Abstract
Hepatocellular carcinoma is the commonest primary liver tumor and its incidence is on an increase.Transplantation and surgical resection are the gold standard curative treatment options but less than 20%patients are surgical candidates because of advanced liver disease and/or co-morbidities.Various interventional radiological procedures have been developed and intensively investigated for treatment of inoperable HCC.This review summarizes the various interventional radiological treatments in HCC including patient selection, procedural considerations and response evaluation. Transarterial chemoembolization, radioembolization and radiofrequency ablation are mainly discussed.
Keywords: HCC, Interventional radiological treatments, TACE, Radioembolization, Radiofrequency ablation
Introduction
Hepatocellular carcinoma (HCC) is the commonest primary tumor of the liver. The incidence of HCC is rapidly increasing [1, 2]. While liver transplantation and resection are the curative treatment modalities, many patients will not be suitable candidates for these treatments due to advanced disease or comorbidities [3–5]. Various interventional radiological treatments are available for patients with unresectable HCC. The interventional treatments have evolved over the last 2 decades and are showing promising results in terms of down staging the disease to transplantation or resection and improving survival and quality of life.
The present review gives a summary of the commonly used interventional radiological treatments for HCC.
Classification of Interventional Therapies for HCC
Interventional therapies for HCC fall into two broad categories
- Transarterial Therapy
- Bland embolization with particles
- Transarterial chemoembolization
- Radioembolization or selective internal radiation therapy
- Percutaneous Ablative Therapy
- Thermal ablation (radiofrequency/microwave/cryo)
- Chemical ablation (ethanol, acetic acid)
Transarterial Therapies
Rationale
Transarterial liver-directed therapies are based on the dual blood supply to the liver and preferential arterial supply to tumors. Almost 75% of the blood flow to the liver is through the portal vein, whereas 25% blood flow is from the hepatic artery. Primary and secondary tumors of the liver derive blood supply from the hepatic artery. Selective delivery of chemotherapeutic agents, bland particles, or radioactive spheres into the hepatic artery branches results in preferable localization into the tumor parenchyma while relatively sparing the normal liver parenchyma. The terminal arterial blockade resulting from embolization causes ischemia and hence tumor necrosis.
Detailed knowledge of normal and variant hepatic arterial anatomy is essential for effective delivery of embolic agents to the tumor parenchyma while preventing nontarget embolization to the gut.
Patient Selection
Transarterial therapies are noncurative treatment modality. They are ideal for intermediate stage HCC in asymptomatic patients with preserved liver function. Contraindications for this therapy are absence of hepatopedal portal flow, encephalopathy, and biliary obstruction; the constellation of bilirubin levels >2 mg/dL (34.2 micromol/L), lactate dehydrogenase levels >425 U/L, and aspartate aminotransferase levels >100 U/L; and tumor burden >50% of the liver, cardiac, or renal insuffiency [6]. Patients with poor performance status (ECOG 2 or more) are at increased risk for morbidity and mortality associated with the procedure.
Bland Embolization with Particle
The rationale of bland embolization with particle is to completely cut off the vascular supply to the tumor leading to tumor necrosis. Few centers across the world continue to use bland embolization as the treatment of HCC with results comparable to chemoembolization. The use of small size particles 40–50 μm is the key for achieving adequate penetration of the tumor microvasculature [7].
Transarterial Chemoembolization
Transarterial chemoembolization (TACE) combines transarterial delivery of chemotherapeutic agents to the tumor bed followed by embolization of the tumor vascularity. In conventional TACE, a mixture of chemotherapeutic drug(s) and lipiodol is injected into the hepatic artery branches supplying the tumor. Lipiodol is ethiodized poppy seed oil. It acts as a carrier for the chemotherapeutic drug and also as a microembolic agent. Lipiodol is retained in the tumor bed because of the absence of Kupffer cells in the tumor parenchyma, whereas normal liver parenchyma is able to clear it [8–10]. Lipiodol was postulated to help retain drug for a longer time in the tumor bed, but this concept has been recently challenged [11]. There is a lot of variation in the technique of TACE, ranging from the use of doxorubicin as a single agent; cisplatin as a single agent; or a combination of cisplatin, doxorubicin, and mitomycin C. The embolic agent used can be polyvinyl alcohol particles or gelfoam slurry [12–14].
Preprocedure Preparation
Patients undergoing chemoembolization are prone to develop nephrotoxicity due to a combination of dehydration, iodinated contrast, chemotherapeutic agents, and tumor lysis syndrome. Good hydration is the key to prevent nephrotoxicity. Another important prophylactic measure is administration of abroad-spectrum antibiotic prior to the procedure. Evaluation by a multidisciplinary team is important to avoid overlooking curative surgical options [15, 16].
Procedural Considerations
The chemoembolization procedure is most commonly performed via the transfemoral route. Transbrachial or transradial route may be selected in case of difficult transfemoral access. A careful review of the triphasic computed tomography (CT) scan generally provides a very good road map about the arterial anatomy including the variations. A detailed celiac and superior mesenteric angiogram is done including imaging in the portal venous phase. Meticulous angiographic technique, use of pressure injectors, and good quality digital subtraction angiography systems are necessary for complete evaluation of the angioarchitecture of the tumor and all the feeders. It is important to target all the arterial feeders for getting a good response [17].
C-arm CT is a recent technical breakthrough in digital subtraction angiography systems wherein it is possible to obtain CT-like images during the angiographic evaluation. This provides critical information about the arterial supply to tumors not visible on conventional digital subtraction angiography. C-arm CT also gives valuable information about blood supply of tumors located in difficult areas such as the caudate lobe [18, 19].
After mapping the complete arterial supply to the tumor, superselective cannulation of the feeding arteries is performed with a microcatheter and the chemoembolic mixture is infused into the feeding arteries. This is followed by embolization of the feeding arteries with either polyvinyl alcohol particles or gelfoam slurry. The end point of embolization is complete stasis (Fig. 1). A completion angiogram is obtained and hemostasis is achieved at the arterial puncture site by manual compression [15].
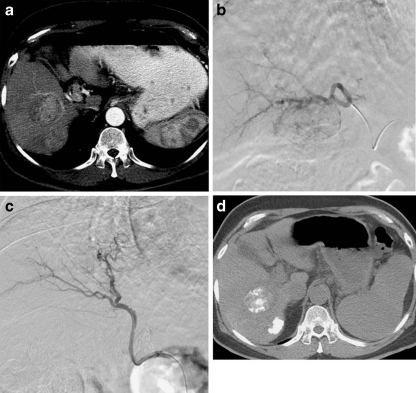
Fig. 1.
a Axial CT scan showing arterial phase enhancing lesion in segment V of right lobe of liver with an adjacent satellite nodule. b Tumor blush after superselective cannulation of feeding vessel. c Postchemoembolization angiogram showing complete obliteration of neoplastic vascularity. d Response evaluation CT scan confirms lipiodol deposition in tumor with sparing of surrounding normal parenchyma
For large tumors and tumors reaching the hepatic surface, angiographic evaluation of the extrahepatic arteries is also performed (Fig. 2). These arteries include the inferior phrenic, intercostals, and internal mammary arteries [17, 20, 21].
Fig. 2.
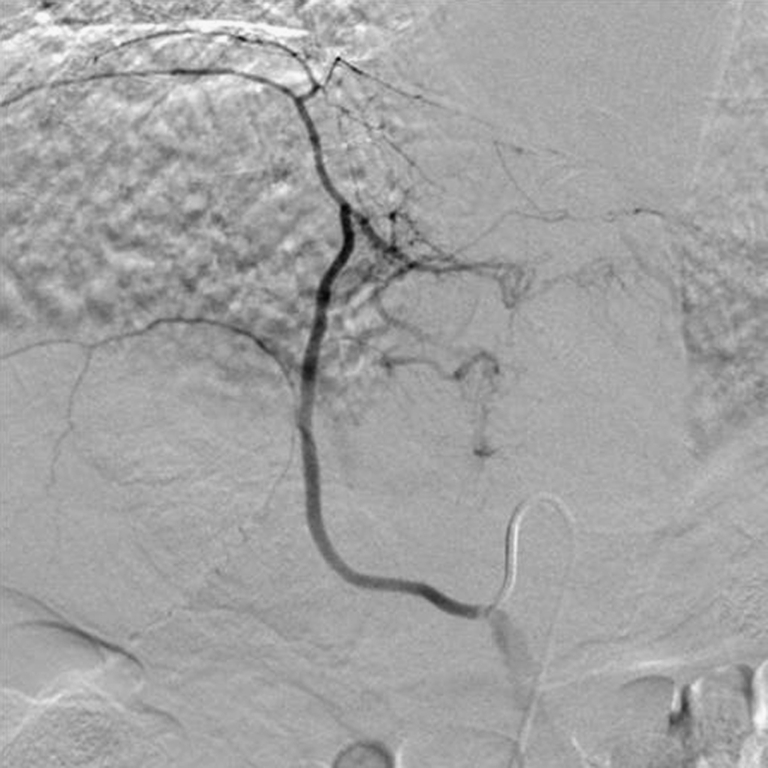
Right inferior phrenic angiogram demonstrating parasitazion of blood supply by large left lobe HCC
Postprocedure Care
Patients receive hydration, intravenous antibiotics, antiemetics, and pain killers. Most patients experience a transient postembolization syndrome comprising of abdominal pain, nausea, fever, leukocytosis, and elevation of liver enzymes. This typically lasts for about a week or two and then subsides. Supportive treatment with nonsteroidal anti-inflammatory drugs and antiemetics is generally the only treatment necessary. Liver abscess and liver failure are the most dreaded complications occurring in <1% of patients [7, 22–24].
Response Evaluation
Response evaluation is typically done at 6 weeks. A plain and postcontrast CT scan of the liver is obtained. Alternatively, a contrast-enhanced MRI can also be performed. Dense lipiodol accumulation and lack of internal enhancement are indicators of complete necrosis. Focal areas of nonopacification with lipiodol and persistent nodular enhancement with washout on portal venous phase indicate residual disease and call for retreatment. Reduction in size can also be documented.
Alpha-fetoprotein levels can also be used for response evaluation (if elevated preprocedure). Recently, the diffusion weighted MRI is being used for response evaluation.
TACE cycles are repeated until complete necrosis is obtained. If there is no response after two cycles of TACE, the therapy is discontinued.
Two major randomized controlled trials (RCTs) have demonstrated improved survival with chemoembolization vs. conservative/medical treatment. The first, performed by Llovet et al [25] in 2002, compared chemoembolization (gelatin sponge and doxorubicin), bland embolization, and conservative treatment. The study was stopped early when survival benefits were demonstrated after several inspections. Two-year survival probabilities were 63% for the chemoembolization group and 27% for the control group. The second RCT, performed by Lo et al [26] in 2002, evaluated chemoembolization and response to cisplatin/lipiodol embolization vs. symptomatic treatment. The 3-year survival rate was 26% in the chemoembolization group vs. 3% in the symptomatic treatment group. Several meta-analyses have been performed to include smaller RCTs and cohort studies, which also concluded that chemoembolization improves survival [27, 28].
Conventional chemoembolization is criticized for the lack of standardization. Various drugs such as doxorubicin or combination such as doxorubicin, cisplatin, and mitomycin-C have been used. Lipiodol and chemotherapeutics agents are considered immixable [9]. Also, the systemic levels of these agents are high.
Use of Drug-Eluting Microspheres
Drug-eluting microspheres are made of polyvinyl alcohol hydrogel. They are biocompatible, hydrophilic, and nonresorbable. They sequester doxorubicin hydrochloride from solution by ion exchange mechanism and release it in tissues again by similar mechanisms. This allows for a sustained release of chemotherapeutics agent over a long period of time (half-life of 150 h for microspheres with a size of 100–300 μm, and the maximum half-life of 1,730 h for microspheres with a size of 700–900 μm) as compared with more rapid release of agents from lipiodol solution (half-life 1 h) in conventional TACE therapy [12]. This increases the contact time of drugs with tumor and lower systemic concentration of the drugs leading to improved objective tumor response as well as decreased systemic side effects and decreased rates of liver failure [29, 30].
Transarterial Radioembolization
Normal hepatic parenchyma has a very poor radiation tolerance. Approximately 50% of the patients who receive a whole-liver radiation dose of 35 Gy—a dose insufficient to induce tumor cell death—develop radiation-induced liver disease. Transarterial radioembolization (TARE) uses beta-emitting radioactive elements Yttrium, Rhenium, or Iodine-131. The preferential deposition of microspheres within the tumor allows selective irradiation of the target tumor rather than the normal hepatic parenchyma, thereby reducing the risk of radiation-induced liver disease. An intratumoral radiation dose of 100–150 Gy is achieved, which is highly effective for tumor destruction. Y-90 bearing microspheres are commercially available in two formulations: glass microsphere (TheraSphere; MDS Nordion, Ottawa, Ontario, Canada) and resin microsphere (SIR-Spheres; Sirtex Medical, Sydney, Australia). Beta-emitting radioisotopes have a very small range of penetration (1–2 mm), and thus act as a point source of radiation. Radioembolization combines the minimal embolic effect on tumor vascularity and cytotoxicity of radiation, thus acting as brachytherapy. The minimal embolic effect is important, as adequate oxygen tension is necessary for optimum effect of radiation.
Planning
Patient Selection
Indications for TARE are similar to TACE. Portal vein thrombosis is not a contraindication to radioembolization. On the contrary, radioembolization has the potential to recanalize portal vein thrombosis resulting from tumor invasion as has been documented in several case reports [31] (Fig. 3).
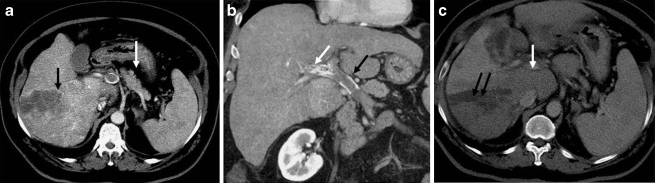
Fig. 3.
a Axial CT scan shows large heterogeneous mass in the right lobe of liver (black arrow) with main portal vein thrombosis (white arrow). b Coronal CT scan shows enhancing tumor thrombus in the right branch of portal vein (white arrow) and a nonenhancing thrombus in main portal vein (black arrow). c Response evaluation CT scan shows complete necrosis of the right lobe HCC with recanalization of the portal vein
Planning Angiogram
A detailed angiogram is performed to map arterial supply to the tumor (Fig. 4). All collaterals to gastrointestinal tract are embolized to prevent intractable radiation ulcer of the stomach and the small bowel [32] (Fig. 12). The vascular supply to the tumor is thus skeletonized. A 5-mCi dose of 99mTc MAA (macroaggregated albumin) is injected into the hepatic artery and a planar scintigraphy is done to calculate the lung shunt fraction. The lungs may tolerate an unintended radiation dose of 30 Gy during a single treatment and a cumulative maximum dose of 50 Gy; higher dose incurs a risk of radiation pneumonitis.
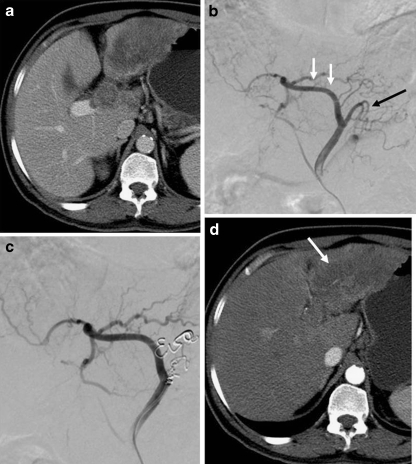
Fig. 4.
a Axial CT scan showing heterogeneous mass in the left lobe of the liver with thrombosis of the left portal vein. b Digital subtraction angiogram (planning angiogram) shows variant arterial supply to the left lobe of the liver in the form of accessory left hepatic artery arising from left gastric artery. Note branches supplying the tumor (white arrow) and branches supplying the stomach (black arrow). c Coil embolization of branches supplying stomach to skeletonize blood supply to tumor. d Response evaluation CT scan after radioembolization shows complete necrosis of the left lobe tumor (white arrow)
Dose Calculation
After calculating volume of the disease with CT volumetry and lung shunt fraction, dose is calculated using specialized software. The desired vial of Y-90 spheres is then ordered and delivered within a week’s time. This vial is calibrated taking into account the exponential decay of Y-90.
Delivery
Delivery of microspheres requires performance of a second angiogram. The Y-90 vial is loaded into a specialized delivery kit by nuclear medicine physician and delivered intra-arterially without any radiation exposure to the operator.
Response Evaluation
Cross-sectional imaging (CCT/MR) is repeated at 6 weeks to evaluate response. The combination of necrosis and change in tumor size is the accurate method to evaluate response. Necrosis is defined as a lack of enhancement (a change in attenuation by less than 10 HU) after the administration of contrast material at CT. A transient thin rim of enhancement represents granulation tissue, whereas a growing enhancing nodule represents treatment failure (Fig. 4).
Side Effects and Complications
The side effects are fatigue, lymphopenia, rise in bilirubin, and rarely liver failure with veno-occlusive disease-like picture. Other reported complications include hepatic abscess especially in DM and after bilioenteric bypass, biliary dyskinesia, and radiation-induced cholecystitis, biloma and biliary necrosis, radiation hepatitis, and gastrointestinal ulceration [32–34].
Compared with chemoembolization, radioembolization has some advantages. Patients with bilobar disease or extensive multifocal HCC can be treated with one treatment. Patients with main portal vein thrombosis can be safely treated with radioembolization [31]. Recanalization of tumor-related portal vein thrombosis has been documented with radioembolization. Radioembolization is also associated with better quality of life as compared with chemoembolization. A recent retrospective analysis demonstrated no difference in tumor response or survival between chemoembolization and radioembolization [31]. There are no RCTs that head-on compare the two treatments.
Percutaneous Ablative Therapy
Historically, percutaneous ethanol injection (PEI) was the primary percutaneous treatment for HCC. With evolution of newer technology, it has largely been replaced with thermal ablation. Three large well-designed RCTs comparing radiofrequency ablation (RFA) with PEI demonstrated increased local recurrence, decreased survival, and increased number of treatments with PEI compared with RFA [35–39].
Radiofrequency Ablation
RFA is currently considered the gold standard among percutaneous ablative therapies. This minimally invasive therapy has the potential to dramatically alter patient outcome.
Principles and Techniques
The principle of RFA is interstitial thermal ablation of tumor. This is achieved by oscillation of high-frequency (200–1,200 kHz) alternating electric current leading to agitation of local ionic milieu.
Schematically, a closed-loop circuit is created by placing a generator, large dispersive electrode (ground pad), a patient, and a needle electrode in series. Both the dispersive electrode and the needle electrode are active, while the patient acts as a resister. Thus, an alternating electric field is created within the tissue of the patient. Given the relatively high electrical resistance of tissue in comparison with the metal electrodes, there is marked agitation of the ions present in the tumor or liver tissue that immediately surrounds the electrode. This ionic agitation creates friction within the body and thus heat that can be tightly controlled through modulation of the amount of radiofrequency energy deposited.
The nature of the thermal damage caused by radiofrequency heating depends on both the tissue temperature achieved and the duration of heating. Thus, an essential objective of ablative therapy is achievement and maintenance of a 50°–100°C temperature throughout the entire target volume for at least 4–6 min. Tissues cannot be heated to greater than 100°–110°C without vaporizing, and this process produces significant gas that serves as an insulator and retards the ability to effectively establish a radiofrequency field. This process coupled with the rapid decrease in heating at a distance from the electrode essentially limits the extent of induced coagulation to no greater than 1.6 cm in diameter. Expandable multitined electrode permits the uniform deposition of energy over a larger volume. Alternate strategies to increase the energy deposited include internally cooled electrode design, pulsing of radiofrequency energy, and preferential cooling of the tissue near the electrode. With these technologic developments it is possible to ablate a lesion with a maximum diameter of 5.0 cm [40].
Patient Selection
RFA is a curative modality. Ideal tumors are smaller than 3 cm in diameter, completely surrounded by hepatic parenchyma, 1 cm or more deep to the liver capsule, and 2 cm or more away from large hepatic or portal veins. Subcapsular liver tumors can be ablated, but there treatment is usually associated with greater procedural and postprocedural pain. Tumors adjacent to large blood vessels are more difficult to ablate completely because of the heat sink effect of vessels. Ablation of tumors adjacent to the larger portal triads causes increased pain and poses the risk of damage to the associated bile ducts. Contraindication to treatment includes sepsis, poor performance status (ECOG >2), and coagulopathies [6].
Procedure
RFA is generally performed percutaneously under ultrasound and CT guidance. It allows precise centering of the electrode on the target, continuous monitoring of distribution of vapor bubbles, fast execution, and determination of the appropriate amount of energy to be given each time. Two types of electrodes are used for RFA, i.e., monopolar and bipolar. Bipolar probe complete the circuit locally; hence, is useful in patients with pacemakers and metallic implants. Complete ablation is a key to successful treatment. For difficult locations such as the diaphragmatic surface or caudate lobe, CT guidance is especially useful.
Surface tumors and tumors close to vessels and porta present special challenge to RFA [41–43]. For lesions at the liver surface or those abutting the colon or stomach, 5% dextrose can be instilled in the plane between lesion and the bowel to prevent thermal injury to these structures. Major portal or hepatic vein branches adjacent to the lesion can be temporarily balloon occluded to prevent the heat sink effect and thereby optimizing the zone of ablation [44–46].
Response Evaluation
A CT scan is performed 1 week after the RFA session. The ablative zone typically appears as a nonenhancing area of low attenuation representing coagulative necrosis. This zone should be larger than the index tumor, centered at the index tumor, and encompass the entire index tumor with an additional circumferential ablative margin, ideally 0.5–1 cm in thickness with sharp demarcation from the normal hepatic parenchyma [47]. A benign perilesional hyperattenuating rim is often visible during the arterial phase of scanning representing inflammatory response to thermal damage [48]. It may persist up to 6 months [49]. Residual unabalated hyper-revascular tumor appears as a nodular or asymmetric enhancing area at the margin of the ablation zone during the arterial phase [50]. Follow-up imaging is done every 3–4 months to look for local tumor progression or for new hepatic and extrahepatic diseases (Fig. 5).
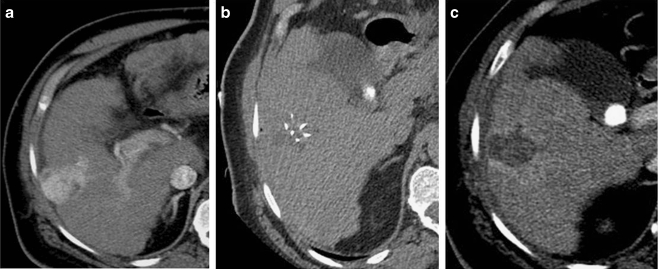
Fig. 5.
a Axial CT scan shows arterial phase enhancing lesion in the right lobe of the liver. b CT-guided RFA using multitined expandable electrode. c Post-RFA response evaluation CT scan shows complete lack of enhancement within the tumor representing good response
Complications
Hepatic abscess is the most common complication after RFA of liver with a reported incidence of 0.3–2% [51–53]. Vascular complications including intraperitoneal bleeding, pseudoaneurysm, portal vein thrombosis, hepatic vein thrombosis and hepatic infarction are reported [52, 54–56]. Bile duct injury, injury to gastrointestinal tract, gallbladder, and diaphragm may rarely occur [54]. Delayed complications include bile duct stricture, biloma, and hemobilia [57]. Tumor seeding along the needle tract, pleura, or peritoneum may occur 3–12 months after RFA with a reported incidence of 0.2–1.4%. It can be minimized by tract ablation during needle withdrawal [58].
Microvawe Abalation and Cryoabalation
Microwave ablation refers to the use electromagnetic method for inducing tumor destruction by using devices with frequencies of at least 900 MHz The potential benefits of microwave technology includes higher intratumoral temperature, larger tumor ablation volumes, faster ablation time, ability to use multiple applicators, improve convection profile, optimal heating of cystic masses, and less procedural pain.
Cryoablation technology uses thaw freeze cycles to kill cancer cells. The potential benefits include reduced procedural pain and ability to visualize an ice ball formation on imaging during treatment. Cryoablation maintains cellular integrity of connective tissue in vessel wall or adjacent visceral lining such as gallbladder, bowel, and kidney [59]. These techniques are not widely practiced in our country.
Combination Therapies
The recent area of research in treatment advances is combination regional treatment combining embolization and RFA. It is based on the hypothesis of increased tumor sensitization to heat kill following chemoembolization. A recent prospective RCT evaluated the combination of PEI/RFA and demonstrated improved tumor response and survival rates in large HCCs compared with RFA alone [41]. A combination of systemic therapy such as sorafenib and loco regional therapies such as TARE, TACE, and RFA are being explored.
Conclusion
Interventional radiology is now playing an important role in the treatment of HCC. These techniques have significantly helped prevent progression of disease in liver transplant candidates and prolong survival in nontransplant candidates. The unique aspects of these therapies are their minimal toxicity profiles and effective tumor response while preserving normal hepatic parenchyma.
References
- 1.El-Serag HB, Mason AC, Key C. Trends in survival of patients with hepatocellular carcinoma between 1977 and 1996 in the United States. Heptology. 2009;33(1):62–65. doi: 10.1053/jhep.2001.21041. [DOI] [PubMed] [Google Scholar]
- 2.Altekruse SF, McGylnn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27(9):1485–1491. doi: 10.1200/JCO.2008.20.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for Hepatocellular carcinoma. Liver Transpl. 2005;11(9):1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 4.Otto G, Heuschen U, Hofmann WJ, Krumm G, Hinz U, Herfarth C. Survival and recurrence after liver transplantation versus liver resection for Hepatocellular carcinoma: a retrospective analysis. Ann Surg. 1998;227(3):424–432. doi: 10.1097/00000658-199803000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long term survival and pattern of recurrence after resection of small Hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235(3):373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dodd GD, 3rd, Soulen MC, Kane RA, Livraghi T, Lees WR, Yamashita Y, et al. Minimally invasive treatment of malignant hepatic tumors: at the threshold of a major breakthrough. Radiographics. 2000;20:9–27. doi: 10.1148/radiographics.20.1.g00ja019. [DOI] [PubMed] [Google Scholar]
- 7.Sakamoto I, Aso N, Nagaoki K, Matsuoka Y, Uetani M, Ashizawa K, et al. Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics. 1998;18(3):605–619. doi: 10.1148/radiographics.18.3.9599386. [DOI] [PubMed] [Google Scholar]
- 8.Kan Z, McCuskey PA, Wright KC, Wallace S. Role of Kupffer cells in iodized oil embolization. Invest Radiol. 1994;29(11):990–993. doi: 10.1097/00004424-199411000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Konno T. Targeting cancer chemotherapeutic agents by the use of lipiodol contrast medium. Cancer. 1990;29(11):990–993. doi: 10.1002/1097-0142(19901101)66:9<1897::aid-cncr2820660907>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki Y, Imoka S, Kasugai H, Fujita M, Kawamoto S, Ishiguro S, et al. A new approach to chemoembolization therapy for hepatoma using ethiodized oil, cisplatin, and gelatin sponge. Cancer. 1987;60(6):1194–1203. doi: 10.1002/1097-0142(19870915)60:6<1194::AID-CNCR2820600607>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 11.Varela M, Real MI, Burrel FA, Sala M, Brunet M, et al. Chemoembolization of Hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007;46(3):474–481. doi: 10.1016/j.jhep.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Olweny CL, Katongole-Mbidde E, Mugerwa J, Kyalwazi SK, Cohen H. Treatment of hepatocellular carcinoma with adriamycin. Preliminary communication. Cancer. 1975;36(4):1250–1257. doi: 10.1002/1097-0142(197510)36:4<1250::AID-CNCR2820360410>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 13.Olweny CL, Kantongole-Mbidde E, Bahendeka S, Otim D, Mugerwa J, Kyalwazi SK. Further experience in treating patients with hepatocellular carcinoma in Uganda. Cancer. 1980;46(12):2717–2722. doi: 10.1002/1097-0142(19801215)46:12<2717::AID-CNCR2820461230>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 14.Lai CL, Wu PC, Chan GC, et al. Doxorubicin versus no antitumor therapy in inoperable Hepatocellular carcinoma. A prospective randomized trial. Cancer. 1988;62(3):479–483. doi: 10.1002/1097-0142(19880801)62:3<479::AID-CNCR2820620306>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 15.Ramsey DE, Kernagis LY, Soulen MC, Geschwind JF. Chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2002;13(9 Pt 2):S211–S221. doi: 10.1016/S1051-0443(07)61789-8. [DOI] [PubMed] [Google Scholar]
- 16.Liapi E, Geogiades CC, Hong K, Geschwind JF. Transcatheter arterial chemoembolization: current technique and future promise. Tech Vasc Interv Radiol. 2007;10(1):2–11. doi: 10.1053/j.tvir.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 17.Liu DM, Salem R, Bui JT, et al. Angiographic and technical considerations in patients undergoing liver-directed therapy. J Vasc Interv Radiol. 2005;16(7):911–935. doi: 10.1097/01.RVI.0000164324.79242.B2. [DOI] [PubMed] [Google Scholar]
- 18.Virmani S, Ryu RK, Sato KT, Lewandowski RJ, Kulik L, Mulcahy MF. Effect of C-arm angiographic CT on transcatheter arterial chemoembolization of liver tumors. J Vasc Interv Radiol. 2007;18(10):1305–1309. doi: 10.1016/j.jvir.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Wallace MJ, Murthy R, Kamat PP, Moore T, Rao SH, Ensor J, et al. Impact of C-arm CT on hepatic arterial interventions for hepatic malignancies. J Vasc Interv Radiol. 2007;18(12):1500–1507. doi: 10.1016/j.jvir.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Convey AM, Brody LA, Maluccio MA, Getrajdman GI, Brown KT. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology. 2002;224(2):542–547. doi: 10.1148/radiol.2242011283. [DOI] [PubMed] [Google Scholar]
- 21.Song SY, Chung JW, Lim HG, Park JH. Nonhepatic arteries originating from hepatic arteries: angiographic analysis in 250 patients. J Vasc Interv Radiol. 2006;17(3):461–469. doi: 10.1097/01.RVI.0000202718.16416.18. [DOI] [PubMed] [Google Scholar]
- 22.Brown DB, Cardella JF, Sacks D, Goldberg SN, Gervais DA, Rajan DK, et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol. 2009;20(Suppl 7):S219–S226. doi: 10.1016/j.jvir.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 23.Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, et al. Prospective cohort study of transarterial chemoembolization for unresectable Hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131(2):461–469. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Brown DB, Geschwind JF, Soulen MC, Millward SF, Sacks D. Society of Interventional Radiology position statement on chemoembolization of hepatic malignancies. J Vasc Interv Radiol. 2009;20(Suppl 7):S317–S323. doi: 10.1016/j.jvir.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Llovet JM, Real MI, Montana X, Planas R, Coll S, Aponte J, et al. Arterial embolization or chemoembolization versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomized controlled trial. Lancet. 2002;359(9319):1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 26.Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, et al. Randomized controlled trial of Transarterial lipiodol chemoembolization for unresectable Hepatocellular carcinoma. Hepatology. 2002;35(5):1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 27.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable Hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37(2):429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 28.Camma C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224(1):47–54. doi: 10.1148/radiol.2241011262. [DOI] [PubMed] [Google Scholar]
- 29.Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010;33(1):41–52. doi: 10.1007/s00270-009-9711-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malagari K, Pomoni M, Kelekis A, Pomoni A, Dourakis S, Spyridopoulos T, et al. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33(3):541–551. doi: 10.1007/s00270-009-9750-0. [DOI] [PubMed] [Google Scholar]
- 31.Salem R, Lewandowski R, Roberts C, Goin J, Thurston K, Abouljoud M, et al. Use of Yttrium-90 glass microspheres (TheraSphere) for the treatment of unresectable hepatocellular carcinoma with portal vein thrombosis. J Vasc Interv Radiol. 2004;15(4):335–345. doi: 10.1097/01.RVI.0000123319.20705.92. [DOI] [PubMed] [Google Scholar]
- 32.Riaz A, Lewandowski R, Kulik L. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol. 2009;20(9):1121–1130. doi: 10.1016/j.jvir.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 33.Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, et al. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138(1):52–64. doi: 10.1053/j.gastro.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 34.Kooby DA, Egnatashivili V, Srinivasan S, Chamsuddin A, Delman KA, Kauh J, et al. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21(2):224–230. doi: 10.1016/j.jvir.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 35.Ishii H, Okada S, Nose H, Okusaka T, Yoshimori M, Takayama T, et al. Local recurrence of hepatocellular carcinoma after percutaneous ethanol injection. Cancer. 1996;77(9):1792–1976. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1792::AID-CNCR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 36.Lencioni RA, Allgaier HP, Cioni D, Olschewski M, Deibert P, Crocetti L, et al. Small hepatocellular carcinoma in cirrhosis: randomized comparison of radiofrequency thermal ablation versus percutaneous ethanol injection. Radiology. 2003;228(1):235–240. doi: 10.1148/radiol.2281020718. [DOI] [PubMed] [Google Scholar]
- 37.Shiina S, Teratani T, Obi S, Sato S, Tateishi R, Fujishima T, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129(1):122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 38.Lin SM, Lin CJ, Lin CC, Hsu CW, Chen YC. Randomized controlled trial comparing percutaneous radiofrequency thermal ablation, percutaneous ethanol injection, and percutaneous acetic acid injection to treat hepatocellular carcinoma of 3 cm or less. Gut. 2005;54(8):1151–1156. doi: 10.1136/gut.2004.045203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orlando A, Leandro G, Olivo M, Andriulli A, Cottone M. Radiofrequency thermal ablation vs. percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009;104(2):514–524. doi: 10.1038/ajg.2008.80. [DOI] [PubMed] [Google Scholar]
- 40.Crocetti L, Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumors. Cardiovasc Intervent Radiol. 2010;33:11–17. doi: 10.1007/s00270-009-9736-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radiofrequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 42.Latteri F, Sandonato L, Marco V, Parisi P, Cabibbo G, Lombardo G, et al. Seeding after radiofrequency ablation of hepatocellular carcinoma in patients with cirrhosis: a prospective study. Dig Liver Dis. 2008;40:684–689. doi: 10.1016/j.dld.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 43.Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, et al. Complications of radiofrequency coagulation of liver tumors. Br J Surg. 2002;89:1206–1222. doi: 10.1046/j.1365-2168.2002.02168.x. [DOI] [PubMed] [Google Scholar]
- 44.Lu DS, Raman SS, Limanond P, Aziz D, Economou J, Busuttil R, et al. Influence of large peritumoral vessels on outcome of radiofrequency ablation of liver tumors. J Vasc Interv Radiol. 2003;14(10):1267–1274. doi: 10.1097/01.RVI.0000092666.72261.6B. [DOI] [PubMed] [Google Scholar]
- 45.Chang I, Mikityansky I, Wary-Cahen D, Pritchard WF, Karanian JW, Wood BJ. Effects of perfusion on radiofrequency ablation in swine kidneys. Radiology. 2004;231(2):500–505. doi: 10.1148/radiol.2312021248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pompili M, Mirante VG, Rondinara G, Fassati LR, Piscaglia F, Agnes S, et al. Percutaneous ablation procedures in cirrhotic patients with hepatocellular carcinoma submitted to liver transplantation: assessment of efficacy at explant analysis and of safety for tumor recurrence. Liver Transpl. 2005;11(9):1117–1126. doi: 10.1002/lt.20469. [DOI] [PubMed] [Google Scholar]
- 47.Choi H, Evelyne M, Charnsangavej C. Radiographic imaging following radiofrequency ablation of liver tumors. In: Ellis LM, Curley SA, Tanabe KK, editors. Radiofrequency ablation for cancer: current indications, techniques, and outcomes. New York: Springer; 2007. pp. 253–267. [Google Scholar]
- 48.Lim HK, Choi D, Lee WJ, Kim SH, Lee SJ, Jang HJ, et al. Hepatocellular carcinoma treated with percutaneous radiofrequency ablation: evaluation with follow-up multiphase helical CT. Radiology. 2001;221:447–454. doi: 10.1148/radiol.2212010446. [DOI] [PubMed] [Google Scholar]
- 49.Goldberg SN, Gazelle GS, Solbiati L, Livraghi T, Tanabe KK, Hahn PF, et al. Ablation of liver tumors using percutaneous RF therapy. AJR Am J Roentgenol. 1998;170:1023–1028. doi: 10.2214/ajr.170.4.9530053. [DOI] [PubMed] [Google Scholar]
- 50.McGhana JP, Dodd GD., 3rd Radiofrequency ablation of liver: current status. AJR Am J Roentgenol. 2001;176:3–16. doi: 10.2214/ajr.176.1.1760003. [DOI] [PubMed] [Google Scholar]
- 51.deBaere T, Risse O, Kuonch V, Dromain C, Sengel C, Smayra T, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003;181:695–700. doi: 10.2214/ajr.181.3.1810695. [DOI] [PubMed] [Google Scholar]
- 52.Kim YS, Rhim H, Lim HK. Imaging after radiofrequency ablation of hepatic tumor. Semin Ultrasound CT MR. 2009;30:49–66. doi: 10.1053/j.sult.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Choi D, Lim HK, Kim MJ, Kim SJ, Kim SH, Lee WJ, et al. Liver abscess after percutaneous radiofrequency ablation for hepatocellular carcinomas: frequency and risk factors. AJR Am J Roentgenol. 2005;184:1860–1867. doi: 10.2214/ajr.184.6.01841860. [DOI] [PubMed] [Google Scholar]
- 54.Akahane M, Koga H, Kato N, Yamada H, Uozumi K, Tateishi R, et al. Complications of percutaneous radiofrequency ablation for hepato-cellular carcinoma: imaging spectrum and management. Radiographics. 2005;25(Suppl 1):S57–S68. doi: 10.1148/rg.25si055505. [DOI] [PubMed] [Google Scholar]
- 55.Tamai F, Furuse J, Maru Y, Yoshino M. Intrahepatic pseudoaneurysm: a complication following radiofrequency ablation therapy for hepatocellular carcinoma. Eur J Radiol. 2002;44:40–43. doi: 10.1016/S0720-048X(01)00436-3. [DOI] [PubMed] [Google Scholar]
- 56.Kim YS, Rhim H, Lim HK, Choi D, Lee WJ, Kim SH. Hepatic infarction after radiofrequency ablation of hepatocellular carcinoma with internally cooled electrode. J Vasc Interv Radiol. 2007;18:1126–1133. doi: 10.1016/j.jvir.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 57.Kim MD, Kim H, Kang SW, Jeong BG. Nontraumatic hepatic artery pseudoaneurysm associated with acute leukemia: a possible complication of pyogenic liver abscess. Abdom Imaging. 2002;27:458–460. doi: 10.1007/s00261-001-0078-8. [DOI] [PubMed] [Google Scholar]
- 58.Jaskolka JD, Asch MR, Kachura JR, Ho CS, Ossip M, Wong F, et al. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol. 2005;16(4):485–491. doi: 10.1097/01.RVI.0000151141.09597.5F. [DOI] [PubMed] [Google Scholar]
- 59.Sung GT, Gill IS, Hsu TH, Meraney AM, Skacel M, Brainard JA, et al. Effect of intentional cryo-injury to the renal collecting system. J Urol. 2003;170(2 Pt 1):619–622. doi: 10.1097/01.ju.0000068722.22186.66. [DOI] [PubMed] [Google Scholar]