Abstract
In part due to their genetic uniformity and stable characteristics, inbred rodents or their F1 progeny are frequently used to study brain aging. However, it is recognized that focus on a single genotype could lead to generalizations about brain aging that might not apply to the species as a whole, or to the human population. As a potential alternative to uniform genotypes, genetically heterogeneous (HET) mice, produced by a four-way cross, were tested in the current study to determine if they exhibit age-related declines in cognitive and psychomotor function similar to other rodent models of brain aging. Young (4 months) and older (23 months) CB6F1 × C3D2F1 mice were administered a variety of tests for cognitive, psychomotor, and sensory/reflexive capacities. Spontaneous locomotion, rearing, and ability to turn in an alley all decreased with age, as did behavioral measures sensitive to muscle strength, balance, and motor coordination. Although no effect of age was found for either startle response amplitude or reaction time to shock stimuli, the old mice reacted with less force to low intensity auditory stimuli. When tested on a spatial swim maze task, the old mice learned less efficiently, exhibited poorer retention after a 66-h delay, and demonstrated greater difficulty learning a new spatial location. In addition, the older mice were less able to learn the platform location when it was identified by a local visual cue. Because there was a significant correlation between spatial and cued discrimination performance in the old mice, it is possible that age-related spatial maze learning deficits could involve visual or motor impairments. Variation among individuals increased with age for most tests of psychomotor function, as well as for spatial swim performance, suggesting that four-way cross mice may be appropriate models of individualized brain aging. However, the analysis of spatial maze learning deficits in older CB6F1 × C3D2F1 mice may have limited applicability in the study of brain aging, because of a confounding with visually cued performance deficits.
Key words: BALB/c, BCCD HET mice, brain aging, C3H, C57BL/6, CB6F1 × C3D2F1, DBA/2, psychomotor function, spatial memory, visual function
Introduction
Based upon their genetic uniformity, stable characteristics, and availability of previous literature for reference, inbred rodents and their F1 hybrids have been used extensively in research on the neurobiology of aging. Indeed, inbred rodents have been maintained specifically for aging studies by the National Institute on Aging (NIA) for more than 30 years. However, many concerns have been raised regarding the focus on particular inbred strains as models for aging studies, as discussed in several reviews (Weindruch and Masoro 1991; Austad 1997; Sprott 1997; McClearn and Hofer 1999a, 1999b; Nadon 2006). Because inbreds and their F1 hybrids represent only a fraction of the species population, conclusions drawn from one strain may be unrepresentative of the species as a whole. Furthermore, the inbreeding process may lead to idiosyncratic phenotypes and strain-specific pathologies that could markedly influence brain aging or have a confounding influence on the measurement of cognitive and psychomotor functions of different age groups. For example, age-related visual system pathology occurs in DBA/2 mice (Chang et al. 1999; Anderson et al. 2002), a phenotype that would confound interpretation of results in tests of visually mediated spatial learning and memory. Such tests comprise important behavioral models that have been used to study hippocampal/cortical brain function during aging (e.g., Barnes 1988; Frick et al. 1995; Gallagher and Rapp 1997).
Whereas outbred rodents have often been used in brain aging research (e.g., Gower and Lamberty 1993; Baxter and Gallagher 1996), this approach may not represent a completely satisfactory alternative to inbred animals. It has been suggested that the extent of genetic heterogeneity, as well as its reproducibility, are less than desirable in commercially maintained outbred rodents (Austad 1997; Lipman 1997). Moreover, gene linkage analysis of neurobiological or behavioral phenotypes would be difficult to achieve in these models. An alternative to the use of either a uniform genotype or an outbred line for the study of brain aging is the use of reproducible segregating populations generated from four to eight inbred strains. Such an approach has been applied successfully in previous biogerontology research (Heller et al. 1998; McClearn and Hofer 1999a). The breeding process used to produce these models employs different F1 parents, resulting in four to eight possible alleles at each gene locus. Genetically heterogeneous (HET) mice, produced by a four-way cross involving well-characterized inbred grandparents (BALB/c, C57BL/6, C3H, and DBA/2), are currently maintained for aging research by the NIA (Miller et al. 1999). These mice have been useful in several studies focused on the identification of aging biomarkers and on the determination of the genetic basis for age-sensitive phenotypes, such as T cell subsets (Miller et al. 1997) and cataract severity (Wolf et al. 2004). Furthermore, these mice are currently being employed to evaluate potential anti-aging interventions in a multi-site, NIA-sponsored initiative, the NIA Interventions Testing Program (Warner et al. 2000; Nadon 2006). Selection of these mice over an inbred strain for this program was based on the rationale that the four-way cross HET mice would afford greater generalizability of results.
Despite the clear advantages outlined above, the CB6F1 × C3D2F1 mice have not as yet been used as models to study brain aging. These HET mice should be particularly useful in approaches designed to identify interrelationships among individualized neural and behavioral phenotypes of aged rodents (e.g., Ingram et al. 1981a; Markowska et al. 1989; Collier and Coleman 1991; Baxter and Gallagher 1996; Forster et al. 1996). In particular, application of this model could allow for quantitative genetic analysis of such data, a dimension of inquiry not easily addressed with currently used rodent models. As an initial step toward such applications, the current studies were designed to determine whether or not the CB6F1 × C3D2F1 mice indeed exhibit age-related declines in cognitive or psychomotor function similar to other rodent models of brain aging, and further, to assess the extent of age-related variability associated with these declines.
Young and old CB6F1 × C3D2F1 male mice were obtained from the NIA and were subjected to a series of age-sensitive behavioral tests that have previously been used by our laboratory to characterize the functional consequences of brain aging in inbred mice and F1 hybrids (Forster and Lal 1991; Forster et al. 1996; Forster and Lal 1999; Sumien et al. 2004). These tests measure a wide range of functions: reflexive capacity, arousal, somatosensory function, auditory function, strength, balance, coordination, simple discrimination, and visually mediated spatial learning and memory. Because previous studies have suggested visual system pathology in the CB6F1 × C3D2F1 population (Wolf et al. 2004), an important goal of this study was to determine if visual dysfunction could influence performance of these mice in the spatial learning and memory task. Thus, performance of mice on a visually cued discrimination task was examined, under different levels of difficulty, and compared with spatial learning performance of the same mice.
Materials and methods
Animals
Male offspring of BALB/cJNia × C57BL/6JNia females and C3H/JNia × DBA/2JNia males were obtained from the NIA and subsequently maintained in the University of North Texas Health Science Center (UNTHSC) vivarium. A total of 20 young (4 months old) and 29 old (23 months old) male CB6F1 × C3D2F1 mice were housed in groups of 3 or 4 in clear polycarbonate cages (2 ×17×12.5 cm), and had ad libitum access to food (NIH-31 diet) and water except during the testing sessions. The ambient temperature was maintained at 23 ± 1°C, under a 12-h light/dark cycle starting at 0600. After a period of acclimation, the mice were given a series of behavioral tests in the following order: spatial learning and memory, locomotor activity, simple reflexes, wire suspension, bridge-walking, coordinated running, auditory and shock startle (sensory reactivity), discriminated avoidance, visible platform. The testing was conducted over a period of approximately 12 weeks. The mice were weighed on a weekly basis, and survival was monitored throughout the study. Food and water intake was measured daily for 1 week prior to behavioral testing.
Locomotor activity
Spontaneous locomotor activity was measured using a Digiscan apparatus (Omnitech Electronics, model RXYZCM-16), as described previously (Forster and Lal 1991). Each mouse was placed in a clear acrylic test cage (40.5× 40.5 ×30.5 cm) that was surrounded by a metal frame lined with photocells. The test cage was enclosed in a dimly lit, sound-attenuating chamber equipped with a fan that provided background noise (80 dB). During a 16-min period, movements in the horizontal plane as well as a vertical plane 7.6 cm above the floor were detected by the photocells and processed by software to yield 14 different variables describing horizontal, vertical, stereotypic, and spatial components of spontaneous activity in the apparatus.
Simple reflexes
Over four consecutive daily sessions, the mice were administered three simple reflex tests. The first test consisted of placing the mouse on a flat smooth surface and recording the latency to move one body length (walk initiation). The second test measured the latency to reverse direction when the mouse was placed in a 3.5-cm wide, 14-cm long, dead-end alley (alley turning). For the third test, the mouse was placed facing downward on a flat surface that was tilted 45°, and the latency to turn 90° in either direction was measured (negative geotaxis).
Wire suspension
The mouse was allowed to grip a horizontal wire with the front paws when suspended 27 cm above a padded surface. The latency to tread (reach the wire with their hind legs) and the latency to fall were recorded and averaged over four consecutive daily sessions (two trials/day).
Bridge walking
Each mouse was tested for the latency to fall or reach a safe platform after being placed on one of four acrylic bridges, each mounted 50 cm above a padded surface. The bridges differed in diameter (small or large) and shape (round or square), providing four levels of difficulty. Each bridge was presented three times, and the measure of performance was the average latency to fall (up to a maximum of 60 s) across all bridges.
Coordinated running
Motor learning and maximum running performance were measured using an accelerating rotorod test described previously (Forster and Lal 1999). The apparatus was a motor-driven treadmill (Accuscan Instruments, Model # AIO411RRT525M) that consisted of a 3-cm diameter nylon cylinder mounted horizontally at a height of 35 cm above a padded surface. In a given trial, the mouse was placed on the cylinder, which then began rotating with increasing speed until the animal fell to a well-padded surface. Ability of the mice to improve running performance was assessed in a series of training sessions (two per day), each consisting of four trials at 10-min intervals. The training sessions continued until the running performance (the average latency to fall from the cylinder) failed to show improvement over three consecutive sessions. The age groups were compared for their average latency to fall on the first seven sessions, and for the final session on which each mouse had reached its maximum stable level of performance.
Spatial learning and memory
Spatial learning and memory were measured using a swim maze test as described previously (Forster et al. 1996). On a given trial, the mouse was allowed to swim in a 120-cm diameter plastic tank filled to 34 cm from the top edge with colored water (non-toxic white paint) and maintained at 24 ± 1°C. An escape was provided by means of a small 10×10-cm platform hidden from view 1.5 cm below the surface of the water. A computerized tracking system recorded the length of the path taken by the mouse to reach the platform, as well as the swimming speed (San Diego Instruments, San Diego, CA, Model # SA-3).
During a pretraining phase, the tank was covered by a black curtain to prevent pre-exposure of the mice to visual cues present outside of the tank. In this way, mice learned the motor components of swimming and climbing onto the platform without learning its location in the tank. On each trial, the mouse was placed at one end of a 10 ×65-cm (width × length) straight alley that had a platform at the other end, and allowed to swim until it reached the platform or a maximum latency of 60 s had elapsed. The mice were given four sessions of pretraining (two per day), each consisting of five trials spaced at 5-min intervals.
After pretraining, the black curtain was removed from above the tank, and the mice were tested for their ability to learn the location of the platform using spatial cues. Testing was divided into three phases: acquisition (eight sessions with the platform in a fixed location), retention (two additional sessions after a 66-h delay interval), and reversal (four sessions with the platform at a new, fixed location). Each session consisted of five trials, at 10-min intervals, during which the mouse had to swim to the platform from one of four different starting points in the tank. Two sessions were conducted per day, separated by a period of at least 2 h, during which the mice were returned to the home cages. After the fifth trial of session 8, a probe trial was given in which the platform was submerged to a depth that prevented the mice from climbing onto it. The platform was raised after 30 s, and the trial was ended when the mouse successfully located it. On this trial, spatial bias for the platform location was evaluated in terms of the (1) percentage of time spent in the platform quadrant, (2) percentage of time spent within 40- and 20-cm diameter annuli surrounding the platform location, and (3) entries into the platform zone itself.
Visible platform test
A test of visually cued learning in the water maze was conducted using the same apparatus as the test for spatial learning and memory. In this test, the safe platform location was identified by a triangular flag (5 cm each side, 11 cm2) that was raised above the surface of the water (6 cm from the water surface to the bottom of the flag). Eight sessions were administered, each consisting of five trials at 10-min intervals. On each trial, the mouse had to swim to the platform from a different starting point in the tank. In addition, the platform was moved to a different location before each trial. Thus, the mouse had to learn to associate the location of the flag with the location of the platform. Subsequently, mice received four additional sessions in which the difficulty of the visual discrimination was varied by using triangular flags of progressively smaller sizes, from 11 cm2 to 0.17 cm2 in area. A smaller flag was introduced at the beginning of the second, third, and fourth sessions.
Sensory reactivity
The musculoskeletal startle reflex to auditory or shock stimuli of various intensities was determined using a standard testing system (SA Lab, San Diego Instruments) that employed an electromagnetic force transducer. For the auditory startle test, a mouse was placed inside an acrylic cylinder and presented with a series of mixed-frequency noise bursts (0, 90, 100, 110, 120 or 140 dB). Each acoustic signal (lasting 20 ms) was presented 12 times in a counterbalanced series, for a total of 72 trials. For the shock startle test, a mouse was placed inside the same acrylic cylinder, and a series of shocks (0, 0.02, 0.04, 0.08, 0.16, 0.24, 0.32, 0.64 or 1.28 mA) were delivered. Each shock stimulus (100 ms in duration and scrambled across eight inputs to the grid floor of the acrylic cylinder) was given five times, for a total of 45 trials. The amplitude of the startle reflex was defined as the peak response to each auditory or shock intensity within a 250-ms time window that began with the stimulus presentation. A measure of “reaction time” used previously (Sumien et al. 2004) was the latency to achieve the peak response following presentation of the 1.28 mA shock stimulus, an intensity that elicited a startle response of maximum amplitude in both young and old mice.
Discriminated avoidance
A T-maze constructed of acrylic (black for the sides and clear for the top) was utilized for the discriminated avoidance task (Forster and Lal 1992; McDonald and Forster 2005). The maze was divided into three compartments: a start box (10×6.3×6 cm), a stem (17.5× 6.3×6 cm) and two goal arms (14.5×6.3×6 cm), each separated by clear acrylic doors. The maze rested on a grid floor wired to deliver 0.27-mA scrambled shock to the feet.
The test consisted of two sessions separated by 24 h. On each training trial, the mouse was placed in the start box, and the start door was removed to signal the beginning of the trial. On the first trial of the first session, the mouse received shock in the first arm entered and was permitted to escape shock by running to the opposite arm, which was then designated the correct arm for the remainder of the session. On subsequent trials, shock was initiated 5 s after the opening of the start door if the mouse had not entered the correct goal arm, or immediately upon entry into the incorrect arm. In either case, the shock continued until the correct goal arm was entered or a maximum of 60 s had elapsed. Upon the mouse’s entry into the correct arm, the door was closed (to prevent departure) and, after 10 s, the mouse was removed (by detaching the goal arm) and allowed to enter a holding cage for 1 min. Training in this fashion continued at 1-min intervals until the mouse had met the criterion of a correct avoidance (defined as running directly to the correct arm within 5 s) on four of the last five training trials. The second session of avoidance training was a reversal such that the mice were required to run to the goal arm opposite that to which they had been trained on the previous day. Ability to learn the avoidance problem was considered inversely proportional to the number of trials required to reach criterion in each of the sessions. The latency to reach the goal on the last trial of the first session (i.e., after the mouse had learned the task) was assessed in order to determine if age affected motivation provided by shock or limited the ability of the old mice to perform the avoidance response.
Statistical analysis of data
The effect of age was assessed using analyses of variance (ANOVA). Food and water intake, locomotor activity, simple reflexes, wire suspension, bridge walking, and discriminated avoidance were considered in separate, single degree-of-freedom ANOVAs, whereas coordinated running, spatial and cued swim maze learning, and startle responses were considered in two-way ANOVAs, with repeated measures on Sessions or Intensity as applicable. Single degree-of-freedom F-tests were performed within the two-way interaction of these analyses to assess differences in age groups on individual sessions or at different intensities. Because age had a significant effect on body weight of the mice, analyses of covariance were done on motor function dependent variables, with body weight during behavioral testing as a covariate. Variance estimates of the age groups were compared using F-max tests to assess the effect of age on variability. Pearson correlation coefficients were calculated to determine the relationship between cued and spatial swim maze performance, and an 18×18 matrix was generated to consider interrelationships among the various behavioral measures in old mice. The alpha level was set at 0.05 for all analyses.
Results
General assessment
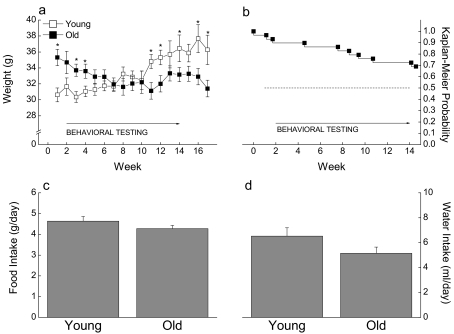
Mice of both age groups were weighed weekly over a period of 17 weeks (Figure 1a). The young mice gained weight during this period whereas the old group showed a weight loss, leading to a significant interaction between Age and Weeks (P < 0.001). Survivorship was followed in the old animals starting from their arrival in the UNTHSC vivarium until the survivors were euthanized (Figure 1b). A steady decrease in survival probability can be observed beginning at 23 months of age, with 70% of animals surviving until the end of the study, when the mice were approximately 25 months of age.
Figure 1.
Effect of age on body weight ±SE (a), Kaplan-Meier survival probability (b), and food (c) and water (d) intake ±SE for 20 young and 29 old CB6F1 × C3D2F1 mice. Food and water intakes were recorded over a period of 1 week prior to behavioral testing, whereas body weights of surviving mice were recorded at weekly intervals. Statistical analysis of the body weight data included only the mice surviving to the end of the experiment. * Significant difference between young and old groups (P < 0.05)
Intake of food and water was measured the week prior to the start of behavioral testing (Figures 1c, d). Young and old mice consumed nearly equivalent amounts of food per day, an observation supported by the absence of a significant difference between the groups (P = 0.195). Even though it appeared that old mice drank less water than the young ones, that difference was not significant (P =0.117).
Locomotor activity
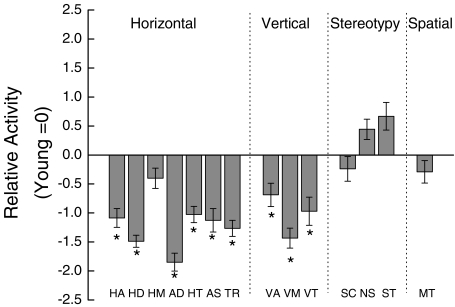
In order to describe the relative magnitudes of age related change in the different measures of spontaneous activity, each measure for the old mice was expressed in units of standard deviation above (+) or below (−) the average for the same measure in the young group (Figure 2). Statistical analyses were performed on the untransformed data. There was no significant difference in the number of horizontal movements (HM) initiated between young and old mice (P = 0.164). However, old mice showed fewer total activity counts (HA), moved more slowly (AS), traversed less distance (HD), had shorter average durations of their movements (AD), and made fewer complete revolutions around the center of the apparatus (TR) than their young counterparts (all P < 0.001). The old mice spent less time rearing (VT), they exhibited fewer vertical activity counts overall (VA), and initiated fewer vertical movements (VM) (all P < 0.029). There was no significant effect of age on amount (SC), duration (ST), or frequency (NS) of stereotypy (repetitive photocell interruptions reflecting stationary grooming or scratching). Finally, there was no effect of age on thigmotaxis, represented by time spent in contact with the inside margin (MT) of the apparatus (P = 0.449).
Figure 2.
Effect of age on horizontal, vertical, stereotypic, and spatial aspects of spontaneous locomotor activity. Each value represents the mean ±SE of 24 old mice expressed in units of standard deviation from the mean for the same measure in the young group of mice. Components of locomotor activity: HA horizontal activity, HD total distance traveled, HM number of horizontal movements, AD average distance per movement, HT time making horizontal movement, AS average speed of movement, TR total number of revolutions, VA vertical activity, VM number of vertical movements, VT time in vertical plane 7.6 cm above the floor, SC stereotypy counts, NS average number of stereotypy, ST time making stereotypic movements, MT margin time. * Significant difference between young and old groups when the untransformed data of young and old groups were compared using ANOVA (P <0.05)
Reflexive and motor performance
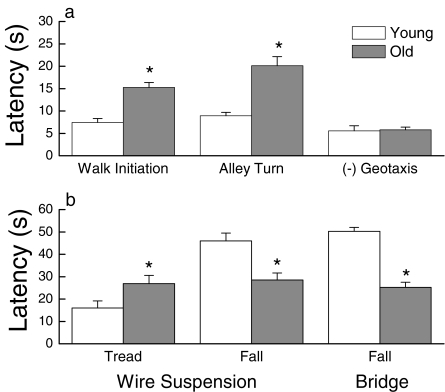
Measures of arousal and reflexive capacity are shown in Figure 3a. One-fold, age-related increases in latency to initiate walking or turn in an alley were observed (all P < 0.001), whereas no effect of age was evident on negative geotaxis (P = 0.839). Figure 3b depicts the effect of age on measures of strength, balance, and coordination. For the wire suspension test, old mice took 75% longer to tread, and fell from the wire 38% faster than the young mice (all P < 0.036). For the bridge test, an age-related decrease in latency to fall of 50% was found (P < 0.001).
Figure 3.
(a) Reflexive activity as measured by latency to walk, to turn in an alley and to turn 90° on a 45° inclined plane [(−) geotaxis] as a function of age. (b) Strength, balance, and coordination as measured by latency to tread and fall on a wire test and fall on a bridge test as a function of age. Each value represents the mean ±SE of 19 young or 28 old mice. * Significant difference between young and old groups (P < 0.05)
Coordinated running
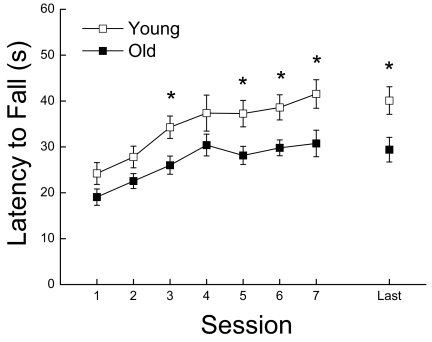
The effects of age on motor learning and maximum running performance are shown in Figure 4. Overall, the young mice performed better than the old mice (P = 0.015); however, both age groups improved at similar rates over sessions (P = 0.214). After reaching their stable maximum level of performance (i.e., the last testing session), old mice continued to fall from the rotorod with an average latency that was 27% shorter than the young mice (P = 0.013).
Figure 4.
Coordinated running performance, measured by latency to fall from a rotorod, as a function of age and testing sessions. Each value represents the mean ±SE of 18 young or 27 old mice. * Significant difference between young and old groups (P < 0.05)
Spatial learning and memory
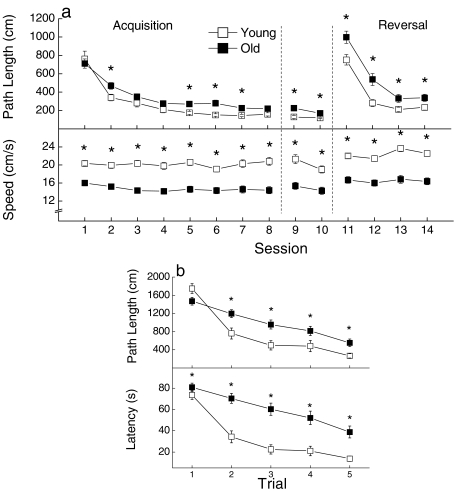
During the pretraining (alley swim) phase (data not shown), latency of both young and old CB6F1 × C3D2F1 mice to swim in the alley to the platform decreased markedly over the first two sessions, and remained stable over the remaining sessions. The old mice took longer to reach the platform than the young on each of the sessions, although the age difference in latency was not significant on the last session (P = 0.066). Analysis of the pretraining data yielded main effects of Age and Session (P < 0.006). Analysis of swim speed, independent of the path length, indicated that an age-related difference in swim speed (about 20%) persisted during all subsequent phases of the spatial swim testing (see Figure 5a, bottom panel).
Figure 5.
(a) Learning and memory for spatial discrimination by young (n = 19) and old (n = 28) mice as measured by path length to reach a hidden platform during acquisition, retention, and reversal sessions (top panel). Swim speed was measured during each of the sessions (bottom panel). Each value represents the mean average path length or swim speed ±SE for the 5 trials conducted within each session. (b) Distance (upper panel) and Latency (lower panel) of the young and old groups to reach the hidden platform during the first session of reversal (Session 11). Each value represents the mean path length or latency ±SE for each trial conducted
During acquisition, retention, and reversal phases, the length of the path taken to reach the hidden platform (Figure 5a, upper panel) was analyzed to assess the efficiency with which the mice located the platform, independently of their speed of swimming (Figure 5a, lower panel). Both young and old mice had nearly equivalent path lengths on the first session, and both could swim to the hidden platform with nearly equal efficiency by the 8th session of the acquisition (learning) phase. However, overall, the older mice were less efficient in learning the location of the platform (P = 0.016). This main effect of age was driven by significant age differences on sessions 2, 5, 6, and 7. Furthermore, young mice exhibited better retention for the location of the platform after a 66-h delay than the old ones (P = 0.007), with significant differences between the two age groups on sessions 9 and 10.
During the reversal phase, both young and old mice learned the new location of the platform, as evidenced by a decrease in path length over sessions 11 through 14. However, the old mice performed more poorly than young mice on each of the sessions, yielding a significant main effect of Age (P < 0.001). To determine whether the age effect was attributable to an initial difference in performance or to a difference in rate of learning the new location, a trial by trial analysis of session 11 was performed (Figure 5b). This analysis suggested that young and old mice traversed similar distances to the platform initially, but differed in ability to improve across all trials during session 11. Except for trial 1, the old mice required longer time (Figure 5b, upper panel) to locate the platform and swam longer distances (Figure 5b, lower panel) than the young ones (all P < 0.047). A significant interaction between Age and Trials supported the conclusion that old mice learned the new platform location at a slower rate than the young ones (P < 0.001).
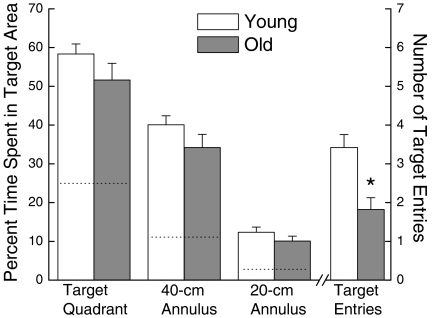
The strength and accuracy of short-term spatial memory after the initial acquisition were assessed with a probe trial performed as the last trial of session 8 (Figure 6). The platform was submerged, and the performance of the mice was determined by the percentage of total time spent in areas around the platform and by the number of entries into the location of the submerged platform itself. The percentages of time spent in the target quadrant and within 20- and 40-cm annuli were significantly greater than chance, indicating that both age groups had acquired a spatial bias for the platform location. The old mice tended to spend less time in the platform quadrant and within the different annuli than the younger ones, although no significant effects of age were found (all P < 0.366). However, the young mice made significantly more target entries than their old counterparts (P = 0.002).
Figure 6.
Performance of young and old mice on a probe trial conducted after the last session of the acquisition phase of spatial swim maze training, when the platform had been lowered below the surface of the water for a period of 30 s. Performance is expressed as the percentage of probe trial time ±SE spent in the target quadrant, in a 40- and 20-cm annulus over the center of the platform position, as well as the number of target entries ±SE made by the mice. The dashed lines represent the percentage of time mice would spend in a specific area due to chance. Each value represents the mean ±SE of 19 young or 27 old mice
Visible platform test
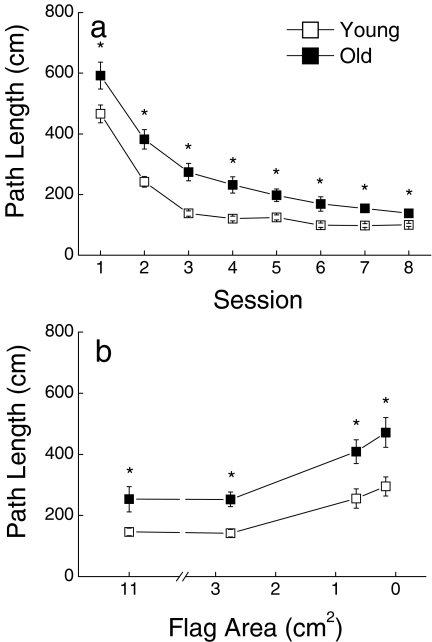
Over the first 8 training sessions, the platform location was cued by an 11-cm2 flag (Figure 7A), and both young and old mice became more efficient at swimming to the platform. However, the older mice consistently performed more poorly than young ones on each of the sessions, resulting in a significant main effect of Age (P < 0.001). There was no significant interaction between Age and Session (P = 0.08) despite the appearance of somewhat faster learning by the young mice over sessions 1–3. In order to determine whether the main effect of age was due to an age-related difference in initial performance, session 1 was considered in a trial by trial manner (data not shown). There was no effect of age on the first trial of the first session, suggesting that both age groups began visible platform training with similar performance.
Figure 7.
(a) Learning of a visible platform task as a function of age as measured by path length to reach a platform when it was identified by local visual cue (a flag). (b) Effects of decreasing flag area on path length during 5 sessions conducted following visible platform training. The flags were equilateral triangles with areas of 11-, 2.75-, 0.66-, and 0.17-cm2. Values represent the mean ±SE of 19 young or 20–24 old mice. * indicates a significant difference between young and old groups (P < 0.05)
Correlation between spatial and visually cued performance
To confirm involvement of visual function in the visible platform test, and to assess the possibility that visual impairment was indeed a cause of impaired cued platform performance by the old mice, the difficulty of the visual discrimination was varied by utilizing flags of decreasing sizes in four subsequent visual platform training sessions (Figure 7b). Deterioration of visible platform performance occurred after introduction of the 0.66- and 0.17-cm2 flags in both young and old groups, with a somewhat greater deterioration evident for old mice. However, ANOVA indicated main effects of Age and Flag (P < 0.001) but no interaction between these factors (P = 0.418), primarily because of the large performance difference in the age groups.
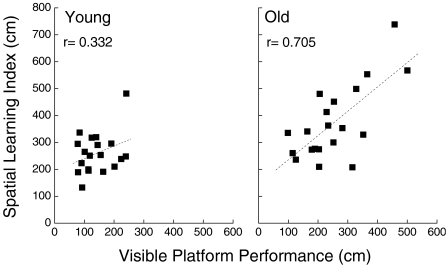
To address the possibility that spatial learning deficits of the CB6F1 × C3D2F1 mice involved impaired visual acuity or other factors not related to spatial performance, the relationship between spatial maze and visible platform performance was examined among individuals within the two age groups (Figure 8). Spatial performance was expressed as an index described previously (Forster et al. 1996; de Fiebre et al. 2006), representing the average path length on sessions 2–4 of acquisition and sessions 12–14 of reversal, whereas cued performance was represented by the average path length on the session after introduction of the 2.75 cm2 flag (the smallest flag that did not disrupt performance of the young and aged mice). When these measures were considered, there was a significant correlation (r = 0.705, P = 0.001) between cued and spatial performance in the old mice, whereas a significant relationship was not detected in younger mice (r = 0.332, P = 0.180). There was no significant correlation between cued platform performance and performance on any of the other tests of cognitive or psychomotor function, although poor cued platform performance did predict long latency for the negative geotaxis reflex in old mice (r = 0.531, P = 0.019).
Figure 8.
Scatter plots showing relationships between spatial (spatial learning index) and cued (visible platform performance) swim maze performance among young (left) and old (right) mice. Pearson correlation coefficients indicated a significant linear relationship for the old mice (P = 0.001)
Auditory and shock startle response
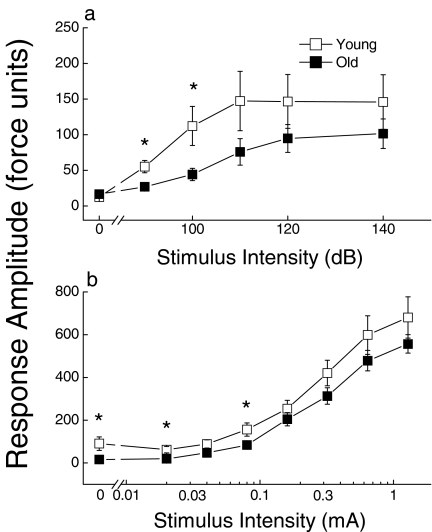
The startle response amplitude of young and old mice to a series of auditory stimuli varying in intensity is presented in Figure 9a. The mean amplitude as well as the variability of the auditory startle response increased with stimulus intensity in both age groups. ANOVA indicated neither a significant overall effect of Age nor an interaction between Age and Intensity (all P > 0.085), although individual comparisons suggested that young mice responded more forcefully than old mice to 90- and 100-dB sounds. The response of the mice to a series of shock stimuli varying in intensity is depicted in Figure 9b. The amplitude of shock startle response showed a relatively small but statistically significant decrease with age (P = 0.037). However, a significant age difference in spontaneous activity under the 0-mA shock condition accounted for the effect of age at most shock intensities. There was no significant effect of age on the latency to achieve peak response upon presentation of the 1.28-mA shock stimulus (reaction time, not shown).
Figure 9.
Effect of age on startle response amplitude (force units) as a function of the intensity of auditory (a) or shock (b) stimuli. Values represent mean ±SE of 19 young or 27 old mice. * Significant difference between young and old groups (P < 0.05)
Discriminated avoidance task
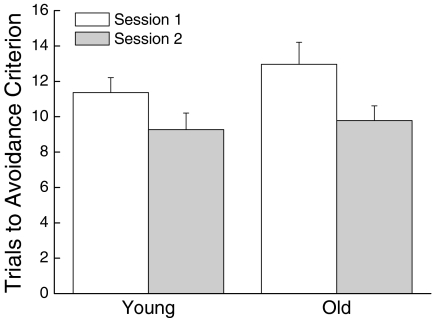
The number of trials to reach the discriminated avoidance criterion on sessions 1 and 2 is shown in Figure 10 for the young and old mice. As suggested in the figure, there was no significant effect of age on the number of trials required to reach the avoidance criterion on either session (both P< 0.388). Analysis of the latency to reach the correct arm of the maze on the last trial of session one (not shown) also failed to reveal a significant effect of age (P = 0.081).
Figure 10.
Effect of age on discriminated escape performance measured as the number of trials to reach a learning criterion during the initial testing session (Initial Learning) and during a second session in which the mice learned to turn in a direction opposite that trained on the previous day (Reversal Learning). Values represent mean ±SE of 19 young or 23 old mice. * Significant difference between young and old mice (P < 0.05)
Age-related variability and interrelationships among behavioral variables
Sample standard deviations of the measures from each behavioral test in this study were tabulated for the young and old mice and summarized in Table 1 for comparison to the effect of age on the group means. In the column labeled Group Mean, an arrow pointed upward indicates that the group mean for the behavioral measure was significantly increased in older mice, whereas an arrow pointed downward indicates that the measure was decreased. Similarly, an arrow pointed upward in the last column indicates that variance in the group of older mice was significantly greater than in the young mice, whereas an arrow pointed downward indicates smaller variance.
Table 1.
Effect of age on behavior: group means and standard deviations.
| Behavioral measure | Group mean | Standard deviation | |
|---|---|---|---|
| Young | Old | ||
| Locomotor activity | |||
| Horizontal (counts) | ↓c | 168.4 | 133.5 |
| Vertical (counts) | ↓ | 43.5 | 42.8 |
| Stereotypy (counts) | − | 90.3 | 93.9 |
| Margin time (s) | − | 12.8 | 12.1 |
| Reflex/motor | |||
| Walking initiation (s) | ↑ | 3.8 | 5.8 ↑ |
| Alley turn (s) | ↑ | 3.3 | 10.7 ↑ |
| (−) geotaxis (s) | − | 5.0 | 3.1 ↓ |
| Auditory startle (force units)a | − | 166.6 | 106.8 ↓ |
| Shock startle (force units)b | − | 418.5 | 218.1 ↓ |
| Psychomotor | |||
| Wire tread (s) | ↑ | 13.7 | 20.7 ↑ |
| Wire fall (s) | ↓ | 15.5 | 16.5 |
| Bridge fall (s) | ↓ | 7.6 | 11.8 ↑ |
| Rotorod fall (s) | ↓ | 12.8 | 14.0 |
| Reaction time (s) | − | 31.7 | 18.9 ↓ |
| Swim speed (cm/s) | ↓ | 2.4 | 3.4 ↑ |
| Learning/memory | |||
| Spatial learning index (cm) | ↑ | 76.0 | 140.7 ↑ |
| Visible platform (cm) | ↑ | 33.5 | 109.7 ↑ |
| Avoidance learning (trials) | − | 3.7 | 6.0 ↑ |
a140 dB stimulus
b1.28 mA stimulus
c↓P < 0.05, decrease with age; ↑P < 0.05, increase with age
For summary measures of the different components of spontaneous locomotor activity, variance was relatively stable, whereas the means for horizontal and vertical activity decreased with age. Variability was decreased with age for auditory and shock startle amplitude measures (for high intensity stimuli) and for reaction time (shock startle). Significant increases with age were found for some reflex/motor functions (walking initiation and alley turning) and some measures of psychomotor function (wire suspension, bridge-walking, and swim speed). Variability was also significantly increased for measures of cognition, namely the spatial learning index, visible platform performance, and learning of the discriminated avoidance task.
A Pearson correlation matrix involving the variables shown in Table 1 was generated to examine the degree of relationship between cognitive and psychomotor impairment among the aged mice. There were no significant correlations of measures of learning and memory across the domains of locomotor activity, reflex/motor, or psychomotor function as listed in Table 1. Moreover, in addition to the correlation between cued (visible platform) and spatial learning noted above, there were very few significant correlations involving different behavioral tests. Notably, among the old animals, latency to fall from the bridge was correlated with performance on the wire suspension test, and treading was significantly correlated with walking initiation.
Body weight of the mice was considered in the correlation analyses to determine if individual differences in body weight might have affected behavioral performance, particularly in the tests of psychomotor function. This analysis revealed a significant correlation between body weight (averaged over weeks 2 through 14) and latency to fall from the bridge. An analysis of covariance on the bridge-walking data, with body weight as a covariate, indicated a residual effect of Age (P < 0.001), suggesting that age differences in weight did not fully account for the apparent decline of motor function measured in this test.
Discussion
The main findings of this study are that: (1) as a group, the four-way cross mice exhibited age-related declines in cognitive and psychomotor function commonly observed in inbred and F1 hybrid mice used previously in brain aging research; (2) the pattern of impairment on tests of different functions is similar, though not identical, to C57BL/6 mice of the same chronological age tested under the same conditions; (3) as predicted, the four-way cross mice exhibited more age-related variation in cognitive behavioral phenotypes than inbred C57BL/6; and (4) age-related impairments in spatial maze performance of the four-way cross mice were confounded by a concurrent robust impairment of visually-cued learning.
Based on mouse literature published within the past 20 years, a clear majority of studies focused on brain aging have employed inbred strains as opposed to various types of genetically more heterogeneous mice. Though it has been argued that basic descriptive information on brain aging is lacking in this species as a whole, inbred C57BL/6 mice are, thus far, clearly the best studied in terms of behavior and structural brain aging (Jucker and Ingram 1997; Jucker et al. 2000). Therefore, a discussion of the similarity between age-related behavioral changes in the C57BL/6 and CB6F1 × C3D2F1 mice relates not only to the applicability of the latter to current brain aging research, but also addresses the generality of aging phenotypes previously studied in the former. The current studies provide preliminary evidence that, at the level of behavior, several commonly described phenotypes in the C57BL/6 mice (and other inbred mice) could be studied in the genetically heterogeneous CB6F1 × C3D2F1 mice of similar chronological age, whereas other aging phenotypes are not evident or do not occur until more advanced age.
In the current study, the four-way cross mice exhibited age-related decrements in performance when given commonly employed tests of psychomotor function involving balance (bridge-walking), coordinated running (rotorod), swimming, and muscle strength (wire suspension). Age-related declines in motor performance measured in similar tests have been described and studied in a variety of inbred mouse strains (e.g., Ingram et al. 1981b; Ingram and Reynolds 1986; Ingram 1988; Forster and Lal 1999; Hengemihle et al. 1999), outbred mice (Gower and Lamberty 1993), and in heterogeneous stock generated from eight inbred strains (McClearn and Hofer 1999a). The magnitude of the apparent age-related losses was similar to that observed at the same chronological ages and under similar test conditions in C57BL/6 mice (Forster and Lal 1991, 1999; Forster et al. 1996; Sumien et al. 2004). The age-related loss of psychomotor functions is also a typical finding in behavioral studies of aging in outbred, inbred, and hybrid rats (Marshall and Berrios 1979; Gage et al. 1984; Spangler et al. 1994; Markowska and Breckler 1999). The pattern of decline in spontaneous locomotor activity in four-way cross mice, involving ambulatory (horizontal) and rearing (vertical) components, was also similar to that previously reported in C57BL/6 (Forster and Lal 1991), as was the decrement in ability to turn in a blind alley and the lack of effect on the negative geotaxis reflex (Forster et al. 1996; Sumien et al. 2004).
A significant correlation between bridge-walking performance and body weight of the old CB6F1 × C3D2F1 mice suggested that weight may have influenced motor performance of this group. However, an analysis of covariance suggested that weight could not fully account for the age-related decrement in bridge-walking performance and, moreover, body weight was not correlated with performance decrements detected on other psychomotor tests such as rotorod, wire suspension, and swimming speed. These results are in accordance with previous findings for C57BL/6, DBA/2, and their F1 hybrids (Forster and Lal 1999). A significant, though modest, relationship between weight and performance on some psychomotor tasks has been reported for aged inbred mice (Ingram and Reynolds 1986) and heterogeneous stock (McClearn and Hofer 1999a).
While the effects at 21 months on spontaneous activity, psychomotor performance, and reflexive function appear similar in C57BL/6 and the four-way cross mice, a different pattern was evident for tests involving startle responses to auditory and shock stimuli, and for learning of a discriminative avoidance response. C57BL/6 mice exhibit an age-related decrease in maximum shock startle amplitude after brief high intensity shocks to the feet (Sumien et al. 2004), whereas no comparable deficit was evident in the group of CB6F1 × C3D2F1 mice tested at the same age in the current study. Reaction time, considered as the latency to achieve peak musculoskeletal response following a brief shock to the feet, was also unaffected by age in the current study but showed a robust increase at the same chronological age in C57BL/6 mice (Sumien et al. 2004).
There was some indication of disrupted auditory startle at relatively low intensities in the old CB6F1 × C3D2F1 mice, although C57BL/6 mice of the same age exhibited a markedly impaired startle response to both low and high intensity auditory stimuli (Sumien et al. 2004). Three of the grandparent genotypes in this four-way cross (C57BL/6, DBA/2, and BALB/c) have relatively severe and progressive age-related hearing loss (AHL) that can be detected by middle age or earlier (e.g., Willott et al. 1984, 1998; Prosen et al. 2003). The AHL phenotype has been linked to three separate genes present in those backgrounds (Erway et al. 1993; Johnson et al. 1997; Willott and Erway 1998), although the current startle data suggest that AHL is attenuated in the CB6F1 × C3D2F1 generation.
There was little or no evidence of impaired avoidance performance in the CB6F1 × C3D2F1 mice at the chronological age tested in this study. The active avoidance task required mice to make a preemptive response involving a simple discrimination (running to the correct arm of the maze), to avoid a punishing stimulus (shock to the feet). Previous investigations have indicated an age-dependent decline in performance of C57BL/6 mice in both the “choice” and preemptive running (avoidance) components of this task (Forster et al. 1988, 1996; Forster and Lal 1992; Dubey et al. 1996; McDonald et al. 2005), and a variety of other studies have reported active avoidance deficits in aging mice and rats, utilizing various testing paradigms (reviewed by Ingram 2001). In particular, it has been noted that older C57BL/6 mice had greater difficulty than young mice in learning a reversal of the correct goal after it had been previously well trained (Dean et al. 1981; Forster and Lal 1992; McDonald and Forster 2005), suggesting an age-related decline in cognitive flexibility. Although similar deficits may become evident in CB6F1 × C3D2F1 mice at more advanced ages, the absence of such impairments, at ages when deficits in other behavioral domains are present, suggests that the age-related impairment in C57BL/6 mice may not be fully generalized to the more heterogeneous population.
The old CB6F1 × C3D2F1 mice tested in the current studies exhibited slower acquisition of a visually mediated spatial swim task, and showed poorer retention over a 66-h interval, when compared to the young group. A probe trial analysis following the initial acquisition indicated that both the young and older mice had learned a spatial strategy for location of the platform, although the older mice appeared to have a smaller spatial bias for the platform location. A larger age-related deficit in spatial learning was evident when the old mice were required to locate a new platform position during a “reversal” phase of the test. Analysis of these data favored the interpretation that the poor reversal learning did not reflect an initial difference in bias for the previous platform location, but rather a difference in ability to learn or remember the new location. The impaired reversal performance of the CB6F1 × C3D2F1 mice in the spatial swim maze could reflect a cognitive inflexibility, although a similar effect is not detected in the context of discriminated avoidance learning.
The older mice in these studies generally swam more slowly than the young mice, suggesting an impairment in their ability to swim efficiently in accordance with other studies (Marshall and Berrios 1979). However, there was no obvious correlation, either across trials or in individual mice, between this motor impairment and the ability to navigate to the platform location using an efficient path.
A different series of studies addressed the possibility that impaired vision, or other factors not related to impaired spatial learning, per se, could account for the spatial deficits displayed by the old mice on the swim task. When the mice were required to locate the platform based on a local visual cue, a robust effect of age was noted over nearly all trials of the test. Furthermore, there was a significant correlation between cued performance and spatial performance of the old mice. Studies of cued swim performance in C57BL/6 mice have not indicated a comparable age-related impairment (Benice et al. 2006; Bennett et al. 2006).
The impaired performance of the older CB6F1 × C3D2F1 mice on the visible platform task could reflect an effect of age on motivation, attention, motor performance, or simple learning. However, it cannot be ruled out that age-related impairments in vision are involved. In a study of age-related cataract and synechia (Wolf et al. 2004), approximately 29% of the male mice of a population of similarly-aged CB6F1 × C3D2F1 mice had anterior segment damage preventing full iris dilation together with a relatively high grade of cataract. This percentage matches an apparent subset of 3–4 of the old mice tested in the current studies with concurrently impaired spatial and cued performance (see Figure 8). Scores of the mice in this subset were primarily responsible for the significant correlation of these two measurements. Nevertheless, an assessment of similar pathology in old mice with cued- and spatial performance deficits would be required to confirm this hypothesis. It is noteworthy that a significant effect of age on spatial swim maze performance was still evident after the four mice with the most severe concurrent cued- and spatial performance deficits were excluded from analysis. This result suggests that spatial learning/memory deficits are present in the aged CB6F1 × C3D2F1 mice, but are contaminated by other deficits in visually mediated performance involving specific genotypes within this population.
Analyses of the interrelationships among the different age-sensitive behaviors indicated that scores of old CB6F1 × C3D2F1 mice on cognitive, psychomotor, and sensory/reflexive tests are not significantly correlated. This result suggests that age-related declines in these different dimensions of behavioral performance occur independently, reflecting an interaction of aging with different neurobiological processes and anatomical targets. Similar findings have been reported previously for C57BL/6 and outbred mice (Gower and Lamberty 1993; Forster et al. 1996) and for commercially outbred, inbred, and F1 hybrid rats (Gage et al. 1989; Markowska et al. 1989; Markowska and Breckler 1999).
The current studies also confirmed a significant age-related increase in performance variability for many measures of learning/memory and psychomotor performance. These included spatial and cued swim maze learning and discriminated avoidance, as well as measures of psychomotor performance, including bridge-walking, wire-suspension, and swimming speed. Although variability in several behaviors decreased with age, nearly all of those behaviors themselves were insensitive to age [e.g., reaction time, auditory and shock startle, (−) geotaxis]. Within the same range of chronological age, C57BL/6 mice did not show a large age difference in estimated variance for any measures of spatial swim performance (de Fiebre et al. 2006), but exhibited age differences in group means comparable to those observed in the current studies for CB6F1 × C3D2F1 mice.
For brain aging research that employs approaches designed to link age-related individual differences in behavioral performance with concurrent neurobiological changes, the use of heterogeneous mice should confer several advantages over use of a single inbred mouse strain. It could be expected that relationships identified in this fashion using CB6F1 × C3D2F1 mice would be more generalizable and less idiosyncratic. Moreover, behavioral performance of individual CB6F1 × C3D2F1 mice shows an apparently greater age-related divergence that, in theory, should facilitate ability of correlative approaches to identify links with important neurobiological determinants. It is also feasible that quantitative approaches could be applied to explore potential genetic linkage of the age-sensitive phenotypes. Thus, in these respects, availability of aged CB6F1 × C3D2F1 mice may indeed fulfill the need for a genetically heterogeneous mouse model useful in approaches focused on the study of individualized brain aging. A significant caveat is that these mice may have limited applicability in the study of age-related deficits in visually mediated spatial learning and memory, due to a confounding with deficits in cued learning that may involve impaired visual acuity.
Acknowledgement
This research was supported by National Institutes of Health-National Institute on Aging grant P01 AG022550.
References
- Anderson MG, Smith RS, Hawes NL, Zabaleta A, Chang B, Wiggs JL, et al. Mutations in genes encoding melanosomal proteins cause pigmentary glaucoma in DBA/2J mice. Nat Genet. 2002;30:81–85. doi: 10.1038/ng794. [DOI] [PubMed] [Google Scholar]
- Austad SN. Issues in the choice of genetic configuration for animal aging models. Exp Gerontol. 1997;32:55–63. doi: 10.1016/S0531-5565(96)00033-2. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Aging and the physiology of spatial memory. Neurobiol Aging. 1988;9:563–568. doi: 10.1016/s0197-4580(88)80114-3. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Gallagher M. Neurobiological substrates of behavioral decline: models and data analytic strategies for individual differences in aging. Neurobiol Aging. 1996;17:491–495. doi: 10.1016/0197-4580(96)00011-5. [DOI] [PubMed] [Google Scholar]
- Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–423. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Bennett JC, McRae PA, Levy LJ, Frick KM. Long-term continuous, but not daily, environmental enrichment reduces spatial memory decline in aged male mice. Neurobiol Learn Mem. 2006;85:139–152. doi: 10.1016/j.nlm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Chang B, Smith RS, Hawes NL, Anderson MG, Zabaleta A, Savinova O, et al. Interacting loci cause severe iris atrophy and glaucoma in DBA/2J mice. Nat Genet. 1999;21:405–409. doi: 10.1038/7741. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Coleman PD. Divergence of biological and chronological aging: evidence from rodent studies. Neurobiol Aging. 1991;12:685–693. doi: 10.1016/0197-4580(91)90122-Z. [DOI] [PubMed] [Google Scholar]
- De Fiebre NC, Sumien N, Forster MJ, de Fiebre CM (2006) Spatial learning and psychomotor performance of C57BL/6 mice: Age sensitivity and reliability of individual differences. AGE 28 (this issue) [DOI] [PMC free article] [PubMed]
- Dean RL, 3rd, Scozzafava J, Goas JA, Regan B, Beer B, Bartus RT. Age-related differences in behavior across the life span of the C57BL/6J mouse. Exp Aging Res. 1981;7:427–451. doi: 10.1080/03610738108259823. [DOI] [PubMed] [Google Scholar]
- Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral functions of the mouse. Arch Biochem Biophys. 1996;333:189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- Erway LC, Willott JF, Archer JR, Harrison DE. Genetics of age-related hearing loss in mice: I. Inbred and F1 hybrid strains. Hear Res. 1993;65:125–132. doi: 10.1016/0378-5955(93)90207-H. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci U S A. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster MJ, Lal H. Neurobehavioral biomarkers of aging: Influence of genotype and dietary restriction. Biomed Environ Sci. 1991;4:144–165. [PubMed] [Google Scholar]
- Forster MJ, Lal H. Within-subject behavioral analysis of recent memory in aging mice. Behav Pharmacol. 1992;3:337–349. doi: 10.1097/00008877-199208000-00010. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Lal H. Estimating age-related changes in psychomotor function: influence of practice and of level of caloric intake in different genotypes. Neurobiol Aging. 1999;20:167–176. doi: 10.1016/S0197-4580(99)00041-X. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Retz KC, Popper MD, Lal H. Age differences in acquisition and retention of one-way avoidance learning in C57BL/6NNia and autoimmune mice. Behav Neural Biol. 1988;49:139–151. doi: 10.1016/S0163-1047(88)90462-1. [DOI] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging. 1995;16:149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Bjorklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5:43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Bjorklund A. Age-related impairments in spatial memory are independent of those in sensorimotor skills. Neurobiol Aging. 1989;10:347–352. doi: 10.1016/0197-4580(89)90047-X. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu Rev Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Gower AJ, Lamberty Y. The aged mouse as a model of cognitive decline with special emphasis on studies in NMRI mice. Behav Brain Res. 1993;57:163–173. doi: 10.1016/0166-4328(93)90132-A. [DOI] [PubMed] [Google Scholar]
- Heller DA, Ahern FM, Stout JT, McClearn GE. Mortality and biomarkers of aging in heterogeneous stock (HS) mice. J Gerontol A Biol Sci Med Sci. 1998;53:B217–B230. doi: 10.1093/gerona/53a.3.b217. [DOI] [PubMed] [Google Scholar]
- Hengemihle JM, Long JM, Betkey J, Jucker M, Ingram DK. Age-related psychomotor and spatial learning deficits in 129/SvJ mice. Neurobiol Aging. 1999;20:9–18. doi: 10.1016/S0197-4580(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Ingram DK. Motor performance variability during aging in rodents. Assessment of reliability and validity of individual differences. Ann N Y Acad Sci. 1988;515:70–96. doi: 10.1111/j.1749-6632.1988.tb32969.x. [DOI] [PubMed] [Google Scholar]
- Ingram DK. Rodent models of age-related memory impairment. In: Hof PR, Mobbs CV, editors. Functional neurobiology of aging. San Diego: Academic; 2001. pp. 373–386. [Google Scholar]
- Ingram DK, London ED, Goodrick CL. Age and neurochemical correlates of radial maze performance in rats. Neurobiol Aging. 1981;2:41–47. doi: 10.1016/0197-4580(81)90058-0. [DOI] [PubMed] [Google Scholar]
- Ingram DK, London ED, Reynolds MA, Waller SB, Goodrick CL. Differential effects of age on motor performance in two mouse strains. Neurobiol Aging. 1981;2:221–227. doi: 10.1016/0197-4580(81)90025-7. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Reynolds MA. Assessing the predictive validity of psychomotor tests as measures of biological age in mice. Exp Aging Res. 1986;12:155–162. doi: 10.1080/03610738608259454. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Erway LC, Cook SA, Willott JF, Zheng QY. A major gene affecting age-related hearing loss in C57BL/6J mice. Hear Res. 1997;114:83–92. doi: 10.1016/S0378-5955(97)00155-X. [DOI] [PubMed] [Google Scholar]
- Jucker M, Ingram DK. Murine models of brain aging and age-related neurodegenerative diseases. Behav Brain Res. 1997;85:1–25. doi: 10.1016/S0166-4328(96)02243-7. [DOI] [PubMed] [Google Scholar]
- Jucker M, Bondolfi L, Calhoun ME, Long JM, Ingram DK. Structural brain aging in inbred mice: potential for genetic linkage. Exp Gerontol. 2000;35:1383–1388. doi: 10.1016/S0531-5565(00)00190-X. [DOI] [PubMed] [Google Scholar]
- Lipman RD. Pathobiology of aging rodents: inbred and hybrid models. Exp Gerontol. 1997;32:215–228. doi: 10.1016/S0531-5565(96)00037-X. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Breckler SJ. Behavioral biomarkers of aging: illustration of a multivariate approach for detecting age-related behavioral changes. J Gerontol A Biol Sci Med Sci. 1999;54:B549–B566. doi: 10.1093/gerona/54.12.b549. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Stone WS, Ingram DK, Reynolds J, Gold PE, Conti LH, et al. Individual differences in aging: behavioral and neurobiological correlates. Neurobiol Aging. 1989;10:31–43. doi: 10.1016/S0197-4580(89)80008-9. [DOI] [PubMed] [Google Scholar]
- Marshall JF, Berrios N. Movement disorders of aged rats: reversal by dopamine receptor stimulation. Science. 1979;206:477–479. doi: 10.1126/science.504992. [DOI] [PubMed] [Google Scholar]
- McClearn GE, Hofer SM. Genes as gerontological variables: genetically heterogeneous stocks and complex systems. Neurobiol Aging. 1999;20:147–156. doi: 10.1016/S0197-4580(99)00046-9. [DOI] [PubMed] [Google Scholar]
- McClearn GE, Hofer SM. Genes as gerontological variables: uniform genotypes. Neurobiol Aging. 1999;20:95–104. doi: 10.1016/S0197-4580(99)00030-5. [DOI] [PubMed] [Google Scholar]
- McDonald SR, Forster MJ. Lifelong vitamin E intake retards age-associated decline of spatial learning ability in apoE-deficient mice. AGE. 2005;27:5–16. doi: 10.1007/s11357-005-4003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald SR, Sohal RS, Forster MJ. Concurrent administration of coenzyme Q10 and alpha-tocopherol improves learning in aged mice. Free Radic Biol Med. 2005;38:729–736. doi: 10.1016/j.freeradbiomed.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Miller RA, Chrisp C, Galecki A. CD4 memory T cell levels predict life span in genetically heterogeneous mice. FASEB J. 1997;11:775–783. doi: 10.1096/fasebj.11.10.9271362. [DOI] [PubMed] [Google Scholar]
- Miller RA, Burke D, Nadon N. Announcement: four-way cross mouse stocks: a new, genetically heterogeneous resource for aging research. J Gerontol A Biol Sci Med Sci. 1999;54:B358–B360. doi: 10.1093/gerona/54.8.b358. [DOI] [PubMed] [Google Scholar]
- Nadon NL. Exploiting the rodent model for studies on the pharmacology of lifespan extension. Aging Cell. 2006;5:9–15. doi: 10.1111/j.1474-9726.2006.00185.x. [DOI] [PubMed] [Google Scholar]
- Prosen CA, Dore DJ, May BJ. The functional age of hearing loss in a mouse model of presbycusis. I. Behavioral assessments. Hear Res. 2003;183:44–56. doi: 10.1016/S0378-5955(03)00211-9. [DOI] [PubMed] [Google Scholar]
- Spangler EL, Waggie KS, Hengemihle J, Roberts D, Hess B, Ingram DK. Behavioral assessment of aging in male Fischer 344 and brown Norway rat strains and their F1 hybrid. Neurobiol Aging. 1994;15:319–328. doi: 10.1016/0197-4580(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Sprott RL. Mouse and rat genotype choices. Exp Gerontol. 1997;32:79–86. doi: 10.1016/S0531-5565(96)00035-6. [DOI] [PubMed] [Google Scholar]
- Sumien N, Heinrich KR, Sohal RS, Forster MJ. Short-term vitamin E intake fails to improve cognitive or psychomotor performance of aged mice. Free Radic Biol Med. 2004;36:1424–1433. doi: 10.1016/j.freeradbiomed.2004.02.081. [DOI] [PubMed] [Google Scholar]
- Warner HR, Ingram DK, Miller RA, Nadon NL, Richardson AG. Program for testing biological interventions to promote healthy aging. Mech Ageing Dev. 2000;155:199–208. doi: 10.1016/S0047-6374(00)00118-4. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Masoro EJ. Concerns about rodent models for aging research. J Gerontol. 1991;46:B87–B88. doi: 10.1093/geronj/46.3.b87. [DOI] [PubMed] [Google Scholar]
- Willott JF, Erway LC. Genetics of age-related hearing loss in mice. IV. Cochlear pathology and hearing loss in 25 BXD recombinant inbred mouse strains. Hear Res. 1998;119:27–36. doi: 10.1016/S0378-5955(98)00029-X. [DOI] [PubMed] [Google Scholar]
- Willott JF, Kulig J, Satterfield T. The acoustic startle response in DBA/2 and C57BL/6 mice: relationship to auditory neuronal response properties and hearing impairment. Hear Res. 1984;16:161–167. doi: 10.1016/0378-5955(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Willott JF, Turner JG, Carlson S, Ding D, Seegers Bross L, Falls WA. The BALB/c mouse as an animal model for progressive sensorineural hearing loss. Hear Res. 1998;115:162–174. doi: 10.1016/S0378-5955(97)00189-5. [DOI] [PubMed] [Google Scholar]
- Wolf N, Galecki A, Lipman R, Chen S, Smith-Wheelock M, Burke D, et al. Quantitative trait locus mapping for age-related cataract severity and synechia prevalence using four-way cross mice. Invest Ophthalmol Vis Sci. 2004;45:1922–1929. doi: 10.1167/iovs.03-0435. [DOI] [PubMed] [Google Scholar]