Abstract
Hilar cholangiocarcinoma provides a surgical challenge. Successful outcome depends upon preoperative imaging, appropriate use of biliary drainage and portal vein embolisation as well as appropriate liver resection with caudate lobe excision and nodal clearance.
Keywords: Biliary tract cancer, Biliary stricture, Liver surgery
Hilar cholangiocarcinoma (HCCC) is a rare tumour involving the hepatic ducts at the liver hilum. The surgical excision of these tumours provides a particular challenge to HPB surgeons as the portal vein and hepatic artery are closely related to the bile duct and varying amounts of liver needs to be excised to ensure complete clearance. The surgery is anatomically challenging. Success rates are higher than for most HPB tumours such as pancreatic adenocarcinomas, and an R0 resection can achieve excellent cure rates in 37–50% of cases [1–4].
Preoperative Assessment
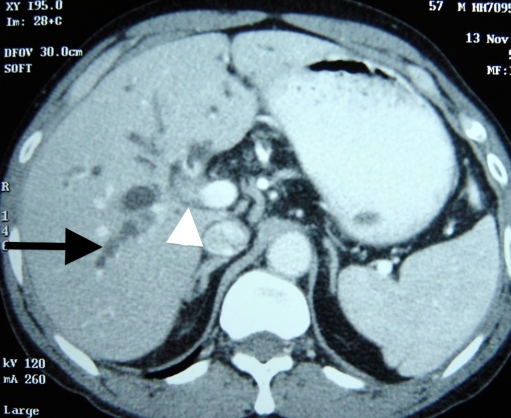
The key to successful resection of these tumours is preoperative assessment and optimization of these patients. As the initial presentation is with obstructive jaundice, ultrasonography is often the primary modality of evaluation. This, however, does not provide enough information to proceed. The initial evaluation should be by a triple-phase CT scan of the liver with an arteriographic phase, venous phase (Fig. 1), and delayed phase. Important information provided by CT is involvement of the hepatic artery and portal vein, some idea of extent of ductal involvement, metastasis to nodes, liver and peritoneal disease and an estimate of liver volume, which is essential for ensuring adequate functional reserve following resection [5, 6].
Fig. 1.
Contrast-enhanced CT scan showing soft tissue mass at hilum adjacent to portal vein (arrowhead) with dilatation of intrahepatic biliary radicals (arrow)
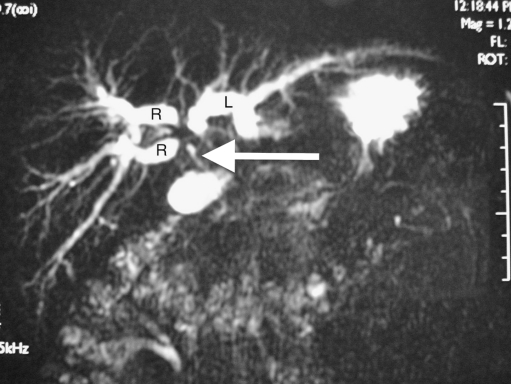
Magnetic resonance cholangiography (MRC) (Fig. 2) is an extremely useful screening investigation to estimate the extent of spread in the biliary tree [7]. MRI with contrast combined with MRC can provide the same information with respect to vascularity as compared to CT scan. Information on involvement is useful in planning pre-operative biliary drainage and type of surgery, and in case of inoperable tumours, approach to palliative stenting [7].
Fig. 2.
MRCP showing dilated intrahepatic biliary radicals with discontinuity between the left (L) and right anterior and posterior (R) biliary radicals. The common bile duct below the tumour (arrow) is collapsed
Biliary Drainage
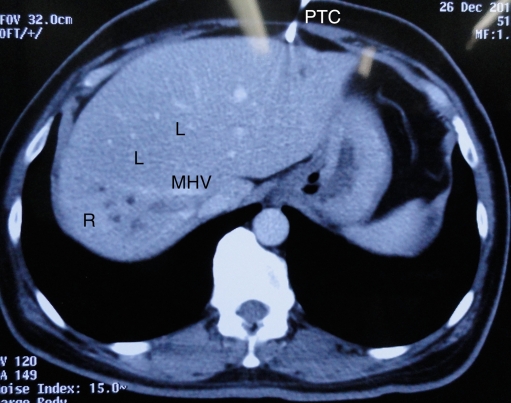
Preoperative biliary drainage is a contentious issue. Nimura [8] demonstrated reduction in morbidity and liver failure when the bilirubin was reduced to 3 mg/dL prior to resection [9]. The Nagoya group recommend percutaneous drainage of each biliary segment prior to surgery, in some cases including the caudate ducts. The advantages of this approach are the prevention of residual sepsis and rapid fall in bilirubin [10, 11]. However, this is extremely tedious and requires great resource utilization. In complete contrast to this certain, centres have performed surgery in patients without any decompression [12]. This ensures a short ‘presentation to surgery’ time and prevents iatrogenic infections that can accompany biliary drainage. The disadvantage of this approach is that the surgeon relies on MRC and CT as imaging for resectability rather than the more accurate cholangiography. Subtle infiltration along one duct wall is often missed by this approach. Further, morbidity in the undrained patient may be higher especially if vascular resections are required. The residual liver volume should not be <40% in the obstructed liver, and trisegmentectomy may be difficult without decompression. The middle path for biliary decompression is to drain the side that is likely to be retained [13]. One drain usually suffices and the fall in bilirubin takes 3–6 weeks. Selective drainage leads to hypertrophy of the drained segment and atrophy of the undrained segment [13] (Fig. 3). Further, accurate cholangiography may be performed.
Fig. 3.
Hypertrophy of the left lobe (L) and atrophy of the right (R) following selective left lobe percutaneous biliary drainage (PTC). Dilated biliary radicals of the un-decompressed right lobe can be seen to the right of the middle hepatic vein (MHV)
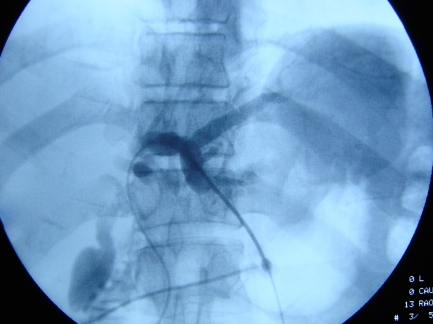
The percutaneous approach under ultrasound guidance provides the most accurate and quickest approach to biliary drainage (Fig. 4). This is in contrast to ERC wherein selective drainage is difficult and sterility is compromised.
Fig. 4.
Percutaneous transhepatic cholangiography showing dye in segment 2 and 3 ducts
Preoperative Diagnosis
Tissue diagnosis is not essential to establish diagnosis before resection. Imaging is highly suggestive of HCCC. However, it is well established that many lesions mimic HCCC, and in up to 10% of cases, the diagnosis may be different. In spite of this, preoperative biopsy is not mandatory. The reason for this is the low tissue yield of cytology from brushings. Modern approaches to increase diagnostic yield include the use of endoscopic ultrasound (EUS)-guided biopsy [14], cholangioscopy [15] and PET CT, especially to differentiate cholangiocarcinoma from benign strictures in patients with primary sclerosing cholangitis [16].
Portal Vein Embolization
Residual liver volume can easily be calculated from the CT scan with available software. An anatomical hemihepatectomy can usually be performed without worry of residual volume. However, in case of extended hepatectomies, there is a need to increase the volume of the residual liver. Portal vein embolization either with coils or glue of the segment to be resected can achieve this [17, 18]. The hypertrophy usually takes 3–6 weeks and a 25% increase in residual volume may be achieved [18]. Although 30% of liver as residual volume is optimal, safe resection leaving only 20% of normal liver may be performed [19].
Surgical Approach
The broad principles of surgery involve resecting the disease with a clear margin along with draining lymph nodes. Initially, local biliary excision was practised; however, recurrence rates were high with this approach [4, 20]. Incomplete margins were seen at the caudate lobe ducts, which are the first branches at the hilum. Therefore, caudate lobe excision is essential for all HCCC (Fig. 5). Upper margins are best defined on cholangiography and require an on table frozen section biopsy for confirmation. The upper margin can be maximized for right trisectionectomies by resecting the hilar plate to the left of the falciform ligament (Fig. 6), leaving the segment 2 and 3 ducts for anastomosis [21], and on the right side by using a transhepatic approach to the second- and third-order ducts [22]. Unfortunately, in spite of these measures, a microscopic positive margin may be seen in a considerable number of cases [1]. In spite of this, a 5-year survival of 32% has been obtained in this series [1]. On the table, stents may be placed and resection margins are marked with clips to enable radiotherapy post-operatively in these cases [23].
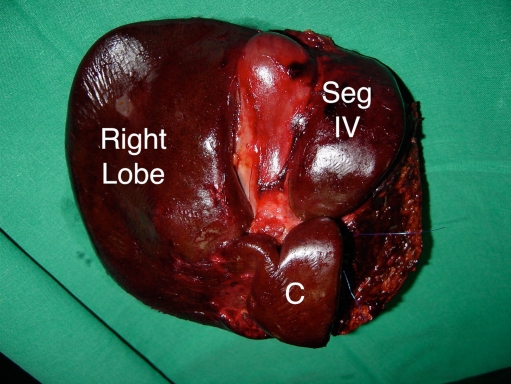
Fig. 5.
Specimen of right trisectionectomy for HCCC. The entire caudate lobe (C) has been resected
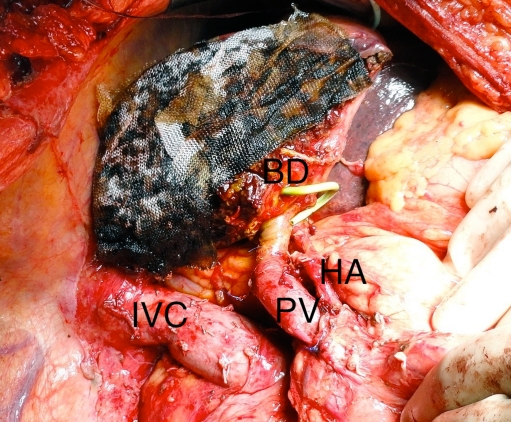
Fig. 6.
Remnant liver following right trisectionectomy for HCCC. The division of the bile duct (BD) is to the left of the falciform ligament. The percutaneous drain is seen emerging from the segment 3 duct
Invasion of the portal vein and hepatic artery branches is not uncommon findings in HCCC. The treatment for portal vein invasion by vascular resection is now well established [17, 24, 25]. Intra-operatively it is difficult to assess whether there is true invasion of the portal vein or just dense adhesion to the adventitia around the vein [25]. However, dissection in this area may breach tumour or lead to bleeding due to accidental breach of the portal vein. In view of this, some authors advocate a ‘no touch’ approach to dissection [2, 24], wherein routine portal vein resection is performed for HCCC with anastomosis. The long extrahepatic portion of the left portal vein makes clamping and reanastomosis safe, and preoperative right portal vein embolization ensures enough volume of the left liver.
Invasion of the artery supplying the remnant liver was long thought to be a contraindication to surgical resection for HCCC. However, many series report arterial resection and reconstruction for these tumours [1, 26]. The true benefit in terms of survival for this aggressive approach is yet to be established [26].
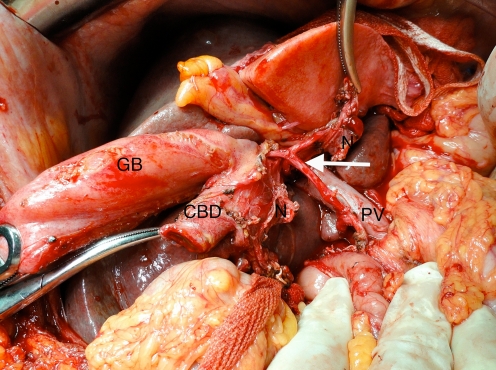
Nodal clearance of the portal sheath, hepatic artery, celiac, retroduodenal and SMA nodes is advocated as a part of this operation (Fig. 7). Nodal involvement reduces the 3-year survival from 50% to 30%. Even in the presence of para-aortic nodes, a 12% 5-year survival may be seen [3].
Fig. 7.
Hilar nodal clearance demonstrating the bared hepatic artery (arrow) and portal vein (PV)
Laparoscopic liver resections have been attempted in a small number of patients with HCCC [27]. The role of preoperative diagnostic laparoscopy in these tumours is unclear in the era of modern imaging [28], the yield being much lower than in patients with cancer of the gallbladder [29].
Liver transplantation was long considered to be contraindicated in HCCC. However, recently, selected cases of unresectable HCCC with no nodal spread have been subjected to liver transplantation following neo-adjuvant chemoradiotherapy with promising results [30]. This approach is under evaluation in clinical trials and is still not considered the standard of care though it promises much for the future [31].
In summary, the surgeon faces many challenges in tackling this rare problem. Preoperative evaluation is the key to performing safe and effective surgery.
References
- 1.Igami T, Nishio H, Ebata T, et al. Surgical treatment of hilar cholangiocarcinoma in the ‘new era’: the Nagoya University experience. J Hepatobiliary Pancreat Sci. 2010;17:449–454. doi: 10.1007/s00534-009-0209-0. [DOI] [PubMed] [Google Scholar]
- 2.Neuhaus P, Jonas S, Bechstein WO, et al. Extended resections for hilar cholangiocarcinoma. Ann Surg. 1999;230:808–818. doi: 10.1097/00000658-199912000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitagawa Y, Nagino M, Kamiya J, et al. Lymph node metastasis from hilar cholangiocarcinoma: audit of 110 patients who underwent regional and paraaortic node dissection. Ann Surg. 2001;233:385–392. doi: 10.1097/00000658-200103000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silva MA, Tekin K, Aytekin F, Bramhall SR, Buckels JA, Mirza DF. Surgery for hilar cholangiocarcinoma: a 10-year experience of a tertiary referral centre in the UK. Eur J Surg Oncol. 2005;31:533–539. doi: 10.1016/j.ejso.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Choi JY, Kim MJ, Lee JM, et al. Hilar cholangiocarcinoma: role of preoperative imaging with sonography, MDCT, MRI, and direct cholangiography. AJR Am J Roentgenol. 2008;191:1448–1457. doi: 10.2214/AJR.07.3992. [DOI] [PubMed] [Google Scholar]
- 6.Aloia TA, Charnsangavej C, Faria S, et al. High-resolution computed tomography accurately predicts resectability in hilar cholangiocarcinoma. Am J Surg. 2007;193:702–706. doi: 10.1016/j.amjsurg.2006.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Zidi SH, Prat F, Guen O, Rondeau Y, Pelletier G. Performance characteristics of magnetic resonance cholangiography in the staging of malignant hilar strictures. Gut. 2000;46:103–106. doi: 10.1136/gut.46.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nimura Y. Preoperative biliary drainage before resection for cholangiocarcinoma (Pro) HPB (Oxford) 2008;10:130–133. doi: 10.1080/13651820801992666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nimura Y, Kamiya J, Nagino M, et al. Aggressive surgical treatment of hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 1998;5:52–61. doi: 10.1007/PL00009951. [DOI] [PubMed] [Google Scholar]
- 10.Maguchi H, Takahashi K, Katanuma A, et al. Preoperative biliary drainage for hilar cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 2007;14:441–446. doi: 10.1007/s00534-006-1192-3. [DOI] [PubMed] [Google Scholar]
- 11.Nagino M, Takada T, Miyazaki M, et al. Preoperative biliary drainage for biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg. 2008;15:25–30. doi: 10.1007/s00534-007-1277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherqui D, Benoist S, Malassagne B, Humeres R, Rodriguez V, Fagniez PL. Major liver resection for carcinoma in jaundiced patients without preoperative biliary drainage. Arch Surg. 2000;135:302–308. doi: 10.1001/archsurg.135.3.302. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy TJ, Yopp A, Qin Y, et al. Role of preoperative biliary drainage of liver remnant prior to extended liver resection for hilar cholangiocarcinoma. HPB (Oxford) 2009;11:445–451. doi: 10.1111/j.1477-2574.2009.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritscher-Ravens A, Broering DC, Knoefel WT, et al. EUS-guided fine-needle aspiration of suspected hilar cholangiocarcinoma in potentially operable patients with negative brush cytology. Am J Gastroenterol. 2009;99:45–51. doi: 10.1046/j.1572-0241.2003.04006.x. [DOI] [PubMed] [Google Scholar]
- 15.Petersen BT. Cholangioscopy for special applications: primary sclerosing cholangitis, liver transplant, and selective duct access. Gastrointest Endosc Clin N Am. 2009;19:579–586. doi: 10.1016/j.giec.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 16.Prytz H, Keiding S, Björnsson E, et al. Dynamic FDG-PET is useful for detection of cholangiocarcinoma in patients with PSC listed for liver transplantation. Hepatology. 2006;44:1572–1580. doi: 10.1002/hep.21433. [DOI] [PubMed] [Google Scholar]
- 17.Palavecino M, Abdalla EK, Madoff DC, Vauthey JN. Portal vein embolization in hilar cholangiocarcinoma. Surg Oncol Clin N Am. 2009;18:257–267. doi: 10.1016/j.soc.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 18.Nagino M, Nimura Y, Kamiya J, et al. Right or left trisegment portal vein embolization before hepatic trisegmentectomy for hilar bile duct carcinoma. Surgery. 1995;117:677–681. doi: 10.1016/S0039-6060(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 19.Kishi Y, Abdalla EK, Chun YS, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540–548. doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- 20.Tsao JI, Nimura Y, Kamiya J, et al. Management of hilar cholangiocarcinoma: comparison of an American and a Japanese experience. Ann Surg. 2000;232:166–174. doi: 10.1097/00000658-200008000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagino M, Kamiya J, Arai T, Nishio H, Ebata T, Nimura Y. ‘Anatomic’ right hepatic trisectionectomy (extended right hepatectomy) with caudate lobectomy for hilar cholangiocarcinoma. Ann Surg. 2006;243:28–32. doi: 10.1097/01.sla.0000193604.72436.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miyazaki M, Kimura F, Shimizu H, et al. Extensive hilar bile duct resection using a transhepatic approach for patients with hepatic hilar bile duct diseases. Am J Surg. 2008;196:125–129. doi: 10.1016/j.amjsurg.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 23.Alexandre JH, Dehni N, Bouillot JL. Stented hepaticojejunostomies after resection for cholangiocarcinoma allow access for subsequent diagnosis and therapy. Am J Surg. 1995;169:428–429. doi: 10.1016/S0002-9610(99)80191-3. [DOI] [PubMed] [Google Scholar]
- 24.Hirano S, Kondo S, Tanaka E, Shichinohe T, Tsuchikawa T, Kato K. No-touch resection of hilar malignancies with right hepatectomy and routine portal reconstruction. J Hepatobiliary Pancreat Surg. 2009;16:502–507. doi: 10.1007/s00534-009-0093-7. [DOI] [PubMed] [Google Scholar]
- 25.Ebata T, Nagino M, Kamiya J, Uesaka K, Nagasaka T, Nimura Y. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann Surg. 2003;238:720–727. doi: 10.1097/01.sla.0000094437.68038.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miyazaki M, Kato A, Ito H, et al. Combined vascular resection in operative resection for hilar cholangiocarcinoma: does it work or not? Surgery. 2007;141:581–588. doi: 10.1016/j.surg.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 27.Bryant R, Laurent A, Tayar C, Cherqui D. Laparoscopic liver resection-understanding its role in current practice: the Henri Mondor Hospital experience. Ann Surg. 2009;250:103–111. doi: 10.1097/SLA.0b013e3181ad6660. [DOI] [PubMed] [Google Scholar]
- 28.Ruys AT, Busch OR, Gouma DJ, Gulik TM. Staging laparoscopy for hilar cholangiocarcinoma: is it still worthwhile? Ann Surg Oncol. 2011;18(9):2647–2653. doi: 10.1245/s10434-011-1576-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber SM, DeMatteo RP, Fong Y, Blumgart LH, Jarnagin WR. Staging laparoscopy in patients with extrahepatic biliary carcinoma. Analysis of 100 patients. Ann Surg. 2002;235:392–399. doi: 10.1097/00000658-200203000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rea DJ, Heimbach JK, Rosen CB, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451–458. doi: 10.1097/01.sla.0000179678.13285.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz JJ, Hutson WR, Gayowski TJ, Sorensen JB. Liver transplantation for cholangiocarcinoma. Transplantation. 2009;88:295–298. doi: 10.1097/TP.0b013e3181adc9e5. [DOI] [PubMed] [Google Scholar]