the formation of isopeptide linkages between the γ-carboxamide group of protein bound glutamine to the ε-amino group of protein bound lysine[32]. TGases are thought to play a role in many cellular processes including inflammation and wound healing[34]. Cysteamine inhibits TGase through a thiol-disulfide exchange by binding to an important cysteine residue in the active site of TGase and blocking the formation of a thioester intermediate[12,33,35,36,37]. This has particular relevance to Huntington's disease, characterised by the accumulation of highly cross-linked insoluble proteins, which may be associated with an up-regulation of TGase. Cystamine mediated an increase in cysteine and GSH levels which may contribute to neuroprotection by reducing the high levels of oxidative stress in diseased brain[38]. Indeed, addition of cystamine to cultured astrocytes resulted in the partial suppression of cell death and a significant reduction in the cleavage of caspase-3[32]
Vanin 1 has a cytoprotective effect against type 1 diabetes in islet cells[31]. Vanin 1-/- mice showed an increase in diabetes which related to an increase in cleaved caspase-3 levels in Vanin 1-/- pancreatic islet cells[31]. Addition of cysteamine significantly reduced the number of caspase-3 positive cells. Moreover, Vanin 1-/- islets were twice as susceptible to cell death as wild-type cells. This was reduced to wild type levels upon addition of cystamine. In this disease model, Vanin 1 may act as a pro-survival molecule through the cysteamine mediated inhibition of caspase-3.
Early studies of Vanin 1 describe a role regulating cell adhesion in the migration of haematopoietic precursors in the thymus [3]. In humans, Vanin 2 is reported to participate in haematopoietic cell trafficking[7]. GPI-80, the product of Vanin 2, is thought to play a role in neutrophil adhesion and migration by modulating β2 integrin function[7,39]. Specifically, GPI-80 crosslinking increases CR3 expression. CR3 (Mac1 or CD11b/CD18) is part of the β2 Integrin family [39,40]. CR3 and GPI-80 where shown to be in very close physical proximity (≤7nm) to one another on adherent neutrophils[40]. During neutrophil adherence, CD14 leaves the vicinity of CR3 allowing GPI-80 to interact, facilitating the movement of neutrophils to a wound site[40]. GPI-80 was also shown to cluster on the forward surface of migrating neutrophils[41] which may increase the level of β2 integrin on the surface of activated human neutrophils and so modulate neutrophil adherence and migration.
A number of genetic studies have suggested that the region encoding the Vanin genes may be associated with cancer [42] and inflammation/infection [43]. However, it is also possible that changes in Vanin gene expression are epiphenomenal to the real mechanism of action. Nonetheless, the overwhelming evidence suggesting that cysteamine has a significant role in inflammation, apoptosis and related cellular processes strongly supports a role for vanin activity in the regulation of these disease pathways.
A Role for Pantothenic Acid in Disease?
Little data are available indicating a role for pantothenic acid in disease. As a CoA precursor, it is unlikely that this molecule is a passive bystander. There is some evidence that the effects of pantothenic acid are wider. For instance, pantothenic acid is thought to increase the wound healing process by facilitating the recruitment of fibroblasts to the affected area[1,44]. Addition of pantothenic acid to human dermal fibroblasts increased not only the number of cells across the edge of the wound but also the speed and distance these cells travelled[44]. It is of note that Vanin 1 itself also has effects at a wound site[15]; a decrease in F4/80+ macrophages occurs in the absence of Vanin 1[28,30,31]. This action of vanin could be beneficial to help rid the wound of pathogens and damaged cells, but whether pantothenate is directly involved is yet to be determined.
Tne Vanins in Cardiovascular Disease
Our interest in the Vanins and their metabolic products stems from our recent observation that Vanin 1 expression shows a strong correlation with cardiovascular phenotypes[45]. Using transcript levels as endophenotypes, we identified Vanin 1 as a quantitative trait locus associated with high density lipoprotein-cholesterol (HDL-C) levels. Vanin 1 exhibits a genetic correlation with HDL-C of 0.28 based on quantitative differences in mean HDL-C levels and variation in Vanin 1 genotype. Given its central role in recycling CoA, an intriguing possiblity is that Vanin-1 is a central regulator of not only stress responses via production of cysteamine but also lipid biosynthesis by controlling the flux through the fatty acid and/or cholesterol biosynthetic pathways (see figure 3). Review of what is known about pantotheinase and its effects provides some credence to this notion.
In this regard, pantethine administration has been shown to lower serum triglycerides, low density lipoprotein (LDL) and Apo-B while increasing HDL-C and Apo-A[46,47]. Wittwer et al. determined the contribution of pantethine and its breakdown products on lipid profiles[17]. Pantethine and cysteamine showed similar cholesterol lowering effects on lipid profiles, whereas pantothenic acid did not[17]. Therefore, cysteamine produced either by the action of Vanin on pantethine (via its reduction to pantetheine) or by direct administration, is likely influencing lipid metabolism. The mechanism by which this occurs is still largely unknown. Early work suggested that cysteamine inhibited HMG-CoA (3-hydroxy-3-methylglutaryl-CoA) causing a reduction in cholesterol synthesis and perhaps a shift towards fatty acid metabolism[48]. Further work suggested that pantethine helps breakdown triglyceride deposits as well as promoting fatty acid catabolism in the liver[47]. More recently the effects of taurine on cholesterol and lipid levels have been reported[49]. Zhang et al. (2003) showed a hypocholesterolemic effect of dietary taurine in overweight adults[50] and in a 2008 study, taurine was shown to reduce the secretion of apolipoprotein B100 and lipids in liver cells[51]. Hence, taurine supplementation may be beneficial for the prevention of artherosclorosis. As cysteamine can be converted to taurine via hypotaurine (see Figure 3), Vanin also may be involved in regulating lipid biosynthesis by regulating the abundance of taurine.
The various data indicating a role for cysteamine in improving lipid profiles suggests that increased Vanin 1 activity may have a protective effect against cardiovascular disease. This, however, contrasts with the negative effects of cysteamine-driven inflammation and oxidative stress. Again, the antithetical effects of cysteamine may be explained by tissue-specific regulation by pantetheinase. We propose that Vanin gene expression is likely to be tightly controlled within a tissue in order to regulate the abundance of cysteamine and pantothenic acid in response to stimuli and the differences between, or even within tissues may be both contrasting and antagonistic.
Emerging Importance
The consequences of Vanin gene family expression remain unclear. Involvement in inflammation is supported, but specific roles in other pathways are not clear. The effects of the Vanin genes seem to be driven by cysteamine, but the possible role of pantothenic acid cannot be ignored. It is likely that the concentration of these two products, which is dependent on pantetheinase activity, is important for maintaining normal cell function so Vanin expression must be tightly controlled. Dysregulation of these genes would disrupt a number of biological pathways setting in motion a cascade of events, contributing to a disease state. The manipulation of Vanin expression or inhibition/administration of cysteamine could be eficacious in the treatment of a number of diseases. With respect to cardiovascular disease, it may be possible to regulate both fatty acid biosynthesis and the stress response simply by regulating Vanin activity.
Highlights.
> vanin pantetheinases are related to biotinidase and are conserved. > product of vanin activity, cysteamine, regulates diverse cellular activities. > vanin genes have been implicated in the eitiology of many diseases. > vanin genes influence cardiovascular disease phenotypes such as lipid profile.
Acknowledgments
FINANCIAL DISCLOSURE
This study was supported by National Institutes of Health grant HL93537 (E.K.M. & L.J.A.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Martin F, Malergue F, Pitari G, et al. Vanin genes are clustered (human 6q22-24 and mouse 10A2B1) and encode isoforms of pantetheinase ectoenzymes. Immunogenetics. 2001;53:296–306. doi: 10.1007/s002510100327. [DOI] [PubMed] [Google Scholar]
- 2.Galland F, Malergue F, Bazin H, et al. Two human genes related to murine Vanin-1 are located on the long arm of human chromosome 6. Genomics. 1998;53:203–213. doi: 10.1006/geno.1998.5481. [DOI] [PubMed] [Google Scholar]
- 3.Aurrand-Lions M, Galland F, Bazin H, et al. Vanin-1, a novel GPI-linked perivascular molecule involved in thymus homing. Immunity. 1996;5:391–405. doi: 10.1016/s1074-7613(00)80496-3. [DOI] [PubMed] [Google Scholar]
- 4.Maras B, Barra D, Dupre S, et al. Is pantetheinase the actual identity of mouse and human vanin-1 proteins? Journal of Clinical Investigation. 1999;113:149–152. doi: 10.1016/s0014-5793(99)01439-8. [DOI] [PubMed] [Google Scholar]
- 5.Pitari G, Malergue F, Martin F, et al. Pantetheinase activity of membrane-bound Vanin-1: lack of free cysteamine in tissues of Vanin-1 deficient mice. Febs Letters. 2000;483:149–154. doi: 10.1016/s0014-5793(00)02110-4. [DOI] [PubMed] [Google Scholar]
- 6.Nitto T, Inoue T, Node K. Alternative spliced variants in the pantetheinase family of genes expressed in human neutrophils. Gene. 2008;426:57–64. doi: 10.1016/j.gene.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki K, Watanabe T, Sakurai S, et al. A Novel Glycosylphosphatidyl Inositol-Anchored Protein on Human Leukocytes: A Possible Role for Regulation of Neutrophil Adherence and Migration. Journal of Immunology. 1999;162:4277–4284. [PubMed] [Google Scholar]
- 8.Udenfriend S, Kodukula K. How Glycosyl-Phosphatidylinositol-Anchored Membrane Proteins are Made. Annual Review of Biochemistry. 1995;64:563–591. doi: 10.1146/annurev.bi.64.070195.003023. [DOI] [PubMed] [Google Scholar]
- 9.Koike S, Takeda Y, Hozumi Y, et al. Immunohistochemical localization in human tissues of GPI-80, a novel glycosylphosphatidyl inositol-anchored protein that may regulate neutrophil extravasation. Cell and Tissue Research. 2002;307:91–99. doi: 10.1007/s00441-001-0481-z. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Takeda Y, Watanabe T, et al. A sandwich ELISA for detection of soluble GPI-80, a glycosylphosphatidyl-inositol (GPI)-anchored protein on human leukocytes involved in regulation of neutrophil adherence and migration--its release from activated neutrophils and presence in synovial fluid of rheumatoid arthritis patients. Microbiology and Immunology. 2001;45:467–471. doi: 10.1111/j.1348-0421.2001.tb02646.x. [DOI] [PubMed] [Google Scholar]
- 11.Wittwer CT, Gahl WA, Butler JD, et al. Metabolism Of Pantethine In Cystinosis. Journal of Clinical Investigation. 1985;76:1665–1672. doi: 10.1172/JCI112152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jansen P, Kamsteeg M, Rodijk-Olthuis D, et al. Expression of the Vanin Gene Family in Normal and Inflamed Human Skin: Induction by Proinflammatory Cytokines. Journal of Investigative Dermatology. 2009:2167–2174. doi: 10.1038/jid.2009.67. [DOI] [PubMed] [Google Scholar]
- 13.Zhang B, Lo C, Shen L, et al. The role of vanin-1 and oxidative stress-related pathways in distinguishing acute and chronic pediatric ITP. Blood. 2011 doi: 10.1182/blood-2010-09-304931. [DOI] [PubMed] [Google Scholar]
- 14.Wittwer CT, Burkhard D, Ririe K, et al. Purification and properties of a pantetheine-hydrolyzing enzyme from pig kidney. Journal of Biological Chemistry. 1983;258:9733–9738. [PubMed] [Google Scholar]
- 15.Martin F, Penet MF, Malergue F, et al. Vanin-1(-/-) mice show decreased NSAID- and Schistosoma-induced intestinal inflammation associated with higher glutathione stores. Journal of Clinical Investigation. 2004;113:591–597. doi: 10.1172/JCI19557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dupre S, Graziani MT, Rosei MA, et al. Enzymatic Breakdown Of Pantethine To Pantothenic Acid And Cystamine. European Journal of Biochemistry. 1970;16:571–&. doi: 10.1111/j.1432-1033.1970.tb01119.x. [DOI] [PubMed] [Google Scholar]
- 17.Wittwer C, Graves C, Peterson M, et al. Pantethine lipomodulation: evidence for cysteamine mediation in vitro and in vivo. Atherosclerosis. 1987;68:41–49. doi: 10.1016/0021-9150(87)90092-x. [DOI] [PubMed] [Google Scholar]
- 18.Daugherty M, Polanuyer B, Farrell M, et al. Complete Reconstitution of the Human Coenzyme A Biosynthetic Pathway via Comparative Genomics. Journal of Biological Chemistry. 2002;277:21431–21439. doi: 10.1074/jbc.M201708200. [DOI] [PubMed] [Google Scholar]
- 19.Coloso RM, Hirschberger LL, Dominy JE, et al. Cysteamine Dioxygenase: Evidence for the Physiological Conversion of Cysteamine to Hypotaurine in Rat and Mouse Tissues. In: Oja SS, Saransaari P, editors. Taurine 6. Springer US; 2006. pp. 25–36. [DOI] [PubMed] [Google Scholar]
- 20.Butler J, Zetz M. Pantethine and Cystamine Deplete Cystine from Cystinotic Fibroblasts via Efflux of Cysteamine-Cysteine Mixed Disulfide. Journal of Clinical Investigation. 1984;74:411–416. doi: 10.1172/JCI111436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood PL, Khan MA, Moskal JR. Cellular thiol pools are responsible for sequestration of cytotoxic reactive aldehydes: Central role of free cysteine and cysteamine. Brain Research. 2007;1158:158–163. doi: 10.1016/j.brainres.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Fox JH, Barber DS, Singh B, et al. Cystamine increases l-cysteine levels in Huntington's disease transgenic mouse brain and in a PC12 model of polyglutamine aggregation. Journal of Neurochemistry. 2004;91:413–422. doi: 10.1111/j.1471-4159.2004.02726.x. [DOI] [PubMed] [Google Scholar]
- 23.Jokay I, Kelemenics K, Gyuris A, et al. S-methylthio-cysteine and cystamine are potent stimulators of thiol production and glutathione synthesis. Life Sciences. 1997;62:PL/27–PL/33. doi: 10.1016/s0024-3205(97)01066-7. [DOI] [PubMed] [Google Scholar]
- 24.Pinto JT, Van Raamsdonk JM, Leavitt BR, et al. Treatment of YAC128 mice and their wild-type littermates with cystamine does not lead to its accumulation in plasma or brain: implications for the treatment of Huntington disease. Journal of Neurochemistry. 2005;94:1087–1101. doi: 10.1111/j.1471-4159.2005.03255.x. [DOI] [PubMed] [Google Scholar]
- 25.Lesort M, Lee M, Tucholski J, et al. Cystamine inhibits caspase activity. Implications for the treatment of polyglutamine disorders. Journal of Biological Chemistry. 2003;278:3825–3830. doi: 10.1074/jbc.M205812200. [DOI] [PubMed] [Google Scholar]
- 26.Hayes JD, McLellan LI. Glutathione and glutathione-dependent enzymes represent a Co-ordinately regulated defence against oxidative stress. Free Radical Research. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 27.Berruyer C, Martin FM, Castellano R, et al. Vanin-1(-/-) mice exhibit a glutathione-mediated tissue resistance to oxidative stress. Molecular and Cellular Biology. 2004;24:7214–7224. doi: 10.1128/MCB.24.16.7214-7224.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berruyer C, Pouyet L, Millet V, et al. Vanin-1 licenses inflammatory mediator production by gut epithelial cells and controls colitis by antagonizing peroxisome proliferator-activated receptor gamma activity. Journal of Experimental Medicine. 2006;203:2817–2827. doi: 10.1084/jem.20061640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meghari S, Berruyer C, Lepidi H, et al. Vanin-1 controls granuloma formation and macrophage polarization in Coxiella burnetii infection. European Journal of Immunology. 2007;37:24–32. doi: 10.1002/eji.200636054. [DOI] [PubMed] [Google Scholar]
- 30.- L, et al. Epithelial Vanin-1 Controls Inflammation-Driven Carcinogenesis in the Colitis-Associated Colon Cancer Model. Inflammatory Bowel Diseases. 2010;16 doi: 10.1002/ibd.21031. [DOI] [PubMed] [Google Scholar]
- 31.Roisin-Bouffay C, Castellano R, Valero R, et al. Mouse vanin-1 is cytoprotective for islet beta cells and regulates the development of type 1 diabetes. Diabetologia. 2008;51:1192–1201. doi: 10.1007/s00125-008-1017-9. [DOI] [PubMed] [Google Scholar]
- 32.Ientile R, Campisi A, Raciti G, et al. Cystamine inhibits transglutaminase and caspase-3 cleavage in glutamate-exposed astroglial cells. Journal of Neuroscience Research. 2003;74:52–59. doi: 10.1002/jnr.10702. [DOI] [PubMed] [Google Scholar]
- 33.Karpuj M, Becher M, Springer J, et al. Prolonged survival and decreased abnormal movements in transgenic model of Huntington disease, with administration of the transglutaminase inhibitor cystamine. Nature Medicine. 2002;8:143–149. doi: 10.1038/nm0202-143. [DOI] [PubMed] [Google Scholar]
- 34.Greenberg C, Birckbichler P, Rice R. Transglutaminases:multifunctional cross-linking enzymes that stabilize tissues. FASEB Journal. 1991;5:3071–3077. doi: 10.1096/fasebj.5.15.1683845. [DOI] [PubMed] [Google Scholar]
- 35.Di Leandro L, Maras B, Schinina ME, et al. Cystamine restores GSTA3 levels in Vanin-1 null mice. Free Radical Biology and Medicine. 2008;44:1088–1096. doi: 10.1016/j.freeradbiomed.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 36.Jeon J, Lee H, Jang G, et al. Different inhibition characteristics of intracellular transglutaminase activity by cystamine and cysteamine. Experimental and Molecular Medicine. 2004;36:576–581. doi: 10.1038/emm.2004.74. [DOI] [PubMed] [Google Scholar]
- 37.Lorand L, Parameswaran K, Stenberg P, et al. Specificity of guinea pig liver transglutaminase for amine substrates. Biochemistry. 1979;18:1756–1765. doi: 10.1021/bi00576a019. [DOI] [PubMed] [Google Scholar]
- 38.Bailey CDC, Johnson GVW. The protective effects of cystamine in the R6/2 Huntington's disease mouse involve mechanisms other than the inhibition of tissue transglutaminase. Neurobiology of Aging. 2006;27:871–879. doi: 10.1016/j.neurobiolaging.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Yoshitake H, Takeda Y, Nitto T, et al. Cross-linking of GPI-80, a possible regulatory molecule of cell adhesion, induces up-regulation of CD11b/CD18 expression on neutrophil surfaces and shedding of L-selectin. Journal of Leukocyte Biology. 2002;71:205–211. [PubMed] [Google Scholar]
- 40.Huang J-B, Takeda Y, Araki Y, et al. Molecular proximity of complement receptor type 3 (CR3) and the glycosylphosphatidylinositol-linked protein GPI-80 on neutrophils: effects of cell adherence, exogenous saccharides, and lipid raft disrupting agents. Molecular Immunology. 2004;40:1249–1256. doi: 10.1016/j.molimm.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura-Sato Y, Sasaki K, Watanabe H, et al. Clustering on the forward surfaces of migrating neutrophils of a novel GPI-anchored protein that may regulate neutrophil adherence and migration. Journal of Leukocyte Biology. 2000;68:650–654. [PubMed] [Google Scholar]
- 42.Huang H, Dong X, Kang MX, et al. Novel Blood Biomarkers of Pancreatic Cancer–Associated Diabetes Mellitus Identified by Peripheral Blood–Based Gene Expression Profiles. American Journal of Gastroenterology. 2010;105:1661–1669. doi: 10.1038/ajg.2010.32. [DOI] [PubMed] [Google Scholar]
- 43.Min-Oo G, Fortin A, Pitari G, et al. Complex genetic control of susceptibility to malaria: positional cloning of the Char9 locus. Journal of Experimental Medicine. 2007;204:511–524. doi: 10.1084/jem.20061252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weimann BI, Hermann D. Studies on wound healing: effects of calcium D-pantothenate on the migration, proliferation and protein synthesis of human dermal fibroblasts in culture. International Journal for Vitamin and Nutrition Research. 1999;69:113–119. doi: 10.1024/0300-9831.69.2.113. [DOI] [PubMed] [Google Scholar]
- 45.Goring HHH, Curran JE, Johnson MP, et al. Discovery of expression QTLs using large-scale transcriptional profiling in human lymphocytes. Nature Genetics. 2007;39:1208–1216. doi: 10.1038/ng2119. [DOI] [PubMed] [Google Scholar]
- 46.Maggi G, Donati C, Criscuoli G. Pantethine: A physiological lipomodulating agent, in the treatment of hyperlipidemias. Current Therapeutic Research. 1982;32:380–386. [Google Scholar]
- 47.Bocos C, Herrera E. Pantethine stimulates lipolysis in adipose tissue and inhibits cholesterol and fatty acid synthesis in liver and intestinal mucosa in the normolipidemic rat. Environmental Toxicology and Pharmacology. 1998;6:59–66. doi: 10.1016/s1382-6689(98)00020-9. [DOI] [PubMed] [Google Scholar]
- 48.Cighetti G, Del Puppo M, Paroni R, et al. Pantethine inhibits cholesterol and fatty acid syntheses and stimulates carbon dioxide formation in isolated rat hepatocytes. J Lipid Res. 1987;28:152–161. [PubMed] [Google Scholar]
- 49.Lombardini J, Militante J. Effects of Taurine Supplementation on Cholesterol Levels with Potential Ramification in Atherosclerosis. In: Oja SS, Saransaari P, editors. Taurine 6. Springer US; 2006. pp. 251–254. [DOI] [PubMed] [Google Scholar]
- 50.Zhang M, Bi LF, Fang JH, et al. Beneficial effects of taurine on serum lipids in overweight or obese non-diabetic subjects. Amino Acids. 2004;26:267–271. doi: 10.1007/s00726-003-0059-z. [DOI] [PubMed] [Google Scholar]
- 51.Yanagita T, Han S-Y, Hu Y, et al. Taurine reduces the secretion of apolipoprotein B100 and lipids in HepG2 cells. Lipids in Health and Disease. 2008;7:38. doi: 10.1186/1476-511X-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]