Abstract
An optimization campaign focused on improving pharmacological activity and physicochemical properties of a recently-identified class of cyclosulfamide-based norovirus inhibitors has been carried out. Dimeric compound 4 was found to be a ~ 10-fold more potent norovirus inhibitor (ED50 0.4 μM) compared to the original hit, however, isonipecotic acid ester derivatives 7e and 10a were shown to have superior therapeutic indices.
Keywords: Norovirus, cyclosulfamide-based inhibitors, cell-based assay
Introduction
The Caliciviridae family of viruses is comprised of the Norovirus, Sapovirus, Vesivirus, and Lagovirus genera [1]. Noroviruses are major medical pathogens which have attracted considerable attention because they are the most common cause of acute viral gastroenteritis outbreaks [2–3]. Due to the highly contagious nature of noroviruses, outbreaks of acute gastroenteritis are common, particularly in crowded settings, such as schools, cruise ships, and hospitals. There are currently no vaccines or specific antiviral agents that target noroviruses. Thus, there is an urgent need for the design and development of anti-norovirus therapeutics.
We have recently reported that cyclosulfamide-based derivatives potently inhibit norovirus replication in a cell-based system [4] and have, furthermore, used a scaffold hopping strategy to identify additional classes of compounds that inhibit noroviruses [5]. The multiple points of diversity present in structure (I) (Figure 1) were used to carry out structural modifications to prospect the entire structure and to obtain preliminary validation for advancing this series of compounds into the lead optimization phase [6]. The results of preliminary studies suggested that modification of R (structure (I), Figure 1) might be a fruitful avenue of investigation for identifying binding sites suitable for further optimization. We envisioned the introduction of a basic functionality in R, and in particular a privileged scaffold such as a piperazine moiety, might yield compounds with superior pharmacological activity and physicochemical properties. We describe herein the results of SAR studies related to new norovirus inhibitors based on the cyclosulfamide core template (I).
Figure 1.

Structure optimization of norovirus inhibitor (I).
Chemistry
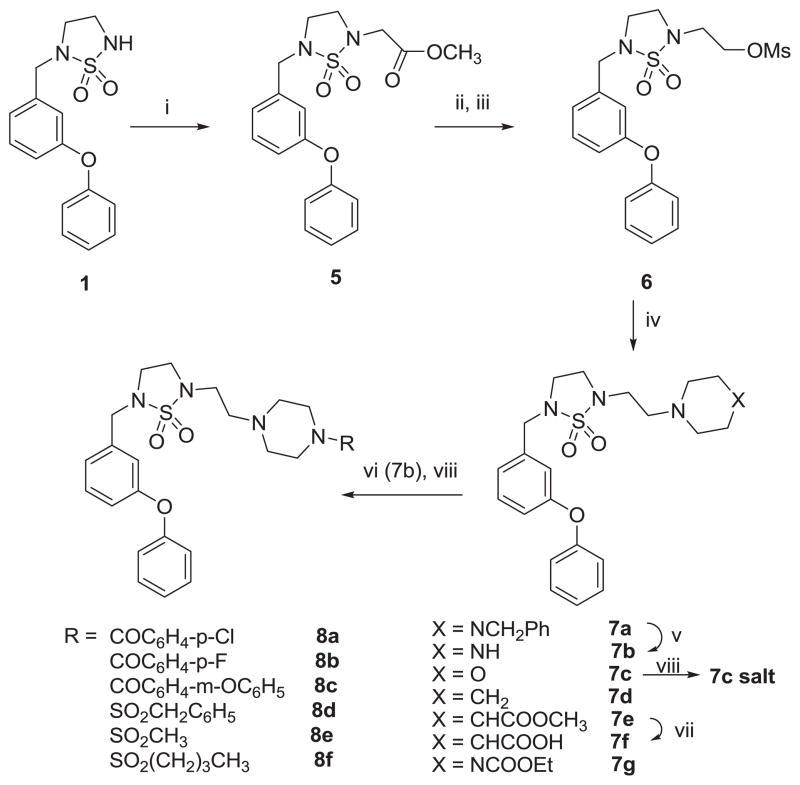
The initial synthetic plan for obtaining tertiary amines 7–8 involved treating compound 1 [4] sequentially with sodium hydride and 1,2-dibromoethane. This resulted in the formation of the desired product 2, along with a dimeric product 3 (Scheme 1).
Scheme 1.
Reaction conditions: i) NaH/DMF, then BrCH2CH2Br; ii) piperazine/K2CO3/DMF.
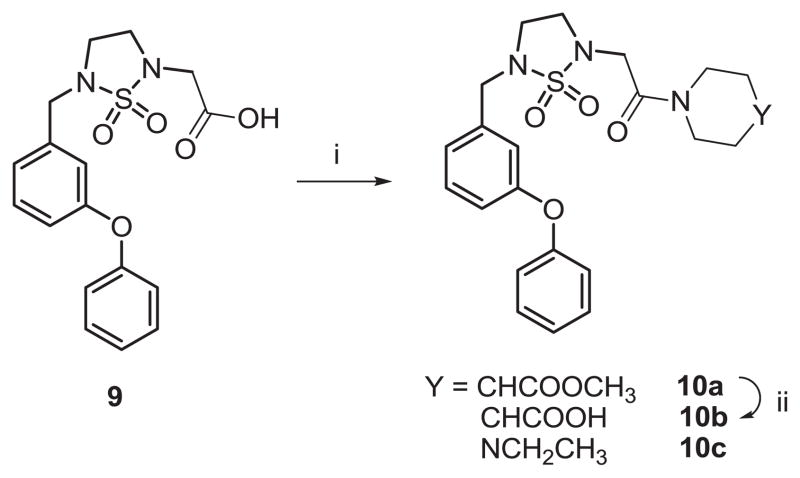
Further reaction of compound 2 with piperazine in the presence of anhydrous potassium carbonate yielded a dimeric compound 4. Thus, an alternative reaction sequence was used to obtain the desired compounds. Compounds 7a–f were readily obtained as illustrated in Scheme 2. Alkylation of compound 1 yielded compound 5, which was converted to mesylate 6 via reduction with lithium borohydride followed by treatment of methanesulfonyl chloride in the presence of triethylamine. The mesylate was subsequently replaced by reacting with N-benzyl piperazine, morpholine, piperidine, or methyl isonipecotate, or ethyl 1-piperazinecarboxylate in the presence of triethylamine to give the corresponding products 7a, 7c–d, 7e and 7g. Catalytic hydrogenation of compound 7a yielded compound 7b which was coupled to an array of EDCI-activated carboxylic acids or sulfonyl chlorides in the presence of triethylamine to give compounds 8a–f. Hydrolysis of compound 7e using 1 M LiOH gave zwitterion 7f. Compounds 7c and 8a–f were treated with 4 M HCl in methanol solution to yield the corresponding hydrochloride salts. The previously synthesized carboxylic acid 9 [4] was activated with EDCI in DMF and coupled with methyl isonipecotate or ethyl piperazine to give tertiary amides 10a and 10c, respectively (Scheme 3). Compound 10b was obtained by hydrolysis of compound 10a.
Scheme 2.
Reaction conditions: i) NaH/DMF, then BrCH2COOCH3; ii) LiBH4/THF/EtOH; iii) MsCl/TEA/CH2Cl2; iv) HN(CH2CH2)2X/TEA/DMF; v) H2/10%Pd-C/MeOH; vi) ROH/EDCl/DMF or RCl/TEA/DMF; vii) 1M LiOH/dixone; viii) 4M HCl/MeOH.
Scheme 3.
Reaction conditions; i) EDCl/DMF, then methyl isonipecotate or ethyl piperazine; ii) 1 M LiOH/1,4-dioxane.
Biochemical Studies
The effects of the synthesized compounds were examined in NV replicon-harboring cells (HG23 cells) and the results are summarized in Table 1. Detailed procedures for studying the antiviral effects using HG23 cells have been reported elsewhere [7–10].
Table 1.
Results and Discussion
Norovirus infections constitute a public health problem. Despite this, only a limited number of studies related to the discovery of norovirus inhibitors have been reported in the literature [11–13]. Our initial foray in this area began with the identification of several hits sharing in common the cyclosulfamide scaffold that exhibited anti-norovirus activity in a cell-based replicon system [4–5] and focused on the optimization of the initial hit (structure (I), Figure 1) via the introduction of structurally-diverse R groups (structure (II), Figure 1). We were particularly interested in R groups, such as the piperazine moiety, a well-known privileged scaffold [14–15], and related heterocyclic amines for the enhancement of potency, as well as aqueous solubility through the formation of the corresponding hydrochloride salts.
Treatment of compound 1 with sodium hydride, followed by 1, 2-dibromoethane, yielded the desired product 2, along with dimeric product 3. Compound 3 exhibited noteworthy anti-norovirus activity; however, it suffered from high toxicity. The intriguing activity of dimer 3 prompted us to synthesize the corresponding piperazine dimer 4 by treating compound 2 with piperazine. Compound 4 displayed an improved profile with an ED50 in the high nanomolar range and a better therapeutic index than compound 3. The observed enhancement of activity via dimerization is a well-documented phenomenon [16–17]. Nevertheless, the non-optimal TD50 of compound 4 provided the impetus for further exploratory work through the synthesis of a series of substituted piperazine, morpholine, piperidine, and isonipecotic acid derivatives (Scheme 2).
It is evident from the results shown in Table 1 that while potency was largely invariant to structural changes, remaining in the 3–10 μM range, the cytotoxicity of the compounds was very sensitive to such changes. Thus, piperazine derivatives 7a–b, 7g, and 8a–f had low therapeutic indices (<10) despite the large variability in the nature of the nitrogen substituent in the piperazine ring. Likewise, piperidine derivative 7d had a low therapeutic index. In contrast, the morpholine (compound 7c) and isonipecotic acid derivatives 7e and 10a showed improved cytotoxicity. These differences in potency and cytotoxicity may be due to the composite effects of multiple factors, including differences in physicochemical properties. These observations will likely be illuminated once the mechanism of action of these compounds is elucidated (in progress).
A few isonipecotic acid and piperazine amide derivatives were also synthesized starting from acid 9 (Scheme 3) to further probe the effect of structure on anti-norovirus activity and cytotoxicity. Compounds 10a and 10c exhibited good therapeutic indices (39 and 24, respectively). Taken together, the results summarized in Table 1 indicate that isonipecotic acid ester derivatives 7e and 10a showed comparable low micromolar potency, as well as a noteworthy improvement in cytotoxicity.
In summary, a set of compounds that incorporate in their structures the cyclosulfamide scaffold with appended substituted heterocyclic amines or amides were found to exhibit low micromolar anti-norovirus activity in a cell-based replicon system. Potency was found to be invariant to structural changes while cytotoxicity was highly sensitive to such changes.
Experimental
General
The 1H spectra were recorded on a Varian XL-300 or XL-400 NMR spectrometer. Melting points were determined on a Mel-Temp apparatus and are uncorrected. High resolution mass spectra (HRMS) were performed at the University of Kansas Mass Spectrometry Lab. Reagents and solvents were purchased from various chemical suppliers (Aldrich, Acros Organics, TCI America, and Bachem). Silica gel (230–450 mesh) used for flash chromatography was purchased from Sorbent Technologies (Atlanta, GA). Thin layer chromatography was performed using Analtech silica gel plates. The TLC plates for the final compounds were eluted using two different solvent systems and were visualized using iodine and/or UV light. Each individual compound was identified as a single spot on TLC plate (purity greater than 95%).
Representative syntheses
2-(2-Bromoethyl)-5-(3-phenoxybenzyl)-1,2,5-thiadiazolidine 1,1-dioxide (2) & 2,2′-ethane-1,2-diylbis[5-(3-phenoxybenzyl)-1,2,5-thiadiazolidine]1,1,1′,1′-tetraoxide (3)
Sodium hydride (60% w/w; 0.27 g; 6.5 mmol) was added slowly to a solution of compound 1 (1.52 g; 5 mmol) in DMF (15 mL) cooled to 0 °C and the reaction mixture was stirred for 30 min. 1,2-Dibromoethane (0.94 g; 5 mmol) was added and the reaction mixture was stirred overnight at room temperature. The solvent was removed in vacuo and the residue was taken up in ethyl acetate (30 mL) and washed with brine (2 × 15 mL). The organic layer was separated, dried over anhydrous sodium sulfate, and concentrated, leaving a crude product which was purified by flash chromatography (silica gel/ethyl acetate/hexanes) to yield compound 2 as a colorless oil (0.91 g; 44% yield). 1H NMR (400 MHz, CDCl3): δ 3.22 (t, J = 6.0 Hz, 2H), 3.45 (t, J = 6.0 Hz, 2H), 4.18 (s, 4H), 6.92–7.13 (m, 3H), 7.10–7.19 (m, 2H), 7.27–7.42 (m, 3H) and compound 3 as white solid (0.59 g; 37% yield), mp 93–95°C. 1H NMR (400 MHz, CDCl3): δ 3.21 (t, J = 6.7 Hz, 2H), 3.45 (t, J = 6.7 Hz, 2H), 4.18 (s, 4H), 6.92–7.18 (m, 11H), 7.25–7.41 (m, 7H). 13C NMR (100 MHz, CDCl3): δ 45.19, 46.17, 46.23, 51.26, 118.48, 119.04, 119.10, 123.44, 123.69, 130.00, 130.26, 137.05, 156.99, 157.82. HRMS (ESI): Calculated for C32H34N4O6S2 [M+Na]+ 657.1818; found 657.1826.
3,3′-(2,2′-(Piperazine-1,4-diyl)bis(ethane-2,1-diyl))bis(1-(3-phenoxybenzyl)1,2,5-thiadiazolidine 1,1-dioxide) (4)
To a solution of compound 5 (0.91 g; 2.2 mmol) in DMF (10 mL) was added piperazine (0.19 g; 2.2 mmol), followed by anhydrous K2CO3 (1.38 g; 10 mmol) and the reaction mixture was stirred at room temperature overnight. The solvent was removed in vacuo and the residue was taken up in ethyl acetate (30 mL) and washed with brine (2 × 20 mL). The organic layer was separated, dried over anhydrous sodium sulfate, and concentrated, leaving a crude product as a colorless oil which was purified by flash chromatography (silica gel/ethyl acetate/hexanes) to yield compound 4 as white solid (0.61g; 37% yield), mp 88–89°C. 1H NMR (400 MHz, CDCl3): δ 2.51 (s, 8H), 2.65 (t, J = 6.3 Hz, 4H), 3.18–3.27 (m, 8H), 3.40 (t, J = 5.5 Hz, 4H), 4.18 (s, 4H), 6.92–7.18 (m, 11H), 7.25–7.41 (m, 7H). 13C NMR (100 MHz, DMSO-d6): δ 44.63, 45.23, 45.59, 50.26, 52.74, 55.60, 117.78, 118.53, 118.58, 123.46, 123.51, 130.07, 130.13, 138.15, 156.49, 156.69. HRMS (ESI): Calculated for C38H47N6O6S2 [M+H]+ 747.2999; found 747.3054.
Methyl [1,1-dioxido-5-(3-phenoxybenzyl)-1,2,5-thiadiazolidin-2-yl]acetate (5)
A solution of compound 1 [4] (1.52 g; 5 mmol) in dry acetonitrile (20 mL) and dry DMF (10 mL) kept at 0°C was treated with sodium hydride (0.24 g; 60% w/w in mineral oil; 6 mmol) and the reaction mixture was stirred for 15 min. Methyl bromoacetate (0.76 g; 5 mmol) was added and the solution was stirred overnight at room temperature. The solvents were removed under high vacuum and the residue was taken up in ethyl acetate (75 mL) and washed with brine (40 mL), 5% HCl (2 × 40 mL), and brine (40 mL). The organic layer was separated, dried over anhydrous sodium sulfate, filtered, and concentrated to give compound 5 as colorless oil (1.6 g; 85% yield). 1H NMR (400 MHz, CDCl3): δ 3.76 (s, 3H), 3.9 (s, 2H), 4.21 (s, 2H), 6.92–7.19 (m, 6H), 7.22–7.40 (m, 3H).
2-[1,1-Dioxido-5-(3-phenoxybenzyl)-1,2,5-thiadiazolidin-2-yl]ethyl methanesulfonate (6)
To a stirred solution of compound 5 (1.88 g; 5 mmol) in dry THF (8 mL) was added dropwise a solution of 2 M lithium borohydride (2.5 mL; 5 mmol), followed by the dropwise addition of absolute ethanol (15 mL). The reaction mixture was stirred overnight at room temperature, cooled in an ice bath, and then acidified with 5% HCl to pH ~4. The solvent was removed and the residue was taken up in ethyl acetate (75 mL) and washed with brine (25 mL). The organic layer was separated, dried over anhydrous sodium sulfate, filtered, and concentrated to give the corresponding alcohol as a colorless oil (1.62 g; 90% yield). 1H NMR (400 MHz, CDCl3): δ 2.2 (t, J = 10.1 Hz, 1H), 3.25 (t, J = 10.0 Hz, 2H), 3.83 (q, J = 13.3 Hz, 2H), 4.2 (s, 2H), 6.92–7.19 (m, 6H), 7.22–7.40 (m, 3H). A solution of the alcohol (1.57 g; 4.5 mmol) in dry THF (8 mL) kept in an ice bath was treated with methanesulfonyl chloride (0.51 g; 4.5 mmol), followed by the dropwise addition of triethylamine (0.91 g; 9 mmol). The ice bath was removed and the reaction mixture was stirred for 5 h at room temperature. The solvent was removed and the residue was taken up in ethyl acetate (60 mL) and washed with 5% HCl (2 × 30 mL) and brine (30 mL). The organic layer was separated, dried over anhydrous sodium sulfate, filtered, and concentrated, leaving a crude product which was purified by flash chromatography (silica gel/ethyl acetate/hexanes) to give compound 6 as a colorless oil (1.92 g; 100% yield). 1H NMR (400 MHz, CDCl3): δ 3.08 (s, 3H), 3.25 (t, J = 10.0 Hz, 2H), 4.2 (s, 2H), 4.4 (t, J = 8.3 Hz, 2H), 6.92–7.19 (m, 6H), 7.22–7.40 (m, 3H).
1-Benzyl-4-{2-[5-(3-Phenoxybenzyl)-1,1-dioxido-1,2,5-thiadiazolidin-2-yl]ethyl}piperazine (7a)
To a solution of compound 6 (2.13 g; 5 mmol) in dry acetonitrile (15 mL) was added 1-benzyl piperazine hydrogen bromide (1.69 g; 5 mmol) and anhydrous K2CO3 (1.24 g; 9 mmol) and the reaction mixture was refluxed for 20 h. The reaction mixture was cooled to room temperature and the solvent was removed. The residue was treated with water (20 mL) and the solution was extracted with ethyl acetate (2 × 50 mL). The organic layer was separated, dried over anhydrous sodium sulfate, filtered, and concentrated, leaving a light yellow oil which was purified by flash chromatography (silica gel/ethyl acetate/hexanes) to yield 7a as a colorless oil (1.02 g; 40% yield).1H NMR (400 MHz, CDCl3): δ 2.4–2.6 (m, 8H), 2.62 (t, J = 9.0 Hz, 2H), 3.2 (m, 4H), 3.4 (t, J = 9.0 Hz, 2H), 3.5 (s, 2H), 4.2 (s, 2H), 6.92–7.19 (m, 6H), 7.22–7.40 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 45.25, 45.32, 46.28, 51.33, 53.18, 53.43, 56.46, 63.18, 118.44, 119.08, 123.47, 123.66, 127.21, 128.37, 129.35, 129.98, 130.22, 137.31, 138.31, 138.19, 157.04, 157.78. HRMS (ESI): Calculated for C28H35N4O3S [M+H]+ 507.2430; found 507.2412.
1-{2-[5-(3-Phenoxybenzyl)-1,1-dioxido-1,2,5-thiadiazolidin-2-yl]ethyl}piperazine (7b)
To a solution of compound 7a (4.3 g; 8.5 mmol) in methanol (25 mL) was added 10% Pd-C (0.2 g) wetted with ethyl acetate (10 mL). The reaction mixture was subjected to 20 psi for 24 h hydrogen gas using a Parr hydrogenator. The catalyst was filtered through Celite and the Celite pad was washed with methanol (10 mL). The combined filtrates were evaporated to give compound 7b as a colorless oil (3.5 g; 99% yield). 1H NMR (400 MHz, CDCl3): δ 1.91 (m, 1H) 2.4–2.6 (m, 8H), 2.62 (t, J = 9.0 Hz, 2H), 3.2 (m, 4H), 3.4 (t, J = 9.0 Hz, 2H), 4.2 (s, 2H), 6.92–7.19 (m, 6H), 7.22–7.40 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 45.15, 45.26, 46.20, 46.33, 51.34, 54.78, 57.09, 118.46, 119.10, 123.49, 123.68, 130.01, 130.24, 137.30, 157.05, 157.80. HRMS (ESI): Calculated for C21H29N4O3S [M+H]+ 417.1960; found 417.1971.
2-(2-Morpholinoethyl)-5-(3-phenoxybenzyl)-1,2,5-thiadiazolidine 1,1-dioxide (7c) [4]
A solution of compound 6 (0.92 g; 2.2 mmol) in 10 mL 95% ethanol was treated with solid sodium bicarbonate (1.0 g; 12 mmol) followed by morpholine (0.19 g; 2.2 mmol) and the reaction mixture was refluxed for 18 h. The reaction mixture was cooled to room temperature, ethanol was removed, and the residue was treated with ethyl acetate (30 mL) and water (30 mL). The layers were separated and the organic layer was washed with brine (30 mL) and dried over anhydrous sodium sulfate. The solvent was removed to yield compound 7c as colorless oil (0.91 g; 99% yield). 1H NMR (400 MHz, CDCl3): δ 2.55 (t, J = 5.01 Hz, 4H), 2.64 (t, J = 8.0 Hz, 2H), 3.20 (q, J = 6.50 Hz, 4H), 3.21 (t, J = 6.3 Hz, 2H), 3.73 (t, J = 4.31 Hz, 4H), 4.18 (s, 2H), 6.93–7.20 (m, 6H), 7.24–7.41 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 45.15, 45.26, 46.20, 46.33, 51.34, 54.78, 57.09, 118.46, 119.10, 123.49, 123.68, 130.01, 130.24, 137.30, 157.05, 157.80. HRMS (ESI): Calculated for C21H28N4O3S [M+H]+ 416.1886; found 416.1857.
2-(3-Phenoxybenzyl)-5-(2-(piperidin-1-yl)ethyl)-1,2,5-thiadiazolidine1,1-dioxide (7d) [4]
A solution of compound 6 (0.82 g; 2 mmol) in 95% ethanol (4 mL) was treated with piperidine (0.17 g; 2 mmol) and solid sodium bicarbonate (0.50 g; 6 mmol) and the reaction mixture was refluxed for 18 h. The reaction mixture was cooled to room temperature and then filtered. The filtrate was concentrated and the residue was taken up in ethyl acetate (30 mL) and water (20 mL) and the layers were separated. The organic layer was washed with brine (30 mL), dried over anhydrous sodium sulfate, filtered, and concentrated, leaving 7d as a yellow oil (0.62 g; 75% yield). 1H NMR (400 MHz, CDCl3): δ 1.38–1.44 (m, 2H), 1.50–1.62 (m, 4H), 2.33–2.50 (m, 4H), 2.60 (t, J = 7.0 Hz, 2H), 3.22 (q, J = 6.7 Hz, 4H), 3.40 (t, J = 5.6 Hz, 2H), 4.18 (s, 2H), 6.93–7.20 (m, 6H), 7.24–7.41 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 24.39, 26.11, 45.31, 45.41, 46.29, 51.35, 54.85, 57.20, 118.44, 119.10, 123.49, 123.66, 129.99, 130.23, 137.36, 157.07, 157.80. HRMS (ESI): Calculated for C22H30N3O3S [M+H]+ 416.2008; found 416.2000.
Methyl N-{2-[5-(3-phenoxybenzyl)-1,1-dioxido-1,2,5-thiadiazolidin-2-yl]ethyl} isonipecotate (7e)
To a solution of compound 6 (2.13 g; 5 mmol) dry acetonitrile (15 mL) was added isonipecotic acid methyl ester (0.71g; 5 mmol) followed by anhydrous K2CO3 (1.24 g; 9 mmol) and the reaction mixture was refluxed for 20 h. The reaction mixture was cooled to room temperature, filtered, and concentrated. The residue was treated with water (20 mL) and the solution was extracted with ethyl acetate (2 × 50 mL). The organic layer was separated, dried over anhydrous sodium sulfate, filtered, and concentrated to give compound 7e as a white solid (2.30 g; 97% yield), mp 59–62°C. 1H NMR (400 MHz, CDCl3): δ 1.62–1.80 (m, 2H), 1.83–1.94 (m, 2H), 2.1 (t, J = 10.0 Hz, 2H), 2.22–2.30 (m, 1H), 2.62 (t, J = 10.0 Hz, 2H), 2.84–2.93 (m, 2H), 3.18–3.23 (m, 4H), 3.4 (t, J = 10.0 Hz, 2H), 3.72 (s, 3H), 4.2 (s, 2H), 6.9–7.10 (m, 4H), 7.12–7.2 (m, 2H), 7.23–7.41 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 28.43, 41.01, 45.29, 45.36, 46.37, 51.33, 51.83, 53.22, 56.76, 118.44, 119.09, 123.47, 123.66, 129.98, 130.23, 137.32, 157.06, 157.79, 175.61. HRMS (ESI): Calculated for C24H32N3O5S [M+H]+ 474.2063; found 474.2046.
N-{2-[5-(3-Phenoxybenzyl)-1,1-dioxido-1,2,5-thiadiazolidin-2-yl]ethyl}isonipecotic acid (7f)
To a solution of compound 7e (0.71 g; 1.5 mmol) in 8 ml 1,4 dioxane was added 1M aqueous lithium hydroxide (6 mL) and the reaction mixture was stirred at room temperature for 1 h. The solvent was removed and the residue was dissolved in water (20 mL) and the pH was adjusted to ~1. The solution was extracted with ethyl acetate (2 × 40 mL). The organic layer was separated, dried over anhydrous sodium sulfate, filtered, and concentrated to give compound 7f as a white solid (0.54 g; 79% yield), mp 108–110°C. 1H NMR (400 MHz, CDCl3): δ 1.62–1.80 (m, 2H), 1.83–1.94 (m, 2H), 2.1 (t, J = 10.0 Hz, 2H), 2.22–2.30 (m, 1H), 2.62 (t, J = 10.0 Hz, 2H), 2.84–2.93 (m, 2H), 3.18–3.23 (m, 4H), 3.4 (t, J = 10.0 Hz, 2H), 4.2 (s, 2H), 6.9–7.10 (m, 4H), 7.12–7.2 (m, 2H), 7.23–7.41 (m, 3H), 10.76 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ 25.24, 42.15, 45.17, 45.41, 50.35, 51.18, 117.84, 118.60, 123.50, 123.54, 130.09, 130.18, 137.99, 156.47, 156.71, 174.64. HRMS (ESI): Calculated for C23H30N3O5S [M+H]+ 460.1906; found 460.1888.
1-Ethoxycarbonyl-4-{2-[5-(3-phenoxybenzyl)-1,1-dioxido-1,2,5-thiadiazolidin-2-yl]ethyl}piperazine (7g)
Compound 7g was prepared using the same procedure as that used in the synthesis of compound 7a to yield 7g as a colorless oil (39% yield). 1H NMR (400 MHz, CDCl3): δ 1.22 (t, J = 9.7 Hz, 3H), 2.43 (t, J = 6.3 Hz, 4H), 2.63 (t, J = 10.0 Hz, 2H), 3.2 (q, J = 11.0 Hz, 4H), 3.36–3.50 (m, 6H), 4.19–4.22 (m, 4H), 6.9–7.10 (m, 4H), 7.12–7.2 (m, 2H), 7.23–7.41 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 14.84, 43.60, 45.23, 45.31, 46.46, 51.33, 53.15, 56.36, 61.60, 118.49, 118.97, 119.10, 123.39, 123.48, 123.70, 130.01, 130.26, 137.21, 155.59, 157.04, 157.83. HRMS (ESI): Calculated for C24H33N4O5S [M+H]+ 489.2172;found 489.2161.
1-(4-Chlorobenzoyl)-4-{2-[5-(3-phenoxybenzyl)-1,1-dioxido-1,2,5-thiadiazolidin-2-yl]ethyl}piperazine (8a)
To a solution of 4-chlorobenzoic acid (0.62 g; 4 mmol) in dry DMF (20 mL) was added EDCI (1.14 g; 6 mmol) and N-hydroxybenzotriazole monohydrate (0.91 g; 6 mmol) and the mixture was stirred for 20 min. Compound 7b (1.66 g; 4 mmol) was added and stirring was continued overnight. The DMF was removed in vacuo and the residue was taken up in ethyl acetate (120 mL) and washed with water (30 mL) and brine (25 mL). The organic layer was separated, dried over anhydrous sodium sulfate, filtered, and concentrated, leaving a crude product which was purified by flash chromatography (silica gel/ethyl acetate/hexanes) to give compound 8a as a white solid (0.94 g; 42% yield), mp 89–91°C. 1H NMR (400 MHz, CDCl3): δ 2.4–2.6 (m, 4H), 2.62 (t, J = 9.0 Hz, 2H), 3.2 (m, 4H), 3.4 (t, J = 9.0 Hz, 2H), 4.2 (s, 2H), 6.9–7.08 (m, 3H), 7.10–7.19 (m, 2H), 7.23–7.41 (m, 8H). 13C NMR (100 MHz, DMSO-d6): δ 41.83, 45.17, 45.39, 50.41, 50.70, 52.18, 117.83, 118.59, 123.48, 123.53, 128.62, 129.79,129.88, 130.08, 130.17, 130.08, 131.11, 137.96, 156.46, 156.70, 161.60, 168.26. HRMS (ESI): Calculated for C28H32ClN4O4S [M+H]+ 555.1833; found 555.1819.
Compounds 8b-d were prepared using the same procedure as that used in the synthesis of compound 8a.
1-(4-Fluorobenzoyl)-4-{2-[5-(3-phenoxybenzyl)-1,1-dioxido-1,2,5-thiadiazolidin-2-yl]ethyl}piperazine (8b)
yellow oil (40% yield). 1H NMR (400 MHz, CDCl3): δ 2.40–2.60 (m, 4H), 2.62 (t, J = 9.0 Hz, 2H), 3.22 (m, 4H), 3.40 (t, J = 9.0 Hz, 2H), 4.21 (s, 2H), 6.9–7.08 (m, 3H), 7.10–7.19 (m, 2H), 7.23–7.41 (m, 8H). 13C NMR (100 MHz, DMSO-d6): δ 41.83, 45.17, 45.39, 50.41, 50.70, 52.18, 115.42, 115.64, 117.83, 118.59, 123.48, 123.53, 129.79, 129.88, 130.08, 130.17, 130.08, 131.11, 137.96, 156.46, 156.70, 161.60, 164.06, 168.26. HRMS (ESI): Calculated for C28H32FN4O4S [M+H]+ 539.2128; found 539.2130.
1-(3-Phenoxybenzoyl)-4-{2-[5-(3-phenoxybenzyl)-1,1-dioxido-1,2,5-thiadiazolidin-2-yl]ethyl}piperazine (8c)
yellow oil (80% yield). 1H NMR (400 MHz, CDCl3): δ 2.52–2.70 (m, 4H), 2.75 (t, J = 9.3 Hz, 2H), 3.12–3.30 (m, 4H), 3.71 (t, J = 9.3 Hz, 2H), 3.50 (s, 2H), 3.82 (s, 2H), 4.21 (s, 2H), 6.92–7.19 (m, 10H), 7.22–7.40 (m, 7H), 7.62 (d, J = 6.1 Hz, 1H). 13C NMR (100 MHz, DMSO-d6): δ 41.80, 43.42, 45.06, 45.31, 50.26, 50.61, 52.21, 60.07, 72.04, 116.67, 117.69, 118.43, 118.87, 119.10, 119.70, 121.64, 123.31, 123.37, 123.81, 129.99, 130.03, 130.26, 136.44, 137.81, 155.95, 156.59, 156.73, 168.12. HRMS (ESI): Calculated for C34H36N4O5S [M+Na]+ 635.2304; found 635.2300.
2-(2-(4-(Benzylsulfonyl)piperazin-1-yl)ethyl)-5-(3-phenoxybenzyl)-1,2,5-thiadiazolidine 1,1-dioxide (8d)
A solution of compound 7b (0.83 g; 2 mmol) in methylene chloride (30 mL) was treated with benzylsulfonyl chloride (0.38 g; 2 mmol) and triethylamine (0.40 g; 4 mmol). The reaction mixture was stirred overnight at room temperature. Methylene chloride (30 mL) was added and the solution was washed with water (2 × 30 mL) and brine (25 mL). The organic layer was separated, dried over anhydrous sodium sulfate, filtered, and concentrated, leaving a crude compound which was purified by flash chromatography (silica gel/ethyl acetate/hexanes) to give compound 8d as a white solid (0.50 g; 43.8% yield), mp 98–100°C, 1H NMR (400 MHz, CDCl3): δ 2.45 (t, J = 6.33 Hz, 4H), 2.62 (t, J = 6.66 Hz, 2H), 3.10–3.21 (m, 8H), 3.25–3.28 (m, 2H), 4.18 (s, 2H), 4.21 (s, 2H), 6.92–7.05 (m, 4H), 7.08–7.16 (m, 2H), 7.22–7.42 (m, 8H). 13C NMR (100 MHz, CDCl3): δ 45.19, 45.61, 46.15, 46.45, 51.35, 53.13, 56.04, 56.99, 118.52, 119.13, 123.52, 123.74, 128.93, 129.00, 130.05, 130.30, 130.96, 137.25, 157.08, 157.85. HRMS (ESI): Calculated for C28H35N4O5S2 [M+H]+ 571.2049; found 571.2042.
Compound 8e, 8f were also prepared using a similar procedure as that used in the synthesis of compound 8d.
1-Methanesulfonyl-4-{2-[5-(3-phenoxybenzyl)-1,1-dioxido-1,2,5-thiadiazolidin-2-yl]ethyl}piperazine (8e)
white solid (58% yield), mp 68–70°C, 1H NMR (400 MHz, CDCl3): δ 2.61–2.73 (m, 6H), 2.80 (s, 3H), 3.2–3.35 (m, 8H), 3.41 (t, J = 10.2 Hz, 2H), 4.2 (s, 2H), 6.92–7.19 (m, 5H), 7.22–7.40 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 34.46, 45.13, 45.67, 45.98, 46.47, 51.29, 52.62, 55.91, 118.49, 119.07, 119.10, 123.49, 123.70, 130.01, 130.26, 137.18, 157.03, 157.80. HRMS (ESI): Calculated for C22H31N4O5S2 [M+H]+ 495.1736; found 495.1723.
1-(n-Butylsulfonyl)-4-{2-[5-(3-phenoxybenzyl)-1,1-dioxido-1,2,5-thiadiazolidin-2-yl]ethyl}piperazine (8f)
light yellow oil (40% yield). 1H NMR (400 MHz, CDCl3): δ 0.98 (t, J = 10.0 Hz, 3H), 1.42 (p, J = 14.0 Hz, 2H), 1.74–1.83 (m, 2H), 2.6 (t, J = 9.3 Hz, 4H), 2.72 (t, J = 10.0 Hz, 2H), 2.9 (t, J = 10.0 Hz, 2H), 3.19–3.22 (m, 4H), 3.30 (t, J = 10.0 Hz, 4H), 3.39 (t, J = 10.0 Hz, 2H), 4.2 (s, 2H), 6.9–7.10 (m, 4H), 7.12–7.2 (m, 2H), 7.23–7.41 (m, 3H). 13C NMR (100 MHz, CDCl3): δ 13.73, 21.88, 25.13, 45.13, 45.56, 45.83, 46.42, 49.00, 51.26, 52.96, 55.98, 118.44, 119.04, 123.45, 123.66, 129.98, 130.23, 137.17, 157.00, 157.76. HRMS (ESI): Calculated for C25H37N4O5S2 [M+H]+ 537.2205; found 537.2217.
Representative preparation of the hydrochloride salts of compounds 7c and 8a–c
Compound 7c (41.7 mg; 0.1 mmol) was dissolved in 1 mL 1, 4-dioxane and treated with 4 M HCl (25 μL; 0.1 mmol) in a sealed vial for 10 min at room temperature. The solvent was evaporated and the residue was re-dissolved in 1 mL 1, 4-dioxane. The solvent was again removed, leaving the hydrochloride salt as a white solid.
Methyl N-[5-(3-phenoxybenzyl)-1,1-dioxido-1,2,5-thiadiazolidin-2-yl]acetyl isonipecotate (10a)
A solution of compound 9 [4] (1.81g; 5 mmol ) in dry dimethyl formamide (20 mL) was treated with EDCI (1.15 g; 6 mmol) and the solution was stirred for 15 min. Isonipecotic acid methyl ester (0.71 g; 5 mmol) was added and the reaction mixture was stirred overnight. The solvent was removed in vacuo and the residue was taken up in ethyl acetate (150 mL) and washed with saturated NaHCO3 (50 mL) and brine (25 mL). The organic layer was separated, dried over anhydrous sodium sulfate, filtered, and concentrated, leaving a light yellow viscous oil which was purified using flash chromatography (silica gel/hexanes/ethyl acetate) to give compound 10a as a white solid (0.53 g; 22% yield), mp 94–97°C. 1H NMR (400 MHz, CDCl3): δ 1.60–1.79 (m, 2H), 1.91–2.05 (m, 2H), 2.51–2.62 (m, 1H), 2.81–3.0 (m, 1H), 3.12–3.30 (m, 4H), 3.5 (t, J = 10.4 Hz, 2H), 3.72 (s, 2H), 3.92 (s, 3H), 4.2 (s, 2H), 4.35–4.42 (m, 1H), 6.92–7.09 (m, 4H), 7.19–7.2 (t, J = 10.0 Hz, 1H), 7.25–7.39 (m, 4H). 13C NMR (100 MHz, CDCl3): δ 27.92, 28.57, 40.77, 41.57, 44.92, 45.37, 46.10, 49.04, 51.51, 52.14, 118.51, 119.06, 119.17, 123.46, 123.73, 130.04, 130.31, 137.14, 157.04, 157.87, 165.20, 174.59. HRMS (ESI): Calculated for C24H29N3O6S [M+Na]+ 510.1675; found 510.1656.
N-[5-(3-Phenoxybenzyl)-1,1-dioxido-1,2,5-thiadiazolidin-2-yl]acetyl isonipecotic acid (10b)
A solution of compound 10a (0.48 g; 1 mmol) in 8 mL 1,4 dioxane was treated with 1M aqueous lithium hydroxide (6 mL) and the reaction mixture was stirred at room temperature for 1 h. The solvent was removed and the residue was dissolved in 20 mL water. The pH was adjusted to ~1 and extracted with ethyl acetate (2 × 40 mL). The organic layer was separated, dried, and evaporated to yield compound 10b as a yellow viscous oil (0.4 g; 85% yield). 1H NMR (400 MHz, DMSO-d6): δ 1.60–1.79 (m, 2H), 1.91–2.05 (m, 2H), 2.51–2.62 (m, 1H), 2.81–3.0 (m, 1H), 3.12–3.30 (m, 4H), 3.5 (t, J = 10.4 Hz, 2H), 3.72 (s, 2H), 3.92 4.2 (s, 2H), 4.35–4.42 (m, 1H), 6.92–7.09 (m, 4H), 7.19–7.2 (t, J = 10.0 Hz, 1H), 7.25–7.39 (m, 4H), 9.32 (s, 1H). 13C NMR (100 MHz, DMSO-d6): δ 27.74, 42.83, 44.03, 46.20, 48.61, 117.29, 117.97, 118.59, 118.68, 122.72, 123.53, 130.10, 130.17, 138.92, 156.50, 156.81, 163.47, 175.23. HRMS (ESI): Calculated for C23H27N3O6S [M+Na]+ 496.1518; found 496.1541.
Compound 10c was prepared using the same procedure as that used for compound 10a.
1-Ethyl-4-[5-(3-Phenoxybenzyl)-1,1-dioxido-1,2,5-thiadiazolidin-2-yl]acetylpiperazine (10c)
Colorless oil (27% yield). 1H NMR (400 MHz, CDCl3): δ 1.22 (t, J = 10.0 Hz, 3H), 3.21–3.40 (m, 5H), 3.41–3.55 (m, 3H), 3.72–3.80 (m, 2H), 3.91 (d, J = 10.0 Hz, 2H), 4.11–4.35 (m, 5H), 4.6 (s, 2H), 6.92–7.2 (m, 6H), 7.22–7.40 (m, 3H). 13C NMR (100 MHz, DMSO-d6): δ 13.23, 43.81, 48.55, 48.78, 49.62, 50.38, 51.25, 51.34, 56.02, 117.29, 117.97, 118.59, 118.68, 122.72, 123.53, 130.10, 130.17, 138.92, 156.50, 156.81, 163.47. HRMS (ESI): Calculated for C23H30N4O4S [M+Na] 481.1885; found 481.1836.
Biochemical Studies
One-day old, 80–90% confluent HG23 cells were treated with varying concentrations of each compound (0 [mock-DMSO]-320 μM) to examine its effects on the replication of NV. At 24 or 48 hrs of treatment, the NV protein or genome were analyzed with Western blot analysis or qRT-PCR, respectively. The ED50s of the compounds for NV genome levels were determined at 24 hr post-treatment. The cytotoxic effects of the compounds on HG23 cells were determined using a cell cytotoxicity assay kit (Promega, Madison, WI) to calculate the median toxic dose (TD50) at 48 hr of treatment.
Acknowledgments
The generous financial support of this work by the National Institutes of Health (AI081891) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bergmann RJ, Gebhardt J, Gould E, Tucker P, Unge T, Hilgenfeld R, Neyts J. Antiviral Research. 2010;87:162–178. doi: 10.1016/j.antiviral.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koo HL, Ajami N, Atmar RL, DuPont HL. Discov Med. 2010;10:61–70. [PMC free article] [PubMed] [Google Scholar]
- 3.Atmar RL. Food Environ Virol. 2010;2:117–126. doi: 10.1007/s12560-010-9038-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dou D, Tiew KC, He G, Mandadapu SR, Aravapalli S, Alliston KR, Kim Y, Chang KO, Groutas WC. Bioorg Med Chem. 2011;19:5975–5983. doi: 10.1016/j.bmc.2011.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dou D, Mandadapu S, Alliston KR, Kim Y, Chang KO, Groutas WC. Bioorg Med Chem. 2011;19:5749–5755. doi: 10.1016/j.bmc.2011.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillespie P, Goodnow RA. Ann Rep Med Chem. 2004;39:293–304. [Google Scholar]
- 7.Chang KO, Sosnovtsev SV, Belliot G, King AD, Green KY. Virology. 2006;2:463–473. doi: 10.1016/j.virol.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 8.Chang KO, George DW. J Virol. 2007;22:12111–12118. doi: 10.1128/JVI.00560-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang KO. J Virol. 2009;83:8587–8595. doi: 10.1128/JVI.00005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim Y, Thapa M, Thuy DH, Chang KO. Antiviral Res. 2011;89:165–173. doi: 10.1016/j.antiviral.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan M, Jiang X. Curr Opin Investig Drugs. 2008;9:146–151. [PubMed] [Google Scholar]
- 12.Guiard J, Fiege B, Kitov PI, Peters T, Bundle DR. Chem Eur J. 2011;17:7438–7441. doi: 10.1002/chem.201003414. [DOI] [PubMed] [Google Scholar]
- 13.Rademacher C, Guiard J, Kitov PI, Fiege B, Dalton KP, Parra F, Bundle DR, Peters T. Chem Eur J. 2011;17:7442–7453. doi: 10.1002/chem.201003432. [DOI] [PubMed] [Google Scholar]
- 14.DeSimone RW, Currie KS, Mitchell SA, Darrow JW, Pippin DA. Comb Chem High Trhoughput Screen. 2004;7:473–494. doi: 10.2174/1386207043328544. [DOI] [PubMed] [Google Scholar]
- 15.Welsh ME, Snyder SA, Stockwell BR. Curr Opin Chem Biol. 2010;14:347–361. doi: 10.1016/j.cbpa.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Posner GH, Chang W, Hess L, Woodard L, Sinishtaj S, Usera AR, Maio W, Rosenthal AS, Kalinda AS, D’Angelo JG, Petersen KS, Stohler R, Chollet J, Santo-Tomas J, Snyder C, Rottmann M, Wittlin S, Brun R, Shapiro TA. J Med Chem. 2008;51:1035–1042. doi: 10.1021/jm701168h. [DOI] [PubMed] [Google Scholar]
- 17.Bolognesi ML, Cavalli A, Valgimigli L, Bartolini M, Rosini M, Andrisano V, Recanatini M, Melchiorre C. J Med Chem. 2007;50:6446–6449. doi: 10.1021/jm701225u. [DOI] [PubMed] [Google Scholar]