Abstract
Two tests often used in aging research, the elevated path test and the Morris water maze test, were examined for their application to the study of brain aging in a large sample of C57BL/6JNia mice. Specifically, these studies assessed: (1) sensitivity to age and the degree of interrelatedness among different behavioral measures derived from these tests, (2) the effect of age on variation in the measurements, and (3) the reliability of individual differences in performance on the tests. Both tests detected age-related deficits in group performance that occurred independently of each other. However, analysis of data obtained on the Morris water maze test revealed three relatively independent components of cognitive performance. Performance in initial acquisition of spatial learning in the Morris maze was not highly correlated with performance during reversal learning (when mice were required to learn a new spatial location), whereas performance in both of those phases was independent of spatial performance assessed during a single probe trial administered at the end of acquisition training. Moreover, impaired performance during initial acquisition could be detected at an earlier age than impairments in reversal learning. There were modest but significant age-related increases in the variance of both elevated path test scores and in several measures of learning in the Morris maze test. Analysis of test scores of mice across repeated testing sessions confirmed reliability of the measurements obtained for cognitive and psychomotor function. Power calculations confirmed that there are sufficiently large age-related differences in elevated path test performance, relative to within age variability, to render this test useful for studies into the ability of an intervention to prevent or reverse age-related deficits in psychomotor performance. Power calculations indicated a need for larger sample sizes for detection of intervention effects on cognitive components of the Morris water maze test, at least when implemented at the ages tested in this study. Variability among old mice in both tests, including each of the various independent measures in the Morris maze, may be useful for elucidating the biological bases of different aspects of dysfunctional brain aging.
Key words: brain aging, bridge walking, elevated path test, balance beam, inbred mice, individual differences, Morris maze, psychomotor function, spatial learning
Introduction
Investigations of the biological processes and targets involved in brain aging have, until recently, mainly focused on laboratory rats as models. This focus evolved based on the extensive use of rats in traditional behavioral neuroscience and the subsequent development and testing of rat analogues of human cognitive and psychomotor decline in aging. There is now substantially increased use of mouse models, based mainly on availability of numerous genetically engineered animals that afford the opportunity to evaluate more specific hypotheses about the causes of brain aging. Unfortunately, behavioral analogues of cognitive and psychomotor aging, as well as their neurobiological bases, have been less well studied in this species (for discussion, see Jucker et al. 2000; Jucker and Ingram 1997). This problem is magnified by the comparatively greater number of mouse genetic backgrounds that may be used in aging research, based on the different genotypes used to produce various transgenic and null mutant mice (Hengemihle et al. 1999).
Whereas mice have increasingly been used in studies of chemical and genetic interventions on cognitive and motor decline in aging, they have also been employed with greater frequency in studies focused on identifying the neurobiological processes of aging through the analysis of individual differences (e.g., Forster et al. 1996; Magnusson 1998; Calhoun et al. 1998; Bernstein et al. 1985; Liu et al. 2003). The latter approach is based on the assumption that differences in cognitive or motor abilities among individuals in a group of old mice should reflect biologically-based differences in brain aging. While this approach has been used extensively as a tool for identifying the specific neurological substrates involved in losses of cognitive and psychomotor performance (e.g., Baxter and Gallagher 1996; Breckler 1993; Collier and Coleman 1991; Gallagher and Rapp 1997; Ingram 1996; Ingram et al. 1981, 1983; Markowska et al. 1989; Olton et al. 1991; Rapp and Amaral 1992; deToledo-Morell et al. 1988), it would appear to be currently most well-developed and widely used in laboratories using rats as experimental models (e.g., Albeck et al. 2003; Bizon et al. 2004; Collier et al. 2004; Gallagher et al. 2006). For example, in Long-Evans rats, spatial memory performance has been analyzed in terms of the age groups, testing parameters, and scaling of behavioral measurements optimal for successful application of this approach, and the relative ranges of performance in young and old rats have been described. Moreover, assumptions of test/retest reliability and independence of cognitive and non-cognitive variables have been addressed (for review, see, Gallagher et al. 2006). In contrast, only a subset of these issues has been addressed in various mouse strains used in aging research (Ingram 1988; Hengemihle et al. 1999).
In the current report, data generated from a relatively large sample of C57BL/6 mice of different ages were used to assess several of the characteristics mentioned above for two behavioral tests previously used extensively in studies of rodent aging. Performance on the elevated path test, used as a measure of balance and sensorimotor coordination in studies of aging (Gage et al. 1989), is highly dependent on cerebellar function (Brunner and Altman 1973) and shows steady decline with age in rodents (Forster et al. 1996; Campbell and Gaddy 1987; Gage et al. 1984; Markowska and Breckler 1999) that appears to be correlated with cerebellar pathology (Zornetzer and Rogers 1983). The Morris water maze test (Morris 1981), which involves the learning and recall of spatial information, has also been used extensively in aging research (e.g., Forster et al. 1996; Albeck et al. 2003; Bizon et al. 2004). A consensus of the literature suggests that progressive impairment of spatial learning/memory, involving structures of the medial temporal lobe, represents one important dimension of cognitive decline in both rodent and human aging (for reviews, see, Gallagher and Rapp 1997; Barnes 1988; Gallagher et al. 2006). With regard to each of these tests as implemented in mice, our studies specifically addressed: (1) sensitivity to age and the degree of interrelatedness among different behavioral measures derived from the same test, (2) the effect of age on the variability and range of performance in young and old mice, and (3) the reliability of individual differences in performance on the tests.
In attempting to analyze individual differences in behavior to identify the neurobiological processes of aging, a basic assumption is that variance in the measures is not restricted by ceiling or floor effects and accurately reflects true individual differences in capacity as opposed to error of measurement (i.e., the differences in performance are reliable). With respect to tests of psychomotor function, such reliability can be readily established using a test/retest method (Ingram 1988). However, reliability of individual differences in measures of spatial learning is intrinsically more difficult to address because the critical measures involve change with time or practice, rather than a stable measure of performance. Thus, attempts to assess reliability using a test/retest method are confounded by instability due to experience with the test procedure. Previous studies have attempted to minimize this problem by retesting animals using a novel task involving similar cognitive capacity (for review, see, Gallagher et al. 2006, this volume). In accordance with this approach, we have previously summarized Morris water maze data into a “spatial learning index” that reflects both initial learning of a hidden platform location and the subsequent learning of a different location (Forster et al. 1996), based on the rationale that such a measure would be more reliable than a measure based on initial acquisition alone. In the current study, we tested the validity of this assumption within the relatively large sample of mice tested in this fashion. Moreover, we further addressed reliability of Morris maze performance by comparing performance of individual mice over stable phases of acquisition and retention testing.
Materials and methods
Animals
Male C57BL/6JNia mice were obtained from the National Institute on Aging. Except for the animals described in the next paragraph, mice were obtained when 5, 15, or 23 months of age. Upon arrival at UNTHSC, mice were injected subcutaneously with a unique identification chip (Allflex, Boulder, Colo.) and were group housed (5–7 per cage). The identification chip was small (2×13 mm) and biologically inert (encased in a glass capsule). Mice were then gradually switched from an NIH-31 diet to a diet low in phytoestrogens (Teklad Global 16% Protein Rodent Diet, #2016S) over the course of 1 week. This diet does not contain soy (minimizing levels of soy isoflavones) nor does it contain alfalfa (minimizing levels of coumestans). Mice were acclimated to this diet and our colony in the central animal care facility at UNTHSC for 4 weeks under a 12-h light/dark schedule with food and water available ad libitum prior to the start of behavioral testing. Thus, mice were 6, 16, or 24 months of age at the start of testing. Each mouse was tested in the elevated path test and, subsequently, in the Morris water maze as described below. The number of mice in each group is described in the Results section.
Additional male C57BL/6JNia mice were obtained when 4 or 18 months of age and used to assess the reliability of the elevated path test data (these mice were control animals for an unpublished intervention study requiring daily oral administration of test compounds). In the reliability study, mice were maintained in the UNTHSC animal care facility on a 12-h light/dark schedule with food (NIH-31) and water available ad libitum. After 1.5 weeks, mice were given daily intragastric administrations of sterile, physiological saline (0.1 ml) throughout their time on study. Starting 1 month after the beginning of saline treatments (when 5 and 19 months of age), mice were tested in the elevated path test as described below. Subsequently, these animals were tested once each month for an additional 5 months (until mice of the two groups were 10 and 24 months old).
Elevated path test (bridge walking)
The apparatus used in this test consisted of four clear acrylic bridges (60 cm long) that were suspended between two platforms located 35.5 cm above a 2.5-cm padded surface. The bridges differed in shape (round versus square) and diameter (2 vs 1 cm), each providing a different degree of difficulty. A different bridge was used on each of four consecutive days of testing (typically Monday–Thursday). In a given trial, a mouse was placed on one of the two platforms for 5 s and was then gently dragged to the center of the bridge. The latency for the mouse to fall from the bridge or to navigate the bridge to either of the platforms was recorded. A maximum latency to fall of 60 s was scored for mice which either had reached the platform in under 60 s or were still on the bridge at 60 s. Mice were given three trials per day with an inter-trial interval of 5 min. The principal measure in the elevated path test was the latency to fall, either examined as the average latency to fall (of three trials) for each bridge individually, or a single overall mean representing the average latency to fall from all four types of bridges.
Morris water maze
During this test, mice were required to learn to navigate a circular tank of water, and to locate and then climb onto a hidden platform using the cues present in the room. The test used was a variation of the Morris maze test previously modified for use with mice (Forster et al. 1996) and further modified to incorporate a single, 30-s probe trial to confirm spatial learning ability following acquisition. Use of a single probe trial, as opposed to multiple probes during acquisition as described by others (Markowska et al. 1993; Gallagher et al. 1993), was implemented to decrease the probability of extinction of the learned behavior and minimize the potentially disruptive effect of the probe trial upon subsequent performance (Frick et al. 1995; Markowska et al. 1993).
The test consisted of four phases. In the first phase (pretraining), mice were acclimated to the water and their ability to swim to and climb onto a platform was assessed in an environment where swimming was confined to a narrow alley and no spatial cues were present. In the acquisition phase of testing, mice learned how to locate the hidden platform using the cues which were present in the room. In the retention phase of testing, memory for the platform location was tested 66 h after the last acquisition trial had been completed. Lastly, in the reversal phase of testing, the ability of a mouse to learn a new location of the hidden platform was assessed.
Apparatus Morris maze testing was done using similar apparatuses in two separate rooms, each arranged to provide similar visual cues. Mice were randomly assigned to the two rooms and a given mouse was always tested in the same room. The Morris maze consisted of a white polyethylene tank of 120 cm diameter filled to a height of 34 cm (15 cm below the top edge of the tank) with water made opaque by the addition of white, nontoxic Crayola paint. Water was kept at 24.0±0.5°C. A platform (10×10 cm) was located 1 cm below the water surface. The platform was designed such that it could be lowered approximately 20 cm (where it was inaccessible to the mouse during the probe trial) and subsequently raised to its normal height via a remote cable. The placement of the platform was standardized for each of the phases of testing as were the spatial cues that were present in each of the two testing rooms. A camera was mounted above the center of the tanks in each room and each was connected to a computer running a video tracking system that recorded behavior. In one room, a SMART tracking system (San Diego Instruments) was used. In the other room, a Polytrack system (San Diego Instruments) was used. Testing consisted of two daily sessions, each consisting of five trials. The two daily sessions were separated by a minimum of 2 h.
Pretraining (straight swim) In this phase of testing, a black, opaque curtain surrounded the tank such that no spatial cues were visible. A 10×60 cm acrylic alley incorporating a 10×10 cm platform at one end and closed on the other was placed in the tank with the platform located 1 cm below the surface of the water. Mice were placed in the water at the closed end of the alley and were allowed to swim to the opposite end of the alley and to escape the water by climbing onto the platform. The time for the mouse to swim to and climb onto the platform was recorded to allow for an assessment of swimming speed. If a mouse was unable to locate the platform within 60 s, it was gently directed to the platform. There was an inter-trial interval of 5 min between each of the five trials. There were four pretraining sessions with sessions 1 and 2 conducted on a Friday (the day immediately after the last day of elevated path testing) and sessions 3 and 4 on the following Monday.
Acquisition Eight spatial training sessions were conducted on the Tuesday through Friday immediately after the last day of pretraining. Each session consisted of five trials except for session 8 that included a sixth trial (the probe trial). The inter-trial interval in this phase of testing was 10 min. In a given trial, mice were placed inside the tank with the base of the tail at one of four predetermined locations along the edge, and were required to locate and to climb onto the platform which was located in the center of one of the quadrants (target quadrant). Immediately upon placing the mouse in the water, the video tracking system was activated and the experimenter assumed a seat in a fixed position relative to the platform. A trial ended either when the animal climbed onto the platform or when the maximum duration of the trial (90 s) expired. If a mouse had not found the platform after 90 s, the experimenter initially tapped on the center of the platform with his or her index finger several times, thereby serving as a visible platform cue. Mice which then did not swim to the platform were gently directed to the platform. After a mouse climbed onto the platform, it was allowed to sit on the platform for 10 s prior to being returned to its holding carrier.During the sixth trial of session 8, a probe trial was conducted. At the start of this trial, the platform was inaccessible to the mouse for the first 30 s of the trial (it was lowered approximately 20 cm below the surface of the water). After 30 s, the platform was raised to its previous position and the trial ended with a successful location of the platform.
Retention Sessions 9 and 10 were referred to as “retention sessions” and were conducted on the Monday following the last day of acquisition testing. Testing in these sessions was identical to testing during the acquisition phase of testing.
Reversal In sessions 11 through 14, conducted on the Tuesday and Wednesday following retention testing, the platform was moved to the opposite side of the tank and closer to the wall of the tank. Testing proceeded in a fashion identical that used in acquisition testing.
Morris maze data A minimum criterion was established in order to exclude from analyses, those mice failing to develop a spatial strategy after extended training. To meet the criterion, a mouse was required to swim over the previous (initial) platform site (platform entry) at least once during the first trial of the reversal (session 11).The principal measure of spatial performance on a given trial was the length of the path on which an animal swam prior to finding the platform (path length). Time to find the platform was also measured for calculation of swimming speed. Maximal spatial learning was estimated by the calculation of a “minimum path” length, the average path length during the final two sessions of acquisition (sessions 7 and 8).We previously summarized Morris maze data into a “spatial learning index” that represented the linear, descending portions of the acquisition and reversal learning curves, and included those sessions that differentiated performance of young and old mice (Forster et al. 1996). In the current study, this total learning index (LI-tot) was the mean of the average path length on sessions 2–4 and in sessions 12–14. Here, we also examined acquisition (sessions 2–4, LI-acq) and reversal (sessions 12–14, LI-rev) learning indices separately.A number of measures of spatial bias were collected during the probe trial in session 8. Specifically, we measured the percent of the time during the probe that the mouse spent in the target quadrant and in an area comprising an annulus of 20 or 40 cm around the center of the platform. Further, we measured the number of times a mouse swam over the platform site (platform entries) as well as the amount of time the mouse spent above the platform site.
Statistical analyses
Main and interactive effects of mouse Age and Test Sessions were assessed by ANOVA for each dependent variable. Where indicated by the outcome of these analyses, individual comparisons between age groups were performed using single degree-of-freedom F tests. Relationships between dependent variables were assessed using Pearson correlation coefficients. Differences in the variances for young and old mice on each measure were assessed via Fmax tests.
Reliability in both the elevated path test and Morris maze was evaluated by calculating an intraclass R statistic (Zar 1984), to assess the degree or relationship among the scores of individuals across the different testing sessions. For the elevated path test, test/retest scores were obtained from a separate group of young and old animals that were tested every month for 5 consecutive months. For data generated by the Morris maze test, test/retest scores were obtained by examining the path lengths in the last three sessions of acquisition (sessions 6–8), plus the path lengths in the two sessions of retention (sessions 9–10), in 34 of the mice of each age which had attained the minimum criterion for learning in the Morris maze. A randomly selected subset of the larger young and old groups was used in this analysis to simplify the calculation and to ensure equal contributions of the age groups to the intraclass R statistic. An additional test of reliability was performed by evaluating the Pearson correlation between acquisition performance during sessions 2–4 with that during sessions 12–14.
Results
Elevated path test
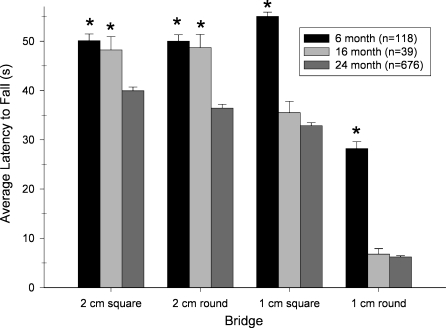
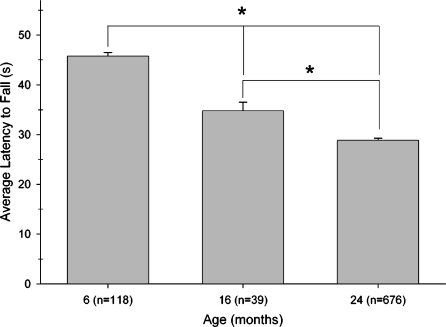
The average latencies for mice of each age to fall from each of the four bridges (in order of difficulty) are presented in Figure 1. That the bridges differed in difficulty was indicated by a main effect of Bridge (F3,2478=283.692; P<.0001). The effect of age to decrease the latency to fall depended on the diameter and shape of the bridge, an observation that was confirmed by a 2-way ANOVA that yielded a significant interaction of Age with Bridge (F6,2478=11.587; P<.0001). Individual comparisons within the Age × Bridge interaction indicated that an effect of age could be detected by 16 months on the 1-cm bridges, whereas the 2-cm bridges could only differentiate 6- and 24-month-olds. However, the 1-cm bridge did not differentiate the 16 and 24-month-old groups, and thus testing on any one bridge was insufficient to differentiate all three age groups. However, when the mean of the average latency to fall from each of the four bridges was calculated for 6, 16 and 24-month-old mice (Figure 2), there was a nearly linear decline as a function of age that was reflected in a significant main effect (F2,826=146.918; P<.0001). Individual comparisons conducted within the Age effect confirmed differences among all three age groups.
Figure 1.
Differences in performance of 6, 16 and 24-month-old C57BL/6 mice on each of the four bridges used in the elevated path (bridge walking) test. Each bar represents the mean±SEM of the average latency to fall from the indicated bridge. Each mouse was given three trials on each bridge with an intertrial interval of 5 min. The number of subjects tested in each age group is presented in parentheses in the figure; * indicates that mice of the indicated age group differed significantly from 24-month-old mice (P<.05, individual comparison within ANOVA)
Figure 2.
Age-related differences in elevated path test scores derived from performance at different levels of difficulty. Each bar represents the mean±SEM of the average latency to fall from the four bridges used in this test. Each mouse was given three trials on each bridge with an intertrial interval of 5 min. The number of subjects tested in each age group is presented in parentheses in the legend for the abscissa. Each of the three age groups differed from each other. * Indicates that a significant difference was found between mice of different ages (P<.05, individual comparison within ANOVA)
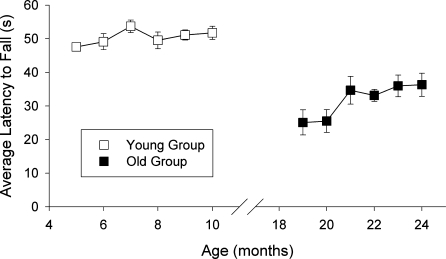
In Figure 3, the test/retest scores are presented for separate groups of young and surviving old mice, each tested every month for 6 consecutive months in the elevated path test. Some improvement was evident in the old group by the third session, suggesting a practice effect in this age group. Performance of the old mice, however, remained stable over the last four monthly testing sessions. Despite some instability in the old group, an intraclass R of 0.653 (P<.001) was calculated when both young and old subjects were considered which supports the assertion that the test is reliable.
Figure 3.
Reliability and stability of elevated path test scores across different age groups. Eleven young and 13 old mice were tested once every month across 4 days in the elevated path test, for a total of 6 months. Data are expressed as the mean average latency to fall (±SEM) across the four bridges for each monthly session. An intraclass R of 0.653 (P.001) was calculated when both young and old subjects were considered
Morris water maze
The different testing rooms had no significant effect on behavior in the Morris water maze and therefore all analyses were done utilizing data combined from the two facilities. The Morris maze data were first examined to assess how many mice of each age group did not attain the minimum criterion for learning (at least one entry into the initial platform site during the first trial of the reversal in session 11). There was no age-related difference in failure to meet criterion, with approximately 13% of mice in each of the three age groups not meeting criterion (6 months: 12.9%; 16 months: 12.8%; 24 months: 12.6%). Data from these mice were excluded from all subsequent analyses.
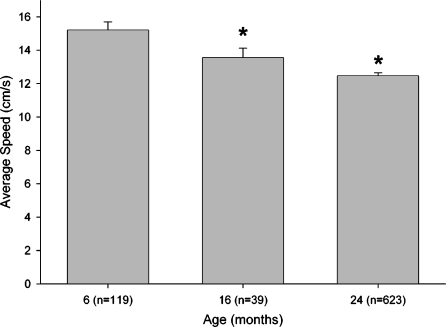
During the last session of pretraining in the Morris maze (Figure 4), the swimming speed of mice of each age was determined. ANOVA revealed a main effect of age (F2,696=10.987; P<.0001) and individual comparisons confirmed that 6-month-old mice swam faster than the 24-month-olds (P<.0001). As with performance in pretraining, the 24-month-old mice also swam slower than 6-month-old mice in each of the three subsequent phases of testing shown in Figure 5 (swimming speed data not shown). Because of the faster swimming speeds of young mice, the distance a mouse swam (path length) before finding the hidden platform in the Morris maze was used in all subsequent analyses of spatial performance.
Figure 4.
Age-related differences in swimming speed during pretraining for Morris maze testing. Each bar represents the mean±SEM swimming speed of mice measured during the last session of pretraining which consisted of four trials in which mice swam for 60 cm down a narrow alley to a submerged platform. The number of subjects tested in each age group is presented in parentheses in the legend for the abscissa. Mice of the 6 months age group swam significantly faster than 24-month-old mice (* P.05)
Figure 5.
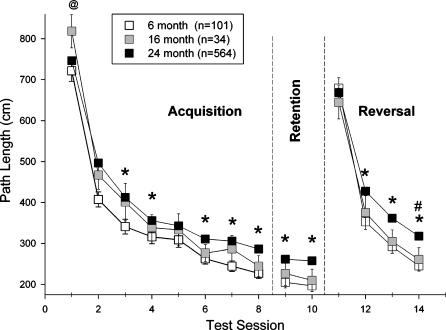
Age-related differences in learning in the Morris maze. Each point represents the mean±SEM distance which mice swam prior to finding the platform (path length) in each session of testing. The number of subjects tested in each age group is presented in parentheses in the legend for the figure. * Indicates that 6-month-old mice differed significantly from 24-month-old mice (P.05); # indicates that 16-month-old mice differed significantly from 24-month-old mice (P.05); @ indicates that 16-month-old mice differed significantly from 6month-old mice (P.05)
In Figure 5, data from the acquisition, retention and reversal phases of Morris maze testing are presented. Each age group showed an overall decrease in path length over sessions 1 through 10, as well as a decrease over sessions 11 through 14, yielding a significant main effect of testing session (F13,9035=182.005; P<.0001). While the average path lengths of the 6- and 24-month-old groups failed to differ on the first session of acquisition and reversal, the performance of 24-month-olds was poorer over most of the subsequent training or reversal sessions, contributing to a significant main effect of age (F2,9035=18.791; P<.0001). Individual comparisons within that main effect confirmed a significant difference between 6- and 24-month-old mice (P<.0001), whereas there was no difference between 6- and 16-month-olds. Comparisons within the Age x Session interaction revealed significant differences between 6- and 24-month-old mice during sessions 2–4 and sessions 6–8 during acquisition testing, differences between 6- and 24-month-old mice in both sessions of retention testing, and differences between mice of these ages in sessions 12–14 during reversal testing. There was also a difference between 6- and 16-month-old mice in session 1 of acquisition and between 16- and 24-month-old mice during session 14 of reversal testing.
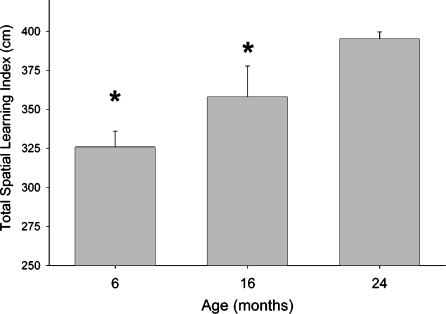
The average Li-tot for 6-, 16- and 24-month-old mice are presented in Figure 6. ANOVA revealed a main effect of age (F2,696=19.497; P<.0001) and individual comparisons confirmed that 24-month-old mice had a significantly larger learning index (representing poorer learning) than both 6-month-old mice (P<.0001) and 16-month-old mice (P<.05).
Figure 6.
Age-related differences in learning ability in the Morris maze. Each bar represents the mean±SEM “total spatial learning index” which was derived from the linear portion of both the acquisition and reversal phases of Morris maze testing. Old (24-month) mice performed more poorly than did both 6-month-old mice as well as 16month-old mice (* P.05)
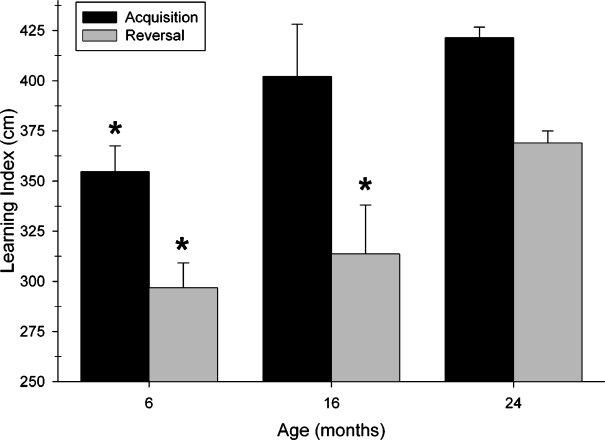
The acquisition and reversal components of the learning index were analyzed separately to determine if they had different sensitivity to the variable of age. The separate learning indices, LI-acq and LI-rev, are presented in Figure 7. For acquisition, there was a main effect of age (F2,696=12.990; P<.0001) and individual comparisons revealed a significant difference between 6- and 24-month-old mice (P<.0001), but not between 6- and 16-month-old mice nor between 16- and 24-month-old mice. For reversal, there was also a main effect of age (F2,696=11.955; P<.0001), although subsequent comparisons revealed a difference between the 6- and 24-month-old mice (P<.0001) as well as between 16- and 24-month-old mice (P<.05) for this measure. There was no difference between 6- and 16-month-old mice.
Figure 7.
Age-related differences in the acquisition and reversal phases of Morris maze testing. Each black bar represents the mean±SEM learning index for the acquisition phase of testing and is the mean of the average path lengths over the five trials of sessions 2–4. Each gray bar represents the mean±SEM learning index for the reversal phase of testing and represents the mean of the average path length during sessions 12–14. Young (6-month-old) mice performed significantly better then 24-month-old mice in the acquisition phase of testing. Old (24-month-old) mice performed significantly worse than both 6- and 16-month-old mice in the reversal phase of testing. * Indicates that mice of the indicated age group differed significantly from 24-month-old mice (P.05)
The different effects of age presented in Figure 7 suggested that performance in the acquisition and reversal phases of testing were independent. To confirm this at the level of individual performance, we calculated the Pearson correlation coefficient between the acquisition component of the learning index and the reversal component of this index. Although the very large number of animals tested renders the calculated correlation coefficient of 0.225 highly significant statistically (P<.0001; Table 1), a correlation of this size indicates that the relationship between these two indices of Morris maze learning is weak (explaining only 5.06% of the variance) and suggests that acquisition and reversal learning abilities in the Morris maze are mostly independent of each other.
Table 1.
Pearson correlations among behavioral measures.
| BW | EP | SPD | Ll-tot | Ll-acq | Ll-rev | MP | RET | %Q | %A40 | %A20 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Body weight (BW) | |||||||||||
| Elevated path (EP) | −0.12 | ||||||||||
| Swim speed (SPD) | 0.16 | 0.15 | |||||||||
| Ll-total (Li-tot) | −0.04 | 0.02 | −0.09 | ||||||||
| LI-acquisition (Li-acq) | −0.05 | 0.04 | −0.05 | 0.75 | |||||||
| LI-reversal (Li-rev) | −0.01 | −0.01 | −0.09 | 0.81 | 0.23 | ||||||
| Minimum path (MP) | −0.07 | 0.05 | −0.05 | 0.30 | 0.24 | 0.23 | |||||
| Retention (RET) | 0.00 | 0.03 | −0.03 | 0.27 | 0.21 | 0.22 | 0.34 | ||||
| % time quad (%Q | 0.10 | 0.00 | 0.14 | −0.25 | −0.16 | −0.23 | −0.29 | −0.33 | |||
| % time Ann40 (%A40) | 0.04 | −0.02 | 0.09 | −0.28 | −0.20 | −0.23 | −0.25 | −0.33 | 0.91 | ||
| % time Ann20 (%A20) | 0.05 | 0.02 | 0.10 | −0.28 | −0.22 | −0.22 | −0.21 | −0.28 | 0.74 | 0.85 | |
| Platform entries | 0.12 | 0.02 | 0.17 | −0.20 | −0.18 | −0.14 | −0.21 | −0.21 | 0.59 | 0.65 | 0.78 |
Entries in italics r2>0.25.
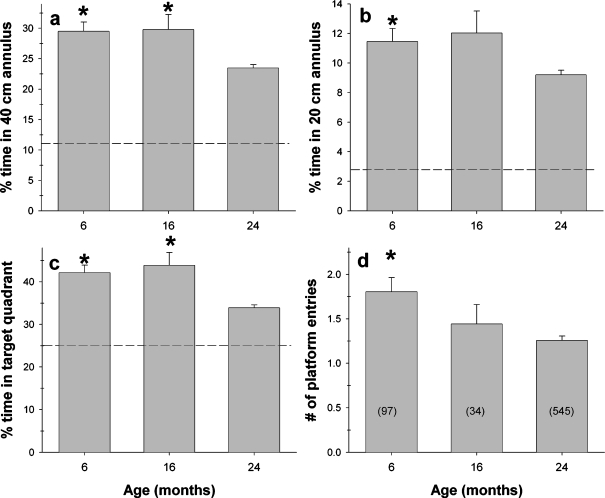
Spatial learning ability was examined during the conduction of a single probe trial during the first 30 s of the last trial of session 8 (Figure 8). In Figure 8a, b, the average percent of time spent within an annulus of 40 or 20 cm (diameter) around the center of the target platform is shown. In both analyses, ANOVA revealed a significant main effect of age (40 cm annulus: F2,673=10.588; P<.0001; 20 cm annulus: F2,673=5.218; P<.01). Individual comparisons revealed that 24-month-old mice spent significantly less time in both the 40 and 20 cm annuli than did either 6- or 16-month-old mice (6 vs 24 months: 40 cm, P<.0001; 20 cm, P<.01; 16 vs 24 month: 40 cm, P<.001; 20 cm, P<.05). In Figure 8c, the percent of time mice of each age group spent within the target quadrant is presented. Again, there was a main effect of age (F2,673=15.645; P<.0001) and subsequent comparisons showed that 24-month-old mice spent significantly less time in the target quadrant than did either 6- or 16-month-old mice (P<.0005). Lastly, in Figure 8d, the average number of times mice of each age group entered the platform site during the probe trial is presented. As with the other indices of spatial learning, there was a main effect of age (F2,673 = 7.892; P<.0005) with 24-month-old mice entering the platform site significantly fewer times than the 6-month-old mice (P<.0001).
Figure 8.
Age-related differences in spatial learning as assessed by a probe trial conducted during the first 30 s of the last trial of session 8. During this 30-s period, the platform was not available to the mouse. The number of mice of each age tested is shown in the bars in panel (d). In panels (a-c), the dotted line indicates the amount of time a mouse would spend in given areas based solely on chance. (a) Percent time spent swimming within a 40-cm annulus of the center of the platform. Old (24 month) mice spend significantly less time in the 40-cm annulus than do either 6-month-old or 16-month-old mice. (b) Percent time spent swimming within a 20-cm annulus of the center of the platform. Old (24 month) mice spend significantly less time in the 20-cm annulus than do either 6- or 16-month-old mice. (c) Percent time spent swimming in the quadrant of the Morris maze where the platform had been located. Old (24 month) mice spend significantly less time in the target quadrant than do either 6-month-old or 16-month-old mice. (d) Number of times a mouse entered the site where the platform had been located. Old (24 month) mice enter the target site significantly fewer times than 6-month-old mice. * Indicates that mice of the indicated age group differed significantly from 24-month-old mice (P<.05)
To address reliability of path length data for individual mice in the different age groups, test/retest scores were obtained by examining the path lengths of mice over periods of testing in which average performance was relatively stable. This period included the last three sessions of acquisition (sessions 6–8) plus the path lengths in the two sessions of retention (sessions 9–10). A significant intraclass R of 0.502 (P<.018) was calculated from these data.
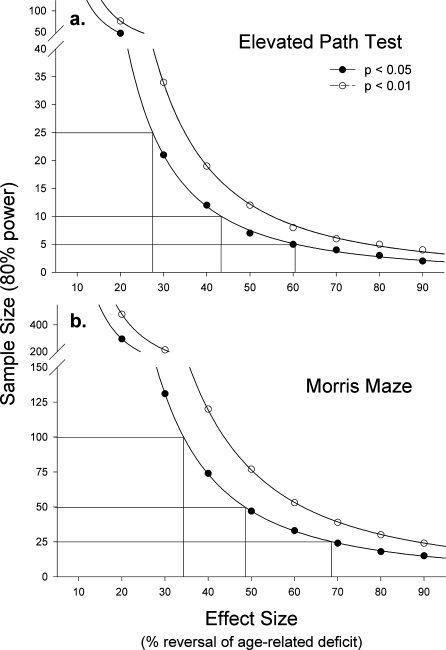
We conducted power analyses to estimate the size of sample that would be needed to detect the effect of an intervention to prevent or reverse age-related deficits in elevated path or Morris maze performance. The results of these analyses are presented in Figure 9. For the elevated path test, a sample size of 25 would be sufficient to detect an effect size of approximately 28% or greater at the P<.05 level with 80% power. Sample sizes of 10 and 5 would be sufficient to detect effect sizes of at least ∼43% and ∼60%, respectively. For the Morris maze test (total learning index), a sample size of 100 would be required to detect an effect size of approximately 35% (at P<.05 and 80% power). Sample sizes of 50 and 25 would be sufficient to detect effect sizes of at least ∼48% and ∼68%.
Figure 9.
Power analyses of the number of mice required to assess the ability of an intervention to reverse or prevent age-related deficits in the (a) elevated path test (latency to fall) or in the (b) Morris maze (total learning index). An effect size of 100% would produce a change in old animals such that their mean score was equal to that of young mice
Correlations among measures
To determine the degree of interrelationship among the different measures assessed in these experiments, a Pearson correlation matrix (Table 1) was generated based on data from the 24-month-old mice. The age-sensitive measures included body weight, latency to fall on the elevated path test (Figure 2), the swim speed during the last session of pretraining (Figure 4), the learning index variables considered in Figure 6 and 7, the probe trial measures considered in Figure 8, and two additional age sensitive measures, the minimum path length (average path length in the last two acquisition sessions) and retention (average path length in the two retention sessions). As discussed above, the large sample size allowed detection of very small correlations. For example, a correlation as low as 0.070 (accounting for 0.5% of the variance) is significant at the P<.05 level. Hence, a criterion of r=0.5 or higher (accounting for at least 25% of the variance) was chosen as an indication of a “strong” relationship between measures. Those correlations reaching this criterion are highlighted in Table 1.
The body weight of mice was not strongly correlated with any measure in the elevated path or Morris maze tests. The principal measure of the elevated path test (mean latency to fall across all bridges) was not strongly correlated with any of the measures from Morris maze testing. As mentioned above, the learning indices calculated separately for the acquisition and reversal phases of Morris maze testing were not strongly correlated with each other. Individually, however, both the acquisition and reversal learning indices were strongly correlated with the total learning index (of which both are a component). All measures of spatial learning collected during the probe trial were strongly correlated with each other. However, none of the measures of spatial learning determined by the probe trial were strongly correlated with the calculated learning indices (total, acquisition or reversal). Lastly, minimum path length, an estimate of the maximal efficiency with which a mouse could find a platform in the Morris maze, and retention scores were correlated neither with each other nor with any other measure of Morris maze learning or elevated path test performance.
Effect of age on variance was considered by application of an Fmax statistic (Table 2) to variance ratios of young (6 month) and old (24 month) C57BL/6 mice in performance on the elevated path test and the various Morris maze measures showing relative independence. For the elevated path test, there was a significant increase in variance of approximately 45%. There were relatively modest increases (12–27%) evident for several measures of Morris maze performance, although no increase was evident for the acquisition learning index or performance on the probe trial. Comparisons between the 24- and 16-month-old groups did not suggest significant differences in variance for the various measures (data not shown).
Table 2.
Effect of age on variance (standard deviation).
| Behavioral measure | 6 months | 24 months | Fmax | P |
|---|---|---|---|---|
| Elevated Path | ||||
| Latency (s) | 6.42 | 9.28 | 2.09 | <0.01 |
| Morris maze | ||||
| LI-total (cm) | 85.26 | 95.85 | 1.48 | <0.01 |
| LI-acquisition (cm) | 109.90 | 119.19 | 1.24 | n.s. |
| LI-reversal (cm) | 103.48 | 130.00 | 1.99 | <0.01 |
| Minimum path (cm) | 100.08 | 126.66 | 1.87 | <0.01 |
| Retention (cm) | 98.69 | 123.21 | 1.78 | <0.01 |
| % time in 20-cm annulus | 6.94 | 6.83 | 1.03 | n.s. |
n.s. Not statistically significant, P>0.05.
Discussion
The large sample of mice available from this project afforded the opportunity to address several issues and assumptions relating to the use of C57BL/6 mice in studies of spatial learning and psychomotor function in aging. First, the current data confirm that chronological age affects both functions, as measured by various components of spatial navigation performance in the Morris water maze test, and in an elevated path test incorporating varying degrees of difficulty. Second, whereas the declines in performance on each of the tests were first detected at the same chronological ages, correlational analyses confirmed that the declines in individuals tend to occur independently of one another. Further, different components of the spatial performance of mice in the water maze could be measured that were sensitive to age, but were also independent of each other based both on correlation analyses and by the differential sensitivity of the measures to age. Additionally, both spatial cognitive and psychomotor performance test results suggested a significant, though imperfect degree of reliability. Lastly, there were small but statistically significant age-related increases in variance for measures of performance in the elevated path test and in most measures of cognitive function in the Morris maze.
Motor tasks requiring rodents to traverse a narrow elevated path (elevated path, balance beam, plank-walking, etc.) have been used historically to analyze neurological deficits (Brunner and Altman 1973) and were incorporated in behavioral test batteries used in early studies characterizing aging in mouse and rat models (Dean et al. 1981; Ingram et al. 1981; Krauter et al. 1981). While many studies have required rodents to traverse a path of fixed shape and diameter, the current studies employed several paths affording different levels of difficulty, in accordance with similar procedures currently used to study psychomotor function in rat models of aging (Gage et al. 1989; Markowska and Breckler 1999; Shukitt-Hale et al. 2006). The current results confirm previous indications that multiple parameters afford greater sensitivity in detecting the progression of motor deficits as a function of age (e.g., Markowska and Breckler 1999). In the current study, the 16-month-old mice performed more similarly to 6-month-old mice on the larger (2 cm) bridges, but performed more similarly to 24-month-old mice on the smaller (1 cm) bridges. When a score reflecting performance on all four bridges was considered, an intermediate performance level was obtained in the 16-month-old group. Based on these findings, the scoring of mice under multiple testing parameters in the elevated path test would seem to provide optimal sensitivity in detecting effects of interventions or subtle differences in individual performance within groups of aged mice.
In experiments to assess the reliability of the elevated path test (Figure 3), improvements in performance across the first 3 months of testing were detected in the old group. This suggests that old mice may have been able to learn behavioral strategies to compensate partially for age-related deficits in psychomotor performance. Although performance improved, the maximal performance of old mice did not reach the level of performance of young mice. Lack of a similar improvement in the performance of young mice may suggest that these animals initially perform at a maximal level for which improvement through practice is not possible.
Reliability of data for individual mice would be critical in approaches attempting to identify neurobiological correlates of individual differences in elevated path performance. While reliability has been reported for a variety of motor tests in C57BL/6 mice (Ingram 1988), this characteristic has not been reported for elevated path performance. Our results suggested acceptable reliability of the elevated path data over several months in which group performance in both young and old mice remained relatively stable. Nevertheless, it could be argued that a higher degree of reliability would be desirable for studies of individual differences.
Numerous studies have reported age-related deficits in the ability of C57BL/6 mice to learn visually-mediated spatial swim tasks (e.g., Forster et al. 1996; Bellush et al. 1996; Liu et al. 2003; Magnusson 2001; Fordyce and Wehner 1993; Bennett et al. 2006), although others have failed to report effects of age (e.g., Calhoun et al. 1998; Means et al. 1993). The current studies confirmed a modest, though readily detectable, impairment in the rate at which old mice learned to swim to the hidden platform in the Morris maze test, after they had been pretrained on the motor components of swimming and climbing onto the platform. After extended spatial training, it was also evident that the maximum extent to which the old mice could navigate a direct path to the platform, independent of their swimming speed, was less than that of younger mice. Analysis of data from a single probe trial confirmed that most of the young and old mice had acquired a spatial strategy for locating the platform after eight training sessions, although the amount of spatial bias for the platform location was greater in the young than in the old mice at that time. After training, memory for the initial platform position was preserved over approximately a 66-h period in both young and old animals, although the old mice were impaired in their subsequent learning of a new platform location (reversal). Several summary measurements were defined for scoring the different aspects of performance of the C57BL/6 mice that were affected by age. Analyses of these measures in different age groups suggested that impairment in the initial acquisition phase of the Morris maze test was evident in mice aged 16 months, whereas other aspects of performance were not affected until later ages.
It should be noted that, in our earlier study (Forster et al. 1996), the reported spatial learning index was based on performance in the first three sessions of both acquisition and reversal, whereas the LI-tot reported here was based on performance in sessions 2–4 of acquisition and in sessions 12–14 of reversal. Although the earlier study suggested that young and old mice differed in the first session of both acquisition and reversal, differences in these sessions were not found in the current study, which incorporates a much larger sample size. Hence, each of the learning indices reported here was calculated based on the linear portion of the acquisition and/or reversal learning curves incorporating those sessions in which young and old mice differed.
The lack of a strong correlation between performance in the elevated path test and Morris maze performance was expected and replicated published reports in different rodent species suggesting that age-related deficits in psychomotor function fail to predict impaired cognitive performance on spatial learning tasks (Gage et al. 1989; Forster et al. 1996; Markowska and Breckler 1999; Markowska et al. 1989; Gallagher and Burwell 1989; Gower and Lamberty 1993). However, it was somewhat surprising to discover that different measures of learning and performance in the Morris maze were not strongly correlated with one another.
It could be argued that the lack of strong correlations among measures based on initial acquisition, reversal learning, and the probe trial, was reflective of a lack of reliability in performance of individual mice across the different phases of the Morris maze testing. Indeed, an important goal of the current studies was to assess reliability by comparing performance of individuals during the initial acquisition and with subsequent learning of a new platform location. While the modest correlation between performances of mice during these two phases could be due to poor reliability, trends observed in group data suggest that deficits in acquisition and reversal could involve different neurobiological substrates, based on their appearance at different chronological ages. Furthermore, intraclass correlation analyses confirmed a modest degree of reliability for more stable phases of water maze performance. It may be the case that learning a new platform location, after a significant amount of training to the initial location, confers a sensitivity to age-related impairment in cognitive flexibility linked to frontal cortical function (Schoenbaum et al. 2006). Cognitive inflexibility has indeed been noted in older C57BL/6 mice tested in various maze learning tasks (Forster and Lal 1992; Dean et al. 1981; McDonald and Forster 2005), and such inflexibility may develop independently of deficient ability in performing tasks involving spatial navigation, per se.
In the current study, spatial performance during initial acquisition failed to predict performance during late acquisition, retention, and on the probe trial, despite the indication from group data that all of the measures reflected impairment with age. Since the latter measures are based on trials conducted after most of the improvement in spatial performance has already occurred, these measures most likely estimate the maximum capacity for localization and/or efficient navigation to the hidden platform, as opposed to the capacity for spatial learning. It seems particularly noteworthy that conducting a probe trial after acquisition may indeed reveal an effect of age, yet that type of measurement may not reflect the same neurobiological dysfunction that is responsible for impairments evident in earlier acquisition.
For C57BL/6 mice, the use of a spatial learning index based only on performance during initial acquisition may be most directly in accordance with measures commonly used in rats to study hippocampal dysfunction in aging (Gallagher et al. 1993). However, it is noteworthy that several other brain regions, in addition to the hippocampus, have been implicated in different aspects of rat and human spatial performance, including subregions of the striatum and the frontal cortex (Devan et al. 1996; Holahan et al. 2005; Kolb et al. 1983; Maguire et al. 1998; for review, see, D’Hooge and De Deyn 2001). Therefore, the apparently independent effects of age on aspects of Morris maze performance of mice, such as late acquisition, the probe test, and reversal, could very well reflect differing degrees of involvement of these regional targets in aging.
A noteworthy finding in this study was that approximately equal numbers (about 13%) of young, middle-aged, and old C57BL/6 mice failed to develop a spatial bias for the platform location, even after 10 training sessions (50 trials). The basis for this observation is not clear, but suggests that a subset of the mice of this strain may simply fail to adopt a spatial strategy in performing on the Morris maze test. Monitoring of mice during all phases of testing did not indicate that any mice were using a taxon or other obvious non-spatial strategy. To provide a degree of assurance that differences in performance among individuals and between groups of mice are reflective of true differences in spatial learning, only those mice for which a spatial strategy could be confirmed were included in subsequent analyses. Other studies have also employed performance criteria to confirm spatial performance in the Morris maze test (Gallagher et al. 1993). It should be noted that this procedure could have the untoward effect of excluding older mice with severe spatial learning deficits; however, in the current studies there was no evidence of a differential effect on the composition the age groups.
It is reasonable to question whether or not motor or visual functions influence the performance of aged C57BL/6 mice, especially in the Morris maze. The oldest mice in these studies had obvious deficits in motor performance in the elevated path test and swam more slowly than the 6-month-old group during the pretraining and subsequent spatial performance components of Morris maze testing. Nevertheless, the severity of these deficits was not correlated with measures of spatial learning based on path length or probe-based measures of spatial bias. Data from the probe trial (Figure 8) demonstrate that on average, 24-month-old mice spend more time near the platform than would be predicted by chance. This would suggest additionally that the majority of 24-month-old mice had sufficient visual acuity to use the cues in the room to find that platform. While the current studies did not specifically evaluate visually cued swim performance in the C57BL/6 mice, a number of other studies have not indicated any obvious age-related impairment in this strain (Bennett et al. 2006; Benice et al. 2006). We also failed to observe a significant age-related impairment in visually-cued performance in an unpublished study. However, it cannot be ruled out that some individuals suffer from subtle impairment of vision not detected in tests involving cue-based navigation. Wolf and co-workers (Wolf et al. 2000, 2005) have reported a significant incidence of cataract in 24-month-old C57BL/6 mice. While no visible opacities were present in the mice tested in these studies, the possible influence of cataract on Morris maze performance of old mice has not been investigated.
Modest, though statistically significant, increases in variance were detected with aging, both for the elevated path test as well as for several measures of cognitive function in the Morris maze. This increase in variance with age is in agreement with other reports (e.g., Gage et al. 1989; Collier and Coleman 1991; Ingram 1988) and could be considered as evidence for individualized brain aging in the C57BL/6 mice. In the context of concurrent reliability, this would tend to confirm the validity of approaches employing this mouse strain to identify neurobiological substrates of age-impaired spatial learning (Forster et al. 1996; Calhoun et al. 1998; Magnusson 1998). Further, C57BL/6 mice may be favored for analyses of this type because they are one of the most common strains on whose background genetic manipulations have been placed. However, because C57BL/6 mice are inbred, genetic variability is not a source of their individualized aging, raising a concern that significant determinants of brain aging may not be identified in such analyses. Indeed, a greater amount of age-related variance in spatial performance seems to be evident in outbred or genetically heterogeneous rodent strains (Gage et al. 1989; Gallagher et al. 1993; Sumien et al. 2006) and among different inbred strains (Jucker et al. 2000). Therefore, studies of individualized brain aging in C57BL/6 mice may be limited in their generalizability, and it should not be expected that results will be in perfect accordance with relationships identified in programs employing outbred rats (Gallagher et al. 2006).
Substrains of the 129 inbred mouse strain are also commonly used in the construction of transgenic mice because their embryonic stem cells are amenable to in vitro manipulations and subsequent germ line integration (Simpson et al. 1997). Hengemihle et al. (1999) have examined age-related psychomotor and spatial learning deficits in 129/SvJ mice. Aged (27-month-old) 129/SvJ mice showed modest deficits in a tightrope test of psychomotor function compared to 5-month-old mice. Unlike our data with C57BL/6 mice in the elevated path test, middle aged (17-month-old) 129/SvJ mice did not perform in the tightrope test of psychomotor function at a level intermediate to that of young and aged mice. In the Morris maze, 129/SvJ mice showed deficits in acquisition of place learning. However, no differences were evident in a probe trial which confounded any conclusions concerning the spatial learning abilities of young and old mice of this strain. The authors noted that variability in old 129/SvJ mice in a visible platform test in the Morris maze correlated with severity of eye pathology common in this strain. For this and other reasons, the authors concluded that 129/SvJ mice may not be appropriate for studies of cognitive aging.
The power calculations depicted in Figure 9 demonstrate that use of a learning index. incorporating both acquisition and reversal learning (LI-tot). should have sufficient power to detect the effects of an intervention with significant potential to attenuate or reverse age-related deficits in cognitive function (i.e., those which produce greater than a 50% change). However, detecting smaller effects using this measure would require relatively large samples. For example, a sample size of 50 would be required to detect a 48% reversal whereas a sample size of 25 would reliably detect a reversal of 68%. Our data suggest that the elevated path test may have greater power in detecting the effects of an intervention to attenuate or reverse age-related deficits in psychomotor function. For example, a sample size as low as 25 may be sufficient to detect an effect size as small as 25%. It should be noted that the sample of 16-month-old mice in the current study was considerably smaller than for either of the other groups, reflecting a reduced need for mice of this age by various projects in our research group. The sample size of the 16-month-old group (34–39 mice) contributed to a somewhat diminished power for detecting effects at the intermediate ages in the current studies.
The mice used in this study were switched from a diet that was high in phytoestrogens to one which was low in phytoestrogens (see Methods) approximately 1 month prior to the start of testing. This was done because of concern that phytoestrogens could affect behavioral outcomes, especially among female mice to be used in other aspects of this project. Dietary soy phytoestrogens have been reported to have anxiolytic effects (Lund and Lephart 2001) as well as effects on spatial learning in a radial arm maze (Lund et al. 2001). Although the current study did not directly test the effects of switching to the low phytoestrogen diet, the water maze and elevated path data from young and aged male C57BL/6 mice presented do not differ appreciably from previous studies (e.g., Forster et al. 1996) suggesting that effects of dietary phytoestrogens, at the concentration present in the standard NIH-31 diet, may be subtle.
In conclusion, the data demonstrate that chronological age affects both spatial/cognitive and psychomotor function of C57BL/6 mice. Correlational analyses suggested not only that declines in elevated path performance occur relatively independently of declines in Morris maze performance, but also that different components of performance in the water maze are independent of each other. We conclude that age-related differences in the elevated path test, as well as in the Morris maze, are large enough to allow the test to be useful for assessing the ability of interventions to reverse or prevent age-related deficits. Further, a modest degree of individualized aging is evident in performance on both tests in aged mice that could be useful for probing the biological bases of age-related cognitive and motor impairments and, perhaps lend insight into the causes of idiopathic dysfunctional brain aging in humans.
Acknowledgements
The authors would like to thank Dr. Kevin Heinrich, Nicole Shelton, Bindu Nair, Angela Money, Daniel Runyan, Tiffany Allen, Eric Martin and Anita Moghe for technical assistance. This work was supported by AG-022550.
Abbreviations
- ANOVA
analysis of variance
- BW
body weight
- EP
elevated path test
- SPD
swim speed
- LI
learning index
- LI-tot
total learning index
- LI-acq
acquisition learning index
- LI-rev
reversal learning index
- MP
minimum path length
- %A20 & %A40
the percent time spent within an annulus of 20 and 40 cm around the platform in the probe trial in session 8
- %Q
the percent time spent in the target quadrant in the probe trial
References
- Albeck D, Mesches MH, Juthberg S, Browning M, Bickford PC, Rose GM, et al. Exogenous NGF restores endogenous NGF distribution in the brain of the cognitively impaired aged rat. Brain Res. 2003;967:306–310. doi: 10.1016/S0006-8993(03)02272-8. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Aging and the physiology of spatial memory. Neurobiol Aging. 1988;9:563–568. doi: 10.1016/s0197-4580(88)80114-3. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Gallagher M. Neurobiological substrates of behavioral decline: models and data analytic strategies for individual differences in aging. Neurobiol Aging. 1996;17:491–495. doi: 10.1016/0197-4580(96)00011-5. [DOI] [PubMed] [Google Scholar]
- Bellush LL, Wright AM, Walker JP, Kopchick J, Colvin RA. Caloric restriction and spatial learning in old mice. Physiol Behav. 1996;60:541–547. doi: 10.1016/S0031-9384(96)80029-1. [DOI] [PubMed] [Google Scholar]
- Benice TS, Rizk A, Kohama S, Pfankuch T, Raber J. Sex-differences in age-related cognitive decline in C57BL/6J mice associated with increased brain microtubule-associated protein 2 and synaptophysin immunoreactivity. Neuroscience. 2006;137:413–423. doi: 10.1016/j.neuroscience.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Bennett JC, McRae PA, Levy LJ, Frick KM. Long-term continuous, but not daily, environmental enrichment reduces spatial memory decline in aged male mice. Neurobiol Learn Mem. 2006;85:139–152. doi: 10.1016/j.nlm.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Bernstein D, Olton DS, Ingram DK, Waller SB, Reynolds MA, London ED. Radial maze performance in young and aged mice: neurochemical correlates. Pharmacol Biochem Behav. 1985;22:301–307. doi: 10.1016/0091-3057(85)90395-8. [DOI] [PubMed] [Google Scholar]
- Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3:227–234. doi: 10.1111/j.1474-9728.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- Breckler SJ. Age-related behavioral and neurochemical deficits: new data analytic strategies. Neurobiol Aging. 1993;14:695–697. doi: 10.1016/0197-4580(93)90076-N. [DOI] [PubMed] [Google Scholar]
- Brunner RL, Altman J. Locomotor deficits in adult rats with moderate to massive retardation of cerebellar development during infancy. Behav Biol. 1973;9:169–188. doi: 10.1016/S0091-6773(73)80154-3. [DOI] [PubMed] [Google Scholar]
- Calhoun ME, Kurth D, Phinney AL, Long JM, Hengemihle J, Mouton PR, et al. Hippocampal neuron and synaptophysin-positive bouton number in aging C57BL/6 mice. Neurobiol Aging. 1998;19:599–606. doi: 10.1016/S0197-4580(98)00098-0. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Gaddy JR. Rate of aging and dietary restriction: sensory and motor function in the Fischer 344 rat. J Gerontol. 1987;42:154–159. doi: 10.1093/geronj/42.2.154. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Coleman PD. Divergence of biological and chronological aging: evidence from rodent studies. Neurobiol Aging. 1991;12:685–693. doi: 10.1016/0197-4580(91)90122-Z. [DOI] [PubMed] [Google Scholar]
- Collier TJ, Greene JG, Felten DL, Stevens SY, Collier KS. Reduced cortical noradrenergic neurotransmission is associated with increased neophobia and impaired spatial memory in aged rats. Neurobiol Aging. 2004;25:209–221. doi: 10.1016/S0197-4580(03)00042-3. [DOI] [PubMed] [Google Scholar]
- D’Hooge R, Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Rev. 2001;36:60–90. doi: 10.1016/S0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- Dean RL, 3rd, Scozzafava J, Goas JA, Regan B, Beer B, Bartus RT. Age-related differences in behavior across the life span of the C57BL/6J mouse. Exp Aging Res. 1981;7:427–451. doi: 10.1080/03610738108259823. [DOI] [PubMed] [Google Scholar]
- Toledo-Morell L, Geinisman Y, Morell F. Individual differences in hippocampal synaptic plasticity as a function of aging: behavioral, electrophysiological and morphological evidence. In: Petit E, Ivy G, editors. Neural plasticity: a lifespan approach. New York: Liss; 1988. pp. 283–328. [Google Scholar]
- Devan BD, Goad EH, Petri HL. Dissociation of hippocampal and striatal contributions to spatial navigation in the water maze. Neurobiol Learn Mem. 1996;66:305–323. doi: 10.1006/nlme.1996.0072. [DOI] [PubMed] [Google Scholar]
- Fordyce DE, Wehner JM. Effects of aging on spatial learning and hippocampal protein kinase C in mice. Neurobiol Aging. 1993;14:309–317. doi: 10.1016/0197-4580(93)90116-S. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Lal H. Within-subject behavioral analysis of recent memory in aging mice. Behav Pharmacol. 1992;3:337–349. doi: 10.1097/00008877-199208000-00010. [DOI] [PubMed] [Google Scholar]
- Forster MJ, Dubey A, Dawson KM, Stutts WA, Lal H, Sohal RS. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc Natl Acad Sci USA. 1996;93:4765–4769. doi: 10.1073/pnas.93.10.4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick KM, Baxter MG, Markowska AL, Olton DS, Price DL. Age-related spatial reference and working memory deficits assessed in the water maze. Neurobiol Aging. 1995;16:149–160. doi: 10.1016/0197-4580(94)00155-3. [DOI] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Bjorklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5:43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Bjorklund A. Age-related impairments in spatial memory are independent of those in sensorimotor skills. Neurobiol Aging. 1989;10:347–352. doi: 10.1016/0197-4580(89)90047-X. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell RD. Relationship of age-related decline across several behavioral domains. Neurobiol Aging. 1989;10:691–708. doi: 10.1016/0197-4580(89)90006-7. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu Rev Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037/0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Colantuoni C, Eichenbaum H, Haberman R, Rapp PR, Tanila H et al (2006) Individual differences in neurocognitive aging of the medial temporal lobe. DOI: 10.1007/s11357-006-9017-5 (this issue) [DOI] [PMC free article] [PubMed]
- Gower AJ, Lamberty Y. The aged mouse as a model of cognitive decline with special emphasis on studies in NMRI mice. Behav Brain Res. 1993;57:163–173. doi: 10.1016/0166-4328(93)90132-A. [DOI] [PubMed] [Google Scholar]
- Hengemihle JM, Long JM, Betkey J, Jucker M, Ingram DK. Age-related psychomotor and spatial learning deficits in 129/SvJ mice. Neurobiol Aging. 1999;20:9–18. doi: 10.1016/S0197-4580(99)00016-0. [DOI] [PubMed] [Google Scholar]
- Holahan MR, Taverna FA, Emrich SM, Louis M, Muller RU, Roder JC, et al. Impairment in long-term retention but not short-term performance on a water maze reversal task following hippocampal or mediodorsal striatal N-methyl-D-aspartate receptor blockade. Behav Neurosci. 2005;119:1563–1571. doi: 10.1037/0735-7044.119.6.1563. [DOI] [PubMed] [Google Scholar]
- Ingram DK. Motor performance variability during aging in rodents. Assessment of reliability and validity of individual differences. Ann NY Acad Sci. 1988;515:70–96. doi: 10.1111/j.1749-6632.1988.tb32969.x. [DOI] [PubMed] [Google Scholar]
- Ingram DK. Brain-behavior linkages in aged rodent models: strategies for examining individual differences. Neurobiol Aging. 1996;17:497–499. doi: 10.1016/0197-4580(96)00003-6. [DOI] [PubMed] [Google Scholar]
- Ingram DK, London ED, Goodrick CL. Age and neurochemical correlates of radial maze performance in rats. Neurobiol Aging. 1981;2:41–47. doi: 10.1016/0197-4580(81)90058-0. [DOI] [PubMed] [Google Scholar]
- Ingram DK, London ED, Waller SB, Reynolds MA. Age-dependent correlation of motor performance with neurotransmitter synthetic enzyme activities in mice. Behav Neural Biol. 1983;39:284–298. doi: 10.1016/S0163-1047(83)90978-0. [DOI] [PubMed] [Google Scholar]
- Jucker M, Ingram DK. Murine models of brain aging and age-related neurodegenerative diseases. Behav Brain Res. 1997;85:1–26. doi: 10.1016/S0166-4328(96)02243-7. [DOI] [PubMed] [Google Scholar]
- Jucker M, Bondolfi L, Calhoun ME, Long JM, Ingram DK. Structural brain aging in inbred mice: potential for genetic linkage. Exp Gerontol. 2000;35:1383–1388. doi: 10.1016/S0531-5565(00)00190-X. [DOI] [PubMed] [Google Scholar]
- Kolb B, Sutherland RJ, Whishaw IQ. A comparison of the contributions of the frontal and parietal association cortex to spatial localization in rats. Behav Neurosci. 1983;97:13–27. doi: 10.1037/0735-7044.97.1.13. [DOI] [PubMed] [Google Scholar]
- Krauter EE, Wallace JE, Campbell BA. Sensory-motor function in the aging rat. Behav Neural Biol. 1981;31:367–392. doi: 10.1016/S0163-1047(81)91455-2. [DOI] [PubMed] [Google Scholar]
- Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, et al. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci USA. 2003;100:8526–8531. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Lephart ED. Dietary soy phytoestrogens produce anxiolytic effects in the elevated plus-maze. Brain Res. 2001;913:180–184. doi: 10.1016/S0006-8993(01)02793-7. [DOI] [PubMed] [Google Scholar]
- Lund TD, West TW, Tian LY, Bu LH, Simmons DL, Setchell KD, et al. Visual spatial memory is enhanced in female rats (but inhibited in males) by dietary soy phytoestrogens. BMC Neurosci. 2001;2:20. doi: 10.1186/1471-2202-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson KR. Aging of glutamate receptors: correlations between binding and spatial memory performance in mice. Mech Ageing Dev. 1998;104:227–248. doi: 10.1016/S0047-6374(98)00076-1. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. Influence of diet restriction on NMDA receptor subunits and learning during aging. Neurobiol Aging. 2001;22:613–627. doi: 10.1016/S0197-4580(00)00258-X. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Burgess N, Donnett JG, Frackowiak RS, Frith CD, O’Keefe J. Knowing where and getting there: a human navigation network. Science. 1998;280:921–924. doi: 10.1126/science.280.5365.921. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Breckler SJ. Behavioral biomarkers of aging: illustration of a multivariate approach for detecting age-related behavioral changes. J Gerontol A Biol Sci Med Sci. 1999;54:B549–B566. doi: 10.1093/gerona/54.12.b549. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Stone WS, Ingram DK, Reynolds J, Gold PE, Conti LH, et al. Individual differences in aging: behavioral and neurobiological correlates. Neurobiol Aging. 1989;10:31–43. doi: 10.1016/S0197-4580(89)80008-9. [DOI] [PubMed] [Google Scholar]
- Markowska AL, Long JM, Johnson CT, Olton DS. Variable-interval probe test as a tool for repeated measurements of spatial memory in the water maze. Behav Neurosci. 1993;107:627–632. doi: 10.1037/0735-7044.107.4.627. [DOI] [PubMed] [Google Scholar]
- McDonald SR, Forster MJ. Lifelong vitamin E intake retards age-associated decline of spatial learning ability in apolipoprotein-E deficient mice. AGE. 2005;27:5–16. doi: 10.1007/s11357-005-4003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Means LW, Higgins JL, Fernandez TJ. Mid-life onset of dietary restriction extends life and prolongs cognitive functioning. Physiol Behav. 1993;54:503–508. doi: 10.1016/0031-9384(93)90243-9. [DOI] [PubMed] [Google Scholar]
- Morris RGM. Spatial localization does not require the presence of local cues. Learn Motiv. 1981;12:239–260. doi: 10.1016/0023-9690(81)90020-5. [DOI] [Google Scholar]
- Olton DS, Markowska A, Breckler SJ, Wenk GL, Pang KC, Koliatsos V. Individual differences in aging: behavioral and neural analyses. Biomed Environ Sci. 1991;4:166–172. [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. Individual differences in the cognitive and neurobiological consequences of normal aging. Trends Neurosci. 1992;15:340–345. doi: 10.1016/0166-2236(92)90051-9. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding changes in orbitofrontal cortex in reversal-impaired aged rats. J Neurophysiol. 2006;95:1509–1517. doi: 10.1152/jn.01052.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukitt-Hale B, Carey A, Simon L, Mark DA, Joseph JA. Effects of Concord grape juice on cognitive and motor deficits in aging. Nutrition. 2006;22:295–302. doi: 10.1016/j.nut.2005.07.016. [DOI] [PubMed] [Google Scholar]
- Simpson EM, Linder CC, Sargent EE, Davisson MT, Mobraaten LE, Sharp JJ. Genetic variation among 129 substrains and its importance for targeted mutagenesis in mice. Nat Genet. 1997;16:19–27. doi: 10.1038/ng0597-19. [DOI] [PubMed] [Google Scholar]
- Sumien N, Sims MN, Taylor HJ, Forster MJ (2006) Profiling psychomotor and cognitive aging in four-way cross mice. DOI: 10.1007/s11357-006-9015-7 (this issue) [DOI] [PMC free article] [PubMed]
- Wolf NS, Li Y, Pendergrass W, Schmeider C, Turturro A. Normal mouse and rat strains as models for age-related cataract and the effect of caloric restriction on its development. Exp Eye Res. 2000;70:683–692. doi: 10.1006/exer.2000.0835. [DOI] [PubMed] [Google Scholar]
- Wolf N, Penn P, Pendergrass W, Remmen H, Bartke A, Rabinovitch P, et al. Age-related cataract progression in five mouse models for anti-oxidant protection or hormonal influence. Exp Eye Res. 2005;81:276–285. doi: 10.1016/j.exer.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. Englewood Cliffs, N.J.: Prentice-Hall; 1984. [Google Scholar]
- Zornetzer SF, Rogers J. Animal models for assessment of geriatric mnemonic and motor deficits. In: Crook T, Ferris S, Bartus R, editors. Assessment in geriatric psychopharmacology. New Caanan: Mark Powley; 1983. pp. 301–322. [Google Scholar]