Abstract
Vascular dementia (VaD), incorporating cognitive dysfunction with vascular disease, ranks as the second leading cause of dementia in the United States, yet no effective treatment is currently available. The challenge of defining the pathological substrates of VaD is complicated by the heterogeneous nature of cerebrovascular disease and coexistence of other pathologies, including Alzheimer’s disease (AD) types of lesion. The use of rodent models of ischemic stroke may help to elucidate the type of lesions that are responsible for cognitive impairment in humans. Endovascular middle cerebral artery (MCA) occlusion in rats is considered to be a convenient and reliable model of human cerebral ischemia. Both sensorimotor and cognitive dysfunction can be induced in the rat endovascular MCA occlusion model, yet sensorimotor deficits induced by endovascular MCA occlusion may improve with time, whereas data presented in this review suggest that in rats this model can result in a progressive course of cognitive impairment that is consistent with the clinical progression of VaD. Thus far, experimental studies using this model have demonstrated a direct interaction of cerebral ischemic damage and AD-type neuropathologies in the primary ischemic area. Further, coincident to the progressive decline of cognitive function, a delayed neurodegeneration in a remote area, distal to the primary ischemic area, the hippocampus, has been demonstrated in a rat endovascular MCA occlusion model. We argue that this model could be employed to study VaD and provide insight into some of the pathophysiological mechanisms of VaD.
Key words: Alzheimer’s disease, hippocampus, ischemia, middle cerebral artery, stroke, vascular dementia
Introduction
Stroke ranks as the third leading cause of death and is the most common cause of permanent disability among people in the United States. Recent epidemiological data suggest that a decline in both stroke incidence and mortality reached a nadir in the early 1990s, and these conditions are now increasing in accordance with a rise in the aging population (Stapf and Mohr 2002). Stroke patients must not only survive the acute stages of infarction, but they must then cope with significant physical and mental impairment. Associated with stroke is a high incidence of deficits in sensorimotor function, as well as deficits in cognitive ability (Phipps 1991).
Ischemic stroke and vascular dementia
Vascular dementia (VaD) incorporates cognitive dysfunction with vascular disease. Epidemiological studies have indicated that the prevalence of dementia in ischemic stroke patients is nine-fold higher than in controls 3 months after the stroke (Pohjasvaara et al. 1998; Madureira et al. 2001) and 4–12 times higher than in controls 4 years after a lacuna infarct (Loeb et al. 1992). Even so, the prevalence of VaD may have been under-estimated, as the incidence of dementia varies considerably depending on which criteria are applied (Pohjasvaara et al. 2000). Vascular cognitive impairment (VCI) has recently been proposed as an umbrella term to include individuals affected with any degree of cognitive impairment resulting from cerebrovascular disease, ranging from mild cognitive impairment to dementia. In the case of VCI, the possible manifestations include memory loss, confusion and, more often, executive dysfunction plus impairments of specialized functions such as language, intentional gesture, or categorical recognition, that may result from stroke (Roman et al. 2004). Hence, VCI would likely encompass the majority of ischemic stroke patients.
Whereas ischemic stroke is itself a major cause of VaD, data from clinical studies suggest that ischemic stroke also amplifies cognitive deficits in patients with Alzheimer’s disease (AD) pathology (Snowdon et al. 1997; Esiri et al. 1999; Zekry et al. 2002). Furthermore, that cerebral ischemia can worsen the effects of AD pathology on cognitive function is also supported by experimental neuropathological data. For example, cerebral ischemia up-regulates the expression of amyloid precursor protein (APP) in rats (Jin et al. 2001a; Nihashi et al. 2001), and enhances the cleavage of Aβ from APP (Saido et al. 1994). Moreover, hyperphosphorylation of tau protein, another hallmark of AD, is induced by global ischemia (Sinigaglia-Coimbra et al. 2002) and focal cerebral ischemia in rats (Wen et al. 2004b). The significant overlap of cerebrovascular disease and AD pathology makes the distinction between VaD and AD less clear, and it is commonly known that the differential diagnosis of VaD and AD on the basis of clinical evidence is, at best, very difficult. Therefore, both experimental and clinical investigations provide evidence that AD and VaD, traditionally considered distinct clinical and pathophysiological entities, could share common features and convergent pathogenic mechanisms. Nevertheless, the coexistence of other pathologies, including Alzheimer’s disease type lesions, and the heterogeneous nature of cerebrovascular disease, make defining the pathological substrates of VaD even more complicated. The study of animal models for ischemic stroke will be essential to more precisely delineate the etiology and mechanisms underlying VaD, to discover potential therapeutic approaches for the treatment of VaD, and to predict the value and effect of therapeutic interventions in human subjects.
Rodent models for cerebral ischemia and VaD
The principal models of ischemic stroke can be divided into two subgroups: global ischemia and focal ischemia. Global models involve blocking the major blood vessels that supply the forebrain, resulting in ischemia over a large proportion of the brain. These models are now generally considered to better model the cerebral consequences of cardiac arrest rather than stroke and, consequently, are used less frequently in stroke research.
Most focal cerebral ischemia models involve occlusion of one major cerebral blood vessel, such as the middle cerebral artery (MCA). The occlusion of the MCA results in varying degrees of reduction of cerebral blood flow in both the striatum and cerebral cortex, depending on the methodological parameters. Models employing MCA occlusion are used extensively because they are considered to be of relevance to human thromboembolic stroke (Traystman 2003). MCA occlusion in rats typically results in extensive neuronal death in the cortex and damage to the caudate putamen ranging from very little to extensive. It has been considered to be a convenient and reliable model of cerebral ischemia in humans (Tamura et al. 1981; Bederson et al. 1986; DeVries et al. 2001).
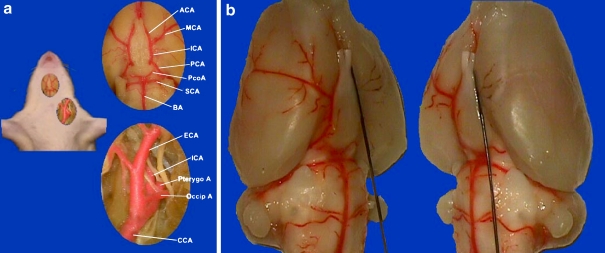
Different techniques have been used for MCA occlusion in rats, including proximal MCA occlusion, distal MCA occlusion, photochemical thrombosis, and endovascular filament MCA occlusion. Among them, an endovascular MCA occlusion model has been extensively used in ischemic stroke research since its development in 1986 (see review by Traystman 2003). This simple technique is relatively noninvasive and has been very popular for studying mechanisms of both cellular injury and neuroprotection. The rat endovascular MCA occlusion model involves inserting a monofilament nylon suture, either 3–0 or 4–0, into the internal carotid artery of rats, and then advancing the suture cranially. This procedure results in blocking the blood flow to the MCA from the internal carotid artery (ICA) as well as collateral circulation from the anterior communicating artery and posterior communicating artery (Figure 1). The suture can either be left in place (permanent occlusion) or removed after a period of 60- or 90-min (transient occlusion). Occlusion of the MCA in rats by this technique, leading to consistent infarctions in the areas it supplies, makes it a useful experimental model of focal cerebral ischemia.
Figure 1.
Endovascular middle cerebral artery occlusion model in Sprague Dawley rats. a. Vascular anatomy of the left carotid artery system and Willis’ Circle. ACA: anterior cerebral artery. ICA: internal carotid artery. CCA: common carotid artery. ECA: external carotid artery. MCA: middle cerebral artery. PCA: posterior cerebral artery. PcoA: posterior communicating artery. SCA: superior cerebellum artery. BA: basilar artery. Occip A: occipital artery. Pterygo A: pterygopalatine artery. Note the potent posterior communicating artery in SD rat. b. With a 3–0 monofilament suture inserted from left ECA and gradually advanced into the intracranial ICA, blood flow was blocked to the left MCA territory
Although this review focuses on the literature involving the endovascular MCA occlusion model in rats, it must be noted that techniques similar to those developed in rats have been successfully applied in mice (Traystman 2003), and cognitive deficits have also been observed in association with MCA occlusion in that species (Hattori et al. 2000). However, different mechanisms could be involved in the cognitive deficits described in mice and rats, due to the difference between these species in cerebrovasculature. In contrast to rats, occlusion of the MCA by an endovascular technique consistently causes hippocampal damage in mice, especially C57BL/6, due to anomalies of the circle of Willis (Kitagawa et al. 1998; Belayev et al. 1999; Ozdemir et al. 1999; McColl et al. 2004). Hence, from the perspective of etiology, the rat model of MCA occlusion may be most applicable to ischemic stroke in humans.
Behavioral studies using rat MCA occlusion models
Both sensorimotor and cognitive impairments have been demonstrated in rats after MCA occlusion. Sensorimotor performance has been quantified by assessing postural abnormalities, coordinated movements, balance, forelimb strength, locomotor activity, or sensory capabilities (DeVries et al. 2001). Spontaneous partial or complete recovery of sensorimotor function has been frequently reported over time after ischemic stroke in this model (Markgraf et al. 1994, 1997; Yonemori et al. 1999; DeVries et al. 2001; Roof et al. 2001; Karhunen et al. 2003). Indeed, the validity of MCA occlusion as a model for human ischemic stroke has been criticized because of the transitory nature of the sensorimotor deficits (Cheng et al. 2004). However, ensuring that the proximal MCA rather than the distal MCA is occluded appears to increase the likelihood of persistent sensorimotor dysfunction (Roof et al. 2001). This is because distal MCA occlusion in rats is more commonly associated with cortical damage alone, whereas endovascular occlusion of the proximal MCA typically results in extensive brain damage in the cerebral cortex as well as the caudate-putamen (Roof et al. 2001). Damage to the latter most likely contributes to persistent sensorimotor deficits after stroke (Reep et al. 2004).
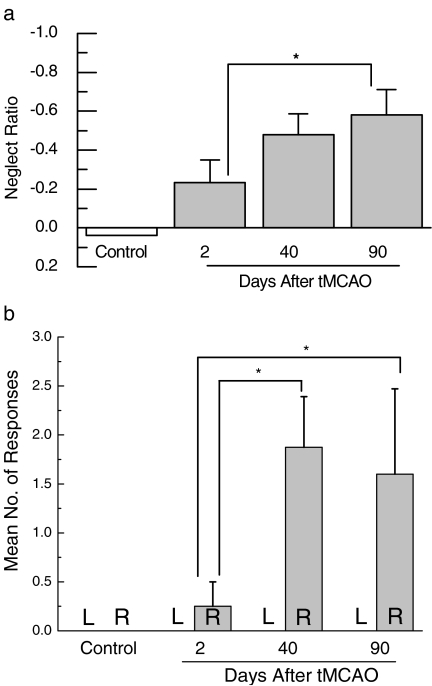
In many reports, rats subjected to MCA occlusion failed to orient toward sensory stimuli contralateral to the damaged hemisphere, a phenomenon that could result from sensory inattention (hemi-neglect), from an inability to initiate the motor responses that signal sensory awareness, or from a combination of deficits in sensorimotor integration (DeVries et al. 2001). In our laboratory, we found not only persistent sensory neglect but even what appears to be a progressive increase in the severity of sensory neglect following transient focal cerebral ischemia induced by 1 hour of MCA occlusion (Figure 2). Widespread caudate damage following proximal MCA occlusion would explain continued sensorimotor impairment (Reep et al. 2004), but the explanation for even less responding to sensory stimulation must lie elsewhere. One possibility that must be considered is that the neuronal and behavioral consequences of MCA occlusion are not limited to those due to the primary infarct insult alone. Ongoing damage to secondary neuronal areas may continue to impact behavioral performance at later testing periods.
Figure 2.
Progressive increase in severity of neglect following transient focal cerebral ischemia. A behavioral test of orienting to visual and tactile stimuli presented to the right or left sides was first performed in separate groups of rats either 2 (n = 11) or 40 days (n = 10) following transient MCA occlusion. The group tested at 40 days was re-tested at 90 days (n = 11). Neglect ratio was calculated using the following formula: (contralateral score - ipsilateral score)/(contralateral + ipsilateral score). A more negative score indicates less responding to contralateral sensory stimulation (a) Neglect ratio ± SE, (b) average number of inappropriate responses ± SE. * P < 0.05 for individual comparison within 2-way ANOVA
In addition to sensorimotor dysfunction, deficits in cognitive function have repeatedly been reported in this model (Yonemori et al. 1996, 1999; Modo et al. 2000; Puurunen et al. 2001; Jolkkonen et al. 2003). For example, impairments of performance in the Morris water maze, considered to be a robust test of hippocampal-dependent spatial learning and memory functions, were found in rats following MCA occlusion (Markgraf et al. 1992, 1994, 1997; Yonemori et al. 1996, 1999; Smith et al. 1997; Stroemer et al. 1998). With the administration of repeated tests, performance differences between MCA occlusion rats and controls tended to persist following the lesion, although the magnitude of the difference was sometimes diminished. A longitudinal study in rats demonstrated both sensorimotor and spatial memory impairment for up to 1 year following transient MCA occlusion, but interestingly, there was no progression of sensorimotor dysfunction from a test at 7 months to the test at 1 year after transient focal cerebral ischemia (Karhunen et al. 2003). Performance in the spatial learning/memory test was improved in both stroke-lesioned and sham animals at 1 year after insult or sham surgery, respectively, compared with the performance obtained at 7 months. However, the improvement in the stroke-lesioned rats was profoundly less than that of the sham group. In a similar behavioral testing paradigm, rats with permanent MCA occlusion exhibited weaker improvement following repeated testing in a spatial memory test at 1 and 2 weeks after stroke when compared with shams (Roof et al. 2001). This pattern is consistent with the presence of cognitive deficits that continue to worsen with time following an experimental stroke.
Several problems may affect the above interpretations. Lesioned and control rats often differ in spatial learning ability on the initial test. Because previous experience with this test has a profound influence on subsequent performance, it is often difficult to assess whether or not progression or recovery occurs following the MCA occlusion. Moreover, because the experimental protocols and indices of performance used in these studies vary considerably, the conclusions are not always consistent. Regardless, almost no studies have suggested recovery of the spatial cognitive impairment over time after MCA occlusion, and a progressive impairment of cognitive function has even been suggested (Markgraf et al. 1992; Stroemer et al. 1998; Roof et al. 2001; Karhunen et al. 2003).
Little or no cognitive impairment has been observed in several experimental studies when animals were tested soon after MCA occlusion. For example, four days after MCA occlusion, Wahl and colleagues (1992) found no disturbances in either vigilance or exploratory behavior measured in a modified open-field test, nor was working memory altered as measured in a Y-maze test. Similarly, Gupta et al. (2002) found no impairment of cognitive function within two weeks after MCA occlusion, as evidenced by insignificant differences between sham-operated and MCA-occluded rats in retention latency in a passive avoidance test, and by a lack of difference in transfer latency in a test of learning and memory using an elevated plus maze. While the information is currently fragmentary and obtained from different studies, the existing literature is consistent with a pattern of progression of cognitive impairment after MCA occlusion in rats. This pattern in rodents is consistent with that of clinical studies in which dementias developed progressively after stroke. The progression of cognitive dysfunction in the endovascular rat MCA occlusion model and in ischemic stroke patients suggests that this rodent model could be used to delineate the neuropathological mechanisms underlying VaD.
Potential neuropathological substrates of VaD defined by MCA occlusion model
MCA occlusion typically results in extensive neuronal death, inducing both necrosis and apoptosis, in the cerebral cortex and caudate putamen. Further, MCA occlusion can also induce AD-related neuropathologies in the primary ischemia area. For example, cerebral ischemia induced by transient endovascular MCA occlusion in rats caused an increase in both amyloid precursor protein (APP) production and BACE 1 activity (Shi et al. 2000; Wen et al. 2004a). In addition, after transient MCA occlusion, the cortex showed high levels of both phosphorylated tau and AD-type tau conformational epitopes (Wen et al. 2004b). Further, this tau hyperphosphorylation occurred predominantly in neurons that were undergoing apoptosis, as evidenced by TUNEL staining (Wen et al. 2004b). These experimental studies provide evidence that support a direct interaction between cerebral ischemia and AD-type pathology, which could contribute to the progression of dementia after ischemic stroke.
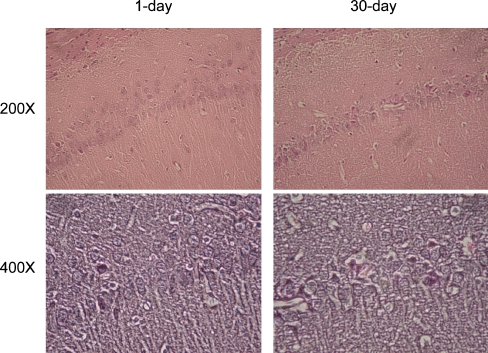
Most studies employing focal cerebral ischemia have focused on cellular changes in the primary infarction areas themselves. While it seems likely that the initial cognitive function impairment observed after endovascular MCA occlusion in rats could be explained by damage to the cerebral cortex and caudate-putamen, the persistence of this impairment, despite the often subsequent spontaneous recovery of sensorimotor function, indicates the possible involvement of other areas related to cognitive function that are distal to the primary infarct area. It is possible that focal cerebral ischemia may also cause delayed neuronal cell death in non-ischemic, remote brain areas that have synaptic contacts to the primary lesion area. For example, after focal ischemic or excitotoxic lesion of the cortex and/or striatum, secondary changes have been observed in the thalamus, substantia nigra pars reticulate, hippocampus, and spinal cord (Block et al. 2005). Following MCA occlusion in rats, atrophy of the ipsilateral substantia nigra and neuronal loss combined with microglial activation in the spinal cord have been described (Tamura et al. 1990; Wu and Ling 1998). Recently, evidence from our laboratory has suggested a delayed neurodegeneration in hippocampus after endovascular MCA occlusion in rats (Figure 4).
Figure 4.
Delayed neurodegeneration in ipsilateral hippocampus after transient MCA occlusion in rats. Hematoxylin and Eosin staining showed extensive cytoplasmic eosinophilia, neuronal shrinkage, and nuclear pyknosis in the ipsilateral CA1 at 30 days, but almost none at 1 day after MCA occlusion. Neurodegeneration was also evident in CA3 and dentate gyrus (data not shown). Upper and lower images are low (200×) and high (400×) magnification, respectively
The hippocampus has long been recognized as playing a vital role in information processing, memory formation, and subsequent regulation of behavior (Bannerman et al. 2004; Lynch 2004). Hippocampal damage has also been identified as a major target in both AD as well as VaD. In VaD, clinicopathological studies have shown that brain infarcts alone were usually insufficient to account for the clinical syndrome of dementia. Studies have indicated that hippocampal atrophy is a better predictor of dementia than the number of the vascular lesions (Mungas et al. 2001; Gainotti et al. 2004). Clinically diagnosed VaD patients frequently show extensive AD-type neuropathological change (Kalaria et al. 2004), and this neuropathological change (e.g., neurofibrillary degeneration) has also been found in the hippocampus of subcortical ischemia patients (Fein et al. 2000). In fact, the designation of “mixed dementia” has been applied to conditions in which cerebrovascular and Alzheimer’s disease pathologies coexist.
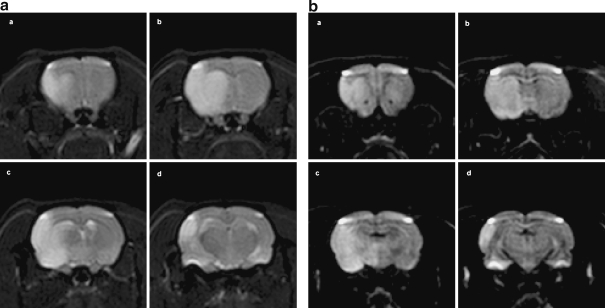
Endovascular MCA occlusion in rats induces very mild cerebral blood flow reduction in hippocampus because it is supplied by the posterior cerebral artery (An et al. 1993; Ozdemir et al. 1999; Tanaka et al. 2000). Despite repeated attempts in our laboratory, we have observed no sign of direct neuronal damage in the ipsilateral hippocampus on day 1 after MCA occlusion in rats. Using MRI to define the extent of brain damage induced by transient MCA occlusion in Sprague Dawley rats, we demonstrated that damage was restricted to the cerebral cortex and caudate putamen at the early time after stroke (Figure 3). However, beginning from day 3 until day 14 after MCA occlusion, other researchers have found delayed apoptotic neuronal death in the ipsilateral hippocampus, with the extent dependent on the length of time since occlusion (Wang et al. 2004). Moreover, our studies have consistently demonstrated a delayed neurodegeneration in the CA1 region of the ipsilateral hippocampus at 1 month after transient MCA occlusion (Figure 4). Other laboratories have reported finding functional impairment in the hippocampus ipsilateral to the side of MCA occlusion. For example, 3 days after transient MCA occlusion, long-term potentiation was not inducible in the ipsilateral hippocampus (Sopala et al. 2000).
Figure 3.
Cerebral cortex and caudate putamen damage induced by transient endovascular middle cerebral artery occlusion in SD rat. (a) T2 and (b) Diffusion-weighted MRI in a same rat after 1 hour left MCA occlusion. Imaging was performed at 4.7 T 33 cm magnet with a Brucket Console (Billerica, MA, USA), using an actively shielded gradient set capable of 220 mT/m. DWI was acquired using a standard pulsed gradient spin echo technique with an echo time (TE) of 33 ms. The gradient pulses were each applied for 9 ms and were separated by 13 ms around the 180° refocusing pulse. The gradient amplitude used was 152 mT/m resulting in a b-value of 1400 s/mm2. T2WI was acquired using a standard spin echo technique with a TE of 75 ms. MCA occlusion induced damage in cerebral cortex, including frontal, parietal and occipital, and caudate putamen, evidenced by both (A) T2 and (B) Diffusion-weighted MRI. Images a, b, c, and d correspond to the cross section of Bregma 1.8, −0.8, −2.8, and −3.8 mm, respectively, from the same rat after MCAO. Note that no damage was shown in hippocampus
There is evidence that endovascular MCA occlusion may initially elicit neuroprotective processes in both the ipsilateral and contralateral hippocampus, which would be consistent with a delayed neurodegeneration. In rats, transient MCA occlusion induced cyclic AMP response element binding protein (CREB) phosphorylation in the CA1 regions of the hippocampus of both the ischemic and non-ischemic hemispheres (Tanaka et al. 2000). Further, increased proliferation of neuronal progenitor cells was shown in the contralateral hippocampus after both transient and permanent MCA occlusion in rats (Jin et al. 2001b; Takasawa et al. 2002). The activation of CREB could contribute to neurogenesis in the hippocampus after focal ischemia (Zhu et al. 2004).
Given the critical involvement of the hippocampus in memory processes, the congruent occurrence following endovascular MCA occlusion of both a progressive decline of cognitive function and a delayed onset of neurodegeneration in the hippocampus (a remote area distal to the primary ischemic lesion), suggests that the delayed neurodegeneration could be a potential neuropathological substrate for VaD.
Mechanisms of delayed neurodegeneration at areas remote to the primary ischemic lesion
The mechanisms that contribute to the neurodegeneration at remote areas distal to the primary ischemic lesion are not fully understood, but suggest the involvement of trans-synaptic retrograde and/or anterograde mechanisms (Ross and Ebner 1990). The elongated morphology of neuronal processes and extensive neuronal network pose a significant challenge for effective intracellular and intercellular communication. Upon damage to neuronal processes and/or soma, the associated neuron must receive accurate and timely information to mount an appropriate response (Hanz and Fainzilber 2004). The selective loss of neurons in neurodegenerative disease is widely thought to involve the process of excitotoxicity, in which glutamate-mediated neuronal damage is elaborated through the excessive stimulation of cell surface receptors. Impairment of inhibitory and excitatory neurotransmission occurs in widespread, structurally intact brain regions after focal ischemic stroke (Que et al. 1999; Redecker et al. 2002). A secondary neuronal damage can be induced by a robust glutamate release from presynaptic neurons in the primary damage area (Dodd 2002). Indeed, delayed transneuronal death of substantia nigra neurons has been demonstrated after excitotoxin induced damage in caudate nucleus. Further, a replacement of inhibitory transmitters prevents the delayed neuronal death, which suggests that the delayed transneuronal degeneration may be produced by neuronal disinhibition consequent to loss of inhibitory inputs (Saji and Reis 1987).
The secondary neurodegeneration remote from the primary lesion area could also result from neuroinflammation induced by the primary ischemic insult. Inflammatory responses in areas relatively remote from the ischemic lesion have been indicated (Block et al. 2005). Further, the inflammatory responses precede the neurodegeneration in the same area, suggesting the role of inflammation in areas remote to the ischemic lesion after stroke (Dihne and Block 2001; Loos et al. 2003).
Summary and conclusions
Endovascular middle cerebral artery occlusion models have been intensively used for both basic as well as translational research to delineate mechanisms underlying cerebral ischemia reperfusion injury and to develop effective therapeutic interventions for the treatment of ischemic stroke. Important advances in experimental stroke research have been made during the last decade. Many stroke researchers have expanded their techniques to assess the cognitive and other behavioral correlates of stroke damage using the endovascular middle cerebral artery occlusion model, especially in rats, and have extended examination of the recovery period to months, even years. Consistently, clinical epidemiology studies have suggested a progressive decline of cognitive function after ischemic stroke, while studies in rodent models have yielded compatible results. More importantly, various treatment options designed to prevent late development of cognitive impairment after onset of stroke have been offered in the studies using the rat endovascular middle cerebral artery occlusion model; notable among them are stem cell implantation (Modo et al. 2002) and environmental enrichment (Dahlqvist et al. 2004). The concurrent decline of cognitive function and the delayed neurodegeneration in hippocampus induced by endovascular MCA occlusion in rats suggests that delayed neurodegeneration in hippocampus or other regions remote to the primary lesion could be potential neuropathological substrates for VaD. Continued study of the long-term consequences of endovascular MCA occlusion in rats may help to further elucidate the type of lesions possibly linked with cognitive impairment in humans, and might provide insight into some of the pathophysiological mechanisms of vascular dementia, as well as AD (Sarti et al. 2002). The ultimate goal of the animal studies is to identify and effectively target therapeutic interventions that may improve the quality of life in stroke survivors.
Briefly, the central thesis that we wish to impart in this review may be summarized as follows. The immediate consequences of endovascular proximal MCA occlusion in rats include neuronal damage to discrete cortical areas and the caudate putamen, accompanied by behavioral impairments that generally persist and sometimes even worsen (see Figure 2). While early assessment of cognitive performance soon after ischemic insult does not always indicate dysfunction, subsequent testing generally reveals deficits. The scope of neuronal damage in the hippocampus, a brain area that is distal to the primary lesions and that is important to cognitive function, also appears to increase with time. Therefore, endovascular proximal MCA occlusion in rats is a model that can induce a pattern of progressive deficits in cognitive and sensorimotor functioning that is clinically relevant.
Acknowledgements
Supported by National Institutes of Health grants AG10485, AG22550, and a grant from American Heart Association (Texas Affiliate).
References
- An G, Lin TN, Liu JS, Xue JJ, He YY, Hsu CY. Expression of c-fos and c-jun family genes after focal cerebral ischemia. Ann Neurol. 1993;33:457–464. doi: 10.1002/ana.410330508. [DOI] [PubMed] [Google Scholar]
- Bannerman DM, Rawlins JN, McHugh SB, Deacon RM, Yee BK, Bast T, Zhang WN, Pothuizen HH, Feldon J. Regional dissociations within the hippocampus-memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–283. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Belayev L, Busto R, Zhao W, Fernandez G, Ginsberg MD. Middle cerebral artery occlusion in the mouse by intraluminal suture coated with poly-L-lysine: neurological and histological validation. Brain Res. 1999;833:181–190. doi: 10.1016/S0006-8993(99)01528-0. [DOI] [PubMed] [Google Scholar]
- Block F, Dihne M, Loos M. Inflammation in areas of remote changes following focal brain lesion. Prog Neurobiol. 2005;75:342–365. doi: 10.1016/j.pneurobio.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Cheng YD, Al-Khoury L, Zivin JA. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx. 2004;1:36–45. doi: 10.1602/neurorx.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlqvist P, Ronnback A, Bergstrom SA, Soderstrom I, Olsson T. Environmental enrichment reverses learning impairment in the Morris water maze after focal cerebral ischemia in rats. Eur J Neurosci. 2004;19:2288–2298. doi: 10.1111/j.0953-816X.2004.03248.x. [DOI] [PubMed] [Google Scholar]
- DeVries AC, Nelson RJ, Traystman RJ, Hurn PD. Cognitive and behavioral assessment in experimental stroke research: will it prove useful? Neurosci Biobehav Rev. 2001;25:325–342. doi: 10.1016/S0149-7634(01)00017-3. [DOI] [PubMed] [Google Scholar]
- Dihne M, Block F. Focal ischemia induces transient expression of IL-6 in the substantia nigra pars reticulata. Brain Res. 2001;889:165–173. doi: 10.1016/S0006-8993(00)03129-2. [DOI] [PubMed] [Google Scholar]
- Dodd PR. Excited to death: different ways to lose your neurones. Biogerontology. 2002;3:51–56. doi: 10.1023/A:1015255312948. [DOI] [PubMed] [Google Scholar]
- Esiri MM, Nagy Z, Smith MZ, Barnetson L, Smith AD. Cerebrovascular disease and threshold for dementia in the early stages of Alzheimer’s disease. Lancet. 1999;354:919–920. doi: 10.1016/S0140-6736(99)02355-7. [DOI] [PubMed] [Google Scholar]
- Fein G, Sclafani V, Tanabe J, Cardenas V, Weiner MW, Jagust WJ, Reed BR, Norman D, Schuff N, Kusdra L, Greenfield T, Chui H. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55:1626–1635. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G, Acciarri A, Bizzarro A, Marra C, Masullo C, Misciagna S, Tartaglione T, Valenza A, Colosimo C. The role of brain infarcts and hippocampal atrophy in subcortical ischaemic vascular dementia. Neurol Sci. 2004;25:192–197. doi: 10.1007/s10072-004-0321-5. [DOI] [PubMed] [Google Scholar]
- Gupta YK, Sinha K, Chaudhary G. Transient focal ischemia induces motor deficit but does not impair the cognitive function in middle cerebral artery occlusion model of stroke in rats. J Neurol Sci. 2002;203–204:267–271. doi: 10.1016/S0022-510X(02)00303-9. [DOI] [PubMed] [Google Scholar]
- Hanz S, Fainzilber M. Integration of retrograde axonal and nuclear transport mechanisms in neurons: implications for therapeutics. Neurosci. 2004;10:404–408. doi: 10.1177/1073858404267884. [DOI] [PubMed] [Google Scholar]
- Hattori K, Lee H, Hurn PD, Crain BJ, Traystman RJ, DeVries AC. Cognitive deficits after focal cerebral ischemia in mice. Stroke. 2000;31:1939–1944. doi: 10.1161/01.str.31.8.1939. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao XO, Eshoo MW, Nagayama T, Minami M, Simon RP, Greenberg DA. Microarray analysis of hippocampal gene expression in global cerebral ischemia. Ann Neurol. 2001;50:93–103. doi: 10.1002/ana.1073. [DOI] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolkkonen J, Gallagher NP, Zilles K, Sivenius J. Behavioral deficits and recovery following transient focal cerebral ischemia in rats: glutamatergic and GABAergic receptor densities. Behav Brain Res. 2003;138:187–200. doi: 10.1016/S0166-4328(02)00241-3. [DOI] [PubMed] [Google Scholar]
- Kalaria RN, Kenny RA, Ballard CG, Perry R, Ince P, Polvikoski T. Towards defining the neuropathological substrates of vascular dementia. J Neurol Sci. 2004;226:75–80. doi: 10.1016/j.jns.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Karhunen H, Pitkanen A, Virtanen T, Gureviciene I, Pussinen R, Ylinen A, Sivenius J, Nissinen J, Jolkkonen J. Long-term functional consequences of transient occlusion of the middle cerebral artery in rats: a 1-year follow-up of the development of epileptogenesis and memory impairment in relation to sensorimotor deficits. Epilepsy Res. 2003;54:1–10. doi: 10.1016/S0920-1211(03)00034-2. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, Yanagihara T. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab. 1998;18:570–579. doi: 10.1097/00004647-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Loeb C, Gandolfo C, Croce R, Conti M. Dementia associated with lacunar infarction. Stroke. 1992;23:1225–1229. doi: 10.1161/01.str.23.9.1225. [DOI] [PubMed] [Google Scholar]
- Loos M, Dihne M, Block F. Tumor necrosis factor-alpha expression in areas of remote degeneration following middle cerebral artery occlusion of the rat. Neuroscience. 2003;122:373–380. doi: 10.1016/S0306-4522(03)00498-6. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Long-term potentiation and memory. Physiol Rev. 2004;84:87–136. doi: 10.1152/physrev.00014.2003. [DOI] [PubMed] [Google Scholar]
- Madureira S, Guerreiro M, Ferro JM. Dementia and cognitive impairment three months after stroke. Eur J Neurol. 2001;8:621–627. doi: 10.1046/j.1468-1331.2001.00332.x. [DOI] [PubMed] [Google Scholar]
- Markgraf CG, Green EJ, Hurwitz BE, Morikawa E, Dietrich WD, McCabe PM, Ginsberg MD, Schneiderman N. Sensorimotor and cognitive consequences of middle cerebral artery occlusion in rats. Brain Res. 1992;575:238–246. doi: 10.1016/0006-8993(92)90085-N. [DOI] [PubMed] [Google Scholar]
- Markgraf CG, Green EJ, Watson B, McCabe PM, Schneiderman N, Dietrich WD, Ginsberg MD. Recovery of sensorimotor function after distal middle cerebral artery photothrombotic occlusion in rats. Stroke. 1994;25:153–159. doi: 10.1161/01.str.25.1.153. [DOI] [PubMed] [Google Scholar]
- Markgraf CG, Johnson MP, Braun DL, Bickers MV. Behavioral recovery patterns in rats receiving the NMDA receptor antagonist MDL 100,453 immediately post-stroke. Pharmacol Biochem Behav. 1997;56:391–397. doi: 10.1016/S0091-3057(96)00231-6. [DOI] [PubMed] [Google Scholar]
- McColl BW, Carswell HV, McCulloch J, Horsburgh K. Extension of cerebral hypoperfusion and ischaemic pathology beyond MCA territory after intraluminal filament occlusion in C57Bl/6J mice. Brain Res. 2004;997:15–23. doi: 10.1016/j.brainres.2003.10.028. [DOI] [PubMed] [Google Scholar]
- Modo M, Stroemer RP, Tang E, Veizovic T, Sowniski P, Hodges H. Neurological sequelae and long-term behavioural assessment of rats with transient middle cerebral artery occlusion. J Neurosci Methods. 2000;104:99–109. doi: 10.1016/S0165-0270(00)00329-0. [DOI] [PubMed] [Google Scholar]
- Modo M, Stroemer RP, Tang E, Patel S, Hodges H. Effects of implantation site of stem cell grafts on behavioral recovery from stroke damage. Stroke. 2002;33:2270–2278. doi: 10.1161/01.STR.0000027693.50675.C5. [DOI] [PubMed] [Google Scholar]
- Mungas D, Jagust WJ, Reed BR, Kramer JH, Weiner MW, Schuff N, Norman D, Mack WJ, Willis L, Chui HC. MRI predictors of cognition in subcortical ischemic vascular disease and Alzheimer’s disease. Neurology. 2001;57:2229–2235. doi: 10.1212/wnl.57.12.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nihashi T, Inao S, Kajita Y, Kawai T, Sugimoto T, Niwa M, Kabeya R, Hata N, Hayashi S, Yoshida J. Expression and distribution of beta amyloid precursor protein and beta amyloid peptide in reactive astrocytes after transient middle cerebral artery occlusion. Acta Neurochir (Wien) 2001;143:287–295. doi: 10.1007/s007010170109. [DOI] [PubMed] [Google Scholar]
- Ozdemir YG, Bolay H, Erdem E, Dalkara T. Occlusion of the MCA by an intraluminal filament may cause disturbances in the hippocampal blood flow due to anomalies of circle of Willis and filament thickness. Brain Res. 1999;822:260–264. doi: 10.1016/S0006-8993(99)01175-0. [DOI] [PubMed] [Google Scholar]
- Phipps MA. Assessment of neurologic deficits in stroke. Acute-care and rehabilitation implications. Nurs Clin North Am. 1991;26:957–970. [PubMed] [Google Scholar]
- Pohjasvaara T, Erkinjuntti T, Ylikoski R, Hietanen M, Vataja R, Kaste M. Clinical determinants of poststroke dementia. Stroke. 1998;29:75–81. doi: 10.1161/01.str.29.1.75. [DOI] [PubMed] [Google Scholar]
- Pohjasvaara T, Mantyla R, Ylikoski R, Kaste M, Erkinjuntti T. Comparison of different clinical criteria (DSM-III, ADDTC, ICD-10, NINDS-AIREN, DSM-IV) for the diagnosis of vascular dementia. National Institute of Neurological Disorders and Stroke-Association Internationale pour la Recherche et l’Enseignement en Neurosciences. Stroke. 2000;31:2952–2957. doi: 10.1161/01.str.31.12.2952. [DOI] [PubMed] [Google Scholar]
- Puurunen K, Jolkkonen J, Sirvio J, Haapalinna A, Sivenius J. Selegiline combined with enriched-environment housing attenuates spatial learning deficits following focal cerebral ischemia in rats. Exp Neurol. 2001;167:348–355. doi: 10.1006/exnr.2000.7563. [DOI] [PubMed] [Google Scholar]
- Que M, Schiene K, Witte OW, Zilles K. Widespread up-regulation of N-methyl-D-aspartate receptors after focal photothrombotic lesion in rat brain. Neurosci Lett. 1999;273:77–80. doi: 10.1016/S0304-3940(99)00598-4. [DOI] [PubMed] [Google Scholar]
- Redecker C, Wang W, Fritschy JM, Witte OW. Widespread and long-lasting alterations in GABA(A)-receptor subtypes after focal cortical infarcts in rats: mediation by NMDA-dependent processes. J Cereb Blood Flow Metab. 2002;22:1463–1475. doi: 10.1097/00004647-200212000-00007. [DOI] [PubMed] [Google Scholar]
- Reep RL, Corwin JV, Cheatwood JL, Vleet TM, Heilman KM, Watson RT. A rodent model for investigating the neurobiology of contralateral neglect. Cog Behav Neurol. 2004;17:191–194. [PubMed] [Google Scholar]
- Roman GC, Sachdev P, Royall DR, Bullock RA, Orgogozo JM, Lopez-Pousa S, Arizaga R, Wallin A. Vascular cognitive disorder: a new diagnostic category updating vascular cognitive impairment and vascular dementia. J Neurol Sci. 2004;226:81–87. doi: 10.1016/j.jns.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Roof RL, Schielke GP, Ren X, Hall ED. A comparison of long-term functional outcome after 2 middle cerebral artery occlusion models in rats. Stroke. 2001;32:2648–2657. doi: 10.1161/hs1101.097397. [DOI] [PubMed] [Google Scholar]
- Ross DT, Ebner FF. Thalamic retrograde degeneration following cortical injury: an excitotoxic process? Neuroscience. 1990;35:525–550. doi: 10.1016/0306-4522(90)90327-Z. [DOI] [PubMed] [Google Scholar]
- Saido TC, Yokota M, Maruyama K, Yamao-Harigaya W, Tani E, Ihara Y, Kawashima S. Spatial resolution of the primary beta-amyloidogenic process induced in postischemic hippocampus. J Biol Chem. 1994;269:15253–15257. [PubMed] [Google Scholar]
- Saji M, Reis DJ. Delayed transneuronal death of substantia nigra neurons prevented by gamma-aminobutyric acid agonist. Science. 1987;235:66–69. doi: 10.1126/science.3798095. [DOI] [PubMed] [Google Scholar]
- Sarti C, Pantoni L, Bartolini L, Inzitari D. Cognitive impairment and chronic cerebral hypoperfusion: what can be learned from experimental models. J Neurol Sci. 2002;203–204:263–266. doi: 10.1016/S0022-510X(02)00302-7. [DOI] [PubMed] [Google Scholar]
- Shi J, Yang SH, Stubley L, Day AL, Simpkins JW. Hypoperfusion induces overexpression of beta-amyloid precursor protein mRNA in a focal ischemic rodent model. Brain Res. 2000;853:1–4. doi: 10.1016/S0006-8993(99)02113-7. [DOI] [PubMed] [Google Scholar]
- Sinigaglia-Coimbra R, Cavalheiro EA, Coimbra CG. Postischemic hyperthermia induces Alzheimer-like pathology in the rat brain. Acta Neuropathol (Berl) 2002;103:444–452. doi: 10.1007/s00401-001-0487-3. [DOI] [PubMed] [Google Scholar]
- Smith SE, Hodges H, Sowinski P, Man CM, Leach MJ, Sinden JD, Gray JA, Meldrum BS. Long-term beneficial effects of BW619C89 on neurological deficit, cognitive deficit and brain damage after middle cerebral artery occlusion in the rat. Neuroscience. 1997;77:1123–1135. doi: 10.1016/S0306-4522(96)00530-1. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Greiner LH, Mortimer JA, Riley KP, Greiner PA, Markesbery WR. Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. Jama. 1997;277:813–817. doi: 10.1001/jama.277.10.813. [DOI] [PubMed] [Google Scholar]
- Sopala M, Frankiewicz T, Parsons C, Danysz W. Middle cerebral artery occlusion produces secondary, remote impairment in hippocampal plasticity of rats - involvement of N-methyl-D-aspartate receptors? Neurosci Lett. 2000;281:143–146. doi: 10.1016/S0304-3940(00)00829-6. [DOI] [PubMed] [Google Scholar]
- Stapf C, Mohr JP. Ischemic stroke therapy. Annu Rev Med. 2002;53:453–475. doi: 10.1146/annurev.med.53.082901.104106. [DOI] [PubMed] [Google Scholar]
- Stroemer RP, Kent TA, Hulsebosch CE (1998) Enhanced neocortical neural sprouting, synaptogenesis, and behavioral recovery with D-amphetamine therapy after neocortical infarction in rats. Stroke 29:2381–2393; discussion 2393–2385. [DOI] [PubMed]
- Takasawa K, Kitagawa K, Yagita Y, Sasaki T, Tanaka S, Matsushita K, Ohstuki T, Miyata T, Okano H, Hori M, Matsumoto M. Increased proliferation of neural progenitor cells but reduced survival of newborn cells in the contralateral hippocampus after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2002;22:299–307. doi: 10.1097/00004647-200203000-00007. [DOI] [PubMed] [Google Scholar]
- Tamura A, Graham DI, McCulloch J, Teasdale GM. Focal cerebral ischaemia in the rat: 1. Description of technique and early neuropathological consequences following middle cerebral artery occlusion. J Cereb Blood Flow Metab. 1981;1:53–60. doi: 10.1038/jcbfm.1981.6. [DOI] [PubMed] [Google Scholar]
- Tamura A, Kirino T, Sano K, Takagi K, Oka H. Atrophy of the ipsilateral substantia nigra following middle cerebral artery occlusion in the rat. Brain Res. 1990;510:154–157. doi: 10.1016/0006-8993(90)90744-V. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Nagata E, Ito D, Suzuki S, Dembo T, Kosakai A, Fukuuchi Y. Persistent CREB phosphorylation with protection of hippocampal CA1 pyramidal neurons following temporary occlusion of the middle cerebral artery in the rat. Exp Neurol. 2000;161:462–471. doi: 10.1006/exnr.1999.7313. [DOI] [PubMed] [Google Scholar]
- Traystman RJ. Animal models of focal and global cerebral ischemia. Ilar J. 2003;44:85–95. doi: 10.1093/ilar.44.2.85. [DOI] [PubMed] [Google Scholar]
- Wahl F, Allix M, Plotkine M, Boulu RG. Neurological and behavioral outcomes of focal cerebral ischemia in rats. Stroke. 1992;23:267–272. doi: 10.1161/01.str.23.2.267. [DOI] [PubMed] [Google Scholar]
- Wang W, Redecker C, Bidmon HJ, Witte OW. Delayed neuronal death and damage of GDNF family receptors in CA1 following focal cerebral ischemia. Brain Res. 2004;1023:92–101. doi: 10.1016/j.brainres.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Wen Y, Onyewuchi O, Yang S, Liu R, Simpkins JW. Increased beta-secretase activity and expression in rats following transient cerebral ischemia. Brain Res. 2004;1009:1–8. doi: 10.1016/j.brainres.2003.09.086. [DOI] [PubMed] [Google Scholar]
- Wen Y, Yang S, Liu R, Brun-Zinkernagel AM, Koulen P, Simpkins JW. Transient cerebral ischemia induces aberrant neuronal cell cycle re-entry and Alzheimer’s disease-like tauopathy in female rats. J Biol Chem. 2004;279:22684–22692. doi: 10.1074/jbc.M311768200. [DOI] [PubMed] [Google Scholar]
- Wu YP, Ling EA. Transsynaptic changes of neurons and associated microglial reaction in the spinal cord of rats following middle cerebral artery occlusion. Neurosci Lett. 1998;256:41–44. doi: 10.1016/S0304-3940(98)00750-2. [DOI] [PubMed] [Google Scholar]
- Yonemori F, Yamada H, Yamaguchi T, Uemura A, Tamura A. Spatial memory disturbance after focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1996;16:973–980. doi: 10.1097/00004647-199609000-00022. [DOI] [PubMed] [Google Scholar]
- Yonemori F, Yamaguchi T, Yamada H, Tamura A. Spatial cognitive performance after chronic focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1999;19:483–494. doi: 10.1097/00004647-199905000-00002. [DOI] [PubMed] [Google Scholar]
- Zekry D, Duyckaerts C, Moulias R, Belmin J, Geoffre C, Herrmann F, Hauw JJ. Degenerative and vascular lesions of the brain have synergistic effects in dementia of the elderly. Acta Neuropathol (Berl) 2002;103:481–487. doi: 10.1007/s00401-001-0493-5. [DOI] [PubMed] [Google Scholar]
- Zhu DY, Lau L, Liu SH, Wei JS, Lu YM. Activation of cAMP-response-element-binding protein (CREB) after focal cerebral ischemia stimulates neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2004;101:9453–9457. doi: 10.1073/pnas.0401063101. [DOI] [PMC free article] [PubMed] [Google Scholar]