Abstract
Optimal care of a patient implies a good professional understanding between all the medical personnel involved in that patient’s care. Similarly a basic understanding of the areas where surgery and pathology interact would go a long way, in clarifying the disease process in the patient. This review aims to cover a few topics in liver lesions, FNAC Vs core biopsy, IHC, Margin examination, and frozen sections, in order to improve the communication between these two specialities.
Keywords: Dysplastic nodules, Core biopsies, Frozen section, IHC
Introduction
In the recent past, there has been a rapid increase in information and technology in both pathology and surgery. This volume of knowledge often leads to reduced interaction between pathologists and surgeons, affecting their ability to communicate and support one another’s role in patient care.
Milestones that mark the history of medicine have been achieved by those who could cross over from one specialty to the other such as Sidney Farber, father of medical oncology, who was initially a pathologist, Samuel Gross, who was initially professor of pathological anatomy and later held the chair of surgery, and whose dictum was ‘A surgeon could not be successful without knowing pathology’. C.D. Haagensen, J.C. Bloodgood, Arthur Purdy Stout, J. Ewing were amongst others. It is in the interest of both surgeons and pathologists to have at least a sideways look at each other’s work, and this review aims to provide the surgeon with hepatobiliary pancreatic interest a window to some advances in pathology.
Cirrhosis and Dysplastic Nodules
Cirrhosis is defined as a diffuse fibrosing disease secondary to liver injury, usually low-grade chronic injury. However, unlike the scar fibrosis that accompanies injury elsewhere, this fibrotic process is dynamic and has a nodular pattern due, in part, to the pressure of the regenerating liver cells. If hepatocyte injury to the entire liver is uniform, as seen in alcohol abuse, and every acinus is uniformly damaged, the fibrosis splits the acini into small fragments, which on regeneration form tiny nodules less than 3 mm in size, that is, micronodular cirrhosis [1]. If the injury to the liver is less uniform, as in hepatitis B, then the fibrosis is irregular, and both preserved and destroyed acini are seen within the larger areas bound by the fibrous bands, that is, macronodular cirrhosis. However, as regeneration, pressure ischaemia, fibrosis, ischaemic cell death, etc. are ongoing processes, the nodules keep remodelling and micronodular cirrhosis can become macronodular cirrhosis over time, and vice versa [2].
The proliferation of reparative capillaries within the fibrous tissue and increase in hepatic arterial branches lead to arterialization. Arterialization leads to fast flow, avoiding the usual percolation of portal blood around the hepatocytes. This results in virtual shunting of blood through the liver depriving the lobules of hepatocytes of their usual share of portal blood, leading to further cellular atrophy and loss.
Hepatocytes that normally have a low turnover rate begin to regenerate rapidly in cirrhotic livers. Frequently multiplying cells are prone to errors in nuclear DNA, and thus cells may be created, which if ‘blessed’ by activated proliferative advantages can form clonal clusters. These hyperactive clones multiply, eventually occupying the whole regenerating nodule. As these are rapidly multiplying, closely packed and hypercellular, these nodules tend to bulge on the cut surface. As they represent a distinct clone, they may also be distinct by way of colour and texture when compared with the surrounding regenerative nodules. Mild dysplasia, with lesser proliferative advantages, would be slow growing, and have time to mature. Cells would have near-normal amount of cytoplasm, less atypia, and would be difficult to distinguish from the surrounding regenerating cells. Without tests of monoclonality, and other molecular markers, many so-called large cell dysplasias actually represent regenerating nodules [3]. Therefore, the incidence of large cell dysplasia showing progression to hepatocellular cancer (HCC) is low and clinically insignificant [4]. In contrast, small cell dysplasia represents fast multiplying cells, more uniform nuclear shape, greater chance of being clonal and is, therefore, much more frequently associated with progression to HCC [5]. These represent clonal clusters of rapidly dividing cells, with less time to develop cytoplasm and mature. Histologically, small cell dysplasia is identified by fairly uniform small cell proliferation and cords that are more often ‘twinned’ and disoriented than single corded. On gross examination, these nodule bulge on the cut surface with a distinctive physical appearance. A small area of high-grade dysplasia when identified within a macroregenerative nodule is a ‘nodule within a nodule’. The rate of transformation of small cell dysplasia into HCC is not a known, nor are the exact genetic mutations that separate a carcinoma from a dysplasia [6]. Therefore to differentiate between small cell dysplasia and small HCC remains a hair splitting academic exercise, currently based on educated guesswork.
As the clonal cells within a high-grade dysplastic nodule continue to multiply they acquire more carcinogenic mutations. They start showing greater architectural,and nuclear atypia, thicker trabecular or adenoid arrangements. They obtain infiltrative ability and invade into the fibrous bands that surround them as well as adjacent vessels. These are features of a full-blown HCC.
The problems in the histological diagnosis of HCC occur at both ends of the spectrum. Tumours that are very well differentiated are difficult to differentiate,on biopsy, from benign lesions such as adenoma, focal nodular hyperplasia and macroregenerative nodules. Focal nodular hyperplasia is identified by its scattered bile ducts and thick vessels, and a large core or a wedge biopsy would have greater chances of including these structures within it, as compared with a thin core [7]. Adenoma, except for its characteristic clinical setting, is near impossible to differentiate from a well-differentiated HCC on biopsy [8].
On the other end of the spectrum are the poorly differentiated tumours whose malignant nature is clearly evident, but showing no clue of their cell of origin. The diagnostic difficulty in these cases is compounded by the problem that serum alpha-fetoprotein is elevated in only 60–70% of HCCs, and may also show elevation in some gastric carcinomas, germ cell tumours and cirrhosis itself. Poorly differentiated tumours would need ancillary studies, such as immunohistochemistry.
When intraoperative biopsies are contemplated, the surgeon should remember that it is advisable to obtain the biopsy sample at the beginning of the surgical procedure to avoid artifacts such as neutrophilic infiltration [9]. Subcapsular regions are usually more fibrosed; hence, a deep-needle biopsy is more representative of the true state of fibrosis within the liver parenchyma [10]. When scattered liver involvement is a possibility, as in granulomatous disease, more than one core is advisable.
Fine-Needle Aspiration Cytology vs. Core Biopsy of HPB Tumours: Which Should be Done?
Pathologists rely on two main features – aberration in cytology and aberration in architecture. Obtaining a diagnosis by fine-needle aspiration cytology relies almost completely on cellular aberration for diagnosis, whereas in the core biopsy the tissue architecture is also available for evaluation.
Cytology is sufficient for diagnosis when the cell type aspirated is sufficiently foreign to the parent tissue; for example, granulomas or epithelial metastasis in a lymph node.
In very well-differentiated cancers, the cytological aberration is slight. Diagnosis of cancer would then rely on demonstration of architectural abnormality such as loss of lobular arrangement, invasive margins and perineural invasion.
Fine-needle aspiration cytology would also be insufficient to differentiate between mildly abnormal cells, as in the inflammatory atypia of chronic pancreatitis, and a well-differentiated adenocarcinoma. A core biopsy would then yield more information to show the loss of the normal lobular architecture and replacement by haphazard invasive patterns, and perhaps the core may contain a nerve with perineural invasion, the sine qua non of Hepatopancreatobiliary cancers [11].
Similarly, in intraoperatively found small, white nodule in the liver, the differentiation between Von Meyenburg complex and metastatic well-differentiated pancreatic cancer depends on very subtle characteristics [12].
Subtle cytological features such as nuclear details, endothelial rimming in HCCs are often lost in the shrinkage that accompanies tissue processing and are often seen better on cytology. Needle core biopsies when accompanied by an aspirate often give complementary information to reach a diagnosis. When an aspirate is not possible, touch preparation of a core biopsy can also give adequate numbers of cells for examination of cytological features.
Core biopsies are also required in firm fibrotic lesions, as dense fibrous stroma yields only a few cells on aspiration.
Aspiration of pancreatic cysts often yields only acellular fluid or few degenerate cells. However, here physical characteristics of the fluid, that is, thin, watery/mucoid and estimation of amylase and CEA levels within the fluid would give clues to indicate if the cyst is a serous cystadenoma, mucinous cyst or pseudocyst.
If inflammatory cells are present in aspirates of pancreatic or biliary lesions, the threshold of atypia to diagnose malignancy has to be raised manyfold, to negate the effects of inflammation induced cellular changes [13]. A core biopsy in these cases may show the characteristic architectural aberrations of malignancy and help establish a diagnosis.
For diffuse parenchymal liver disease, the histological diagnosis depends predominantly on architectural disarray within the liver parenchyma. So, fine-needle aspiration has no role.
For image-guided biopsies, the radiologist may employ a coaxial system, wherein a fine cannula may be placed close to the lesion through which cytology samples may be collected easily followed by multiple passes of a Tru-cut needle, maximizing tissue yield with little chance of spillage and tract seeding.
Immunohistochemistry
Aberrant DNA produces aberrant RNA, which in turn produces aberrant protein resulting in aberrant cell morphology. Aberrant morphology is the level at which routine light microscopy is done. When aberrant cell morphology is not diagnostic, colour-labelled antibodies,ie immunohistochemistry[IHC]) is used to detect the protein, by antigen–antibody reaction. Thus small quantities of protein are highlighted on cell membrane/cytoplasm/nuclear membrane. If this protein is also too scant to be detected, then molecular probes are used to detect the aberrant RNA (polymerase chain reaction techniques). When the RNA also cannot be satisfactorily identified, then DNA probes are used.
IHC studies with a panel of antibodies are enough to establish the diagnosis, in most undifferentiated tumours. Subtypes of cytokeratin, CK7 and CK20 [14] are both usually negative in HCC, whereas CK7 is positive in carcinomas of the stomach, lung, ovary, breast and cholangiocarcinoma. CK20 positivity on the other hand is fairly specific for colorectal carcinomas. CDX2 is also a specific marker for intestinal differentiation, both large and small, whereas TTF-1 nuclear staining and Napsin A is specific for lung [15]. HepPar-1 is a sensitive and specific marker for hepatocytes. Glypican has emerged as a robust marker for HCC and does not stain benign liver [16]. CD34 stains the endothelial wrapping around the neoplastic clusters. Alpha-fetoprotein stains less than 50% of HCC, usually the well-differentiated ones, where identification is usually not a problem.
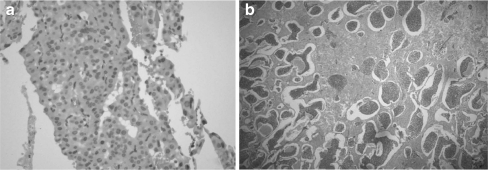
Poorly differentiated HCC and neuroendocrine tumours have overlapping features, as both show trabecular patterns, rich vasculature and the lesional cells in both can be small and uniform.(Fig. 1). On IHC,HCCs are identified by the hepatocyte-specific antibody glypican, while,neuroendocrine tumours are positive for chromogranin and synaptophysin. IHC with proliferation markers (MIB-1/Ki67) helps grade and prognosticate neuroendocrine tumours.
Fig. 1.
Two cases of primary neuroendocrine tumours of the liver (a) mimicking hepatocellular carcinoma on morphology (b) mimicking a hemangioma on imaging studies
It is often seen that it is not a single IHC marker that guides the diagnosis, but a permutation and combination of the various markers available, and a deduction made on the IHC patterns thus displayed.
‘Theranostics’ is a chimera of therapy and diagnostics. These tests identify the tumour sensitivity to specific targeted therapy; for example, HER2-neu positivity and herceptin therapy, CD20 and rituximab, and C-kit positivity and imatinib. Development of similar molecular tests will help to tailor and personalize therapy in the long run.
Margin Examination
The Halsteadian radical approach to surgery is slowly losing its charm and radical resections are slowly giving way to smaller and more organ sparing surgery, thereby reducing the margin between the tumour and normal tissue. Surgery is often sandwiched between neoadjuvant and adjuvant chemotherapy, and therefore smaller surgical specimens are being studied with partial and irregular involution of the tumour, making margin examination important. Tumour-free margin is an important prognostic factor and therefore it is imperative that an accurate margin examination be done by the pathologist. As the pathologists reduce all 3-dimensional masses into 2-dimensional sections on a slide, with every section/slice, they have to, perforce, choose to leave out two dimensions of the six-sided specimen. Resection margins have to be chosen in order of importance.
Colouring the six sides in different colours helps in preserving the 3-dimensional aspect of the tumour, even when reduced to 2-dimensional sections, giving a more accurate anatomical assessment in relation to the structures adjacent to the tumour. This also offers a feedback to the radiologist.
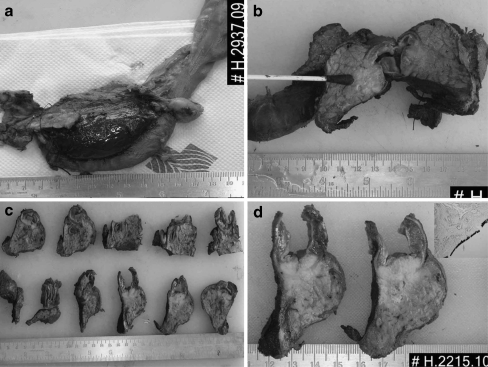
In pancreaticoduodenectomies, R0 surgery offers the only opportunity for cure. Recurrence rates after R0 and R1 resections are fairly similar, suggesting that positive margins are underrecognized and underreported. Meta-analysis of various studies shows wide variation in R0 and R1 rates [17]. Studies with the highest R1 rates identify the subgroup with the longest survival, that is, the true R0 group [16]. True R1 identification can identify the group that could benefit from adjuvant therapy. Standard surgical pathological examination protocols, with colouring of the surfaces, offer the best method to examine the margins of these complex specimens (Fig. 2).
Fig. 2.
Margin examination in a pancreaticoduodenectomy specimen, with colouring of surfaces
Similarly, in low rectal adenocarcinomas, the presence of tumour below the level of the anterior peritoneal reflection, the completeness of the mesorectal excision and the presence of tumour within 1 mm of the circumferential margin are now recognized to be important prognostic markers [18].
R1 rates, being the detection of microscopic residual tumour, do not reflect the quality of surgery, but reflect high quality of gross and microscopic assessment of the specimen and the biological behaviour of the tumour.
Use of standardized grossing and reporting protocols offer the best analysis of surgical specimens and enables true comparison of treatment efficacy.
Frozen Sections
Dr. Louis B. Wilson developed the technique of frozen section evaluation at the request of Dr. William Mayo, surgeon and one of the founders of the Mayo Clinic, in 1905, creating one more example of successful cooperation between a surgeon and a pathologist [19].
Frozen sections are appropriately obtained to:
render a diagnosis that has impact on the immediate surgical procedures being contemplated.
determine biopsy adequacy when the only purpose of the procedure is to obtain sufficient material for diagnosis.
stage malignant neoplasms intraoperatively, especially in cases where this may alter the scope of the surgical procedure.
assess adequacy of excision (checking the margins).
(rarely) evaluate certain specimens containing fatty substances or sugars, which would be dissolved during the process of fixation, dehydration and embedding [19]
Inappropriate use of frozen sections would include any request that has no bearing on the immediate surgical care of the patient, that is, those requested to satisfy the curiosity of the surgeon, appease the anxiety of the patient or simply speed up the diagnostic work-up of the patient [20].
The quality of the slides produced by frozen section is of lower quality than formalin-fixed, paraffin-embedded sections.. Study of frozen section requires experience; knowledge of clinical and intraoperative features; an ability to quickly sieve through several differential diagnosis; the capacity to make quick decisions under pressure; an attitude that is conservative, but not excessively so and a keen awareness of the limitations of the method, mainly sampling error, freezing artifacts and the lack of availability of special studies [21].
Biopsies from the periphery or surface of lesions may represent only capsular tissue, which at times may be up to 1 cm thick. Adenomatous lesions may harbour invasive carcinomas only in the base. Features of pancreatic carcinomas imperceptibly mix with those of chronic pancreatitis at their expanding margins.
Fatty tissues freeze, at very low temperatures, but at that temperature the areas of interest could shatter, compromising microscopy [21]. Frozen ice crystals in lymph nodes can cause architectural distortions mimicking metastasis. Small bits in saline for too long can cause waterlogging of the tissue.
Lack of the usual tissue shrinkage can give benign endothelial cells, histiocytes and ganglion cells menacing proportions. (A surgeon breathing down your neck has the same effect!) Sutures and staples cause the sections to split. Tissue once frozen partially carries its artifacts into subsequently made paraffin sections, which are therefore, suboptimal.
Aspiration smears/imprints provide information that cannot be seen in sectioned tissue either on permanent or frozen section, and provide details to fill any void that may be created by the frozen section.
In summary, although pathology and surgery are widely different fields, improving communication and understanding the strengths and limitations of these specialties lead to optimal patient care.
References
- 1.Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, Sobin LH. The morphology of cirrhosis: recommendations on definition, nomenclature, and classification by a working group sponsored by the World Health Organization. J Clin Pathol. 1978;31:395–414. doi: 10.1136/jcp.31.5.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fauerholdt L, Schlichting P, Christensen E, Poulsen H, Tygstrup N, Juhl E. Conversion of micronodular cirrhosis into macronodular cirrhosis. Hepatology. 1983;3:928–931. doi: 10.1002/hep.1840030607. [DOI] [PubMed] [Google Scholar]
- 3.Hytiroglou P, Theise ND, Schwartz M, Mor E, Miller C, Thung SN. Macroregenerative nodules in a series of adult cirrhotic liver explants: issues of classification and nomenclature. Hepatology. 1995;21(3):703–708. [PubMed] [Google Scholar]
- 4.Lee RG, Tsamandas AC, Demetris AJ. Large cell change (liver cell dysplasia) and hepatocellular carcinoma in cirrhosis: matched case–control study, pathological analysis, and pathogenetic hypothesis. Hepatology. 1997;26(6):1415–1422. doi: 10.1002/hep.510260607. [DOI] [PubMed] [Google Scholar]
- 5.Ferrell L. Hepatocellular nodules in the cirrhotic liver: diagnostic features and proposed nomenclature. In: Ferrell L, editor. Diagnostic problems in liver pathology, pathology: state of the art reviews. Philadelphia: Hanley & Belfus; 1994. pp. 105–117. [PubMed] [Google Scholar]
- 6.Sherman M. Pathogenesis and screening for hepatocellular carcinoma. Clin Liver Dis. 2004;8(2):419–443. doi: 10.1016/j.cld.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 7.Robert D, Odze JR Goldblum. Surgical pathology of the GI tract, Liver. Saunders, Elsevier: Biliary tract and Pancreas; 2009. pp. 1294–1297. [Google Scholar]
- 8.Hytiroglou P, Theise N. Differential diagnosis of hepatocellular nodular lesions. Semin Diagn Pathol. 1998;15:285–299. [PubMed] [Google Scholar]
- 9.Christoffersen P, Poulsen H, Skeie E. Focal liver cell necroses accompanied by infiltration of granulocytes arising during operation. Acta Hepatosplenol. 1970;17:240–245. [PubMed] [Google Scholar]
- 10.Petrelli M, Scheuer PJ. Variation in subcapsular liver structure and its significance in the interpretation of wedge biopsies. J Clin Pathol. 1967;20:743–748. doi: 10.1136/jcp.20.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hyland C, Kheir SM, Kashlan MB. Frozen section diagnosis of pancreatic carcinoma: a prospective study of 64 biopsies. Am J Surg Pathol. 1981;5:179–191. doi: 10.1097/00000478-198103000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Hornick JL, Lauwers GY, Odze RD. Immunohistochemistry can help distinguish metastatic pancreatic adenocarcinomas from bile duct adenomas and hamartomas of the liver. Am J Surg Pathol. 2005;29:381–389. doi: 10.1097/01.pas.0000149710.01559.fe. [DOI] [PubMed] [Google Scholar]
- 13.Geisinger KR, Stanley MW, Raab SS, et al. Modern cytopathology. Philadelphia: Churchill Livingstone; 2004. pp. 546–553. [Google Scholar]
- 14.Chu P, Wu E, Weiss LM. Cytokeratin 7 and cytokeratin 20 expression in epithelial neoplasms: a survey of 435 cases. Mod Pathol. 2000;13(9):962–972. doi: 10.1038/modpathol.3880175. [DOI] [PubMed] [Google Scholar]
- 15.Saqi A, Alexis D, Remotti F, Bhagat G. Usefulness of CDX2 and TTF-1 in differentiating gastrointestinal from pulmonary carcinoids. Am J Clin Pathol. 2005;123:394–404. doi: 10.1309/UKN6PVRKXHG422DA. [DOI] [PubMed] [Google Scholar]
- 16.Yamauchi N, Watanabe A, Hishinuma M, et al. The glypican-3 oncofetal protein is a promising diagnostic marker for hepatocellular carcinoma. Mod Pathol. 2005;18:1591–1598. doi: 10.1038/modpathol.3800436. [DOI] [PubMed] [Google Scholar]
- 17.Menon KV, Gomez D, Smith AM, Anthoney A, Verbeke CS. Impact of margin status on survival following pancreatoduodenectomy for cancer: the Leeds Pathology Protocol (LEEPP) HPB (Oxford) 2009;11(1):18–24. doi: 10.1111/j.1477-2574.2008.00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tilney HS, Rasheed S, Northover JM, Tekkis PP. The influence of circumferential resection margins on long-term outcomes following rectal cancer surgery. Dis Colon Rectum. 2009;52(10):1723–1729. doi: 10.1007/DCR.0b013e3181b54fbd. [DOI] [PubMed] [Google Scholar]
- 19.Gal AA. The centennial anniversary of the frozen section technique at the Mayo Clinic. Arch Pathol Lab Med. 2005;129(12):1532–1535. doi: 10.5858/2005-129-1532-TCAOTF. [DOI] [PubMed] [Google Scholar]
- 20.Zarbo RJ, Schmidt WA, Bachner P, Howanitz PJ, Meier FA, Schifman RB, et al. Indications and immediate patient outcomes of pathology intraoperative consultations. College of American Pathologists/Centers for Disease Control and Prevention Outcomes Working Group Study. Arch Pathol Lab Med. 1996;120(1):19–25. [PubMed] [Google Scholar]
- 21.Rosai Juan (2004) Rosai and Ackerman’s Surgical Pathology. 9th ed, Elsevier Inc, (vol.1)