Abstract
Common complaints of the elderly involve impaired cognitive abilities, such as loss of memory and inability to attend. Although much research has been devoted to these cognitive impairments, other factors such as disrupted sleep patterns and increased daytime drowsiness may contribute indirectly to impaired cognitive abilities. Disrupted sleep–wake cycles may be the result of age-related changes to the internal (circadian) clock. In this article, we review recent research on aging and circadian rhythms with a focus on the senescence-accelerated mouse (SAM) as a model of aging. We explore some of the neurobiological mechanisms that appear to be responsible for our aging clock, and consider implications of this work for age-related changes in cognition.
Key words: aging, C-Fos, circadian rhythms, mouse, running wheel, SAMP8, suprachiasmatic nucleus
Cognitive impairments are well-known complaints of the elderly. Despite their obvious clinical importance, many questions remain unanswered. One important issue concerns whether cognitive impairments are due to direct alterations in the cognitive function, or to other difficulties that may in turn result in cognitive deficits. For example, the elderly often report poor night-time sleep quality, daytime sleepiness, lack of energy, and difficulty adjusting to a time shift (increased “jet lag”). Could these effects contribute to cognitive impairments?
Although a confluence of factors may lead to poor sleep quality, etc., it is likely that one general culprit is aging of the internal (circadian) clock that regulates these behaviors (Munch et al. 2005; Turek et al. 1995; Weitzman et al. 1982). Moreover, evidence suggests that our internal clock may also mediate cognitive functioning (Antoniadis et al. 2000; Brock 1991, Stone 1989; van Gool 1986; van Someren et al. 1993a). In this article, we review recent research on aging and circadian rhythms, with a focus on the senescence-accelerated mouse line. We explore some of the neurobiological mechanisms that appear to be responsible for our aging clock, and consider implications of this work for the study of age-related changes in cognition.
Circadian rhythms are predictable and regularly occurring daily changes in behavior and physiological states. The term circadian, literally meaning “around a day,” has its origins in two Latin words: Circa (around) and dies (day). Two defining characteristics of circadian rhythms are their continued persistence in the absence of external cues in the environment and their ability to be entrained (synchronized) by environmental cues. With respect to the former property, it is the endogenous (self-sustaining) nature of circadian rhythms that engenders an internal clock. With respect to the latter property, the major external cue that serves to synchronize the circadian rhythms of humans and other mammals is light, but almost any periodic event can act as a Zeitgeber (“time-giver”).
Research on the biological basis of circadian rhythms in humans and other mammals implicates the suprachiasmatic nucleus (SCN) of the anterior hypothalamus as the brain region responsible for the generation and maintenance of circadian rhythms (Herzog and Schwartz 2002; Klein et al. 1991). Support for the SCN as the master circadian clock comes from a wide variety of sources. Perhaps the most direct evidence is the rhythmic pattern of cell activity that is observed with in vivo and in vitro SCN preparations (Gillete 1991; Schwartz 1991). Furthermore, both complete and partial lesions of the SCN produce an array of rhythm disruptions that are reversible with transplants of fetal SCN tissue (Kaufman and Menaker 1993; Lehman et al. 1987; Lehman et al. 1991; Ralph et al. 1990; Ralph et al. 1993). Not only do these tissue transplants restore rhythm to the SCN, but the endogenous period of the restored rhythm matches the period of the donor (Ralph et al. 1990).
The SCN can be divided into two major areas, which have been given different labels depending on the animal species (Abrahamson and Moore 2001; Moore 1995; Moore 1996). In the mouse, the two divisions are referred to as “core” and “shell” (Abrahamson and Moore 2001; Moore 1995; Moore 1996). The core, which receives direct retinal inputs, shows cell activity that is primarily light-induced (Cai et al. 1997; Guido et al. 1999b; Schwartz et al. 1995; Schwartz et al. 2000; Takashi 1993). This cell activity follows the behavioral pattern of the animal’s phase responses to light pulses, suggesting that cells of the core are involved in entrainment to the environmental light-dark cycle (Aronin and Schwartz 1991; Colwell and Foster 1992; Schwartz et al. 2000). The shell of the SCN does not receive direct retinal input, and displays spontaneous rhythmic cell activity in constant conditions. For this reason, the shell may be important in the generation of free-running (endogenous) rhythms (Guido et al. 1999a; Schwartz et al. 2000).
Age-related disruptions of circadian rhythms are a common occurrence in many species, including humans, and are characterized by changes in both behavior and physiology (Brock 1991; Ingram et al. 1982; Miles and Dement 1980; van Gool 1986). In elderly humans, rhythm disturbances include fragmented sleep–wake patterns, weak coupling with environmental rhythms, reduced amplitude of daily body temperature rhythms, alterations in the daily rhythm of hormone secretion, high levels of nighttime activity, and reduced daytime cognitive performance (Brock 1991; van Someren et al. 1993b; van Someren et al. 1996; Weitzman et al. 1982). Similar types of behavioral changes occur in aged animals (Valentinuzzi et al. 1997; Witting et al. 1994). These changes in circadian rhythms may, at least in part, contribute to the cognitive and physical impairments observed in aged individuals (Antoniadis et al. 2000).
Age-related disruptions of circadian rhythms may be due to alterations in the SCN. Neurobiological changes of the SCN that have been associated with circadian rhythm disruptions in aged animals have included a reduction in the number of cells and the volume of the SCN (Brock 1991; Swaab et al. 1985), a decrease in total and rhythmic expression of mRNA for vasoactive intestinal polypeptide in the SCN (Kawakami et al. 1997; Krajnak et al. 1998), and attenuated c-Fos expression induced by light (Cai et al. 1997; Guido et al. 1999b; Schwartz et al. 1995; Schwartz et al. 2000; Takahshi 1993). Although these findings do not discount other possible age-related changes outside the SCN, they do suggest that some changes within the SCN itself could be involved in the circadian rhythm disruptions observed in aged individuals.
Senescence-accelerated mouse as a model of aging
Over the past few years, our laboratory has been investigating age-related changes in the circadian rhythms of an inbred strain of mouse that shows accelerated aging. Two major lines of senescence-accelerated mouse (SAM) have been developed by selective breeding. One line is prone to the early onset of age-related pathologies and behavioral impairments (SAMP), whereas the other line shows a normal rate of aging (SAMR) (Takeda et al. 1991). Because SAMP and SAMR mice are derived from the same background strain (AKR/J), studies on the biological differences of the two lines could highlight mechanisms involved in aging. Both SAMP and SAMR lines have been further divided into sub-lines that have different types of pathology (Takeda et al. 1991). Of the 9 SAMP sub-lines, the P8 and P10 have exaggerated age-related impairments of learning and memory and circadian rhythms as compared to the R1 sub-line (Flood and Morley 1998; Miyamoto 1997; Miyamoto 2004; Miyamoto et al. 1986; Takeda 1999; Yagi et al. 1988). The SAMP8 tend to have more accelerated impairments of learning and memory, and the deficits are more pronounced than the SAMP10 (Miyamoto 1997). Furthermore, previous research has highlighted the age-related hippocampal changes in the SAMP8, whereas much of the focus of research in the SAMP10 has been on neocortical changes, although this distinction is not absolute (Flood and Morley 1998; Han et al. 2004; Morley et al. 2004; Sano et al. 2004; Shimada 1999; Shimada et al. 2003). Because we were interested in the possible relationship between circadian rhythm disruptions and hippocampal learning and memory deficits, we used the SAMP8 rather than the SAMP10 for our studies.
Neurochemical, molecular, and genetic studies of the SAMP8 have begun to define the neurobiological changes that may be responsible for age-related cognitive impairments in this animal model. These findings have recently been reviewed (Butterfield and Poon 2005). A number of genes and proteins are abnormally expressed in aged SAMP8, but most are related to a few important functions, including neuroprotection, signal transduction, immune function, energy metabolism and cytoskeletal elements (Butterfield and Poon 2005; Nomura et al. 2004; Takahashi and Goto 2004). These findings suggest that aged SAMP8 suffer from increased oxidative stress, possibly leading to altered protein formation and neurochemical changes (Butterfield and Poon 2005). For most of these genes and proteins, further research is needed to link the particular changes to age-related cognitive impairments and circadian rhythm irregularities. However, some transmitters and proteins have been associated with the age-related learning and memory impairments. Changes in acetylcholine and glutamate transmitter systems make the aged SAMP8 more sensitive to pharmacological manipulations that alter learning and memory performance (Flood and Morley 1998; Fujiwara et al. 2004). SAMP8 also exhibit age-related increases in β-amyloid (Aβ) levels. Moreover, the increase in Aβ may lead to cognitive deficits, because reducing the circulating levels of Aβ reduces oxidative stress and improves learning and memory (Kumar et al. 2000; Poon et al. 2004). Together with changes in other Alzheimer’s disease related markers, such as apolipoprotein E and presenilin-2, the SAMP8 seems to be an attractive animal model for studying the cognitive impairments associated with Alzheimer’s disease, as well as normal aging.
Because of the number of neurobiological changes, the SAMP8 represents a different type of animal model of Alzheimer’s disease compared to transgenic mouse lines that over express Aβ or Tau, two hallmarks of Alzheimer’s disease. Transgenic models of Alzheimer’s disease provide researchers with a way to specifically assess the effects of increased levels of the Aβ or Tau proteins, and determine whether increases in either of these proteins lead to learning impairments. On the other hand, the SAMP8 may more closely represent the complexity of the disease because of its multifactorial nature. Furthermore, although transgenic models may demonstrate the cognitive impairment of the disease, SAMP8 may mimic more symptoms, including the circadian rhythm alterations, as well as the cognitive impairments.
Age-related changes in circadian rhythm of SAMP8
Our initial study of circadian rhythms characterized age-related changes in SAMP8 using wheel-running activity (McAuley et al. 2002). Assessment of circadian rhythms using wheel-running activity is analogous to the rest–activity rhythms used to examine circadian rhythms in humans (van Someren et al. 1996, 1997). In this study, mice of three ages (2 , 7 and 12 months) were allowed free access to running wheels for a period of 16 days. For the first 6 days of testing, mice were maintained on a 12:12 light:dark cycle that matched the light:dark cycle of their colony room. For the remaining 10 days, mice were housed in constant darkness to assess characteristics of their endogenous (“free running”) rhythm.
In general, all ages of SAMP8 showed a daily rhythmic pattern of wheel-running activity characteristic of the circadian rhythms of nocturnal animals. However, there were clear age differences that were apparent by 7 months of age; older mice tended to run less and exhibited more fragmented activity patterns. When compared to young mice, aged mice ran less, and this difference was observed primarily during the night when mice are normally most active. The amount of daytime activity also increased between 2 and 7 months, but then decreased between 7 and 12 months. When expressed as a proportion of total activity, 7- and 12-month-old SAMP8 were more active during the day than 2-month-old SAMP8. Consistent with these results, analysis of periodograms constructed for each animal showed dramatic changes in the amplitude of the activity rhythms. The amplitude quantifies the relative strength of an underlying rhythm at a given period (i.e., 24 hours). Overall amplitude of the 24-hour rhythm was substantially reduced in both 7- and 12-month-old mice relative to 2-month-old mice.
Reports of age-related changes to the period of the circadian rhythm are inconsistent across a variety of animals, varying from shortening to no change to lengthening (Asai et al. 2000; Davis and Viswanathan 1998; Duffy et al. 1999; McAuley et al. 2002; Morin 1988; Pang et al. 2004; Sanchez-Barcelo et al. 1997). Although a previous study found that aged SAMP8 had a longer period of the circadian rhythm than young SAMP8 (Asai et al. 2000), we found no evidence of age-related changes to the circadian period (McAuley et al. 2002). When light cues were available, all ages of SAMP8 were accurately synchronized to (entrained by) the 12:12 light:dark cycle. Mean periods (tau) of activity rhythms during the light:dark cycle were within 0.1 hr of 24 hours for all ages. When light cues were eliminated (constant darkness or DD), free-running periods were significantly less than 24 hours (23.56±0.06, 23.58±0.05, and 23.69±0.06 hours for the 2-, 7-, and 12-month-old mice respectively). A trend was observed for lengthening of the free-running period with age, but this effect was not significant. We have since replicated this lack of change in the free-running period in a longitudinal study of SAMP8 (Pang et al. 2004). However, a previous study found a lengthening of the free-running period in SAMP8, although the effect was relatively small—on the order of 0.3 hours (Asai et al. 2000). A conservative conclusion from this work is that age-related alterations in circadian period are rather weak and do not show consistent changes in the SAMP8.
In summary, our investigations of circadian rhythms in the SAMP8 reveal age-related rhythm disruptions that appear as early as 7 months. Aged SAMP8 show decreased amount of wheel-running activity, decreased rhythm amplitude, and increased fragmentation. Contrary to previous reports, we did not find any age-related differences in free-running period. Overall, these findings in the SAMP8 are similar to the age-related rhythm disruptions reported for healthy elderly adults, individuals with senile dementia, and for other species (McAuley et al. 2002; Satlin et al. 1991; van Gool 1986; van Someren et al. 1993a). Patients with senile dementia, in particular, show excessive levels of nighttime activity, in relation to healthy elderly of the same age (Satlin et al. 1991; van Gool 1986; van Someren et al. 1993a). Because the night is the normally inactive period for humans, this finding in dementia patients may be analogous to the increased daytime activity observed in aged SAMP8. These similarities support the use of the SAMP8 as an animal model of circadian rhythm disruptions associated with human aging and Alzheimer’s disease.
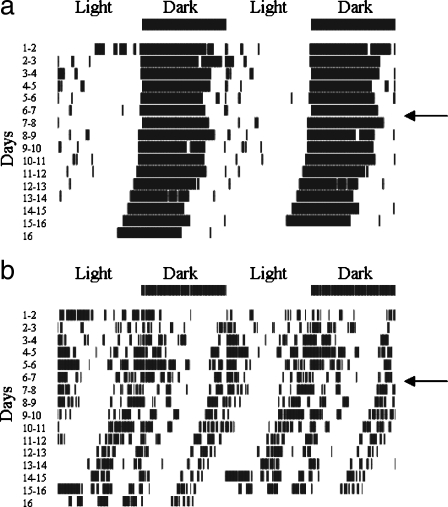
Rhythm splitting One aspect of the activity rhythms of the aged SAMP8 appears to be particularly unusual. Concomitant with the decrease in amplitude of the 24-hour rhythm was a somewhat surprising increase in the amplitude of a secondary peak near 12 hours in the older mice (McAuley et al. 2002). The amplitude of the secondary peak increased between 2 and 7 months, without further change between the 7- and 12-month-old mice.The basis for this 12-hour rhythm can be observed in the double-plotted actograms (Figure 1). Young SAMP8 typically have a behavioral pattern in which running activity starts just before the lights turn off and continues for most of the dark period, until a reduction in running occurs just before the lights turn on. In 7- and 12-month-old mice, there appeared to be two bouts of activity; one near the beginning of the dark period and the other near the beginning of the light period. The presence of dual activity bouts observed in 7- and 12-month-old SAMP8 is a pattern similar to that observed in young adult hamsters following extended periods in constant conditions (either light or dark). This finding has been called “rhythm splitting” (Pittendrigh and Daan 1976), and can occur in other mammals and birds (Aschoff 1967; Gwinner 1974; Hoffmann 1971; Pittendrigh 1960), but the phenomenon has been most studied in hamsters. In the hamster, rhythm splitting occurs when a single daily bout of locomotor activity dissociates into two periodic components. Given enough time, the two components become synchronized so that each component is cycling 180° apart (in anti phase).One explanation of splitting describes two mutually coupled oscillators with two stable modes of entrainment (Pittendrigh and Daan 1976). The two oscillators are referred to as the E and M oscillators, with the E oscillator controlling the onset of activity in the evening and the M oscillator controlling the offset of activity in the morning. In the normally unsplit circadian rhythm, the E and M oscillators are mutually entrained, with the M oscillator lagging the E oscillator by several hours. Under certain conditions, the E and M oscillators can split, first free-running independently before re-entraining with a 180° phase relationship. Evidence from hamsters with split circadian rhythms suggests that the two oscillators may be functionally localized to the left and right hemispheres of the SCN. First, unilateral SCN lesions in split hamsters abolish the splitting, and produce a single bout of locomotion (Daan and Berde 1978). Second, the SCN hemispheres are in antiphase with one another, as assessed by expression of genes involved in circadian rhythm such as Per1 (de la Iglesia et al. 2000). This anti phase relation is only observed for hamsters demonstrating split rhythms.The effect of age on rhythm splitting has been examined in hamsters (Duncan and Deveraux 2000; Morin 1988). In the hamster, the incidence of spontaneous rhythm splitting in constant light decreased with age, in contrast to the results from our study. However, hints of an increase in “split-like” activity rhythms in aged SAMP8 can be seen in another study. Miyamoto observed that a large proportion of activity during the light phase tended to occur primarily during the first 3–4 hours of the light period (Miyamoto 1997). This activity in addition to the normal activity observed during the dark phase is consistent with a split rhythm. Furthermore, the same study showed spontaneous motor activity that appears to show a split-like activity rhythm in the 8- and 12-month old SAMP8, but not younger subjects, although the author does not comment on this observation (Miyamoto 1997). These results, together with those of our studies, strongly suggest that aging in the SAMP8 increased the incidence of split-like activity rhythms in contrast to the decreased incidence in aged hamsters (Duncan and Deveraux 2000; Morin 1988). Three possible explanations for the differences are: (1) species differences, (2) the manner in which split rhythms were produced (i.e., constant dark vs constant light), and (3) different processes that may be occurring in hamsters and SAMP8 mice.We monitored c-Fos expression in the SCN to address the latter possibility, and in particular we were interested in two issues (Miller et al. 2005). First, we wanted to determine whether the reduced 24-hour rhythm amplitude in aged SAMP8 might be associated with a decrease of SCN neuronal activity. Second, we wanted to examine whether hemispheric asymmetries existed in c-Fos expression similar to that observed in young hamsters with split circadian rhythms (de la Iglesia et al. 2000). If c-Fos expression in the two SCN hemispheres were cycling 180° out of phase, it would provide evidence that split rhythms in aged SAMP8 were similar to that found in young hamsters.To assess the endogenous rhythms of the SCN, mice were kept in the dark for 14 (CT2) or 26 (CT14) hours prior to sacrifice. CT2 corresponds to 2 hours after the lights would normally turn on, and CT14 is 2 hours after the lights would normally have turned off; times that correspond respectively to the highest and lowest levels of spontaneous c-Fos expression in the SCN (Sumova et al. 1998). No differences in the number of c-Fos-ir cells were observed across the three ages of SAMP8, even though regional differences were observed in the SCN shell and core, and more c-Fos-ir cells were observed at CT2 than at CT14. The difference between CT2 and CT14 was greater in the shell than in the core, consistent with the idea that the shell of the SCN is important in the endogenous rhythm (Guido et al. 1999a; Schwartz et al. 2000). Differences in c-Fos expression for time of day and SCN sub-regions are in agreement with previous reports in rats (Guido et al. 1999b; Sumova et al. 1998; Sumova et al. 2000). Our results suggest that the reduction in activity rhythm amplitude observed in aged SAMP8 was not due to a reduction in SCN neuronal activity.To investigate whether hemispheric asymmetries were more likely in aged SAMP8, a correlational analysis was performed on the left- and right-hemisphere cell counts in the shell of the SCN. The core was not analyzed because, on the whole, its cell counts were lower and less variable than counts from the shell across CT2 and CT14. The regression lines for all three ages were similar, and had slopes close to 1 (2-month = 0.94, 7-month = 1.05, 12-month = 1.04). Slopes near 1 provide evidence that the hemispheres of the SCN are not oscillating 180° out of phase as observed during rhythm splitting in hamsters. Based on this finding, the “split-like” activity rhythms observed in aged SAMP8 (McAuley et al. 2002; Pang et al. 2004) appear to be fundamentally different from rhythm splitting in young hamsters (Aschoff 1967; de la Iglesia et al. 2000; Hastings et al. 1991; Pittendrigh 1960; Pittendrigh and Daan 1976).The major conclusion resulting from these studies is that age-related changes in circadian rhythms as measured by wheel-running activity are not caused by disruptions of SCN activity. SCN activity of aged SAMP8 was as robust and rhythmic as that of young SAMP8. The lack of impairments in SCN activity suggests that disruption of activity rhythms may be due to an inability of the SCN to consolidate neuronal signals, resulting in a reduction in the strength of the output of the SCN. Another possibility is the change in the amplitude of activity rhythms is due to age-related impairments in the coupling between the pacemaker (SCN) and “down-stream” targets. Peripheral systems may be impaired in their ability to read or synchronize with an output signal from the SCN. One place to examine may be the subparaventricular zone, since this area is one of the major targets of the SCN (Watts 1991; Watts and Swanson 1987; Watts et al. 1987).Although we found no evidence to support the hypothesis that age-related changes in SCN activity account for circadian rhythm disruptions, drawing a definitive conclusion may be premature for several reasons. First, it is possible that age-related changes in the SCN may manifest itself as alterations in the amount (intensity) of c-Fos expressed, rather than the number of c-Fos-ir cells. Second, c-Fos may not be the best marker to demonstrate reduced activity or hemispheric asymmetries. Reductions in the circadian fluctuations of glucose metabolism (Wise et al. 1988), number of arginine-vasopressin (AVP) neurons (Roozendaal et al. 1987), and in vitro neuronal activity (Satinoff et al. 1993; Watanabe et al. 1995) have been demonstrated with increased age. Third, split activity rhythms in hamsters are also accompanied by hemispheric asymmetries in levels of Per, Bmal, and AVP (de la Iglesia et al. 2000). It would be important to examine rhythms of these markers in the SCN of the SAMP8 before concluding that the SCN is not responsible for the disruption of activity rhythms in the aged SAMP8. Finally, c-Fos expression was assessed at only two time points, and we cannot discount the possibility that increasing the number of time points might reveal a difference in the overall pattern of activity that was not captured by two time points.
Figure 1.
Double plotted actograms of a 2-month (a) and a 7-month (b) SAMP8 mouse. Running-wheel activity is represented by the small vertical lines. During days 1-6, mice were housed in a 12:12 hr light:dark cycle. During days 7–16, mice were located in a constant dark room. The transition from a light–dark cycle to constant dark is denoted by the arrow. The 2-month-old mouse exhibits an unsplit circadian rhythm with a single bout of activity each day that occurs during the dark phase. The 7-month mouse shows a split circadian rhythm, with two bouts of activity each day. One bout of activity occurs around the beginning of the dark phase and another takes place around the beginning of the light phase. The activity rhythms of 12-month SAMP8 were similar to those noted for the 7-month mouse (not shown)
Early predictors of age-related circadian rhythm disruptions Identifying the neural systems responsible for age-related rhythm disruptions is important for developing treatment or prevention regimens. Intervention regimens are also most effective when instituted as early as possible. In this regard, much research has been devoted to identifying markers that can be used to predict future disease or age-related impairments.With this in mind, we performed a cross-sequential study to identify predictors of future circadian rhythm disruption (Pang et al. 2004). Although the cross-sequential design has elements of both longitudinal and cross-sectional studies, the focus here is on the results from the longitudinal component, as this is the most relevant regarding predictors of circadian rhythm disruptions. The SAMP8 are an ideal model for longitudinal studies because of their accelerated rate of senescence.Circadian rhythms were assessed in SAMP8 mice at 2, 7, and 12 months of age using wheel-running activity. Similar to our previous cross-sectional study, wheel-running rhythms were relatively intact at 12 months in some mice, while rhythms were fragmented at 12 months in other mice. The progression of changes in circadian rhythms also varied with two general patterns. Some mice progressed from a stable rhythm at 2 months to disrupted activity rhythms at 7 months, and then back to a more stable rhythm at 12 months that was similar to the rhythm at 2 months. Other mice showed a more uniform decline in rhythmicity across the lifespan.Given the disruptions in circadian rhythms at 7 and 12 months, the longitudinal component of the experimental design allowed identification of measures at 2 months that potentially predict circadian rhythm disruptions later in life. The measures we examined included light activity, dark activity, proportion of light activity, total activity in constant dark conditions, observer perceptual ratings of the actograms concerning the presence/absence of double bouts of activity, rhythm amplitude, and free-running period. Overall, the best predictor of future circadian rhythm disruption was proportion of light activity at 2 months (a measure of rhythm fragmentation). Specifically, proportion of light activity predicted several measures of disruptions at 7 months of age, including rhythm amplitude (r=−0.723) and perceptual ratings of actograms (r=+0.742). Proportion of light activity at 2 months was also positively correlated with proportion of light activity (r=+0.895) and negatively correlated with amount of dark activity (r=−0.910) at 12 months.
Summary of circadian rhythm changes with increased age In summary, our studies on aging and circadian rhythms have demonstrated that the SAMP8 is a viable animal model to investigate mechanisms responsible for age-related circadian rhythm disruptions. Various age-related changes in circadian rhythms are present in SAMP8 that are similar to those observed with humans, most notably increased fragmentation and a large reduction in rhythm amplitude (van Someren et al. 1993b, 1996; Weitzman et al. 1982). The exact mechanism of this rhythm disruption in SAMP8 does not appear to reside in the activity of SCN neurons, although more studies are necessary to adequately rule out this brain structure. Finally, our longitudinal studies show that circadian rhythm disruption in middle age does not necessarily lead to further disruption later in life. Some animals were found to spontaneously revert back to a pattern of activity that resembled young mice. Understanding the reasons for this spontaneous improvement will lead to possible interventions that might be used clinically. As it seems that interventions are often better applied early on, we also found that proportion of light activity in the young SAMP8 was a good predictor for future circadian rhythm disruption. This predictor could prove useful in targeting those individuals who are more susceptible to future rhythm disruptions for clinical interventions.
Modulation of cognition by circadian rhythms
In addition to the fact that age-related changes in circadian rhythms are important in their own right, there is increasing evidence that circadian rhythm disruptions may contribute to some of the cognitive impairments observed in aging (Antoniadis et al. 2000; Brock 1991; Stone 1989; van Gool 1986; van Someren et al. 1993a). However, the brain systems involved in the interaction of circadian rhythms and cognition are not well understood. Although indirect effects of disrupted circadian rhythms can impact cognitive abilities (i.e., general sleepiness), disrupted rhythms may also have a more direct influence on brain areas important in cognition, such as the hippocampus and cortex.
Several systems in the hippocampus show daily cycles. Acetylcholine, norepinephrine, and serotonin systems in the hippocampus fluctuate with a circadian rhythm (Brunel and de Montigny 1987; Holmes et al. 1995; Holmes et al. 1997; Krajnak et al. 2003; Leitch et al. 2003; Masuda et al. 2005; Matsumoto et al. 1981; Mizuno et al. 1991; Mizuno et al. 1994; Moore and Traynor 1976; Weiner at al. 1992). The neurotrophin BDNF and its high affinity receptor TrkB also show daily changes, with higher levels during the active night period (Berchtold et al. 1999; Dolci et al. 2003; Pollock et al. 2001; Schaaf et al. 2000; Sei et al. 2003). These fluctuations in molecular systems may underlie functional changes in the hippocampus that follow the day–night cycle. For example, daily rhythms have been reported for theta rhythms and long-term potentiation (Bliss and Lomo 1973; Morris et al. 1986; Welsh et al. 1985), a type of synaptic plasticity that may underlie learning and memory (Bliss and Lomo 1973; Morris et al. 1986). At present, it is unclear whether rhythmic fluctuations in hippocampal function are driven by rhythms from the SCN (i.e., hormonal fluctuations such as glucocorticoids may influence the hippocampus) or are endogenous to the hippocampus. Regardless of whether rhythmic fluctuations are intrinsic to the hippocampus or are driven from the extrahippocampal structures, the demonstration of these rhythms in the hippocampus suggests a direct influence of circadian rhythms in learning and memory.
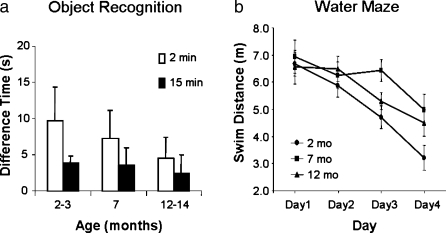
An important issue that we are starting to address is whether aging disrupts the hippocampal circadian rhythms as it does wheel-running activity. If so, do disruptions of hippocampal circadian rhythms contribute to age-related memory impairments? Our preliminary studies have examined the time course of age-related memory impairments in the SAMP8 to determine if they match age-related changes in circadian rhythms. Memory for a familiar object (Figure 2a) showed progressive impairment at 7 and 12 months of age as compared to 2-month SAMP8 for a retention interval of 2 minutes. With a 15-minute retention interval, performance for all three ages was similar, but the results suggest a possible floor effect. In contrast, spatial learning in 7- and 12-month-old SAMP8 was similarly impaired compared to the 2-month-old mice (Figure 2b). Both tasks are hippocampal-dependent, but measure different types of learning and memory.
Figure 2.
Age-related memory impairment was observed in SAMP8 for object recognition (a) and spatial memory (b). In the object recognition task, mice were exposed to an object. After 2 or 15 minutes, mice were presented with a novel item and the previously presented object. The difference in time spent exploring the novel and familiar object represents the memory for the familiar object. For the 2-minute retention, memory for the familiar object was poorer as age increased (a). With the 15-minute retention, all ages had poor memory, and the lack of an effect of age may be due to a floor effect. Age-related impairments were also observed learning a spatial task (b). The distance required to swim to a hidden platform decreased with training for all ages. However, learning the location of the escape platform was worse for the aged mice than for the young mice. Error bars represent the standard error of the mean
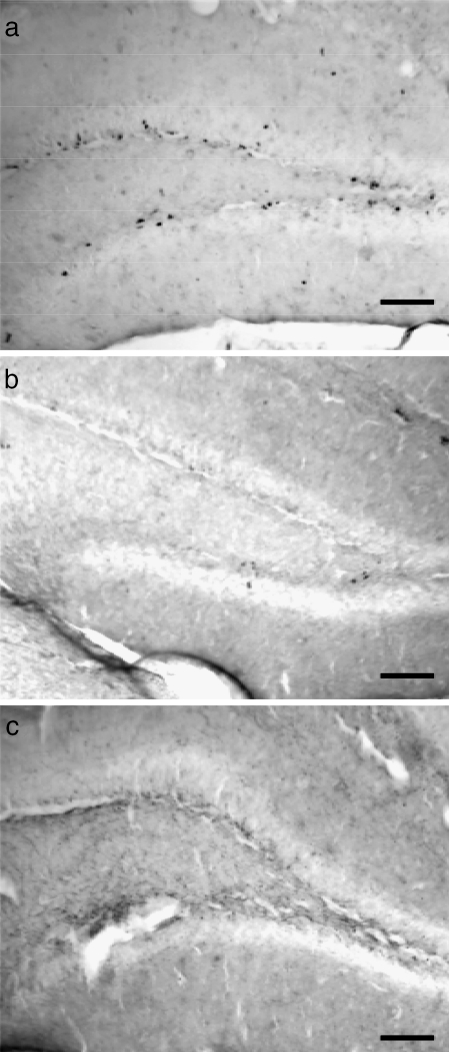
In conjunction with these behavioral studies, we have started to investigate possible mechanisms for the memory impairments. New neurons and glia can functionally integrate into the adult hippocampal network (Song et al. 2005; van Praag et al. 1999; van Praag et al. 2002). Therefore, it has been hypothesized that hippocampal cell proliferation is important for acquisition of new memories, and the reduction of hippocampal neurogenesis may be involved in the memory impairments observed in aged individuals (Gould et al. 1999a, 1999b; Shors et al. 2001; van Praag et al. 1999). Additionally, the rate of cell proliferation in the hippocampus is influenced by circadian rhythms (Goergen et al. 2002; Holmes et al. 2004). We have recently begun to examine age-related changes in hippocampal cell proliferation in SAMP8. Cell proliferation as measured by BrdU incorporation was high at 2 months but was dramatically reduced by 5 months of age (Figure 3). Further reduction was found between 5 and 7 months, such that very few BrdU-labeled cells were observed in SAMP8 of 7-month or older. What is noteworthy is that the time course of the decline in hippocampal cell proliferation is similar to the time course for age-related spatial learning impairments, but quite different than either the circadian rhythm disruption or object recognition impairment. For both circadian rhythms and object recognition, the middle age group (7–8 months) show disruptions that are not as severe as the old age group (12+ months). In contrast, hippocampal cell proliferation and spatial learning on the water maze are affected by age as much at 7 months as they are at 12 months. These preliminary results suggest that it is unlikely that the disruption of circadian rhythms is responsible for the decline in hippocampal neurogenesis or spatial learning. The results also suggest that hippocampal neurogenesis may be more closely linked to spatial learning than object recognition. However, further work is clearly needed to confirm these preliminary results.
Figure 3.
Cell proliferation in the hippocampus decreased with age. Bromo-deoxyuridine (BrdU) was used to label new proliferating cells in the dentate gyrus. Cell proliferation was dramatically reduced between 2 months (a) and 5 months (b). Although proliferation was already low by 5 months of age, further reduction to an almost non-existent level was observed in 7 months or older SAMP8 (c). Photomicrographs were taken with a 10× objective lens. Scale bar represents 100 μm in all panels
Conclusions
Overall, our studies support the view that SAMP8 is a good animal model to study aging and circadian rhythms. SAMP8 show a variety of circadian rhythm disruptions with increased age, including a reduction of the rhythm amplitude (strength), an increase in the strength of a secondary 12-hour rhythm, and a general increase in activity during the normally inactive light phase of the day (increased rhythm fragmentation). Some of these disruptions are observed in other species, and have relevance to common complaints in elderly humans. However, in SAMP8 these disruptions do not appear to be associated with age-related changes in SCN cell activity (as measured by expression of c-Fos). It is possible that other gene products or areas downstream from the SCN may be responsible for the circadian rhythm disruptions that we observed.
In longitudinal studies of SAMP8, it is noteworthy that the appearance of circadian rhythm disruptions does not necessarily mean that disruptions will be present later in life. Circadian rhythms of some mice improved, rather than declined, with increasing age. Obviously, mechanisms involved in the spontaneous improvement of circadian rhythms are of interest because they may form the basis of interventions that can reverse or slow age-related declines in behavior. As it is always better to prevent age-related impairments prior to the initial decline, predictors of circadian rhythm disruptions may be useful for targeting susceptible populations for preventative actions. We found that the best predictor of future circadian rhythm disruptions was an unusual amount of activity during the normally inactive phase.
Interventions have been attempted to reverse some of the age-related impairments of circadian rhythm. Melatonin has been administered as a treatment because of the possible age-related reduction in light input to the pineal gland, resulting in less melatonin secretion from the pineal. Melatonin administration in SAMP8 was not helpful in ameliorating the age-related changes in re-entrainment, although it seems to have effects on young as well as aged SAMR1 (Asai et al. 2000; Shibata et al. 2000). This result contrasts with the positive effects that melatonin has in stabilizing sleep and activity patterns in humans, although the effects of melatonin in humans are relatively mild (Asayama et al. 2003; Zisapel 1999). If aging reduces visual inputs to the pineal gland or SCN, bright-light therapy might also rescue the disruption of circadian rhythm. Indeed, bright-light treatment improves circadian rhythms or sleep-wake cycles in both SAMP8 and humans with Alzheimer’s disease (Dowling et al. 2005; Miyamoto et al. 1998; Yamadera et al. 2000).
Finally, we have started to investigate age-related changes in learning and memory, and possible mechanisms by which circadian rhythm disruption might have a negative impact on cognition. Our preliminary studies suggest that impairments in object recognition and circadian rhythm disruptions start to appear at similar ages in the SAMP8, and reduction in hippocampal neurogenesis and impairments of spatial learning progress similarly, with the latter two preceding the former two measures. Further studies linking age-related changes in circadian rhythms and cognition are necessary, and will be important in informing new treatments for the elderly.
Acknowledgements
We are grateful for the help of Eric Beck, Kara Hensley, Brandi Patton, and Elyssa Winzeler in conducting the behavioral tests. This research was supported by funds from the National Institutes of Health and Mrs. Dorothy Price.
References
- Abrahamson EE, Moore RY. Suprachiasmatic nucleus in the mouse: Retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- Antoniadis EA, Ko CH, Ralph MR, McDonald RJ. Circadian rhythms, aging and memory. Behav Brain Res. 2000;111(1–2):25–37. doi: 10.1016/s0166-4328(00)00145-5. [DOI] [PubMed] [Google Scholar]
- Aronin N, Schwartz WJ. A new strategy to explore molecular mechanisms of suprachiasmatic nucleus function. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus: The Mind’s Clock. New York: Oxford University Press; 1991. pp. 445–456. [Google Scholar]
- Asai M, Ikeda M, Akiyama M, Oshima I, Shibata S. Administration of melatonin in drinking water promotes the phase advance of light–dark cycle in senescence-accelerated mice, SAMR1 but not SAMP8. Brain Res. 2000;876:220–224. doi: 10.1016/s0006-8993(00)02661-5. [DOI] [PubMed] [Google Scholar]
- Asayama K, Yamadera H, Ito T, Suzuki H, Kudo Y, Endo S. Double blind study of melatonin effects on the sleep-wake rhythm, cognitive and non-cognitive functions in Alzheimer type dementia. J Nippon Med Sch = Nihon Ika Daigaku Zasshi. 2003;70(4):334–341. doi: 10.1272/jnms.70.334. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Circadian rhythms in birds. In: Snow DW, editor. Proceedings of XIV International Ornithological Conference. Oxford: Blackwell; 1967. pp. 85–105. [Google Scholar]
- Berchtold NC, Oliff HS, Isackson P, Cotman CW. Hippocampal BDNF mRNA shows a diurnal regulation, primarily in the exon III transcript. Brain Res Mol Brain Res. 1999;71(1):11–22. doi: 10.1016/s0169-328x(99)00137-0. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock MA. Chronobiology and aging. J Am Geriatr Soc. 1991;39(1):74–91. doi: 10.1111/j.1532-5415.1991.tb05909.x. [DOI] [PubMed] [Google Scholar]
- Brunel S, Montigny C. Diurnal rhythms in the responsiveness of hippocampal pyramidal neurons to serotonin, norepinephrine, gamma-aminobutyric acid and acetylcholine. Brain Res Bull. 1987;18(2):205–212. doi: 10.1016/0361-9230(87)90191-2. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Poon HF. The senescence-accelerated prone mouse (SAMP8): a model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp Gerontol. 2005;40(10):774–783. doi: 10.1016/j.exger.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Cai A, Lehman MN, Lloyd JM, Wise PM. Transplantation of fetal suprachiasmatic nuclei into middle-aged rats restores diurnal fos expression in host. Am J Physiol Regul Integr Comp Physiol. 1997;272:R422–R428. doi: 10.1152/ajpregu.1997.272.1.R422. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Foster RG. Photic regulation of Fos-like immunoreactivity in the suprachiasmatic nucleus of the mouse. J Comp Neurol. 1992;324:135–142. doi: 10.1002/cne.903240202. [DOI] [PubMed] [Google Scholar]
- Daan S, Berde C. Two coupled oscillators: simulations of the circadian pacemaker in mammalian activity rhythms. J Theor Biol. 1978;70(3):297–313. doi: 10.1016/0022-5193(78)90378-8. [DOI] [PubMed] [Google Scholar]
- Davis FC, Viswanathan N. Stability of circadian timing with age in Syrian hamsters. Am J Physiol. 1998;275(4 Pt 2):R960–R968. doi: 10.1152/ajpregu.1998.275.4.R960. [DOI] [PubMed] [Google Scholar]
- Iglesia HO, Meyer J, Carpino A, Jr, Schwartz WJ. Antiphase oscillation of the left and right suprachiasmatic nuclei. Science. 2000;290(5492):799–801. doi: 10.1126/science.290.5492.799. [DOI] [PubMed] [Google Scholar]
- Dolci C, Montaruli A, Roveda E, Barajon I, Vizzotto L, Grassi Zucconi G, et al. Circadian variations in expression of the trkB receptor in adult rat hippocampus. Brain Res. 2003;994(1):67–72. doi: 10.1016/j.brainres.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Dowling GA, Hubbard EM, Mastick J, Luxenberg JS, Burr RL, Someren EJW. Effect of morning bright light treatment for rest-activity disruption in institutionalized patients with severe Alzheimer’s disease. Int Psychogeriatr. 2005;17(2):221–236. doi: 10.1017/S1041610205001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy JF, Viswanathan N, Davis FC. Free-running circadian period does not shorten with age in female Syrian hamsters. Neurosci Lett. 1999;271(2):77–80. doi: 10.1016/s0304-3940(99)00519-4. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Deveraux AW. Age-related changes in circadian responses to dark pulses. Am J Physiol Regul Integr Comp Physiol. 2000;279(2):R586–R590. doi: 10.1152/ajpregu.2000.279.2.R586. [DOI] [PubMed] [Google Scholar]
- Flood JF, Morley JE. Learning and memory in the SAMP8 mouse. Neurosci Biobehav Rev. 1998;22(1):1–20. doi: 10.1016/s0149-7634(96)00063-2. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y, Takahashi H, Kirai K, Miyamoto M. Involvement of the glutamatergic system in behavioral disorders in senescence-accelerated mice (SAMP8) In: Nomura Y, Takeda T, Okuma Y, editors. The senescence-accelerated mouse (SAM): an animal model of senescence. Amsterdam: Elsevier BV; 2004. pp. 303–308. [Google Scholar]
- Gillete MV. SCN electrophysiology in vitro: Rhythmic activity and endogenous clock properties. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus: The Mind’s Clock. New York: Oxford University Press; 1991. pp. 125–143. [Google Scholar]
- Goergen EM, Bagay LA, Rehm K, Benton JL, Beltz BS. Circadian control of neurogenesis. J Neurobiol. 2002;53(1):90–95. doi: 10.1002/neu.10095. [DOI] [PubMed] [Google Scholar]
- Gould E, Beylin A, Tanapat P, Reeves A, Shors TJ. Learning enhances adult neurogenesis in the hippocampal formation. Nat Neurosci. 1999;2(3):260–265. doi: 10.1038/6365. [DOI] [PubMed] [Google Scholar]
- Gould E, Tanapat P, Hastings NB, Shors TJ. Neurogenesis in adulthood: a possible role in learning. Trends Cogn Sci. 1999;3(5):186–192. doi: 10.1016/s1364-6613(99)01310-8. [DOI] [PubMed] [Google Scholar]
- Guido ME, Guido LB, Goguen D, Robertson HA, Rusak B. Daily rhythm of spontaneous immediate-early gene expression in the rat suprachiasmatic nucleus. J Biol Rhythms. 1999;14:275–280. doi: 10.1177/074873099129000687. [DOI] [PubMed] [Google Scholar]
- Guido ME, Goguen D, De GL, Robertson HA, Rusak B. Circadian and photic regulation of immediate-early gene expression in the hamster suprachiasmatic nucleus. Neuroscience. 1999;90:555–571. doi: 10.1016/s0306-4522(98)00467-9. [DOI] [PubMed] [Google Scholar]
- Gwinner E. Testosterone induces “splitting” of circadian locomotor activity rhythms in birds. Science. 1974;185(145):72–74. doi: 10.1126/science.185.4145.72. [DOI] [PubMed] [Google Scholar]
- Han J-x, Ding X-r, Yu J-c, Yu T, Lu M-x, Wang S. Aging and acupuncture effects on hippocampal gene expression profile of SAMP10. In: Nomura Y, Takeda T, Okuma Y, editors. The senescence-accelerated mouse (SAM): an animal model of senescence. Amsterdam: Elsevier BV; 2004. pp. 379–382. [Google Scholar]
- Hastings JW, Rusak B, Boulos Z. Circadian rhythms: the physiology of biological timing. In: Prosser CL, editor. Neural and integrative animal physiology. New York: John Wiley & Sons, Inc.; 1991. pp. 435–546. [Google Scholar]
- Herzog ED, Schwartz WJ. Invited review: A neural clockwork for encoding circadian time. J Appl Physiol. 2002;92(1):401–408. doi: 10.1152/japplphysiol.00836.2001. [DOI] [PubMed] [Google Scholar]
- Hoffmann K. Splitting of circadian rhythms in tree shrews. In: Menaker M, editor. Biochronometry. Washington, DC: National Academy of Sciences; 1971. [Google Scholar]
- Holmes MC, French KL, Seckl JR. Modulation of serotonin and corticosteroid receptor gene expression in the rat hippocampus with circadian rhythm and stress. Brain Res Mol Brain Res. 1995;28(2):186–192. doi: 10.1016/0169-328x(94)00207-u. [DOI] [PubMed] [Google Scholar]
- Holmes MC, French KL, Seckl JR. Dysregulation of diurnal rhythms of serotonin 5-HT2C and corticosteroid receptor gene expression in the hippocampus with food restriction and glucocorticoids. J Neurosci. 1997;17(11):4056–4065. doi: 10.1523/JNEUROSCI.17-11-04056.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J Neurosci Res. 2004;76(2):216–222. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- Ingram DK, London ED, Reynolds MA. Circadian rhythmicity and sleep: effects of aging in laboratory animals. Neurobiol Aging. 1982;3(4):287–297. doi: 10.1016/0197-4580(82)90017-3. [DOI] [PubMed] [Google Scholar]
- Kaufman CM, Menaker M. Effect of transplanting suprachiasmatic nuclei from donors of different ages into completely SCN lesioned hamsters. J Neural Transplant Plast. 1993;4(4):257–265. doi: 10.1155/NP.1993.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami F, Okamura H, Tamada Y, Maebayashi Y, Fukui K, Ibata Y. Loss of day-night differences in VIP mRNA levels in the suprachiasmatic nucleus of aged rats. Neurosci Lett. 1997;222(2):99–102. doi: 10.1016/s0304-3940(97)13355-9. [DOI] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM. Suprachiasmatic nucleus: The mind’s clock. New York: Oxford; 1991. [Google Scholar]
- Krajnak K, Kashon ML, Rosewell KL, Wise PM. Aging alters the rhythmic expression of vasoactive intestinal polypeptide mRNA but not arginine vasopressin mRNA in the suprachiasmatic nuclei of female rats. J Neurosci. 1998;18(12):4767–4774. doi: 10.1523/JNEUROSCI.18-12-04767.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajnak K, Rosewell KL, Duncan MJ, Wise PM. Aging, estradiol and time of day differentially affect serotonin transporter binding in the central nervous system of female rats. Brain Res. 2003;990(1–2):87–94. doi: 10.1016/s0006-8993(03)03441-3. [DOI] [PubMed] [Google Scholar]
- Kumar VB, Farr SA, Flood JF, Kamlesh V, Franko M, Banks WA, et al. Site-directed antisense oligonucleotide decreases the expression of amyloid precursor protein and reverses deficits in learning and memory in aged SAMP8 mice. Peptides. 2000;21(12):1769–1775. doi: 10.1016/s0196-9781(00)00339-9. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci. 1987;7(6):1626–1638. doi: 10.1523/JNEUROSCI.07-06-01626.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, Silver R, Bittman EL. Anatomy of suprachiasmatic nucleus grafts. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus: The Mind's Clock. New York: Oxford University Press; 1991. pp. 349–374. [Google Scholar]
- Leitch MM, Ingram CD, Young AH, McQuade R, Gartside SE. Flattening the corticosterone rhythm attenuates 5-HT1A autoreceptor function in the rat: Relevance for depression. Neuropsychopharmacology. 2003;28(1):119–125. doi: 10.1038/sj.npp.1300016. [DOI] [PubMed] [Google Scholar]
- Masuda J, Mitsushima D, Funabashi T, Kimura F. Sex and housing conditions affect the 24-h acetylcholine release profile in the hippocampus in rats. Neuroscience. 2005;132(2):537–542. doi: 10.1016/j.neuroscience.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Kimura K, Fujisawa A, Uyama O, Yoneda S, Imaizumi M, et al. Diurnal variations in monoamine contents in discrete brain regions of the mongolian gerbin (Meriones unguiculatus) J Neurochem. 1981;37(3):792–794. [PubMed] [Google Scholar]
- McAuley JD, Miller JP, Beck E, Nagy ZM, Pang KCH. Age-related disruptions in circadian timing: evidence for “split” activity rhythms in the SAMP8. Neurobiol Aging. 2002;23:625–632. doi: 10.1016/s0197-4580(01)00344-x. [DOI] [PubMed] [Google Scholar]
- Miles LE, Dement WC. Sleep and aging. Sleep. 1980;3(2):1–220. [PubMed] [Google Scholar]
- Miller JP, McAuley JD, Pang KC. Spontaneous fos expression in the suprachiasmatic nucleus of young and old mice. Neurobiol Aging. 2005;26(7):1107–1115. doi: 10.1016/j.neurobiolaging.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Miyamoto M. Characteristics of age-related behavioral changes in senescence-accelerated mouse SAMP8 and SAMP10. Exp Gerontol. 1997;32(1–2):139–148. doi: 10.1016/s0531-5565(96)00061-7. [DOI] [PubMed] [Google Scholar]
- Miyamoto M. Emotional disorders and memory deficits in senescence-accelerated mice, SAMP8 and SAMP10. In: Nomura Y, Takeda T, Okuma Y, editors. The senescence-accelerated mouse (SAM): an animal model of senescence. Amsterdam: Elsevier BV; 2004. pp. 99–106. [Google Scholar]
- Miyamoto M, Kiyota Y, Yamazaki N, Nagaoka A, Matsuo T, Nagawa Y, et al. Age-related changes in learning and memory in the senescence-accelerated mouse (SAM) Physiol Behav. 1986;38(3):399–406. doi: 10.1016/0031-9384(86)90112-5. [DOI] [PubMed] [Google Scholar]
- Miyamoto M, Takahashi H, Ohta H, Sakamoto J. Animal model of brain aging: senescence-accelerated mouse (SAM) CNS Drug Rev. 1998;4(4):361–375. doi: 10.1111/j.1527-3458.1998.tb00076.x. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Endo Y, Arita J, Kimura F. Acetylcholine release in the rat hippocampus as measured by the microdialysis method correlates with motor activity and exhibits a diurnal variation. Neuroscience. 1991;44(3):607–612. doi: 10.1016/0306-4522(91)90081-x. [DOI] [PubMed] [Google Scholar]
- Mizuno T, Arita J, Kimura F. Spontaneous acetylcholine release in the hippocampus exhibits a diurnal variation in both young and old rats. Neurosci Lett. 1994;178(2):271–274. doi: 10.1016/0304-3940(94)90776-5. [DOI] [PubMed] [Google Scholar]
- Moore RY. Organization of the mammalian circadian system. Ciba F Symp. 1995;183:88–99. [PubMed] [Google Scholar]
- Moore RY. Entrainment pathways and the functional organization of the circadian system. Prog Brain Res. 1996;111:103–119. doi: 10.1016/s0079-6123(08)60403-3. [DOI] [PubMed] [Google Scholar]
- Moore RY, Traynor ME. Diurnal rhythms in pineal N-acetyltransferase and hippocampal norepinephrine: Effects of water deprivation, blinding and hypothalamic lesions. Neuroendocrinology. 1976;20(3):250–259. doi: 10.1159/000122489. [DOI] [PubMed] [Google Scholar]
- Morin LP. Age-related changes in hamster circadian period, entrainment, and rhythm splitting. J Biol Rhythms. 1988;3(3):237–248. [Google Scholar]
- Morley JE, Banks WA, Kumar VB, Farr SA. The SAMP8 mouse as a model for Alzheimer disease: Studies from Saint Louis University. In: Nomura Y, Takeda T, Okuma Y, editors. The senescence-accelerated mouse (SAM): an animal model of senescence. Amsterdam: Elsevier BV; 2004. pp. 23–28. [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. Nature. 1986;319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Munch M, Knoblauch V, Blatter K, Schroder C, Schnitzler C, Krauchi K, et al. Age-related attenuation of the evening circadian arousal signal in humans. Neurobiol of Aging. 2005;26(9):1307–1319. doi: 10.1016/j.neurobiolaging.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Okuma Y, Hosoi T, Nomura J. Biochemical changes in the brain of the senescence-accelerated mouse P8 and P10. In: Nomura Y, Takeda T, Okuma Y, editors. The senescence-accelerated mouse (SAM): an animal model of senescence. Amsterdam: Elsevier BV; 2004. pp. 91–97. [Google Scholar]
- Pang KCH, Miller JP, McAuley JD. Circadian rhythms in SAMP8: A longitudinal study of the effects of age and experience. Neurobiol Aging. 2004;25:111–123. doi: 10.1016/s0197-4580(03)00029-0. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS. Circadian rhythms and the circadian organization of living systems. Cold Spring Harbor Symp Quant Biol. 1960;25:155–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. J Comp Physiol. 1976;106:333–355. [Google Scholar]
- Pollock GS, Vernon E, Forbes ME, Yan Q, Ma YT, Hsieh T, et al. Effects of early visual experience and diurnal rhythms on BDNF mRNA and protein levels in the visual system, hippocampus, and cerebellum. J Neurosci. 2001;21(11):3923–3931. doi: 10.1523/JNEUROSCI.21-11-03923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon HF, Joshi G, Sultana R, Farr SA, Banks WA, Morley JE, et al. Antisense directed at the Abeta region of APP decreases brain oxidative markers in aged senescence accelerated mice. Brain Res. 2004;1018(1):86–96. doi: 10.1016/j.brainres.2004.05.048. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247(4945):975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Joyner AL, Lehman MN. Culture and transplantation of the mammalian circadian pacemaker. J Biol Rhythms. 1993;8(Suppl):S83–S87. [PubMed] [Google Scholar]
- Roozendaal B, Gool WA, Swaab DF, Hoogendijk JE, Mirmiran M. Changes in vasopressin cells of the rat suprachiasmatic nucleus with aging. Brain Res. 1987;409(2):259–264. doi: 10.1016/0006-8993(87)90710-4. [DOI] [PubMed] [Google Scholar]
- Sanchez-Barcelo EJ, Megias M, Verduga R, Crespo D. Differences between the circadian system of two strains of senescence-accelerated mice (SAM) Physiol Behav. 1997;62(6):1225–1229. doi: 10.1016/s0031-9384(97)00208-4. [DOI] [PubMed] [Google Scholar]
- Sano A, Uezu K, Flood JF, Farr SA, Morley JE, Euzu E, et al. Age-related changes in hippocampal theta rhythm in SAMP8 mouse. In: Nomura Y, Takeda T, Okuma Y, et al., editors. The senescence-accelerated mouse (SAM): an animal model of senescence. Amsterdam: Elsevier BV; 2004. pp. 321–324. [Google Scholar]
- Satinoff E, Li H, Tcheng TK, Liu C, McArthur AJ, Medanic M, et al. Do the suprachiasmatic nuclei oscillate in old rats as they do in young ones? Am J Physiol : Regul Integr Comp Physiol. 1993;265(5 Pt 2):R1216–R1222. doi: 10.1152/ajpregu.1993.265.5.R1216. [DOI] [PubMed] [Google Scholar]
- Satlin A, Teicher MH, Lieberman HR, Baldessarini RJ, Volicer L, Rheaume Y. Circadian locomotor activity rhythms in Alzheimer’s disease. Neuropsychopharmacology. 1991;5(2):115–126. [PubMed] [Google Scholar]
- Schaaf MJ, Duurland R, Kloet ER, Vreugdenhil E. Circadian variation in BDNF mRNA expression in the rat hippocampus. Brain Res : Mol Brain Res. 2000;75(2):342–344. doi: 10.1016/s0169-328x(99)00314-9. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ. SCN metabolic activity in vivo. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus: The Mind's Clock. New York: Oxford University Press; 1991. pp. 144–156. [Google Scholar]
- Schwartz WJ, Aronin N, Takeuchi J, Bennett MR, Peters RV. Towards a molecular-biology of the suprachiasmatic nucleus-photic and temporal regulation of c-fos gene-expression. Semin Neurosci. 1995;7(1):53–60. [Google Scholar]
- Schwartz WJ, Carpino A, Jr, Iglesia HO, Baler R, Klein DC, Nakabeppu Y, et al. Differential regulation of fos family genes in the ventrolateral and dorsomedial subdivisions of the rat suprachiasmatic nucleus. Neuroscience. 2000;98(3):535–547. doi: 10.1016/s0306-4522(00)00140-8. [DOI] [PubMed] [Google Scholar]
- Sei H, Fujihara H, Ueta Y, Morita K, Kitahama K, Morita Y. Single eight-hour shift of light-dark cycle increases brain-derived neurotrophic factor protein levels in the rat hippocampus. Life Sci. 2003;73(1):53–59. doi: 10.1016/s0024-3205(03)00251-0. [DOI] [PubMed] [Google Scholar]
- Shibata S, Asai M, Oshima I, Ikeda M, Yoshioka T. Melatonin normalizes the re-entrainment of senescence accelerated mice (SAM) to a new light–dark cycle. In: Olcese J, editor. Melatonin after four decades. New York: Kluwer Academic/Plenum; 2000. pp. 261–270. [PubMed] [Google Scholar]
- Shimada A. Age-dependent cerebral atrophy and cognitive dysfunction in SAMP10 mice. Neurobiol Aging. 1999;20(2):125–136. doi: 10.1016/s0197-4580(99)00044-5. [DOI] [PubMed] [Google Scholar]
- Shimada A, Keino H, Satoh M, Kishikawa M, Hosokawa M. Age-related loss of synapses in the frontal cortex of SAMP10 mouse: a model of cerebral degeneration. Synapse. 2003;48(4):198–204. doi: 10.1002/syn.10209. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Song H, Kempermann G, Wadiche LO, Zhao C, Schinder AF, Bischofberger J. New neurons in the adult mammalian brain: synaptogenesis and functional integration. J Neurosci. 2005;25(45):10366–10368. doi: 10.1523/JNEUROSCI.3452-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone WS. Sleep and aging in animals. Relationships with circadian rhythms and memory. Clin Geriatr Med. 1989;5(2):363–379. [PubMed] [Google Scholar]
- Sumova A, Travnickova Z, Mikkelsen JD, Illnerova H. Spontaneous rhythm in c-fos immunoreactivity in the dorsomedial part of the rat suprachiasmatic nucleus. Brain Res. 1998;801:254–258. doi: 10.1016/s0006-8993(98)00619-2. [DOI] [PubMed] [Google Scholar]
- Sumova A, Travnickova Z, Illnerova H. Spontaneous c-fos rhythm in the rat suprachiasmatic nucleus: Location and effect of photoperiod. Am J Physiol : Regul Integr Comp Physiol. 2000;279(6):R2262–R2269. doi: 10.1152/ajpregu.2000.279.6.R2262. [DOI] [PubMed] [Google Scholar]
- Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain Res. 1985;342(1):37–44. doi: 10.1016/0006-8993(85)91350-2. [DOI] [PubMed] [Google Scholar]
- Takahashi JS. Circadian-clock regulation of gene expression. Curr Opin Genetics Dev. 1993;3(2):301–309. doi: 10.1016/0959-437x(93)90038-q. [DOI] [PubMed] [Google Scholar]
- Takahashi R, Goto S. Altered gene expression in the brain of senescence-accelerated mouse SAMP8. In: Nomura Y, Takeda T, Okuma Y, editors. The senescence-accelerated mouse (SAM): an animal model of senescence. Amsterdam: Elsevier BV; 2004. pp. 85–90. [Google Scholar]
- Takeda T. Senescence-accelerated mouse (SAM): a biogerontological resource in aging research. Neurobiol Aging. 1999;20(2):105–110. doi: 10.1016/s0197-4580(99)00008-1. [DOI] [PubMed] [Google Scholar]
- Takeda T, Hosokawa M, Higuchi K. Senescence-accelerated mouse (SAM): a novel murine model of accelerated senescence. J Am Geriatr Soc. 1991;39(9):911–919. doi: 10.1111/j.1532-5415.1991.tb04460.x. [DOI] [PubMed] [Google Scholar]
- Turek FW, Penev P, Zhang Y, Reeth O, Takahashi JS, Zee P. Alterations in the circadian system in advanced age. Ciba Found Symp. 1995;183:212–226. doi: 10.1002/9780470514597.ch12. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol. 1997;273(6 Pt 2):R1957–R1964. doi: 10.1152/ajpregu.1997.273.6.R1957. [DOI] [PubMed] [Google Scholar]
- Gool WA. Aging and circadian rhythms. Prog Brain Res. 1986;70:255–277. doi: 10.1016/S0079-6123(08)64309-5. [DOI] [PubMed] [Google Scholar]
- Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96(23):13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praag H, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415(6875):1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someren EJ, Mirmiran M, Swaab DF. Non-pharmacological treatment of sleep and wake disturbances in aging and Alzheimer’s disease: Chronobiological perspectives. Behav Brain Res. 1993;57(2):235–253. doi: 10.1016/0166-4328(93)90140-l. [DOI] [PubMed] [Google Scholar]
- Someren EJ, Mirmiran M, Swaab DF. Non-pharmacological treatment of sleep and wake disturbances in aging and Alzheimer’s disease: chronobiological perspectives. Behav Brain Res. 1993;57(2):235–253. doi: 10.1016/0166-4328(93)90140-l. [DOI] [PubMed] [Google Scholar]
- Someren EJ, Hagebeuk EE, Lijzenga C, Scheltens P, Rooij SE, Jonker C, et al. Circadian rest-activity rhythm disturbances in Alzheimer’s disease. Biol Psychiatry. 1996;40(4):259–270. doi: 10.1016/0006-3223(95)00370-3. [DOI] [PubMed] [Google Scholar]
- Someren EJ, Lijzenga C, Mirmiran M, Swaab DF. Long-term fitness training improves the circadian rest-activity rhythm in healthy elderly males. J Biol Rhythms. 1997;12(2):146–156. doi: 10.1177/074873049701200206. [DOI] [PubMed] [Google Scholar]
- Watanabe A, Shibata S, Watanabe S. Circadian rhythm of spontaneous neuronal activity in the suprachiasmatic nucleus of old hamster in vitro. Brain Res. 1995;695(2):237–239. doi: 10.1016/0006-8993(95)00713-z. [DOI] [PubMed] [Google Scholar]
- Watts AG. The efferent projections of the suprachiasmatic nucleus: Anatomical insights into the control of circadian rhythms. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus: The Mind’s Clock. New York: Oxford; 1991. pp. 77–106. [Google Scholar]
- Watts AG, Swanson LW. Efferent projections of the suprachiasmatic nucleus: II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J Comp Neurol. 1987;258(2):230–252. doi: 10.1002/cne.902580205. [DOI] [PubMed] [Google Scholar]
- Watts AG, Swanson LW, Sanchez-Watts G. Efferent projections of the suprachiasmatic nucleus: I. Studies using anterograde transport of Phaseolus vulgaris leucoagglutinin in the rat. J. Comp Neurol. 1987;258(2):204–229. doi: 10.1002/cne.902580204. [DOI] [PubMed] [Google Scholar]
- Weiner N, Clement HW, Gemsa D, Wesemann W. Circadian and seasonal rhythms of 5-HT receptor subtypes, membrane anisotropy and 5-HT release in hippocampus and cortex of the rat. Neurochem Int. 1992;21(1):7–14. doi: 10.1016/0197-0186(92)90062-v. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Moline ML, Czeisler CA, Zimmerman JC. Chronobiology of aging: temperature, sleep-wake rhythms and entrainment. Neurobiol Aging. 1982;3(4):299–309. doi: 10.1016/0197-4580(82)90018-5. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Richardson GS, Dement WC. A circadian rhythm of hippocampal theta activity in the mouse. Physiol Behav. 1985;35(4):533–538. doi: 10.1016/0031-9384(85)90136-2. [DOI] [PubMed] [Google Scholar]
- Wise PM, Cohen IR, Weiland NG, London ED. Aging alters the circadian rhythm of glucose utilization in the suprachiasmatic nucleus. Proc Natl Acad Sci USA. 1988;85(14):5305–5309. doi: 10.1073/pnas.85.14.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witting W, Mirmiran M, Bos NP, Swaab DF. The effect of old age on the free-running period of circadian rhythms in rat. Chronobiol Int. 1994;11(2):103–112. doi: 10.3109/07420529409055896. [DOI] [PubMed] [Google Scholar]
- Yagi H, Katoh S, Akiguchi I, Takeda T. Age-related deterioration of ability of acquisition in memory and learning in senescence accelerated mouse: SAM-P/8 as an animal model of disturbances in recent memory. Brain Res. 1988;474(1):86–93. doi: 10.1016/0006-8993(88)90671-3. [DOI] [PubMed] [Google Scholar]
- Yamadera H, Ito T, Suzuki H, Asayama K, Ito R, Endo S. Effects of bright light on cognitive and sleep-wake (circadian) rhythm disturbances in Alzheimer-type dementia. Psychiatry Clin Neurosci. 2000;54(3):352–353. doi: 10.1046/j.1440-1819.2000.00711.x. [DOI] [PubMed] [Google Scholar]
- Zisapel N. The use of melatonin for the treatment of insomnia. Biol Signals Recept. 1999;8(1–2):84–89. doi: 10.1159/000014574. [DOI] [PubMed] [Google Scholar]