Abstract
Hepatocellular carcinoma (HCC) often occurs in patients with chronic liver disease or cirrhosis. Liver transplantation for hepatocellular carcinoma has the potential to eliminate both the tumor as well as the underlying cirrhosis and is the ideal treatment for HCC in cirrhotic liver as well as massive HCC in noncirrhotic liver. Limitations in organ availability, necessitate stringent selection of patients who would likely to derive most benefit. Selection criteria have considered tumor size, number, volume as well as biological features. The Milan criteria set the benchmark for tumors that would benefit from liver transplantation but were found to be excessively restrictive. Modest expansion in criteria has also been shown to be associated with equivalent survival. Microvascular invasion is the single most important adverse prognostic factor for survival. Living donor liver transplantation has expanded donor options and has the advantage of lower waiting period and not impacting the non-HCC waiting list. Acceptable outcomes have been obtained with living donor liver transplantation for larger and more numerous tumors in the absence of microvascular invasion. Downstaging of tumors to prevent progression while waiting for an organ or for reduction in size to allow enrolment for transplantation has met with variable success.
Keywords: Hepatocellular carcinoma, Liver transplantation, Living donor, Milan criteria, Alpha-fetoprotein, Chemoembolization, Radiofrequency ablation, Microvascular invasion
Introduction
Hepatocellular carcinoma (HCC) is the commonest primary malignant tumor of the liver cells. In some ways HCC is a rather unique tumor: a large majority of cases are found in patients who have chronic liver disease, and there are well-defined environmental and genetic factors associated with its occurrence. Due to its more frequent detection in diseased livers, HCC may be considered a tumor in a tumor-generating environment.
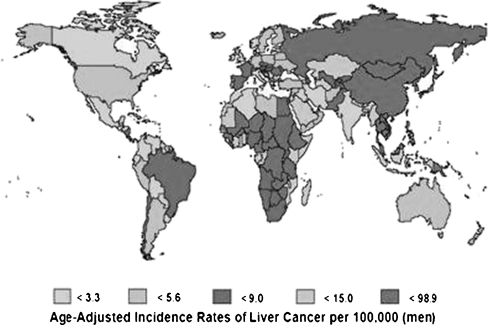
HCC is the seventh most common cancer and third to fourth most common cause of cancer-related deaths worldwide [1, 2]. The incidence of HCC in a population closely parallels the incidence of chronic liver disease and cirrhosis. The incidence is highest in the Far East (Taiwan, Korea, China) where hepatitis B virus infection is prevalent, while in the western hemisphere it is moderate and usually related to hepatitis C virus or alcohol intake [3]. The reported incidence in India could be deceptively low as a result of under-reporting (Fig. 1).
Fig. 1.
Worldwide incidence of HCC
Clinical features of HCC are usually myriad and can be extremely subtle leading to a delay in detection in the absence of active surveillance of high-risk groups. About 5–15% of patients with cirrhosis can have HCC at the time of initial diagnosis of liver disease. Recommended surveillance strategies usually hinge on periodic imaging (often by an abdominal ultrasound every 6 months) and blood levels of the tumor marker alpha-fetoprotein (AFP). There is no global consensus on the cut-off level of AFP because the marker has less than optimal sensitivity and specificity. Groups in Japan and increasingly in the rest of the world have looked at other markers with interest. Prominent among these is des-gamma carboxy-prothrombin (DCP) or protein induced by vitamin K absence (PIVKA II) to increase the accuracy of surveillance programs [4]. Suspicious lesions on surveillance need further characterization by more advanced imaging such as contrast-enhanced CT scan, MRI, or contrast ultrasound with contrast agents. Lesions more than 2 cm usually can be detected and reliably diagnosed based on their imaging characteristics (arterial hypervascularity and early wash-out in portal phase) on two imaging modalities irrespective of AFP levels. Smaller lesions (1–2 cm) may need histological confirmation to differentiate from regenerating nodules and dysplastic nodules, which are considered precursor lesions for early HCC [5]. While the risk of biopsy (bleeding and track seeding) has often been overstated, it has the ability to provide valuable prognostic information such as histological grade, microvascular invasion (mVI), and genetic and molecular markers of aggressiveness. Lesions less than 1 cm are often too small to characterize or target and need frequent imaging to monitor their progression.
Rationale for Liver Transplantation in HCC
Surgical resection of the involved segment, section or lobe is the traditional treatment for HCC with curative intent. Although recent advances in liver surgery have reduced blood loss, infectious morbidity and operative mortality after major liver resection, patients who have chronic liver disease tolerate loss of liver volume much more poorly (morbidity 10–40% and mortality of 0–20%) than those without (morbidity 5–15% and mortality 0–10%) depending on the extent of liver resection, status of liver disease, and experience of the center [6–10].
Parenchyma preserving techniques and techniques to augment the future liver remnant volume (FLRV) enhance the early outcome in noncirrhotics or early cirrhotics but not disease-free survival (DFS) or overall survival (OS) in the decompensated cirrhotic patient. Loco-regional therapies (either individually or in combination) have shown promise in achieving equivalent survival as surgical resection with minimal morbidity in selected small HCC with compensated cirrhosis, but have technical limitations in the tumor volume and locations that can be effectively treated. Intrahepatic recurrence of HCC may be true recurrence or de novo tumorigenesis in the damaged hepatocytes; both of these account for a post-treatment recurrence rate of 10–50% within 2 years and up to 70% at 5 years depending on the extent of tumor volume, completeness of resection, or ablation [11].
Liver transplantation (LT) has the potential to eliminate the tumor itself as well as the tumor-generating environment of cirrhosis and provide the widest surgical margin possible. Therefore, it not only reduce true recurrences but also protect from de novo tumorigenesis. In noncirrhotic patients with tumors too large for resection despite FLRV manipulation, total hepatectomy and consequently LT may be the only curative option available. In summary, in the absence of extrahepatic spread, for large HCC in noncirrhotics and HCC in decompensated cirrhotics, LT is likely to be the best curative option at least from the oncological stand-point.
History and Evolution of Liver Transplantation for HCC
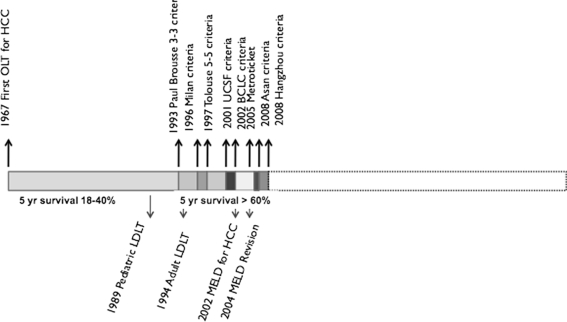
Liver transplantation for HCC is almost as old as LT itself. The early series of Thomas Starzl from Denver had four HCC patients including two children. The longest survival achieved was 16 months and only two patients survived more than 1 year [12]. Early series of LT from various centers in the 70s and 80s yielded poor results and prompted the US Department of Health to declare HCC as a contraindication to LT in 1989. The unflattering outcomes of LT for HCC (high early recurrence and 18–40% 5-year OS) [13] could be largely attributed to inclusion of patients with large tumors, evolutionary phase of modern imaging, and evolving surgical techniques (Fig. 2).
Fig. 2.
Events in evolution of LT for HCC
By the late 1980s with improving outcomes of LT, there was an increasing demand on the limited availability of organs for transplantation. The philosophy of allocating scarce organs to those who were likely to derive the greatest benefit came into being at the disadvantage of patients with HCC. The Paul-Brousse group from France was among the first to propose that in selected patients of HCC (uninodular or binodular tumor ≤3 cm without portal vein thrombus) good DFS and acceptable 3-year survival (47%) was achievable with LT. For this selected group called 3–3 criteria, DFS was significantly superior with LT as compared to resection [14]. These and other similar criteria established that in selected patients LT for HCC could yield acceptable outcomes that are superior to resection.
The game-changer for LT in patients with HCC came in the form of experience from the Milan group that revealed a 4-year DFS of 75% and OS of 83% for patients with a single HCC ≤5 cm or <3 HCC ≤3 cm each on pretransplant imaging [15]. These criteria thereafter known as the Milan criteria have been used elsewhere with equivalent results and were accepted by the United Network of Organ Sharing (UNOS) for graft allocation to patients with HCC in 1998. They have since served as the benchmark based on which decision to allocate organs for patients with HCC is made. With more experience with LT for HCC, it become apparent that equivalent results as the Milan group were achievable with slightly larger tumor volume both in explanted livers and based on preoperative imaging. Experience from University of San Francisco in particular showed survival that was superior (5-year survival 75.2%) to that achieved by the Milan group even for patients with single tumors ≤6.5 cm or ≤3 tumors, ≤4.5 cm with a total tumor volume ≤8 cm (UCSF Criteria) [16]. The UCSF criteria have been validated in some other centers; however, multicenter data from Europe included in the Metroticket project have failed to support inclusion of larger tumors due to lower survival (OS and DFS) [17].
The Mayo end-stage liver disease (MELD) score, based on creatinine, international normalized ratio (INR) and bilirubin levels, was incorporated by UNOS for allocation of organs in 2002. Patients with HCC, who had a higher chance of mortality and progression on the waiting list, were accorded 24 points (single HCC <2 cm) or 29 (as per Milan criteria). These were subsequently revised to 20 and 29, none and 24, and none and 22, respectively, recognizing an undue increase in LT for HCC and the absence of tumor in explant specimen in patients listed on imaging criteria in the MELD priority era [18, 19]. Further 10% points are allotted for every 3 months spent on the waiting list.
Living donor liver transplantation (LDLT) emerged as a technique to overcome the severe graft shortage and high waiting list mortality faced by pediatric recipients in the early 1990s. With increased experience, this technique was extended to adults using the donor’s left lobe initially and then the right lobe as a graft. This technique was embraced with prodigious fervor by surgeons in Japan, Taiwan, Hong Kong, and Korea, where LT activity until then was impeded by poor deceased organ donation rates as well as in some centers in Europe and the US to expand the donor pool. The ethical justification for LDLT in HCC was the same as that for other indications and focused on the principle of double equipoise where significant benefit to the recipient is achieved without imperiling the safety of the donor [20, 21]. Since the mid 1990s, LDLT has been introduced for patients with unresectable HCC in Asia, where the incidence of HCC is six to tenfold higher than the west and deceased donor organs are rarely available. Multicenter studies (Table 1) and nationwide registries using non-uniform selection criteria revealed actuarial OS of 68% and DFS of 61% at 5 years in Japan [22] and 73% OS and 55% DFS at 3 years in Korea [23], which were only marginally inferior to the Milan experience. Groups from Asia, where LDLT is dominant, have demonstrated acceptable survival by extending inclusion criteria beyond UCSF criteria and incorporating levels of tumor markers such as AFP and PIVKA II. The rationale for extending the criteria in LDLT centers has been that the living donor organ is a private gift and does not deprive another individual of a transplantable organ even if it is used in a patient with larger tumors. Moreover, if acceptable recipient survival can be achieved, the benefit to the recipient, who otherwise has no hope of receiving a deceased donor organ, justifies the risk to the living donor. Opponents of LDLT for HCC argue that absence of waiting period may allow tumors with poor biology to be transplanted without a period to manifest their behavior and that the early regenerative cytokine environment within the partial graft may accelerate growth of any occult tumor cells [24]. Despite size, tumor volume, and absence of extrahepatic disease being the predominant factors in consideration of patients with HCC for LT, molecular, genetic markers, and markers of invasiveness may have a significant role to play in determining the outcomes after transplantation. Functional allelic imbalance [25], high tumor grade (G2 and G3) [26], and mVI [27] have been predictive of early recurrence without adjusting for tumor volume. The correlation between tumor volume and mVI has also not been consistently demonstrated to be linear.
Table 1.
Multicenter results: LDLT for HCC
Extended Criteria: Rationale and the Metroticket Concept—How Far Is Too Far?
The Milan criteria set the benchmark of outcomes for LT in patients with HCC and have been endorsed across many studies and the UNOS. These criteria have subsequently been termed ‘conventional’ and any criteria beyond these size and number limits deemed ‘extended’.
Rationale for Extended Criteria
Since the initial publication describing the Milan experience, major leaps have been made in the fields of liver transplantation. This led to the debate whether the Milan criteria in their original form may be too restrictive and unnecessarily deny many patients a lifesaving procedure. Limitations of the Milan criteria have emerged as experience with the procedure has increased. In the Milan study, size and number of tumors was determined on contrast-enhanced CT scan and hepatic angiography at varying time before LT (median 143 days) [15]. Because it is well known that best preoperative imaging techniques can understage as many as 20% cases and progression of tumor between imaging and LT cannot be ruled out [16, 28, 29], the Milan cohort may have included patients that exceeded size and number criteria. A retrospective analysis of data from Pittsburgh revealed that 25–49% of patients beyond conventional criteria experienced long-term survival and more than 50% patients with HCC beyond the conventional criteria remained recurrence-free at 3.3 years [29]. Also, it was increasingly realized that size and number alone are insufficient to determine post-LT prognosis of patients and incorporation of other factors such as microvascular invasion and tumor markers is important. The introduction and evolution of LDLT also challenged paradigms of utility because the living donor graft is a private gift. A recent meta-analysis revealed that the Milan criteria were significantly predictive of better biological characteristics (lower microvascular invasion rate, lower proportion of poorly differentiated histology, lower AFP levels, lower incidence of microsatellites) [30]. The analysis favored LT for patients within Milan criteria than those outside it.
Extended Criteria
A pathology-based study from the UCSF reported that survival was superior (5-year survival 75.2%) to that achieved by the Milan group even for patients with single tumors ≤6.5 cm or ≤3 tumors, ≤4.5 cm with a total tumor volume ≤8 cm [16]. External validation of the UCSF criteria on explant histology has been reported. The UCSF study has been criticized for being retrospective, explant pathology based, having significant size overlap with Milan criteria, and having included patients who were subjected to a downstaging protocol concurrently. The same group later prospectively validated the UCSF criteria in 168 patients based on preoperative imaging [31]. Using the UCSF criteria, an additional 5–20% HCC patients could be considered for LT. However, in a French multicenter study, 5-year survival of only 48% could be achieved in patients with HCC beyond conventional criteria but within UCSF criteria [32]. The Milan group recently reviewed the explant pathological data in 1,556 patients (of the Metroticket project) of which 1,112 were found to have HCC beyond Milan criteria. Despite this, in 283 patients who had the sum of largest tumor diameter in centimeters and number of tumors ≤7 (up to 7 criteria), a 5-year survival of 71% was achieved [17, 33]. Faced with low rates of deceased donor liver transplantation (DDLT) and prolonged waiting periods, LDLT is often the only realistic option for patients with HCC in some parts of the world. After reporting excellent outcomes in patients without HCC, many centers have been routinely offering LDLT to larger tumors with good biological characteristics in the absence of macrovascular invasion and extrahepatic metastasis. Multicenter study from Korea indicated a 3-year survival of 62.6 and 58.5% for tumors beyond Milan and UCSF, respectively, with LDLT and without adjustment for presence of macrovascular invasion [23]. In a report by the Japanese Liver Transplantation Study Group, 653 patients who underwent LDLT for HCC at 49 centers were analyzed. Patients whose tumor size exceeded Milan criteria (on pathology) had OS of 78, 63.6, and 60.3% and recurrence rates were 17.7, 34.3, and 37.3% at 1, 3 and, 5 years, respectively. However, those with tumors outside the Milan criteria on preoperative imaging but who had AFP ≤200 ng/mL and PIVKA II ≤1,000 mAu/mL had a 5-year DFS of 78.7% [22]. At the Asan Medical Centre in Seoul, patients with HCC ≤5 cm, ≤6 tumors, who had no gross vascular invasion (Asan criteria) had excellent outcome. Based on preoperative imaging, patients with HCC fulfilling the Asan criteria had 5-year OS and recurrence rate of 76.3% and 20% as compared to 18.9 and 73.6% with larger and numerous tumors. The Asan criteria provided superior discriminant potential is selection of patients for LT than the Milan or UCSF criteria. When combined with the preoperative AFP cut-off level of 3,000 ng/mL, 3-year recurrence rates were 12.4% within criteria and 80.4% outside criteria [34]. Data from Hangzhou reveal that patients who have well or moderately differentiated HCC ≤8 cm without macrovascular invasion and AFP ≤400 ng/mL (Hangzhou criteria) have the 1-, 3-, 5-year OS and DFS of 92.8, 70.7, 70.7% and 83.7, 65.6, 62.4%, respectively, an 49.9, 27.0, 18.9%, and 25.8, 12.5, 4.7%, respectively, for those patients exceeding Hangzhou criteria. Patients exceeding Milan but fulfilling Hangzhou criteria had better outcome than those outside Hangzhou criteria [35]. Similar criteria have been reported by individual centers with results equivalent to the Milan criteria [36] (Table 2).
Table 2.
Extended criteria for LT in HCC
| Center [reference] | Details | Results |
|---|---|---|
| UCSF, USA [16] | Single ≤6.5 cm | 1-year OS 90% |
| Two lesions ≤4.5 cm | 5-year OS 75.2% | |
| If total diameter ≤8 cm | ||
| Asan Center, Korea [34] | Diameter ≤5 cm | 5-year recurrence 15% |
| Lesions ≤6 cm | 5-year OS 76.3% | |
| No gross vascular invasion | ||
| Hangzhou, China [35] | No gross vascular invasion | 1-year DFS 83.7% |
| Lesion <8 cm OR | 1-year OS 92.8% | |
| Lesion ≥8 cm if AFP ≤400 and well-differentiated | 5-year DFS 62.4% | |
| 5-year OS 70.7% | ||
| Padua, Italy [33] | Sum of number of lesions and diameter ≤7 cm | 1-year recurrence 4% |
| 5-year recurrence 14% |
How Far Is Too Far?
While several studies have attempted to push the envelope while enrolling patients with HCC for LT particularly using LDLT, most of them are retrospective and based on explant data, which are not available at time of inclusion and very few have been prospectively and externally validated [30]. The fundamental questions to be answered are what constitutes an acceptable outcome and how much does extension of criteria impact the non-HCC waiting list. A Markov mathematical model revealed that unless a 5-year survival of at least 61% could be achieved, performing LT for patients with tumors beyond Milan criteria put other patients without HCC at a risk of dying without LT [37]. This survival rate may increase to 71% in regions with severe organ shortage and reduce to 25% in regions where the shortage is not so acute. A study from Italy found that unless the 5-year survival was less than 30%, the non-HCC waiting list was not significantly affected by transplanting for more extensive tumors [38].
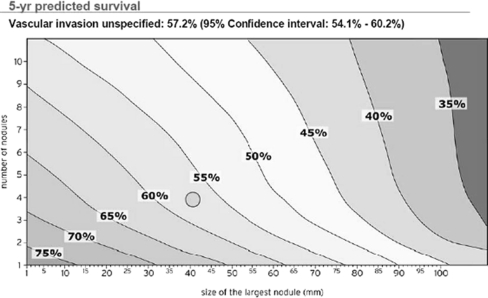
Although debatable, it is widely regarded that a 5-year OS of 50% is the minimum level at which the donor risk can be rationalized with recipient benefit [39]. It has been demonstrated time and again that performing LT for more extensive HCC beyond the Milan criteria comes at a price (reduced DFS and OS). This is illustrated in the Metroticket project, which combines LT data for HCC from 24 centers in Europe. The results are plotted as a Cartesian contour plot illustrating 5-year survival after LT according to size and number of nodules based on explant liver analysis. This has led to the development of a ‘HCC forecast chart’ (Fig. 3) and ‘Metroticket calculator’ to predict 5-year survival based on size and number of tumors on pretransplant morphological staging. Clearly, the core issue is whether we can afford the price (lower DFS and OS) by transplanting more extensive HCC [1]. It is broadly agreed that limited extension of conventional morphological criteria in patients receiving DDLT is acceptable only in regions where organ shortages are not critical. Whenever possible these tumors should be subjected to downstaging modalities on the waiting list. Performance of LDLT for extended criteria should be restricted to a center with long waiting periods for DDLT, which has demonstrated significant proficiency and donor safety record. Selection of these patients should be based on morphological parameters and tumor markers ideally supplemented and refined by biological and histological features with a view to obtain best possible outcome [36].
Fig. 3.
The HCC weather forecast Cartesian plot
Issues and Controversies
Liver transplantation for HCC is still in evolution. Several questions remain to be answered and controversies to be resolved:
How best to diagnose and stage HCC in chronic liver disease?
Is a biopsy necessary for diagnosis and listing of patients with HCC for LT?
Should LT be performed for HCC in the noncirrhotic liver?
What is the best staging for patients with HCC who are transplant candidates?
How to select patients with HCC for liver transplantation—morphology, pathology, or biology?
Is it possible to predict microvascular invasion?
How to manage patients with HCC on DDLT waiting list?
Does pretransplant downstaging impact outcome after liver transplantation?
Should criteria for transplantation in HCC differ for DDLT and LDLT?
Does the type of donor graft matter?
What is the best immunosuppression for patients with HCC after liver transplantation?
How should recurrences be monitored and managed after transplantation?
Diagnosis and Staging of HCC before LT
Despite technological advances in cross-sectional imaging techniques (CT, MRI and USG), concerns regarding the dependence on imaging techniques to determine appropriateness of HCC for LT abound. A retrospective analysis of data from UNOS revealed that cross-sectional pre-LT imaging overestimated tumor burden in more than 25% cases when compared with explant pathological examination [40].
For de novo lesions in a cirrhotic liver, enhancement in the late arterial phase (wash-in) and wash-out on portal venous and delayed phases on contrast-enhanced cross-sectional imaging has a high positive predictive value (PPV) for diagnosis of HCC but a relatively inferior negative predictive value (NPV). Two of three lesions >1 cm that do not demonstrate characteristic wash-out can still be HCC and need an additional imaging modality for reliable diagnosis. Hypovascular lesions cannot be reliably diagnosed with currently available imaging techniques without a confirmatory biopsy [41, 42]. When even 1 HCC is detected, dynamic contrast CT or MRI that provides complete anatomical coverage of the liver is recommended. The sensitivity of MRI (85%) is superior to CT (68%), but reverse is true as regards specificity (93% CT and 82% MRI) [43]. On balance, MRI is considered superior to other modalities for HCC <2 cm. The choice depends on patient preference, breath-hold time, and available expertise.
For extrahepatic staging before LT, CT, or MRI of the chest, abdomen, and pelvis is routinely recommended. Current data do not support the use of 18FDG-PET scanning due to its poor sensitivity for small well-differentiated HCC and background uptake by the cirrhotic liver. Emerging evidence seems to suggest that 18FDG-avid HCC may harbor poor prognostic features such as high grade and microvascular invasion that may herald poorer recurrence-free survival after LT. Some workers have even proposed the use of 18FDG-PET to refine morphologic criteria before LT [44, 45]. Dual-tracer PET (18FDG + 11C-acetate) may fare better due to superior avidity of well-differentiated HCC for acetate; however, its application is limited by high costs, excessive radiation exposure, and availability [46, 47].
Biopsy: Is It Necessary?
Traditionally, diagnosis of a malignant tumor needs histological confirmation before curative or palliative treatment can be planned. For HCC these paradigms have been challenged and arterial hypervascularity with wash-out on delayed phase in lesions >1 cm has been considered sufficient to make a diagnosis of HCC.
The problem with this approach to diagnose HCC without preoperative histological confirmation has major implications:
Diagnosis of HCC in a cirrhotic liver attracts priority points (such as in UNOS-MELD system) on DDLT waiting lists to allow timely LT.
Undeserved allocation of organs, based on a false-positive radiological diagnosis of HCC, diverts transplantable organs away from more deserving candidates without a diagnosis of HCC.
In 10–30% [48, 49] of patients transplanted for radiologically diagnosed HCC, the explanted liver may not reveal the presence of tumor when subjected to pathological examination. The false-positivity rate may be even higher for smaller tumor volume at LT.
The specificity and positive predictive value of a tumor biopsy is 100%, while sensitivity and accuracy is 86–93% (83% for lesions ≤1 cm and 66% when finer than 20 G needle is used) [50, 51]. In a multivariate analysis, location of lesion in upper posterior section and size ≤1 cm was associated with lower sensitivity and accuracy of tumor biopsy [52]. Moreover, in 2–11% the quality of biopsy was unsuitable for making a definite diagnosis [53]. One of the main concerns associated with the performance of a tumor biopsy has been the associated complication rate. Studies on tumor biopsy have usually included patients with normal coagulation and not commented on the number of patients unsuitable for the procedure. The risk of bleeding associated with biopsy varies between 0 and 7.9%, and the risk of tumor seeding of the tract was 0–5% (0.9% per year) [54]. The time for detection of seeding can be between 3 and 48 months after the biopsy. Limited data suggest that size of needle, number of needle passes, subcapsular location, and tumor size do not impact the rate of seeding; however, isolated studies have indicated that the use of a coaxial cutting needle [55] in combination with simultaneous tumor ablation [56] can significantly reduce seeding potential.
There is therefore a definite place for pretransplant biopsy in the diagnosis of patients with HCC awaiting LT as long as risks of bleeding and seeding are minimized:
Lesions >1 cm with elevated AFP with equivocal imaging features of HCC
Lesions >1 cm without AFP elevation with equivocal imaging features of HCC
Selecting Patients with HCC for LT: Morphology, Pathology, or Biology?
One of the tempestuous and unresolved issues in LT for HCC is how to select patients for LT. Because the paucity of donor organs does not allow LT to be performed for all patients with HCC, selection of patients who would derive the greatest benefit from LT has become mandatory. Pretransplant assessment of tumor volume based on best available imaging can be inaccurate in more than 25% of patients, and 10–15% of patients with small tumors detected on imaging may not reveal any tumor on pathological assessment of their explanted liver after LT [40].
Most early series selected patients based on their appearance (size of tumor, number of tumors, and tumor volume). The landmark Milan criteria and the subsequent extension as UCSF criteria commonly followed at most centers are purely based on morphological characteristics. Excellent DFS and OS reported by these centers have been validated and duplicated elsewhere. Among morphologic criteria, whether size, number, or total volume is more relevant for selection remains unresolved. A recently reported meta-analysis [57] studied recurrence and survival data in patients who underwent LT for HCC with reference to the size and number of tumor data available before LT. Patients with total tumor diameter (Ttotal) ≥10 cm have a probability of OS 1/5 compared to those with Ttotal <10 cm, and those with Ttotal ≥9 cm have 1/2 probability of DFS as compared to those with Ttotal <9 cm. Considering the diameter of the largest nodule (Tmax), it become apparent that Tmax influences DFS more than OS. Patients with Tmax ≥5 cm had DFS 1/4 compared to those with Tmax <5 cm. The number of tumors did not appear to impact either DFS or OS in the meta-analysis.
Despite the importance being given to appearance of HCC before LT in selection, it is the biological behavior that ultimately will determine the post-transplant outcomes. There is increasing recognition that not all patients with similar tumor morphology before LT would have uniform outcomes. Efforts to refine these morphology-based selection systems have included microvascular invasion, grade, encapsulation, mRNA AFP, and AFP level, but have failed to improve on morphology-based criteria [36]. Also, acquisition of this information mandates a biopsy that is associated with risk of tumor seeding. Japanese, Korean, and Chinese groups have included serum levels of AFP or PIVKA II along with their center-specific extended criteria for selection of patients for LDLT with acceptable long-term DFS and OS.
Pathological features have received significant attention as potential markers of poor prognosis after LT in patients with HCC. Prominent among these are the presence of mVI, satellites, aneuploidy, and grade. Various studies have reported on the impact of tumor grade on pretransplant biopsy [58]. In a heterogeneous cohort including patients with tumors within and outside Milan criteria, those with well and moderately differentiated HCC had a 5-year actuarial DFS of 92% and OS of 75%, which was significantly better than those with poor differentiation. In another study, 5-year survival for HCC after LT was significantly better for well and moderately differentiated tumors than poorly differentiated ones of the same size. mVI or emboli [59] has been consistently associated with poor DFS after OLT. Surrogate markers of mVI like tumor size and 18FDG avidity have been used to refine selection criteria for LT with mixed success. Combined with size >2 cm, poor differentiation was associated with significantly more mVI in patients with HCC undergoing LT than those with smaller and well-differentiated lesions [60].
Biomarkers correlating with patient’s prognosis or response to therapy are critical in modern cancer treatment. Recent studies [61] have correlated several biomarkers such as gene expression, micro-RNAs, and methylation with outcome in patients with HCC. At the University of Pittsburgh, an index of cumulative mutational damage called fractional allelic imbalance (FAI) was evolved by studying nine tumor-associated gene loci. Vascular invasion and FAI >20% were most important predictors of DFS, and FAI >40% had a hazard ratio of 19 for recurrence [25]. Five biomarkers have shown consistent correlation with prognosis: epithelial cell adhesion molecule (EpCAM), G3 proliferation subclass, miR-26 miRNA precursor expression status, and two prognostic gene signatures in non-tumorous hepatic tissue [62–66].
Vascular endothelial growth factor (VEGF) and Ang2 have also been independently associated with prognosis in large cohorts of patients with HCC [67]. Most of these markers have not yet been externally validated to allow selection of patients beyond conventional morphologic criteria who have better tumor biology to benefit from LT.
It is clear that much more work needs to be done before an ideal selection system can be evolved. Conceptually, this system will very likely comprise some combination of morphological and biological features including molecular and genetic markers.
Is Microvascular Invasion Predictable?
It is well accepted that the presence of microvascular invasion is a major factor that influences DFS after resection or LT for HCC. However, the presence of mVI is reliably diagnosed only on histopathological assessment that is not available at the time of clinical decision making. Some studies have reported a correlation between size of lesion [60], number of lesions [68], and histological grade [69] with mVI. Japanese groups have found a consistent correlation with morphologic growth pattern and presence of mVI in resected specimen [69, 70]. Correlation between pretransplant imaging and presence of mVI has not been consistent. However, a recent study demonstrated a strong association between FDG avidity on PET scan and poor histological grade [45], which was claimed by the authors to be a marker for mVI.
Biochemical markers as predictors of mVI have been studied widely. A preoperative DCP level of >300–400 was associated with a significantly higher detection of mVI in surgical specimens [71, 72].
While imaging and biochemical features associated mVI have been extensively studied, these data are retrospective and usually derived from small groups of patients. The search for a reliable method of predicting mVI before LT still continues.
Should LT be Performed for HCC in the Noncirrhotic Liver?
The occurrence of HCC on noncirrhotic liver is uncommon (10–15%). HCC in noncirrhotic liver (NCHCC) and its fibrolamellar variant (FLHCC) is seen more in younger adults as compared to HCC in cirrhotic liver [73–75].
On long-term follow-up, surgery offers the only chance of cure. Liver resection can be considered only for patients with well-compensated cirrhosis or normal liver tissue. Due to the subtlety of clinical features, most NCHCCs are larger in size than those in cirrhotic livers. Advances in radiology, future liver remnant volume manipulation, and surgical techniques have made it possible to extend the limits of safe liver resection even up to 80% in NCHCC with 0–6% operative mortality and a 1-year and 5-year DFS and OS of 62–97% and 25–81% and 49–84% and 24–59%, respectively [76–79]. Complete resection (R0) has the greatest impact on DFS and OS after surgery. Other factors influencing outcome are AFP level, poor pathological features, and blood transfusion.
Data on LT for NCHCC are scarce and initial results were unflattering. Increasing experience has led to the conclusion that LT for NCHCC can yield 5-year DFS and OS of 49% [80, 81]. Criteria used to select cirrhotic patients for LT cannot be directly applied to NCHCC because most of these lesions are large at time of detection. The European Liver Transplant Registry data suggest that macrovascular invasion and node positivity are important determinants of poor survival after LT [81].
Clearly, NCHCC is an underutilized indication for LT. The ideal NCHCC for primary LT is one with two nodules, absence of nodal or macrovascular invasion, and where R0 resection is unlikely. Salvage LT may be offered to a select group with good biological features that recur in the liver more than 12 months after index resection.
Staging of HCC before LT: Which is the Best System?
The purpose of cancer staging is to determine patient prognosis and to determine appropriate interventions. Due to its predominant occurrence in diseased livers, it is logical that any staging system for HCC must take into account the extent of tumor as well as underlying liver function.
The TNM classification is irrelevant to patients with HCC who are LT candidates because LT is clearly ruled out for N + and M + lesions leaving T as the only relevant factor. The Liver Cancer Study Group Japan staging and Japan integrated staging have not been externally validated, but take into account underlying liver damage, Child–Pough score, and extent of tumor involvement [82].
The Cancer of Liver Italian Project has been prospectively validated and includes tumor morphology, Child–Pough score, presence of portal vein thrombosis, and AFP levels [83]. It has been found to be useful in predicting prognosis of patients with HCC. From the liver physician’s perspective, the Barcelona Clinic Liver Cancer staging system [84] is considered ideal because it comprises tumor morphology, vascular invasion, and presence of portal hypertension, Child–Pough score, as well as performance status of the patient. It is important to understand that none of these systems have been validated in the context of patients with HCC who are candidates for LT.
Downstaging of HCC before LT—Does It Work?
One of the areas of great debate in the management has been the use of neo-adjuvant therapies with a view to make curative interventions (resection or transplantation) possible. It is well known that the risk of HCC progression directly correlates with the time on the waiting list for LT. The fundamental principle of downstaging therapy is to allow selection of a subset of tumors with favorable biology that are likely to respond to treatment and have better outcome after LT. Transarterial therapies (chemo-embolization and Y90 radio- embolization) and percutaneous ablative therapies (radiofrequency, alcohol, acetic acid, and high-intensity focused ultrasound) have been employed for patients with two putative benefits:
To prevent progression beyond transplant criteria
To reduce tumor burden to make LT possible within accepted criteria
No level 1 study, to date, has demonstrated that these putative benefits are achievable with neo-adjuvant therapy. However, percutaneous radiofrequency ablation (RFA) has been shown to provide equal survival to resection [85, 86] in compensated cirrhotic patients with a small HCC with better safety and trans-arterial chemo-embolization [87, 88] enhances survival in HCC patients according to level 1 studies. There are conflicting data on the impact of downstaging therapy on DFS and OS after LT for HCC. An early study, describing the use of transarterial chemo-embolization (TACE) before LT, failed to demonstrate improvement in DFS after LT despite a demonstrable reduction in tumor volume being achieved in nearly 50%. However, patients with HCC >3 cm who responded to TACE experienced better 5-year DFS of 71% as compared to those that did not undergo TACE (49%) or those who did not demonstrate response to TACE (22%) [89]. In another study from the pre-MELD era [90], 46% patients with HCC >5 cm dropped out of the waiting list despite TACE, but those with HCC 5–7 cm who had LT after TACE experienced better survival than those with lesions >7 cm or those who did not receive TACE.
In a prospective study from UCSF with intention to treat (ITT) analysis [91], entry and response criteria were strictly defined and a 3-month period of observation was obligatory before DDLT or LDLT was performed. Limited resection, RFA (percutaneous or laparoscopic), and TACE were the downstaging therapies applied, alone or in combination, but resection was abandoned during the study. Using ITT analysis, 70% could be downstaged to criteria acceptable for LT and 57% had undergone LT at time of publication. There was waiting list death of 4.9% and two-thirds of these deaths could be directly related to downstaging procedure. Of the 35 patients who underwent LT, 13 had complete tumor necrosis on explant analysis and the 1-year and 4-year post-transplant survival was 96, and 92%, respectively [92]. None had recurrence with a median follow-up of 25 months.
Transarterial chemo-infusion (TACI), a variant of TACE, has been used with excellent results at Stanford University. The use of TACI prevented progression in 87% cases on the LT waiting list and downstaged nearly 50% larger tumors to within Milan criteria [93].
Resection of Transplantable HCC as a Bridge to Transplantation—Is It a Reasonable Strategy?
Local resection as a downstaging or bridging modality for transplantable HCC before transplantation is controversial. With the advent of minimal access surgery and refinement in management of liver disease, the morbidity and mortality associated with resection is declining. The advantages of doing a resection prior to LT are manifold:
The diagnosis is confirmed.
Biological markers, grade, and microvascular invasion can be assessed.
Tumor volume can be reduced with histological margin confirmation before LT.
However, whether resection should be used as a downstaging therapy or bridge to LT is still debatable. Data from France [94] have shown that it may not be a reasonable strategy to resect transplantable HCC because the outcome of a salvage LT in terms of DFS, OS, and operative mortality is inferior to a primary LT. However, a study from Hong Kong [95] revealed that after resection less than half patients developed recurrence in the liver after a follow-up of 48 months and nearly 80% of those could be transplanted and progression of liver disease without recurrence occurred in less than 5%. A recent French study [96] on liver resection for transplantable HCC used both laparoscopic and open resection techniques to report intrahepatic recurrence of 54% at a mean follow-up of 4.8 years. Of these recurrences, 77% were transplantable though only 44% underwent LT. With intention to treat analysis and close imaging monitoring after resection, 61% patients can be salvaged with LT if they recur. Good 5-year survival of 70% could be achieved after salvage LT after resection. Robotic liver resections may add to the accuracy and safety of resection of small tumors in cirrhotic patients with HCC and be used as bridging therapies in future [97]. From a cost-effectiveness standpoint, primary LT was most cost-effective as compared to salvage LT after resection or loco-regional therapy [98].
How Should Patients with HCC on LT Waiting List be Managed?
It is estimated that the total dropout rate is about 22%, comprising a monthly dropout rate of 4% with a tumor-related dropout rate of 3% per month [6]. Neo-adjuvant strategies are implemented prior to treatment with a view to improve outcome. Bridging strategies aim to allow patients to wait longer on the waiting list as well as help in selecting those with better tumor biology who would fare better after LT.
Without the use of neo-adjuvant loco-regional treatment (LRT), the annual dropout rate for T2 HCC on the LT waiting list is estimated to be 0–10%. Progression-related dropout rate depends on initial tumor size, multifocality, AFP levels, and response to LRT in treated patients. In a study of patients with HCC from Taiwan [99], T1 lesions progressed at 2.1 and 5.3% and T2 lesions progressed at 3 and 6.8% at 3 and 6 months, respectively, when only a strategy of observation was observed for patients awaiting LT.
In the UCSF study [100], pretransplant LRT reduced dropout rates (HR 0.4). Two decision analyses support pretransplant LRT depending on the waiting time and type of treatment [101, 102]. In the Barcelona clinic study, the dropout rates were 10, 20, and 50% at 6, 12, and 24 months, respectively, for untreated patients. In the Paul-Brousse study of T2 lesions awaiting LT, TACE significantly reduced dropout rates only when waiting times for transplantation were between 4 and 9 months. The ITT analysis also reveals a survival advantage for treated patients over untreated patients. Transarterial radio-embolization (TARE) with Y90 microspheres is a recent addition to the list of LRT for HCC, but data for its use are scarce. A retrospective study [103] demonstrated increased time to progression and greater AFP reduction after TARE as compared to TACE but failed to detect a survival advantage. Until more data are available, TARE continues to be investigational as a downstaging tool.
It is clear from the above that for T2 lesions within conventional criteria, observation alone is reasonable if waiting times are between 3 and 9 months. However, for T2 lesions approaching 5 cm where waiting time exceeds 6 months, LRT should be used to reduce dropout rates [104].
Should Criteria for LT in HCC Patients Differ between DDLT and LDLT?
When recipients with HCC are considered, DDLT is justifiable only when there is significant benefit to the recipient because a deceased donor organ is a social resource. The use of a deceased donor organ in extended criteria for HCC recipients potentially diverts a precious resource from a needy patient without HCC who is more likely to have a better outcome. Criteria for allocation of deceased donor organs have therefore been restrictive despite moderate expansion being associated with equivalent outcome in selected series.
The principle of double equipoise is the only justification for LDLT to be performed in any clinical situation. To put it simply, LDLT is ethically justifiable only if the benefit to the recipient is high and the risk to the donor is low. It has been argued that since a living donor organ is a private gift, further expansion of criteria for recipients with HCC receiving living donor organs is justifiable.
Many experienced centers performing LDLT have made incremental changes in inclusion morphologic criteria for consideration of LDLT and reported equivalent post-LT DFS and OS as conventional criteria without a corresponding increase in donor risk. While this suggests that modest expansion in size and number criteria may be justifiable, it opens the question whether further expansion of criteria for patients in countries with little or no access to DDLT is acceptable.
Currently, there is no consensus on what is the minimum acceptable recurrence rate and recipient survival for expansion of morphological criteria or for acceptable donor risk that would make LDLT ethically justifiable. Moreover, what constitutes an acceptable risk to the donor and benefit to the recipient varies with the access to DDLT, acceptance of LDLT, and potential donor risks within the community. Most consider a minimum 3-year DFS of >50% and 5-year OS of 50% [39] and maximum donor mortality risk of 0.1% for the left lobe, and 0.3% [105] mortality as reasonable for performance of LDLT in patients with HCC. Decision to perform LDLT for larger and multicentric HCC also needs to factor in social and utility issues as long as risk to the donor is not elevated. It is clear that in less experienced centers where donor safety falls short of acceptable standards, LDLT for larger or multicentric HCC is clearly unjustifiable [106].
In summary, in experienced centers where access to DDLT is limited, modestly expanded morphologic criteria beyond UCSF may be acceptable indications for LDLT, provided risk to donor is not elevated.
Does the Type of Donor Graft Matter?
Apart from the threat of small-for-size dysfunction and slightly elevated risk of biliary complications, there is broad consensus that partial grafts from living donors are acceptable alternatives to whole organs from deceased donors [107]. While waiting may be an option for other patients, those with HCC frequently drop out due to tumor progression during the long wait for an organ. In countries where the organ shortage is even more marked, LDLT is often the only realistic option for a timely LT.
Cytokines and growth factors that play an integral role in hepatocyte regeneration have been shown to increase tumor cell proliferation in vitro. It is hypothesized that early regenerative processes (hypertrophy and hyperplasia) when a partial graft is implanted, will enhance growth and proliferation of any occult tumor cells [108, 109]. Moreover, the impact of immunosuppression on enhancing tumor growth has also been reported.
The issue whether DDLT or LDLT is better for HCC is unsettled (Table 3). A study from Hong Kong revealed a higher rate of recurrence with LDLT (29%) as compared to DDLT (0%) for patients with HCC. However, in the LDLT group there was a selection bias for LDLT including salvage LT after resection, larger tumors, and no pretransplant TACE [110]. When ITT analysis was performed, survival advantage was apparent in the LDLT group. Analysis of data from the adult-to-adult living donor liver transplant cohort of nine centers revealed that the use of LDLT significantly reduced waiting times but those that underwent LDLT had higher recurrence rates (29%) than DDLT (0%) and tended to have lower OS although not significantly (HR 0.82) [111]. However, on closer perusal there were larger tumor volumes, higher mean AFP (at enrolment and at transplant) in LDLT groups, and many patients received living donor grafts because they were not eligible to receive deceased donor grafts. Data from a Korean multicenter study [23] revealed superior survival for patients who underwent LDLT than those who underwent DDLT although the DDLT group had significantly fewer patients. A recently published ITT analysis [112] revealed no significant difference in outcomes between DDLT and LDLT. In the LDLT group, time to recurrence was significantly longer than in the DDLT group. However, there was a trend toward poor outcome in LDLT for tumors exceeding Milan or UCSF criteria. Another study divided patients undergoing LDLT into three groups according to graft-to-recipient weight ratio (GRWR) and found similar DFS across the groups [113], thereby dispelling the hypothesis that rapid regeneration could increase the rate of HCC recurrence.
Table 3.
Comparison of data of LDLT or DDLT for HCC
| Author [reference] | Conclusion |
|---|---|
| Hwang et al. [23] | Better survival and lower recurrence rate in LDLT |
| Lo et al. [110] | Higher recurrence with LDLT due to selection bias |
| Fisher et al. [111] | Higher 3-year recurrence with LDLT |
| Bhangui et al. [112] | Comparable survival |
| Longer time to recurrence in LDLT | |
| Lower time to LT in LDLT | |
| Trend to worse outcomes with LDLT for extended criteria |
It is thus evident that the use of living donor graft in patients with HCC who need LT at experienced centers confers an advantage by reducing time to transplantation with similar or even superior outcomes (in some studies) to DDLT as long as similar criteria are used for patient selection.
Immunosuppression Strategies in Patients with HCC: Which is Best?
Experimental data have demonstrated that apart from the mammalian target of rapamycin (mTOR) inhibitors (sirolimus and everolimus), immunosuppressant medications promote the growth of malignant cells including those of HCC. Cyclosporin A (CsA) promotes invasiveness and affects DNA repair in malignant cells, while tacrolimus promotes proliferation in animal models [114–116]. With exposure to these calcineurin inhibitors, tumor-doubling time is significantly reduced in human studies [117].
The rationale for use of mTOR inhibitors for immunosuppression in patients with HCC undergoing LT is that mTOR is expressed in over 60% of HCCs [118, 119]. Sirolimus inhibits hepatoma cell proliferation and downregulates vascular endothelial growth factor (vEGF) expression [120, 121]. Sirolimus-treated rats develop fewer recurrences of extrahepatic metastasis and experience enhanced survival as compared to controls [122]. A few case reports [123, 124] and single-center studies [125–127] have demonstrated improved recurrence-free survival with the use of sirolimus from the time of transplantation. However, in the absence of level 1 studies, these results cannot be considered devoid of bias in selection or reporting. In a recent study, multivariate analysis [128] revealed improved survival with sirolimus-based maintenance immunosuppression in patients with HCC (hazard ratio 0.53, 95% CI).
The view is that mTOR inhibitors (in particular sirolimus) are not only effective in prevention of allograft rejection but may also have antineoplastic effect in patients transplanted for HCC, but its use has been associated with increased hepatic arterial (black box warning) and wound complications. However, the risk of increased arterial and would complications has not been upheld by recent data both from single and multicenter studies [129].
The Silver trial—an open-label, multicenter-randomized control trial designed to compare sirolimus containing immunosuppression with mTOR inhibitor-free regimen in patients undergoing liver transplantation for HCC—is in the recruitment phase [130]. The outcome of this trial is expected to clarify the controversy.
How Should Recurrences be Monitored and Managed after Transplantation?
Despite various attempts at refinement of selection criteria with a view to increase DFS, 30–50% patients with HCC recur within 2 years. Only 20% of recurrences occur 3 years after LT [131, 132]. However, it is believed that a few recurrences would be amenable to cure. This has been questioned where a policy of active surveillance (imaging and AFP) for recurrence is allowed treatment with curative intent for nearly a third of recurrent HCC after LT [133].
Isolated hepatic recurrences occur in 15–20% and are most likely to be amenable to cure. Options for isolated recurrences can be resection, transplantation, or LRT. In a study from Japan, resection led to better outcomes than RFA for recurrent HCC after LT [134] and can be performed safely [135]. The presence of vascular invasion in the original explant reduced chances of complete resection in the event of recurrence [136]. The use of TACE for post-LT recurrences is well-tolerated but has not shown to provide superior outcomes to RFA.
Retransplantation for HCC recurrence is controversial and has been restricted to a few reports [137]. A very select group who develops de novo HCC as a part of progression or recurrent disease may merit consideration as primary HCC.
The imaging modalities for hepatic and extrahepatic staging used in the pretransplant setting are applicable for monitoring recurrence after LT. Patients with tumors who have increased risk of recurrence such as size beyond Milan criteria, microvascular invasion, microsatellitosis, and AFP >400 mg/dL should have cross-sectional imaging studies at least every 6-monthly for 3–5 years post-transplant. Increased frequency of imaging or use in low-risk HCC is not cost-effective.
The serum level of AFP has been extensively studied as a marker of recurrence after LT. At a cut-off level of 20 ng/mL, the sensitivity and the specificity of AFP were 68 and 100%, while at a cut-off level of 10 ng/mL, they were 78 and 98%, respectively [138]. There are insufficient data to recommend the use of other biomarkers. The AFP levels should be monitored 3-monthly for 2 years and 6-monthly thereafter. A level of ≥20 merits liver-specific or systemic imaging for treatable recurrences.
Optimizing Outcomes of LT for HCC
Performing LT for HCC is worthwhile only if significant survival advantage can be achieved over other palliative therapies. In DDLT this is important so that scarce organs are not diverted away from needier non-HCC patients, and in LDLT this is important so that the risk albeit small to the living donor is ethically justifiable. To this end a multidisciplinary and multilevel strategy needs to be in place. The key components include patient selection, reduction of peri-operative morbidity, mortality, and recurrence, early detection, and treatment of recurrence. Centers performing LT for HCC need to have an experienced multidisciplinary team consisting of surgeons, hepatologists, and oncologists in place to evaluate and guide every step of the process. A recent report from Switzerland outlined five strategies to optimize outcome of LT for HCC [139]:
Select patients with low baseline circulating tumor cells
Decrease peritransplant release of cells
Prevent engraftment of circulating tumor cells
Use anticancer drugs after LT
Tune immunity to clear tumor cells after LT
Employing these strategies, survival equivalent to nontumor indications after LT is achievable for patients with HCC.
What is New in LT for HCC?
With increasing experience with LT for HCC, 5–10% of LT across the world are for patients with HCC. Paradigms for selection of patients are being constantly refined with regard to morphological criteria and markers of invasiveness and biological aggressiveness with a view to reduce recurrence in the post-transplant period.
Efforts to use drugs to prevent proliferation of occult residual tumor cells after transplant have attracted attention. Sorafenib, the multiple kinase inhibitor, has been shown to enhance survival in advanced HCC [140]. Its use following curative-intent treatments for HCC is being investigated in the phase III STORM study. It is expected that adjuvant use of sorafenib following LT for HCC with high-risk of recurrence may be associated with better DFS, but needs to be investigated in a clinical trial. Safety of use in early post-LT period remains to be proved.
Another related exciting area is the tailoring of the tumor immune response with a view to eliminate occult tumor cells. Adaptive transfer of natural killer (NK cells) cells has been demonstrated to reduce the growth of tumor cells. Infusion of NK cells in post-transplant scenario needs further evaluation [141].
How We Do It?
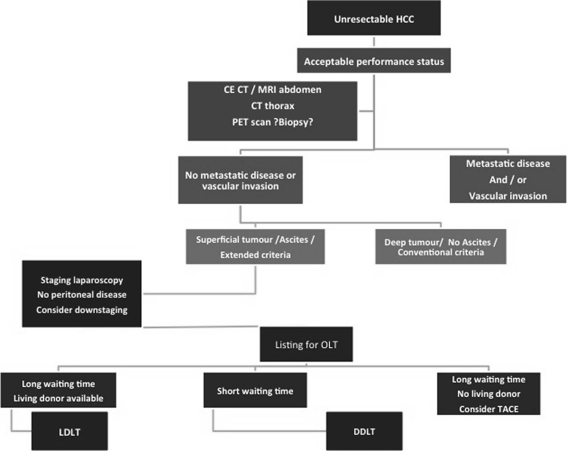
At the Medanta Institute of Liver Transplantation and Regenerative Medicine, Gurgaon, 10% of LT are performed for patients with HCC. Majority of LT at the center involve LDLT due to a chronic deceased donor organ shortage and unrealistic waiting periods for deceased donor grafts. Patients with HCC are diagnosed based on imaging studies, and tumor biopsy is avoided for diagnosis in potentially transplantable patients. Our preference for diagnosing and liver staging is multidetector CT angiography using upward of a 64-slice scanner with 1 mm sections acquired in unenhanced, arterial, portal venous, and delayed phases. For extrahepatic staging besides the abdominal CT, the 18FDG-PET scan of the whole body is performed (dual-tracer or C11-acetate PET is not available currently). Ascitic fluid, if any, is sampled for the presence of malignant cells. If the primary lesion in not FDG-avid, a high-resolution CT scan of the thorax and a Tc99 skeletal scan are performed. Once extrahepatic involvement and/or macrovascular invasion are ruled out, medically fit individuals are considered LT candidates. Serum AFP is recorded before LT. For patients younger than 65 years, LT is considered irrespective of tumor size and number as long as macrovascular invasion and metastasis are ruled out. For older patients, the UCSF criteria are followed for enrollment. Pretransplant TACE is performed only for patients with mild-to-moderate liver dysfunction with maximum tumor diameter >8 cm in whom LT is likely to be delayed by >3 months due to unavailability of a suitable living donor. In donor selection, those with projected lower GRWR (<0.7) are avoided. During the conduct of LT, recipient is subjected to staging laparoscopy or laparotomy before donor surgery commences to confirm absence of peritoneal involvement by the tumor. During recipient hepatectomy, the lymphatic tissue from the porta hepatis is excised and the diseased liver is explanted with minimal handling to avoid shedding of cells into circulation. The portal and hepatic veins are divided with endovascular cutting stapler to avoid spillage during their division. Meticulous care is taken to reduce blood loss and avoid breach of tumor capsule in surface tumors. After transplant, sirolimus-based immunosuppression is introduced 3 months after surgery. Monitoring for recurrence involves AFP estimation as well as abdominal ultrasonography 3-monthly for 2 years and 6-monthly thereafter. Contrast-enhanced CT scan is performed at 6-month intervals for 2 years and then annually. For high-risk tumors (size outside UCSF, enrollment AFP >1,000 ng/mL, or microvascular invasion), CT scan is performed 3-monthly for 1 year and 6-monthly thereafter for 5 years. 18FDG-PET is performed annually for high-risk HCC that are FDG-avid at enrollment (Fig. 4).
Fig. 4.
Unresectable HCC: how we do it
References
- 1.Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127(Suppl 1):S5–S16. doi: 10.1053/j.gastro.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, et al. Global patterns of cancer incidence and mortality rates and trends. Canc Epidemiol Biomarkers Prev. 2010;19:1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 4.Ishii M, Gama H, Chida N, Ueno Y, Shinzawa H, Takagi T, Toyota T, Takahashi T, Kasukawa R. Simultaneous measurements of serum alpha-fetoprotein and protein induced by vitamin K absence for detecting hepatocellular carcinoma. South Tohoku District Study Group. Am J Gastroenterol. 2000;95(4):1036–1040. doi: 10.1111/j.1572-0241.2000.01978.x. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 6.Llovet JM, Fuster J, Bruix J. Intention-to-treat analysis of surgical treatment for early hepatocellular carcinoma: resection versus transplantation. Hepatology. 1999;30:1434–1440. doi: 10.1002/hep.510300629. [DOI] [PubMed] [Google Scholar]
- 7.Okada S, Shimada K, Yamamoto J, Takayama T, KosugeT YS, et al. Predictive factors for postoperative recurrence of hepatocellular carcinoma. Gastroenterology. 1994;106:1618–1624. doi: 10.1016/0016-5085(94)90419-7. [DOI] [PubMed] [Google Scholar]
- 8.Minagawa M, Makuuchi M, Takayama T, Kokudo N. Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg. 2003;238:703–710. doi: 10.1097/01.sla.0000094549.11754.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Intrahepaticrecurrence after curative resection of hepatocellular carcinoma: long-term results of treatment and prognostic factors. Ann Surg. 1999;229:216–222. doi: 10.1097/00000658-199902000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adachi E, Maeda T, Matsumata T, Shirabe K, Kinukawa N, Sugimachi K, Tsuneyoshi M. Risk factors for intrahepaticrecurrence in human small hepatocellular carcinoma. Gastroenterology. 1995;108:768–775. doi: 10.1016/0016-5085(95)90450-6. [DOI] [PubMed] [Google Scholar]
- 11.Wong R, Frenette C, Gish R. Hepatocellular carcinoma: locoregional and targeted therapies. Gastroenterol Clin North Am. 2011;40(3):599–610. doi: 10.1016/j.gtc.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Starzl TE, Porter KA, Brettschneider L, Penn I, Bell P, Putnam CW, McGuire RL. Clinical and pathologic observations after orthotopic transplantation of the human liver. Surg Gynecol Obstet. 1969;128(2):327–339. [PMC free article] [PubMed] [Google Scholar]
- 13.Penn I. Hepatic transplantation for primary and metastasis cancer of the liver. Surgery. 1991;110:726–735. [PubMed] [Google Scholar]
- 14.Bismuth H, Chiche L, Adam R, Castaing D, Diamond T, Dennison A. Liver resection versus transplantation for hepatocellular carcinoma in cirrhotic patients. Ann Surg. 1993;218(2):145–151. doi: 10.1097/00000658-199308000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mazzaferro V, Regalia E, Doci R, Andreola S, PulvirentiA BF, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 16.Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33(6):1394–1403. doi: 10.1053/jhep.2001.24563. [DOI] [PubMed] [Google Scholar]
- 17.Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL, Adam R, Neuhaus P, Salizzoni M, Bruix J, Forner A, Carlis L, Cillo U, Burroughs AK, Troisi R, Rossi M, Gerunda GE, Lerut J, Belghiti J, Boin I, Gugenheim J, Rochling F, Hoek B, Majno P, Metroticket Investigator Study Group Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective exploratory analysis. Lancet Oncol. 2009;10(1):35–43. doi: 10.1016/S1470-2045(08)70284-5. [DOI] [PubMed] [Google Scholar]
- 18.Freeman RB, Wiesner RH, Edwards E, Harper A, Merion R, Wolfe R. Results of the first year of the new liver allocation plan. Liver Transpl. 2004;10:7–15. doi: 10.1002/lt.20024. [DOI] [PubMed] [Google Scholar]
- 19.Sharma P, Balan V, Hernandez JL, Harper AM, Edwards EB, Rodriguez-Luna H, Byrne T, et al. Liver transplantation for hepatocellular carcinoma: the MELD impact. Liver Transpl. 2004;10:36–41. doi: 10.1002/lt.20012. [DOI] [PubMed] [Google Scholar]
- 20.Miller CM. Ethical dimensions of living donation: experience with living liver donation. Transplant Rev (Orlando) 2008;22:206–209. doi: 10.1016/j.trre.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Singer PA, Lantos JD, Whitington PF, Broelsch CE, Siegler M. Equipoise and the ethics of segmental liver transplantation. Clin Res. 1988;36(6):539–545. [PubMed] [Google Scholar]
- 22.Todo S, Furukawa H, Japanese Study Group on Organ Transplantation Living donor liver transplantation for adult patients with hepatocellular carcinoma: experience in Japan. Ann Surg. 2004;240(3):451–459. doi: 10.1097/01.sla.0000137129.98894.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hwang S, Lee SG, Joh JW, Suh KS, Kim DG. Liver transplantation for adult patients with hepatocellular carcinoma in Korea: comparison between cadaveric donor and living donor liver transplantations. Liver Transpl. 2005;11(10):1265–1272. doi: 10.1002/lt.20549. [DOI] [PubMed] [Google Scholar]
- 24.Pomfret EA, Lodge JP, Villamil FG, Siegler ML. Should we use living donor grafts for patients with hepatocellular carcinoma? ethical considerations. Liver Transpl. 2011;17(Suppl 2):S128–S132. doi: 10.1002/lt.22356. [DOI] [PubMed] [Google Scholar]
- 25.Dvorchik I, Schwartz M, Fiel MI, Finkelstein SD, Marsh JW. Fractional allelic imbalance could allow for the development of an equitable transplant selection policy for patients with hepatocellular carcinoma. Liver Transpl. 2008;14(4):443–450. doi: 10.1002/lt.21393. [DOI] [PubMed] [Google Scholar]
- 26.Tamura S, Kato T, Berho M, Misiakos EP, O'Brien C, Reddy KR, Nery JR, Burke GW, Schiff ER, Miller J, Tzakis AG. Impact of histological grade of hepatocellular carcinoma on the outcome of liver transplantation. Arch Surg. 2001;136(1):25–30. [PubMed] [Google Scholar]
- 27.Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33(5):1080–1086. doi: 10.1053/jhep.2001.23561. [DOI] [PubMed] [Google Scholar]
- 28.Roayaie S, Frischer JS, Emre SH, Fishbein TM, Sheiner PA, Sung M, et al. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533–539. doi: 10.1097/00000658-200204000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsh JW, Dvorchik I. Liver organ allocation for hepatocellular carcinoma: are we sure? Liver Transpl. 2003;9:693–696. doi: 10.1053/jlts.2003.50086. [DOI] [PubMed] [Google Scholar]
- 30.Mazzaferro V, Bhoori S, Sposito C, Bongini M, Langer M, Miceli R, Mariani L. Milan criteria in liver transplantation for hepatocellular carcinoma: an evidence-based analysis of 15 years of experience. Liver Transpl. 2011;17(Suppl 2):S44–S57. doi: 10.1002/lt.22365. [DOI] [PubMed] [Google Scholar]
- 31.Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSF-expanded criteria based on preoperative imaging. Am J Transplant. 2007;7(11):2587–2596. doi: 10.1111/j.1600-6143.2007.01965.x. [DOI] [PubMed] [Google Scholar]
- 32.Decaens T, Roudot-Thoraval F, Hadni-Bresson S, MeyerC GJ, Durand F, et al. Impact of UCSF criteria according to pre- and post-OLT tumor features: analysis of 479 patients listed for HCC with a short waiting time. Liver Transpl. 2006;12:1761–1769. doi: 10.1002/lt.20884. [DOI] [PubMed] [Google Scholar]
- 33.D'Amico F, Schwartz M, Vitale A, Tabrizian P, Roayaie S, Thung S, Guido M, Rio MJ, Schiano T, Cillo U. Predicting recurrence after liver transplantation in patients with hepatocellular carcinoma exceeding the up-to-seven criteria. Liver Transpl. 2009;15(10):1278–1287. doi: 10.1002/lt.21842. [DOI] [PubMed] [Google Scholar]
- 34.Lee SG, Hwang S, Moon DB, Ahn CS, Kim KH, Sung KB, Ko GY, Park KM, Ha TY, Song GW. Expanded indication criteria of living donor liver transplantation for hepatocellular carcinoma at one large-volume center. Liver Transpl. 2008;14(7):935–945. doi: 10.1002/lt.21445. [DOI] [PubMed] [Google Scholar]
- 35.Zheng SS, Xu X, Wu J, Chen J, Wang WL, Zhang M, Liang TB, Wu LM. Liver transplantation for hepatocellular carcinoma: Hangzhou experiences. Transplantation. 2008;85(12):1726–1732. doi: 10.1097/TP.0b013e31816b67e4. [DOI] [PubMed] [Google Scholar]
- 36.Prasad KR, Young RS, Burra P, Zheng SS, Mazzaferro V, Bog Moon D, Freeman RB. Summary of candidate selection and expanded criteria for liver transplantation for hepatocellular carcinoma: a review and consensus statement. Liver Transpl. 2011;17(Supp l 2):S81–S89. doi: 10.1002/lt.22380. [DOI] [PubMed] [Google Scholar]
- 37.Volk ML, Vijan S, Marrero JA. A novel model measuring the harm of transplanting hepatocellular carcinoma exceeding Milan criteria. Am J Transplant. 2008;8:839–846. doi: 10.1111/j.1600-6143.2007.02138.x. [DOI] [PubMed] [Google Scholar]
- 38.Vitale A, Volk ML, Gambato M, Zanus G, D’Amico F, Carraro A, et al. Estimation of the harm to the waiting list as a crucial factor in the selection of patients with hepatocellular carcinoma for liver transplantation. Transplant Proc. 2010;42:1194–1196. doi: 10.1016/j.transproceed.2010.03.089. [DOI] [PubMed] [Google Scholar]
- 39.Broelsch CE, Frilling A, Malago M. Should we expand the criteria for liver transplantation for hepatocellular carcinoma—yes, of course? J Hepatol. 2005;43:569–573. doi: 10.1016/j.jhep.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 40.Freeman RB, Mithoefer A, Ruthazer R, Nguyen K, Schore A, Harper A, Edwards E. Optimizing staging for hepatocellular carcinoma before liver transplantation: a retrospective analysis of the UNOS/OPTN database. Liver Transpl. 2006;12:1504–1511. doi: 10.1002/lt.20847. [DOI] [PubMed] [Google Scholar]
- 41.Luca A, Caruso S, Milazzo M, Mamone G, Marrone G, Miraglia R, et al. Multidetector-row computed tomography (MDCT) for the diagnosis of hepatocellular carcinoma in cirrhotic candidates for liver transplantation: prevalence of radiological vascular patterns and histological correlation with liver explants. Eur Radiol. 2010;20:898–907. doi: 10.1007/s00330-009-1622-0. [DOI] [PubMed] [Google Scholar]
- 42.Sangiovanni A, Manini MA, Iavarone M, Romeo R, Forzenigo LV, Fraquelli M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010;59:638–644. doi: 10.1136/gut.2009.187286. [DOI] [PubMed] [Google Scholar]
- 43.Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, Duca P. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006;101:513–523. doi: 10.1111/j.1572-0241.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 44.Lee JW, Paeng JC, Kang KW, Kwon HW, Suh KS, Chung JK, et al. Prediction of tumor recurrence by 18F-FDGPET in liver transplantation for hepatocellular carcinoma. J Nucl Med. 2009;50:682–687. doi: 10.2967/jnumed.108.060574. [DOI] [PubMed] [Google Scholar]
- 45.Kornberg A, Freesmeyer M, Bärthel E, Jandt K, KatenkampK SJ, et al. 18F-FDG-uptake of hepatocellular carcinoma on PET predicts microvascular tumor invasion in liver transplant patients. Am J Transplant. 2009;9:592–600. doi: 10.1111/j.1600-6143.2008.02516.x. [DOI] [PubMed] [Google Scholar]
- 46.Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med. 2003;44:213–221. [PubMed] [Google Scholar]
- 47.Ho CL, Chen S, Yeung DW, Cheng TK. Dual-tracer PET/CT imaging in evaluation of metastatic hepatocellular carcinoma. J Nucl Med. 2007;48:902–909. doi: 10.2967/jnumed.106.036673. [DOI] [PubMed] [Google Scholar]
- 48.Wiesner RH, Freeman RB, Mulligan DC. Liver transplantation for hepatocellular cancer: the impact of the MELD allocation policy. Gastroenterology. 2004;127(suppl 1):S261–S267. doi: 10.1053/j.gastro.2004.09.040. [DOI] [PubMed] [Google Scholar]
- 49.Compagnon P, Grandadam S, Lorho R, Turlin B, Camus C, Jianrong Y, et al. Liver transplantation for hepatocellularcarcinoma without preoperative tumor biopsy. Transplantation. 2008;86:1068–1076. doi: 10.1097/TP.0b013e318187754c. [DOI] [PubMed] [Google Scholar]
- 50.Caturelli E, Bisceglia M, Fusilli S, Squillante MM, Castelvetere M, Siena DA. Cytological vs. microhistological diagnosis of hepatocellular carcinoma: comparative accuracies in the same fine-needle biopsy specimen. Dig Dis Sci. 1996;41:2326–2331. doi: 10.1007/BF02100122. [DOI] [PubMed] [Google Scholar]
- 51.Caturelli E, Solmi L, Anti M, Fusilli S, Roselli P, Andriulli A, et al. Ultrasound guided fine needle biopsy of early hepatocellular carcinoma complicating liver cirrhosis: a multicentre study. Gut. 2004;53:1356–1362. doi: 10.1136/gut.2003.032359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Durand F, Regimbeau JM, Belghiti J, Sauvanet A, Vilgrain V, Terris B, et al. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol. 2001;35:254–258. doi: 10.1016/s0168-8278(01)00108-8. [DOI] [PubMed] [Google Scholar]
- 53.Müllhaupt B, Durand F, Roskams T, Dutkowski P, Heim M. Is tumor biopsy necessary? Liver Transpl. 2011;17(Suppl 2):S14–S25. doi: 10.1002/lt.22374. [DOI] [PubMed] [Google Scholar]
- 54.Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592–1596. doi: 10.1136/gut.2008.149062. [DOI] [PubMed] [Google Scholar]
- 55.Maturen KE, Nghiem HV, Marrero JA, Hussain HK, Higgins EG, Fox GA, Francis IR. Lack of tumor seeding of hepatocellular carcinoma after percutaneous needle biopsy using coaxial cutting needle technique. AJR Am J Roentgenol. 2006;187:1184–1187. doi: 10.2214/AJR.05.1347. [DOI] [PubMed] [Google Scholar]
- 56.Germani G, Pleguezuelo M, Stigliano R, Burroughs AK. Risk of seeding is reduced by associating diagnostic biopsy with percutaneous ablation for hepatocellular carcinoma. Gut. 2009;58:734–735. [PubMed] [Google Scholar]
- 57.Germani G, Gurusamy K, Garcovich M, Toso C, Fede G, Hemming A, Suh KS, Weber A, Kenneth Burroughs A. Which matters most: number of tumors, size of the largest tumor, or total tumor volume? Liver Transpl. 2011;17(Suppl 2):S58–S66. doi: 10.1002/lt.22336. [DOI] [PubMed] [Google Scholar]
- 58.Gouw AS, Balabaud C, Kusano H, Todo S, Ichida T, Kojiro M. Markers for microvascular invasion in hepatocellular carcinoma: where do we stand? Liver Transpl. 2011;17(Suppl 2):S72–S80. doi: 10.1002/lt.22368. [DOI] [PubMed] [Google Scholar]
- 59.Lee HH, Joh JW, Park JH, Lee KW, Heo JS, Choi SH, Kim SJ, Lee SK. Microvascular tumor embolism: independent prognostic factor after liver transplantation in hepatocellular carcinoma. Transplant Proc. 2005;37(2):1251–1253. doi: 10.1016/j.transproceed.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 60.Esnaola NF, Lauwers GY, Mirza NQ, Nagorney DM, Doherty D, Ikai I, Yamaoka Y, Regimbeau JM, Belghiti J, Curley SA, Ellis LM, Vauthey JN. Predictors of microvascular invasion in patients with hepatocellular carcinoma who are candidates for orthotopic liver transplantation. J Gastrointest Surg. 2002;6(2):224–232. doi: 10.1016/s1091-255x(01)00015-4. [DOI] [PubMed] [Google Scholar]
- 61.Hoshida Y, Villanueva A, Kobayashi M, Peix J, Chiang DY, Camargo A, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamashita T, Forgues M, Wang W, Kim JW, Ye Q, Jia H, et al. EpCAM and alpha-fetoprotein expression defines novel prognostic subtypes of hepatocellular carcinoma. Cancer Res. 2008;68:1451–1461. doi: 10.1158/0008-5472.CAN-07-6013. [DOI] [PubMed] [Google Scholar]
- 63.Yamashita T, Ji J, Budhu A, Forgues M, Yang W, Wang HY, et al. EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology. 2009;136:1012–1024. doi: 10.1053/j.gastro.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boyault S, Rickman DS, Reyniés A, Balabaud C, Rebouissou S, Jeannot E, et al. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 65.Ji J, Shi J, Budhu A, Yu Z, Forgues M, Roessler S, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361:1437–1447. doi: 10.1056/NEJMoa0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Budhu A, Forgues M, Ye QH, Jia HL, He P, Zanetti KA, et al. Prediction of venous metastases, recurrence, and prognosis in hepatocellular carcinoma based on aunique immune response signature of the liver microenvironment. Canc Cell. 2006;10:99–111. doi: 10.1016/j.ccr.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 67.Llovet JM, Peña C, Shan M, Lathia C, Bruix J (2008) Biomarkers predicting outcome of patients with advanced hepatocellular carcinoma (HCC) randomized in the phase III SHARP trial. Paper presented at: 59th Annual Meeting of the American Association for the Study of Liver Diseases; October 31–November 4, 2008; San Francisco, CA
- 68.Parfitt JR, Marotta P, Alghamdi M, Wall W, Khakhar A, Suskin NG, et al. Recurrent hepatocellular carcinoma after transplantation: use of a pathological score on explanted livers to predict recurrence. Liver Transpl. 2007;13:543–551. doi: 10.1002/lt.21078. [DOI] [PubMed] [Google Scholar]
- 69.Pawlik TM, Delman KA, Vauthey JN, Nagorney DM, Ng IO, Ikai I, et al. Tumor size predicts vascular invasion and histologic grade: implications for selection of surgical treatment for hepatocellular carcinoma. Liver Transpl. 2005;11:1086–1092. doi: 10.1002/lt.20472. [DOI] [PubMed] [Google Scholar]
- 70.Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375–1382. doi: 10.1245/s10434-008-9846-9. [DOI] [PubMed] [Google Scholar]
- 71.Shirabe K, Aishima S, Taketomi A, Soejima Y, Uchiyama H, Kayashima H, et al. Prognostic importance of the gross classification of hepatocellular carcinoma in living donor-related liver transplantation. Br J Surg. 2011;98:261–267. doi: 10.1002/bjs.7311. [DOI] [PubMed] [Google Scholar]
- 72.Fujiki M, Takada Y, Ogura Y, Oike F, Kaido T, Teramukai S, Uemoto S. Significance of des-gamma-carboxy prothrombin in selection criteria for living donor liver transplantation or hepatocellular carcinoma. Am J Transplant. 2009;9:2362–2371. doi: 10.1111/j.1600-6143.2009.02783.x. [DOI] [PubMed] [Google Scholar]
- 73.Cherqui D, Boudjema K, Celebic A, Laurent A, Roudot-Thoraval F. Résection hépatique pour carcinome hépatocellulaire sur foie sain et pathologique en France. Résultats de l’enqueˆte AFC sur la période 1990–2005. In: Boudjema K, Cherqui D, editors. Cancer Hépatocellulaire. Rueil-Malmaison: Wolters Kluwer; 2006. pp. 183–218. [Google Scholar]
- 74.Rahbari NN, Mehrabi A, Mollberg NM, Müller SA, KochM BMW, Weitz J. Hepatocellular carcinoma: current management and perspectives for the future. Ann Surg. 2011;253:453–469. doi: 10.1097/SLA.0b013e31820d944f. [DOI] [PubMed] [Google Scholar]
- 75.Trevisani F, Frigerio M, Santi V, Grignaschi A, Bernardi M. Hepatocellular carcinoma in non-cirrhotic liver: a reappraisal. Dig Liver Dis. 2010;42:341–347. doi: 10.1016/j.dld.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 76.Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521–527. [PubMed] [Google Scholar]
- 77.Torzilli G, Montorsi M, Fabbro D, Palmisano A, Donadon M, Makuuchi M. Ultrasonographically guided surgical approach to liver tumours involving the hepatic veins close to the caval confluence. Br J Surg. 2006;93:1238–1246. doi: 10.1002/bjs.5321. [DOI] [PubMed] [Google Scholar]
- 78.Kokudo N, Makuuchi M. Current role of portal vein embolization/hepatic artery chemoembolization. Surg Clin North Am. 2004;84:643–657. doi: 10.1016/j.suc.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 79.Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, et al. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540–548. doi: 10.1097/SLA.0b013e3181b674df. [DOI] [PubMed] [Google Scholar]
- 80.Mergental H, Porte RJ. Liver transplantation for unresectable hepatocellular carcinoma in patients without liver cirrhosis. Transpl Int. 2010;23:662–667. doi: 10.1111/j.1432-2277.2010.01076.x. [DOI] [PubMed] [Google Scholar]
- 81.Mergental H, Adam R, Kalicinski P, Ericzon BG, Friman S, Köningsrainer A, et al. Liver transplantation for hepatocellular carcinoma in non-cirrhotic livers [abstract] Liver Transpl. 2007;13(suppl 1):S119. [Google Scholar]
- 82.Toyoda H, Kumada T, Kiriyama S, Sone Y, Tanikawa M, Hisanaga Y, et al. Comparison of the usefulness of three staging systems for hepatocellular carcinoma (CLIP, BCLC, and JIS) in Japan. Am J Gastroenterol. 2005;100:1764–1771. doi: 10.1111/j.1572-0241.2005.41943.x. [DOI] [PubMed] [Google Scholar]
- 83.The Cancer of the Liver Italian Program(CLIP) investigators Prospective validation of the CLIP score: a new prognostic system for patients with cirrhosis and hepatocellular carcinoma. Hepatology. 2000;31:840–845. doi: 10.1053/he.2000.5628. [DOI] [PubMed] [Google Scholar]
- 84.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 85.Shiina S, Teratani T, Obi S, et al. A randomized controlled trial of radiofrequency ablation with ethanol injection for small hepatocellular carcinoma. Gastroenterology. 2005;129:122–130. doi: 10.1053/j.gastro.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 86.Lin SM, Lin CJ, Lin CC, et al. Radiofrequency ablation improves prognosis compared to ethanol injection for hepatocellular carcinoma ≤4 cm. Gastroenterology. 2004;127:1714–1723. doi: 10.1053/j.gastro.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 87.Lo CM, Ngan H, Tso WK, et al. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164–1171. doi: 10.1053/jhep.2002.33156. [DOI] [PubMed] [Google Scholar]
- 88.Llovet JM, Real MI, Montana X, et al. Arterial embolization or chemoembolization versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomized controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 89.Majno PE, Adam R, Bismuth H, et al. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 2004;239:150–159. doi: 10.1097/00000658-199712000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roayaie S, Frischer JS, Emre SH, et al. Long-term results with multimodal adjuvant therapy and liver transplantation for the treatment of hepatocellular carcinomas larger than 5 centimeters. Ann Surg. 2002;235:533–539. doi: 10.1097/00000658-200204000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yao FY, Hirose R, Laberge J, et al. A prospective study on downstaging of hepatocellular carcinoma prior to liver transplantation. Liver Transpl. 2005;11:1505–1514. doi: 10.1002/lt.20526. [DOI] [PubMed] [Google Scholar]
- 92.Yao FY, Kerlan R, Davern TJ, et al. Excellent long-term outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis [abstract] Hepatology. 2007;46(Suppl):309A. doi: 10.1002/hep.22412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luna W, Sze DY, Ahmed A, Ha BY, Ayoub W, Keeffe EB, Cooper A, Esquivel C, Nguyen MH. Transarterial chemoinfusion for hepatocellular carcinoma as downstaging therapy and a bridge toward liver transplantation. Am J Transplant. 2009;9(5):1158–1168. doi: 10.1111/j.1600-6143.2009.02576.x. [DOI] [PubMed] [Google Scholar]
- 94.Adam R, Azoulay D, Castaing D, Eshkenazy R, Pascal G, Hashizume K, Samuel D, Bismuth H. Liver resection as a bridge to transplantation for hepatocellular carcinoma on cirrhosis: a reasonable strategy? Ann Surg. 2003;238(4):508–518. doi: 10.1097/01.sla.0000090449.87109.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235(3):373–382. doi: 10.1097/00000658-200203000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cherqui D, Laurent A, Mocellin N, Tayar C, Luciani A, Nhieu JT, Decaens T, Hurtova M, Memeo R, Mallat A, Duvoux C. Liver resection for transplantable hepatocellular carcinoma: long-term survival and role of secondary liver transplantation. Ann Surg. 2009;250(5):738–746. doi: 10.1097/SLA.0b013e3181bd582b. [DOI] [PubMed] [Google Scholar]
- 97.Panaro F, Piardi T, Cag M, Cinqualbre J, Wolf P, Audet M. Robotic liver resection as a bridge to liver transplantation. JSLS. 2011;15(1):86–89. doi: 10.4293/108680811X13022985131417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Landman MP, Feurer ID, Pinson CW, Moore DE. Which is more cost-effective under the MELD system: primary liver transplantation, or salvage transplantation after hepatic resection or after loco-regional therapy for hepatocellular carcinoma within Milan criteria? HPB (Oxford) 2011;13(11):783–791. doi: 10.1111/j.1477-2574.2011.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huo TI, Huang YH, Su CW, Lin HC, Chiang JH, Chiou YY, et al. Validation of the HCC-MELD for dropout probability in patients with small hepatocellular carcinoma undergoing locoregional therapy. Clin Transplant. 2008;22:469–475. doi: 10.1111/j.1399-0012.2008.00811.x. [DOI] [PubMed] [Google Scholar]
- 100.Yao FY, Bass NM, Nikolai B, Merriman R, Davern TJ, Kerlan R, et al. A follow-up analysis of the pattern and predictors of dropout from the waiting list for liver transplantation in patients with hepatocellular carcinoma: implications for the current organ allocation policy. Liver Transpl. 2003;9:684–692. doi: 10.1053/jlts.2003.50147. [DOI] [PubMed] [Google Scholar]
- 101.Llovet JM, Mas X, Aponte JJ, Fuster J, Navasa M, Christensen E, et al. Cost effectiveness of adjuvant therapy for hepatocellular carcinoma during the waiting list for liver transplantation. Gut. 2002;50:123–128. doi: 10.1136/gut.50.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Aloia TA, Adam R, Samuel D, Azoulay D, Castaing D. A decision analysis model identifies the interval of efficacy for transarterial chemoembolization (TACE) in cirrhotic patients with hepatocellular carcinoma awaiting liver transplantation. J Gastrointest Surg. 2007;11:1328–1332. doi: 10.1007/s11605-007-0211-2. [DOI] [PubMed] [Google Scholar]
- 103.Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, et al. Radioembolization results in longer time-to progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497–507. doi: 10.1053/j.gastro.2010.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Majno P, Lencioni R, Mornex F, Girard N, Poon RT, Cherqui D. Is the treatment of hepatocellular carcinoma on the waiting list necessary? Liver Transpl. 2011;17(Suppl 2):S98–S108. doi: 10.1002/lt.22391. [DOI] [PubMed] [Google Scholar]
- 105.Pruett TL, Tibell A, Alabdulkareem A, Bhandari M, Cronin DC, Dew MA, Dib-Kuri A, Gutmann T, Matas A, McMurdo L, Rahmel A, Rizvi SA, Wright L, Delmonico FL. The ethics statement of the Vancouver Forum on the live lung, liver, pancreas, and intestine donor. Transplantation. 2006;81(10):1386–1387. doi: 10.1097/01.tp.0000214976.36526.e3. [DOI] [PubMed] [Google Scholar]
- 106.Grant D, Fisher RA, Abecassis M, McCaughan G, Wright L, Fan ST. Should the liver transplant criteria for hepatocellular carcinoma be different for deceased donation and living donation? Liver Transpl. 2011;17(Suppl 2):S133–S138. doi: 10.1002/lt.22348. [DOI] [PubMed] [Google Scholar]
- 107.Chan SC, Fan ST, Lo CM, Liu CL, Wei WI, Chik BH, Wong J. A decade of right liver adult-to-adult living donor liver transplantation: the recipient mid-term outcomes. Ann Surg. 2008;248(3):411–419. doi: 10.1097/SLA.0b013e31818584e6. [DOI] [PubMed] [Google Scholar]
- 108.Efimova EA, Glanemann M, Liu L, et al. Effects of human hepatocyte growth factor on the proliferation of human hepatocytes and hepatocellular carcinoma cell lines. Eur Surg Res. 2004;36:300. doi: 10.1159/000079915. [DOI] [PubMed] [Google Scholar]
- 109.Schweinitz D, Faundez A, Teichmann B, et al. Hepatocyte growth-factor-scatter factor can stimulate post-operative tumor-cell proliferation in childhood hepatoblastoma. Int J Cancer. 2000;85:151. doi: 10.1002/(SICI)1097-0215(20000115)85:2<151::AID-IJC1>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 110.Lo CM, Fan ST, Liu CL, Chan SC, Ng IO, Wong J. Living donor versus deceased donor liver transplantation for early irresectable hepatocellular carcinoma. Br J Surg. 2007;94(1):78–86. doi: 10.1002/bjs.5528. [DOI] [PubMed] [Google Scholar]
- 111.Fisher RA, Kulik LM, Freise CE, Lok AS, Shearon TH, Brown RS, Jr, Ghobrial RM, Fair JH, Olthoff KM, Kam I, Berg CL, A2ALL Study Group Hepatocellular carcinoma recurrence and death following living and deceased donor liver transplantation. Am J Transplant. 2007;7(6):1601–1608. doi: 10.1111/j.1600-6143.2007.01802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bhangui P, Vibert E, Majno P, Salloum C, Andreani P, Zocrato J, Ichai P, Saliba F, Adam R, Castaing D, Azoulay D. Intention-to-treat analysis of liver transplantation for hepatocellular carcinoma: living versus deceased donor transplantation. Hepatology. 2011;53(5):1570–1579. doi: 10.1002/hep.24231. [DOI] [PubMed] [Google Scholar]
- 113.Hwang S, Lee SG, Ahn CS, Kim KH, Moon DB, Ha TY, Park KM, Song GW, Jung DH, Kim BS, Moon KM. Small-sized liver graft does not increase the risk of hepatocellular carcinoma recurrence after living donor liver transplantation. Transplant Proc. 2007;39(5):1526–1529. doi: 10.1016/j.transproceed.2007.03.066. [DOI] [PubMed] [Google Scholar]
- 114.Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530–534. doi: 10.1038/17401. [DOI] [PubMed] [Google Scholar]
- 115.Herman M, Weinstein T, Korzets A, Chagnac A, Ori Y, Zevin D, et al. Effect of cyclosporin A on DNA repair and cancer incidence in kidney transplant recipients. J Lab Clin Med. 2001;137:14–20. doi: 10.1067/mlc.2001.111469. [DOI] [PubMed] [Google Scholar]
- 116.Schumacher G, Oidtmann M, Rosewicz S, Langrehr J, Jonas S, Mueller AR, et al. Sirolimus inhibits growth of human hepatoma cells in contrast to tacrolimus which promotes cell growth. Transplant Proc. 2002;34:1392–1393. doi: 10.1016/s0041-1345(02)02899-3. [DOI] [PubMed] [Google Scholar]
- 117.Yokoyama I, Carr B, Saitsu H, Iwatsuki S, Starzl TE. Accelerated growth rates of recurrent hepatocellular carcinoma after liver transplantation. Cancer. 1991;68:2095–2100. doi: 10.1002/1097-0142(19911115)68:10<2095::aid-cncr2820681002>3.0.co;2-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hui IC, Tung EK, Sze KM, Ching YP, Ng IO. Rapamycin and CCI-779 inhibit the mammalian target of rapamycin signalling in hepatocellular carcinoma. Liver Int. 2010;30:65–75. doi: 10.1111/j.1478-3231.2009.02117.x. [DOI] [PubMed] [Google Scholar]
- 119.Sahin F, Kannangai R, Adegbola O, Wang J, Su G, Torbenson M. mTOR and P70 S6 kinase expression in primary liver neoplasms. Clin Cancer Res. 2004;10:8421–8425. doi: 10.1158/1078-0432.CCR-04-0941. [DOI] [PubMed] [Google Scholar]
- 120.Shirouzu Y, Ryschich E, Salnikova O, Kerkadze V, Schmidt J, Engelmann G. Rapamycin inhibits proliferation and migration of hepatoma cells in vitro. J Surg Res. 2010;159:705–713. doi: 10.1016/j.jss.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 121.Schumacher G, Oidtmann M, Rueggeberg A, Jacob D, Jonas S, Langrehr JM, et al. Sirolimus inhibits growth of human hepatoma cells alone or combined with tacrolimus, while tacrolimus promotes cell growth. World J Gastroenterol. 2005;11:1420–1425. doi: 10.3748/wjg.v11.i10.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Semela D, Piguet AC, Kolev M, Schmitter K, Hlushchuk R, Djonov V, et al. Vascular remodeling and antitumoral effects of mTOR inhibition in a rat model of hepatocellular carcinoma. J Hepatol. 2007;46:840–848. doi: 10.1016/j.jhep.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 123.Stippel DL, Kasper HU, Schleimer K, Töx U, Bangard C, Hölscher AH, Beckurts KT. Successful use of sirolimusin a patient with bulky ovarian metastasis of hepatocellular carcinoma after liver transplantation. Transplant Proc. 2005;37:2185–2187. doi: 10.1016/j.transproceed.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 124.Elsharkawi M, Staib L, Henne-Bruns D, Mayer J. Completeremission of postransplant lung metastases from hepatocellular carcinoma under therapy with sirolimus and mycophenolate mofetil. Transplantation. 2005;79:855–857. doi: 10.1097/01.tp.0000154913.88193.ff. [DOI] [PubMed] [Google Scholar]
- 125.Chinnakotla S, Davis GL, Vasani S, Kim P, Tomiyama K, Sanchez E, et al. Impact of sirolimus on the recurrence of hepatocellular carcinoma after liver transplantation. Liver Transpl. 2009;15:1834–1842. doi: 10.1002/lt.21953. [DOI] [PubMed] [Google Scholar]
- 126.Zimmerman MA, Trotter JF, Wachs M, Bak T, Campsen J, Skibba A, Kam I. Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl. 2008;14:633–638. doi: 10.1002/lt.21420. [DOI] [PubMed] [Google Scholar]
- 127.Toso C, Meeberg GA, Bigam DL, Oberholzer J, Shapiro AM, Gutfreund K, et al. De novo sirolimus-based immunosuppression after liver transplantation for hepatocellular carcinoma: long-term outcomes and side effects. Transplantation. 2007;83:1162–1168. doi: 10.1097/01.tp.0000262607.95372.e0. [DOI] [PubMed] [Google Scholar]
- 128.Toso C, Merani S, Bigam DL, Shapiro AM, Kneteman NM. Sirolimus-based immunosuppression is associated with increased survival after liver transplantation for hepatocellular carcinoma. Hepatology. 2010;51:1237–1243. doi: 10.1002/hep.23437. [DOI] [PubMed] [Google Scholar]
- 129.Liang W, Wang D, Ling X, Cao AA, Kong Y, Shang Y, Guo Z, He X (2011) Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl; doi:10.1002/lt.22441 [Epub ahead of print] [DOI] [PubMed]
- 130.Schnitzbauer AA, Zuelke C, Graeb C, Rochon J, Bilbao I, Burra P, Jong KP, Duvoux C, Kneteman NM, Adam R, Bechstein WO, Becker T, Beckebaum S, Chazouillères O, Cillo U, Colledan M, Fändrich F, Gugenheim J, Hauss JP, Heise M, Hidalgo E, Jamieson N, Königsrainer A, Lamby PE, Lerut JP, Mäkisalo H, Margreiter R, Mazzaferro V, Mutzbauer I, Otto G, Pageaux GP, Pinna AD, Pirenne J, Rizell M, Rossi G, Rostaing L, Roy A, Turrion VS, Schmidt J, Troisi RI, Hoek B, Valente U, Wolf P, Wolters H, Mirza DF, Scholz T, Steininger R, Soderdahl G, Strasser SI, Jauch KW, Neuhaus P, Schlitt HJ, Geissler EK. A prospective randomised, open-labeled, trial comparing sirolimus-containing versus mTOR-inhibitor-free immunosuppression in patients undergoing liver transplantation for hepatocellular carcinoma. BMC Canc. 2010;10:190. doi: 10.1186/1471-2407-10-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Regalia E, Fassati LR, Valente U, et al. Pattern and management of recurrent hepatocellular carcinoma after liver transplantation. J Hepatobiliary Pancreat. 1998;5:29–34. doi: 10.1007/pl00009947. [DOI] [PubMed] [Google Scholar]
- 132.Roayaie S, Schwartz JD, Sung MW, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10:534–540. doi: 10.1002/lt.20128. [DOI] [PubMed] [Google Scholar]
- 133.Sandroussi C, Guba M, Sandhu L, Dubay D, Ghanekar E, Selzner M, et al. Liver transplantation for HCC: characterization of recurrence and outcomes with aggressive multimodal treatment [abstract] Am J Transplant. 2010;10(suppl 4):452. [Google Scholar]
- 134.Taketomi A, Fukuhara T, Morita K, Kayashima H, Ninomiya M, Yamashita Y, et al. Improved results of a surgical resection for the recurrence of hepatocellular carcinoma after living donor liver transplantation. Ann Surg Oncol. 2010;17:2283–2289. doi: 10.1245/s10434-010-0999-y. [DOI] [PubMed] [Google Scholar]
- 135.Marangoni G, Faraj W, Sethi H, Rela M, Muiesan P, Heaton N. Liver resection in liver transplant recipients. Hepatobiliary Pancreat Dis Int. 2008;7:590–594. [PubMed] [Google Scholar]
- 136.Kornberg A, Küpper B, Tannapfel A, Katenkamp K, Thrum K, Habrecht O, Wilberg J. Long-term survival after recurrent hepatocellular carcinoma in liver transplant patients: clinical patterns and outcome variables. Eur J Surg Oncol. 2010;36:275–280. doi: 10.1016/j.ejso.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 137.Chen GH, Fu BS, Yang Y, Cai CJ, Lu MQ, Li H, et al. Early liver retransplantation versus late liver retransplantation: analysis of a single-center experience. Chin Med J (Engl) 2008;121:1992–1996. [PubMed] [Google Scholar]
- 138.Yamashiki N, Sugawara Y, Tamura S, Tateishi R, Yoshida H, Kaneko J, et al. Postoperative surveillance with monthly serum tumor markers after living-donor liver transplantation for hepatocellular carcinoma. Hepatol Res. 2010;40:278–286. doi: 10.1111/j.1872-034X.2009.00591.x. [DOI] [PubMed] [Google Scholar]
- 139.Toso C, Mentha G, Majno P. Liver transplantation for hepatocellular carcinoma: five steps to prevent recurrence. Am J Transplant. 2011;11(10):2031–2035. doi: 10.1111/j.1600-6143.2011.03689.x. [DOI] [PubMed] [Google Scholar]
- 140.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 141.Ohira M, Ohdan H, Mitsuta H, et al. Adoptive transfer of TRAIL expressing natural killer cells prevents recurrence of hepatocellular carcinoma after partial hepatectomy. Transplantation. 2006;82:1712–1719. doi: 10.1097/01.tp.0000250935.41034.2d. [DOI] [PubMed] [Google Scholar]