Abstract
Mutations in Gjb2 and Gjb6 genes, coding for connexin26 (Cx26) and Cx30 proteins respectively, are linked to about half of all cases of human autosomal non-syndromic prelingual deafness. Molecular mechanisms of the hearing impairments, however, are unclear. Most cochlear gap junctions (GJs) are co-assembled from Cx26 and Cx30 and deletion of either one of them causes deafness. Our previous studies have shown that normal hearing is possible in the absence of the Cx30 gene when Cx26 is over-expressed. To further test unique functional requirements for various types of connexins in the hearing, we investigated whether the hearing in the conditional Cx26 (cCx26) null mice could be rescued by genetically over-expressing Cx30.
Multiple lines of control and experimental mouse models were used. Auditory brainstem response (ABR) measurements showed normal hearing in targeted gene deletion mice when the deleted Cx26 or Cx30 was transgenically expressed from integrated bacterial artificial chromosome (BAC), demonstrating the effectiveness of the BAC rescue approach. In contrast, severe hearing loss was found in cCx26 null mice in which Cx30 was over-expressed. Morphology observations were consistent with the ABR data. Cochleae of cCx26 null mice with and without the transgenic over-expression of Cx30 both showed the typical immature feature of postnatal cochlear development-the closed tunnel of Corti. Immunolabeling data and Western blot quantification indicated that the Cx26 protein expression preceded that of Cx30 during the early postnatal period in the cochlea. Null expression of Cx26 may therefore uniquely result in a transient period when a total elimination of GJs in functionally-important regions of the developing cochlea is possible. We conclude that Cx26 plays an essential role in the development of the auditory sensory epithelium and its unique developmental functions required for normal hearing is not replaceable by Cx30.
Keywords: connexin26, connexin30, gap junctions, hereditary deafness, hearing rescue, mouse model, functional studies
Introduction
Connexins (Cxs) are a family of membrane proteins constituting the building blocks of gap junctions (GJs) that form intercellular channels to facilitate ionic and biochemical communication among neighboring cells. Mutations in Gjb2 (gene coding for Cx26) and Gjb6 (gene coding for Cx30) are one of the most common forms of human genetic defects [1,2,3]. In almost all ethnic populations examined so far mutations in Cx26 [1,4], and sometime in Cx30 as well [5,6], have been linked to a significant portion (~30–50%) of inherited non-syndromic deafness cases. Functional null mutations of Cx26 [7,8] and Cx30 (e.g., e.g., GJB6-D13S1830) [6] are the most common deafness-causing mutations found in deaf populations [9]. The corresponding animal models of homozygous Cx26 null [10,11] and Cx30 null [12] mice also show profound nonsyndromic deafness resembling human phenotypes. It is clear that both Cx26 and Cx30 play essential roles in normal cochlear functions. However, molecular mechanisms of deafness caused by mutations of these Cxs are still not clear.
Six Cx subunits assemble together to form GJ hemi-channels and two hemi-channels dock in GJ plaques of the two neighboring cells to generate complete GJs. Almost all GJs in the cochlea are co-assembled from Cx26 and Cx30 [13,14,15]. It has been speculated that Cx26 and Cx30 play similar functional roles in the cochlea. However, the numbers of deafness-linked human Cx26 mutations far exceed the reported Cx30 mutations (http://davinci.crg.es/deafness/). In addition, homomeric GJs constituting only Cx26 is sufficient for normal hearing in the deaf Cx30 null mutant mice when the protein level of Cx26 is increased to the wild type (WT) level [16]. The result of complete hearing rescue in the Cx30 null mutant mice by genetically over-expressing Cx26 indicates a dominate role for Cx26 in the inner ear. These results support that Cx26 and Cx30 are not functionally-equal building blocks for GJs in the cochlea. Our published paper showed that Cx26 play essential roles in cochlear development [11]. However, it is unclear whether Cx30 play any significant role in the cochlear development. To further test unique functional requirements for major connexins in the hearing, we investigated whether the hearing in the cCx26 null mice could be rescued by genetically over-expressing Cx30 in the cochlea. Results support that Cx26 plays an essential role in the postnatal development of the normal cochlear sensory epithelium, the organ of Corti. Protein expression of Cx26 preceded Cx30 temporally and spatially in many key regions of the cochlea. Therefore, significant portion of cochlear GJs were constituted by homomeric Cx26 during early cochlear development. We concluded that at least the developmental functions contributed by Cx26 are not replaceable by Cx30.
Material and Method
Transgenic and gene deletion mice used in the study
The experimental protocol for animal use was approved by the Institutional Animal Care and Use Committee of the Emory University. Five lines of gene deletion and transgenic mice were used to generate various types of mice used in the study. Gjb6−/− [12] mice, BACCx26 mice [16], mice that are homozygous for the loxP sequence on both sides of the Gjb2 gene (Gjb2loxp/loxp mice) and mice in which one of the two Foxg1 alleles was replaced with the Cre recombinase gene (Foxg1cre/+ mice) [11] were previously validated and described in details. The BACCx30 mice are a new line of mice generated for this study. Details about generation and validation of the BACCx30 mice are given in supplemental materials. Interbreeding of these mice generated Gjb2loxp/loxP;Foxg1cre/+ mice (cCx26 null mice), BACCx30;Gjb2loxp/WT;Foxg1cre/+ mice, BACCx26;Gjb6+/−, BACCx26;Gjb2loxp/WT;Foxg1cre/+. These four lines of mice were used in three breeding groups to generate the mice needed in this study (Table 1). For genotyping, pups born were screened for the presence of targeted modification or presence of markers. Oligo primers used for genotyping of mice used in this study are given in the supplemental materials. Genotyping were determined by PCR amplifications from genomic DNA extracted from mouse tail tips using PuregeneTM mouse tail kit (Gentra, Minneapolis, MN). The genotypes of BACCx30 founder mice were further confirmed by Southern blot hybridizations with a specific probe to DNA fragments (Supplementary Figs. 1&2).
Table 1.
Three mouse breeding groups used in the study and the data summary.
| Row number |
Genotype of mice | # of mice |
% of predicted genotype in mouse pups |
% of actual genotype in mouse pups |
ABR test results for hearing |
|---|---|---|---|---|---|
| Group1: Breeding of BACCx30;Gjb2loxp/WT;Foxg1Cre/+ mice and Gjb2loxp/loxP mice for testing overexpressing Cx30 to rescue hearing in cCx26 null mice. Following genotypes presented in rows 1–6 are possible. | |||||
| 1 | BACCx30;Gjb2loxp/loxp;Foxg1cre/+ | 34 | 12.5% | 15.2% | All were deaf |
| 2 | BACCx30; Gjb2loxp/WT;Foxg1cre/+ | 21 | 12.5% | 9.4% | All showed normal hearing |
| 3 | BACCx30;Gjb2loxp/WT and BACCx30;Gjb2loxp/loxp | 76 | 25% | 34.1% | All showed normal hearing |
| 4 | Gjb2loxp/loxp;Foxg1cre/+ | 26 | 12.5% | 11.6% | All were deaf |
| 5 | Gjb2loxp/WT;Foxg1cre/+ | 27 | 12.5% | 12.3% | All showed normal hearing |
| 6 | Gjb2loxp/loxp and Gjb2loxp/WT | 39 | 25% | 17.5% | All showed normal hearing |
| Total numbers of mice tested | 223 | ||||
| Group2: Breeding of BACCx30;Gjb6+/− mice and Gjb6−/− mice for testing overexpressing Cx30 to rescue hearing in Cx30 null mice. Following genotypes presented in rows 7–9 are possible. | |||||
| 7 | BACCx30;Gjb6−/− or BACCx30;Gjb6+/− | 79 | 50% | 54.1% | All showed normal hearing |
| 8 | Gjb6−/− | 37 | 25% | 25.3% | All were deaf |
| 9 | Gjb6+/− | 30 | 25% | 20.5% | All showed normal hearing |
| Total numbers of mice tested | 146 | ||||
| Group3: Breeding of BACCx26;Gjb2loxp/WT;Foxg1Cre/+ mice and Gjb2loxp/loxP mice for testing overexpressing Cx26 to rescue hearing in cCx26 null mice. Following genotypes presented in rows 10–14 are possible. | |||||
| 10 | BACCx26;Gjb2loxp/loxp;Foxg1cre/+ or BACCx26;Gjb2loxp/WT;Foxg1cre/+ | 40 | 25% | 23.8% | All showed normal hearing |
| 11 | BACCx26; Gjb2loxp/loxp and BACCx26; Gjb2loxp/WT | 40 | 25% | 23.8% | All showed normal hearing |
| 12 | Gjb2loxp/loxp; Foxg1cre/+ | 25 | 12.5% | 14.9% | All were deaf |
| 13 | Gjb2loxp/WT;Foxg1cre/+ | 20 | 12.5% | 11.9% | All showed normal hearing |
| 14 | Gjb2loxp/WT and Gjb2loxp/loxp | 43 | 25% | 25.6% | All showed normal hearing |
| Total numbers of mice tested | 168 | ||||
Western blots analyzing protein expression of Cx26 and Cx30
Details of Western blot procedures were given in our publications [16,17]. We therefore only give a briefly description here. Total proteins were extracted using RIPA lysis buffer by following the manufacturer’s instructions (Upstate Biotechnology Cell Signaling System, Lake Placid, NY). Proteins extracted from two cochleae of the same animal were used in each lane. The protein concentrations were measured by using a bicinchoninic acid protein assay kit (Pierce, Rockford, IL). Proteins were separated by electrophoresis on a 12% sodium dodecyl sulphate polyacrylamide gel. After transferring to nitrocellulose membrane, Cxs were detected by conventional Western blotting procedures using polyclonal antibodies against Cx26 (0.5 µg/ml) and/or Cx30 (1 µg/ml, Invitrogen Inc, Carlsbad, CA). Equal amount of protein (5 µg) was loaded in each lane. The amount of protein loading/lane was further checked by Western blotting of a house-keeping protein (actin, dilution factor 1:2000, Chemicon/Millipore, Billerica, MA). Protein bands on the blots were visualized by enhanced chemiluminescence (Super-Signal, Pierce, Rockford, IL) exposed to X-ray films (Hyper Film, Amersham Biosciences, Piscataway, NJ).
Cochlear histology, immunolabeling of the cochlear samples and objective evaluation of hearing sensitivities
For immunolabeling, cochlear sections and whole mount cochlear samples were labeled with antibodies against Cx26 (1:200), Cx30 (1:100, both purchased from Invitrogen, Carlsbad, CA). After washing three times with PBS, the samples were labeled with appropriate secondary antibodies conjugated to either Cy2 or Cy3 (1:500 dilution, Jackson ImmunoLab Inc., West Grove, PA). Labeled cochlear samples were mounted in an anti-fade medium (Molecular Probes Inc., Eugene, OR) and examined with a confocal microscope (Zeiss LSM). Negative controls were processed similarly with primary antibodies substituted by equal volume of PBS. Details of preparing resin cochlear section [18] and immunolabeling [17] preparations were given in our publications.
Hearing sensitivities were measured objectively by testing the auditory brainstem responses (ABRs) evoked by short-duration sounds presented repetitively (tone bursts). Details of the testing methods were given in our previously published papers [16]. The ABR thresholds were measured visually based on the appearance of a series of wave II in the ABR responses obtained at increasing or decreasing sound intensities. The genotype of mice was unknown to the ABR testers. ABR thresholds at the three most sensitive frequencies (8 kHz, 12 kHz and 18 kHz) in mice were averaged. Comparing to WT controls, we defined severe hearing loss as a threshold increase of more than 20 dB. Deafness was defined when thresholds elevations averaged at the three frequencies were more than 60 dB.
Results
Results of this study were generated from three groups of mice generated from three breeding colonies (Table 1). Genotyping results showed that the percentages of confirmed mouse genotypes followed the predicted Mendelian inheritance ratio of alleles closely (Table 1). We first investigated whether the hearing of Gjb6−/− mice could be rescued by expressing the Cx30 gene (Gjb6) from the BAC. This was to test the efficacy of the BACCx30 transgene, which was later used for hearing rescue in the cCx26 null mice later. Mice generated from interbreeding BACCx30;Gjb6+/− and Gjb6−/− mice (group2 in the Table 1) were used for this purpose. The breeding of Gjb6+/− and Gjb6−/− mice should produce 50% Gjb6−/− mice and 50% Gjb6+/− mice respectively, although we were not able to distinguish the two genotypes due to the presence of Gjb6 allele in the BAC (row 7 in the Table 1). However, all 79 mice with the BACCx30 transgene in this group showed normal hearing. If there is no hearing rescue at all, the prediction is that about half (~39) of mice should be deaf. The fact that none of the BACCx30 expressing mice in this group showed hearing loss strongly support that Gjb6 transgenically expressed from the BAC was sufficient to restore hearing for the Cx30 null mice. With these positive control results available to validate the BACCx30 mice, we tested a total of 223 mice from the offspring generated from the breeding of BACCx30;Gjb2loxp/WT;Foxg1Cre/+ mice and Gjb2loxp/loxP mice. Results showed that cCx26 null mice were always deaf, regardless of whether Cx30 was over-expressed from the BAC (results presented in rows 1&4 in the Table 1). As expected, hearing of all other mice was normal as long as one allele of Cx26 was intact (results presented in rows 2,3,5 &6).
To further test whether hearing of the deaf cCx26 null mice could be rescued at all, we conducted another series of positive control experiments by transgenically expressing the Cx26 from a modified BAC construct. The BACCx26 mice were validated in our previously published studies [16]. This group of mice (group 3 in the Table 1) were generated by interbreeding BACCx26;Gjb2loxp/WT;Foxg1cre/+ mice and Gjb2loxp/loxp mice. In the presence of BACCx26, genotypes of homozygous and heterozygous cCx26 null couldn’t be distinguished in the forty mice we tested (row 10 in Table 1). However, conditional homozygous Gjb2−/− should constitute half of the mice in which both BACCx26 and Foxg1cre were present (about 20, row 10 of Table 1). ABR testing results indicated that all mice with both the BACCx26 and Foxg1cre expressions (row 10 of Table 1) had normal hearing, therefore supporting transgenic expression of Cx26 from the BAC was able to rescue hearing in the cCx26 null mice. We also found that the hearing was normal for all other mice in the group 3 as long as there is one Gjb2 allele (rows 11–14 in the Table 1). These mice also showed expected percentages of genotypes calculated by the Mendelian inheritance rule. Combining results obtained from the three groups (Table 1), we concluded that over-expressing Cx30 was not sufficient to restore the hearing of cCx26 null mice.
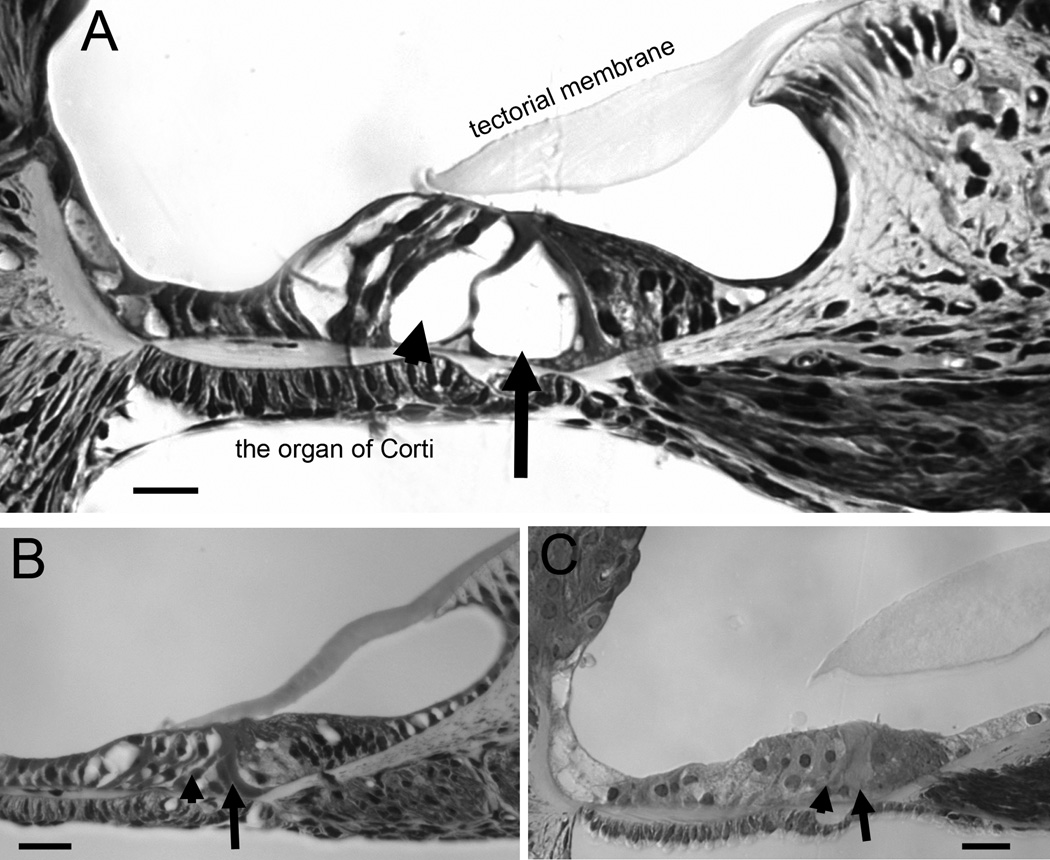
Consistent with hearing tests, morphology observations indicated that the cochleae of cCx26 null mice with the BACCx30 the transgene (rows 1 in Table 1) still showed abnormality in the postnatal development of the organ of Corti. Tunnel of Corti (arrows in Fig. 1) and Nuel’s space (arrowheads in Fig. 1) were open in the WT mice (arrow in the Fig. 1A). In contrast, both of these features of normal cochlear development were not demonstrated in the adult cCx26 null mice (arrowhead and arrow in the Fig. 1B) and in the cCx26 null mice with the presence of the BACCx30 transgene (arrowhead and arrow in the Fig. 1C).
Figure 1.
Comparison of the morphology of cochlear sections (middle turn) obtained from WT (A), cCx26 null (B) and BACCx30;Cx26 null mice. Arrowheads show the location of the Nuel’s space. Arrows point to the location of the tunnel of Corti. Scale bars represent approximately 200 µm.
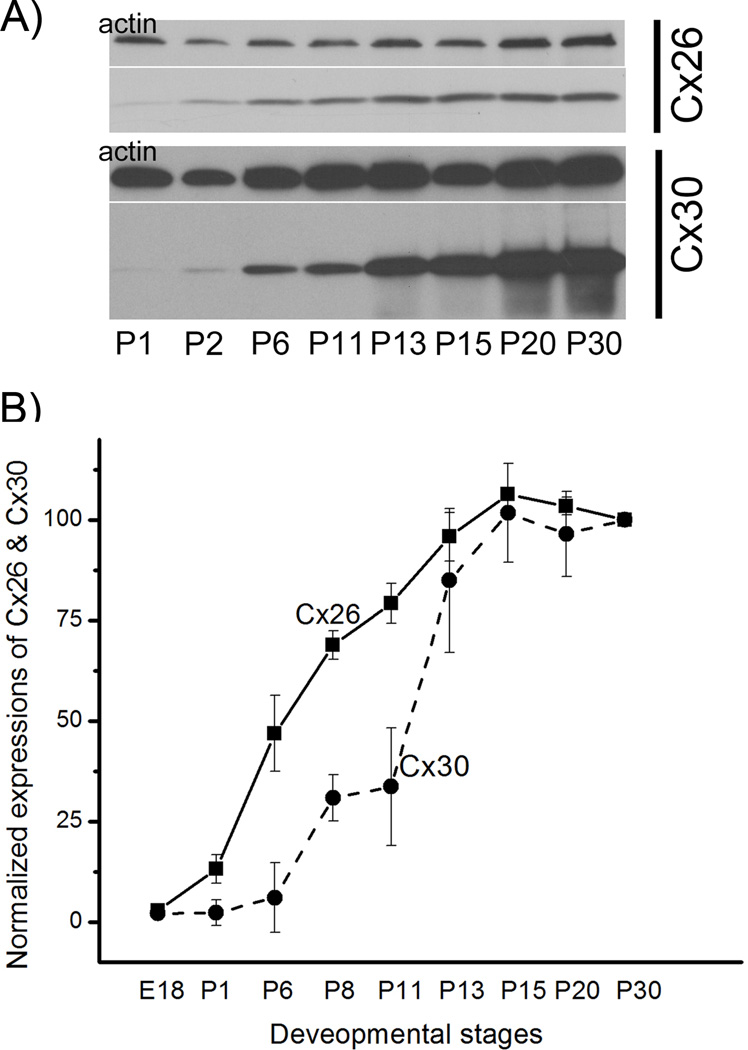
In searching for underlying mechanisms to help us understand the relative contributions of Cx26 and Cx30 in cochlear development, we compared the temporal protein expressions of the two Cxs. Protein levels of Cx26 and Cx30 in the cochlea were measured at a series of developmental stages by Western blotting (Fig. 2 A) from postnatal day 1 (P1) to postnatal day 30 (P30). The expressions were quantified by normalizing to the level of actin (a house-keeping gene) at corresponding developmental stages (Fig. 2B). Expressions of both Cx26 and Cx30 increased gradually in the developing cochlea and their levels saturated around P15. However, Cx26 protein in the cochlea (solid line in the Fig. 2B, n=5) started to increase earlier than that of Cx30 (dashed line in the Fig. 2B, n=5). The relative amount of Cx30 was significantly lower than Cx26 between P6 and P11 (p<0.01 by student t-tests), indicating that the Cx30 expression lagged behind Cx26 at the time period just before endocochlear potential starts to form in the cochlea of mice.
Figure 2.
Quantification of the protein expression levels of Cx26 and Cx30 in the cochlea of WT mice at various postnatal developmental stages. Two examples of Western blotting for Cx26 and Cx30 respectively at P1, P2, P6, P11, P13, P15 and P20 and P30 are given (A). Bands on the upper row are results obtained with actin, a house keeping protein used for normalization purpose. Normalized levels of Cx26 and Cx30 are given in (B). Vertical bars represent standard errors (n=5).
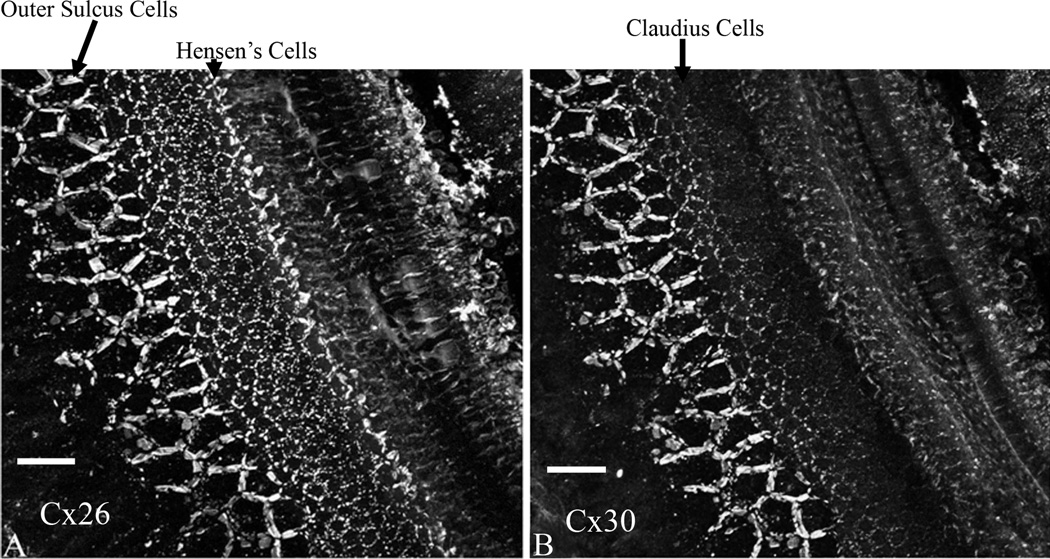
We next used immunolabeling to find out the differences in spatial expressions between the two Cxs at P8, which was at a time point when they showed the largest difference based on Western blotting results (Fig. 2B). Results obtained from whole-mount preparations of cochlear epithelium demonstrated that Cx26 (Fig. 3A) and Cx30 (Fig. 3B) immunoreactivities were similar in the outer sulcus cell region. In contrast, Cx30 immunolabeling was significantly lower in the Claudius cells, and undetected in the Hensen’s cells (comparing Fig. 3 A&B). These results suggested that most GJs in the regions of the Hensen’s cells, Claudius cells and inner sulcus cells were homomeric Cx26 GJs before P8. Therefore, Cx26 null mutation would completely eliminate GJs specifically in these regions of the organ of Corti.
Figure 3.
Immunolabeling results for Cx26 (A) and Cx30 (B) obtained from whole-mount cochlear preparations at P8. The preparation is viewed from the top. Various types of cochlear supporting cells are labeled. Bars represent approximately 200 µm.
Discussion
It is well established that Cx26 and Cx30 are co-localized in the majority of the GJ plaques (85–95% except in the Deiters’ cells [17]) of the adult cochlea in mice. No other Cxs are expressed in the cells where Cx26 and Cx30 are co-expressed [14,15]. The genome distance on chromosome 14 between the two genes is approximately 20,000 bps apart. The relatively short distance made it possible for the two genes to share the control under the same set of regulatory elements for gene expressions [19]. The fact that the two genes share nearly 80% homology at the protein level further hinted that they may play similar roles in the cochlear GJs. However, current experimental data are lacking for testing these hypotheses.
Homozygous human deletion mutations of either Cx26 or Cx30 result in deafness [1,6]. The corresponding homozygous Cx26 and Cx30 null mice are also deaf [10,12]. Both Cxs are therefore necessary for normal hearing. In the absence of the Cx30 gene, however, Cx26 expressed at a sufficiently high level is able to provide GJ functions required for normal hearing if the copy number of Gjb2 is increased by a genetic approach, indicating that normal hearing can be achieved by Cx26 alone when it is over-expressed [16]. These results suggest that boosting the Cx26 protein level by pharmacological agents may provide an alternative therapeutic strategy to the traditional gene therapy approach for treating deafness caused by the Cx30 null expression [16]. Because many more genetic deafness cases are caused by Cx26 mutations, it is intriguing to find out whether homomeric Cx30 GJs alone expressed at a sufficiently high level is adequate for rescuing the hearing in Cx26 mutant mice. The answer to this question is the basis to know whether a similar intervention on gene expression approach may be applied for treating Cx26-null deafness. A major aim of the study is to answer this question by over-expressing Cx30 gene in the absence of the Gjb2 in the cochlea of cCx26 null mice.
The morphology of the organ of Corti was normal in the Cx30 null mice and hair cells degenerate slowly over a time period of >6 months after mice are deaf [18]. The endocochlear potential (EP), a major driving force for mechanotransduction of the auditory hair cells, is never developed in the Gjb6−/− mice [12]. These results suggest that Cx30 is required for producing sufficient quantities of GJs together with Cx26 in the cochlea and for the initiation and maintenance of the EP [12]. In contrast, relatively little is known for the specific functional roles that the cochlear Cx26 plays in the inner ear. This paper showed that over-expression of Cx30 was not able to rescue the deaf phenotype of the cCx26 null mice, as determined by both morphological examinations and objective ABR hearing tests, while two lines of control mice generated positive results as expected. We also found that, for the first time, the Cx26 is the only GJ protein detected by immunolabeling in many key supporting cells in the organ of Corti in the early postnatal period younger than P11. Null expression of Cx26 may therefore uniquely result in a nearly total elimination of GJ-mediated intercellular communication among inner sulcus cells, Claudius cells and Hansen’s cells during a critical period of cochlear development (Fig. 3). Because over-expressing Cx30 was unable to rescue hearing for cCx26 null mice and Cx30 expression is not detected by immunolabeling in these cells in the same postnatal period in the cochlea, we concluded that the essential role in the development of the auditory sensory epithelium played by Cx26 is unique and is not replaceable by Cx30.
There could be multiple consequences for a lack of GJ-mediated intercellular communication during the early postnatal period in the cochlea. Mice do not hear until after P12 [20]. High concentration of extracellular potassium in the endolymph and high positive potential of the EP, both are essential for hearing in mammals, rapidly develop a few days before the onset of hearing (P4-P11) [21]. The absence of GJs in the supporting cells of the organ of Corti during this critical period may therefore greatly reduce the proposed K+ recycling [22] and intercellular diffusion of other molecules essential for normal cellular activities and survival (e.g., glucose [23]). Many nutrients and signaling molecules needed for normal development of the inner ear may be in a short supply. These deficiencies may eventually result in an immature organ of Corti (Fig. 1) with non-functional supporting cells and hair cells. Cx26 has 226 amino acids and the reported Cx26 mutations that are linked to deafness are close to 200 (http://davinci.crg.es/deafness/). In contrast, there are less than 10 currently reported Cx30 mutations that are linked to deafness. A dominant role of Cx26 in the critical development period of the organ of Corti may help provide an explanation for a predominant role of Cx26 in genetic deaf caused by Cx mutations.
It has been speculated that two major Cx genes in the cochlea are under the control of the same regulatory elements for gene expressions [19]. Our immunolabeling and Western blot quantification analysis (Figs. 2&3) suggest that Cx26 and Cx30 have dramatically different temporal and cellular expression patterns during early postnatal cochlear development. The expression of Cx26 preceded that of Cx30 in inner sulcus cells, Claudius cells and Hensen’s cells. The differential expression of Cx26 and Cx30 in early postnatal organ of Corti indicated that the two Cxs do not necessarily use the exact same set of regulatory elements for their gene expressions. Therefore, different regulatory mechanism/regions are likely to govern their developmental expressions.
Highlights.
Specific functional roles of Gjb2 and Gjb6 genes in the inner ear are not clear.
We tested whether the cochlear functions played by Cx26 could be replaced by Cx30.
Overexpressing Cx30 didn’t rescue hearing, while multiple positive controls worked.
Cx26 was the only Cx expressed in many key cell types in developing organ of Corti.
The unique developmental functions of Cx26 are not replaceable by Cx30.
Supplementary Material
Acknowledgements
This study was supported by following grants to X.Lin from National Institute on Deafness and other Communicative Disorders: NIDCD 4R33DC010476, R01DC010204 and RO1 DC006483. Tang also received grant support from NIDCD (R21 DC008672).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interest statement: The authors declare that they have no competing financial interests.
References
- 1.Kelsell DP, Dunlop J, Stevens HP, Lench NJ, Liang JN, Parry G, Mueller RF, Leigh IM. Connexin 26 mutations in hereditary non-syndromic sensorineural deafness. Nature. 1997;387:80–83. doi: 10.1038/387080a0. [DOI] [PubMed] [Google Scholar]
- 2.Estivill X, Fortina P, Surrey S, Rabionet R, Melchionda S, D'Agruma L, Mansfield E, Rappaport E, Govea N, Mila M, Zelante L, Gasparini P. Connexin-26 mutations in sporadic and inherited sensorineural deafness. Lancet. 1998;351:394–398. doi: 10.1016/S0140-6736(97)11124-2. [DOI] [PubMed] [Google Scholar]
- 3.Alexander DB, Goldberg GS. Transfer of biologically important molecules between cells through gap junction channels. Curr Med Chem. 2003;10:2045–2058. doi: 10.2174/0929867033456927. [DOI] [PubMed] [Google Scholar]
- 4.Guilford P, Ben Arab S, Blanchard S, Levilliers J, Weissenbach J, Belkahia A, Petit C. A non-syndrome form of neurosensory-recessive deafness maps to the pericentromeric region of chromosome 13q. Nat Genet. 1994;6:24–28. doi: 10.1038/ng0194-24. [DOI] [PubMed] [Google Scholar]
- 5.Grifa A, Wagner CA, D'Ambrosio L, Melchionda S, Bernardi F, Lopez-Bigas N, Rabionet R, Arbones M, Monica MD, Estivill X, Zelante L, Lang F, Gasparini P. Mutations in GJB6 cause nonsyndromic autosomal dominant deafness at DFNA3 locus. Nat Genet. 1999;23:16–18. doi: 10.1038/12612. [DOI] [PubMed] [Google Scholar]
- 6.del Castillo I, Villamar M, Moreno-Pelayo MA, del Castillo FJ, Alvarez A, Telleria D, Menendez I, Moreno F. A deletion involving the connexin 30 gene in nonsyndromic hearing impairment. N Engl J Med. 2002;346:243–249. doi: 10.1056/NEJMoa012052. [DOI] [PubMed] [Google Scholar]
- 7.Liu XZ, Xia XJ, Ke XM, Ouyang XM, Du LL, Liu YH, Angeli S, Telischi FF, Nance WE, Balkany T, Xu LR. The prevalence of connexin 26 ( GJB2) mutations in the Chinese population. Hum Genet. 2002;111:394–397. doi: 10.1007/s00439-002-0811-6. [DOI] [PubMed] [Google Scholar]
- 8.Denoyelle F, Weil D, Maw MA, Wilcox SA, Lench NJ, Allen-Powell DR, Osborn AH, Dahl HH, Middleton A, Houseman MJ, Dode C, Marlin S, Boulila-ElGaied A, Grati M, Ayadi H, BenArab S, Bitoun P, Lina-Granade G, Godet J, Mustapha M, Loiselet J, El-Zir E, Aubois A, Joannard A, Petit C, et al. Prelingual deafness: high prevalence of a 30delG mutation in the connexin 26 gene. Hum Mol Genet. 1997;6:2173–2177. doi: 10.1093/hmg/6.12.2173. [DOI] [PubMed] [Google Scholar]
- 9.Hoang Dinh E, Ahmad S, Chang Q, Tang W, Stong B, Lin X. Diverse deafness mechanisms of connexin mutations revealed by studies using in vitro approaches and mouse models. Brain Res. 2009 doi: 10.1016/j.brainres.2009.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen-Salmon M, Ott T, Michel V, Hardelin JP, Perfettini I, Eybalin M, Wu T, Marcus DC, Wangemann P, Willecke K, Petit C. Targeted ablation of connexin26 in the inner ear epithelial gap junction network causes hearing impairment and cell death. Curr Biol. 2002;12:1106–1111. doi: 10.1016/s0960-9822(02)00904-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Chang Q, Tang W, Sun Y, Zhou B, Li H, Lin X. Targeted connexin26 ablation arrests postnatal development of the organ of Corti. Biochem Biophys Res Commun. 2009;385:33–37. doi: 10.1016/j.bbrc.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teubner B, Michel V, Pesch J, Lautermann J, Cohen-Salmon M, Sohl G, Jahnke K, Winterhager E, Herberhold C, Hardelin JP, Petit C, Willecke K. Connexin30 (Gjb6)-deficiency causes severe hearing impairment and lack of endocochlear potential. Hum Mol Genet. 2003;12:13–21. doi: 10.1093/hmg/ddg001. [DOI] [PubMed] [Google Scholar]
- 13.Lautermann J, ten Cate WJ, Altenhoff P, Grummer R, Traub O, Frank H, Jahnke K, Winterhager E. Expression of the gap-junction connexins 26 and 30 in the rat cochlea. Cell Tissue Res. 1998;294:415–420. doi: 10.1007/s004410051192. [DOI] [PubMed] [Google Scholar]
- 14.Ahmad S, Chen S, Sun J, Lin X. Connexins 26 and 30 are co-assembled to form gap junctions in the cochlea of mice. Biochem Biophys Res Commun. 2003;307:362–368. doi: 10.1016/s0006-291x(03)01166-5. [DOI] [PubMed] [Google Scholar]
- 15.Forge A, Becker D, Casalotti S, Edwards J, Marziano N, Nevill G. Gap junctions in the inner ear: comparison of distribution patterns in different vertebrates and assessement of connexin composition in mammals. J Comp Neurol. 2003;467:207–231. doi: 10.1002/cne.10916. [DOI] [PubMed] [Google Scholar]
- 16.Ahmad S, Tang W, Chang Q, Qu Y, Hibshman J, Li Y, Sohl G, Willecke K, Chen P, Lin X. Restoration of connexin26 protein level in the cochlea completely rescues hearing in a mouse model of human connexin30-linked deafness. Proc Natl Acad Sci U S A. 2007;104:1337–1341. doi: 10.1073/pnas.0606855104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun J, Ahmad S, Chen S, Tang W, Zhang Y, Chen P, Lin X. Cochlear gap junctions coassembled from Cx26 and 30 show faster intercellular Ca2+ signaling than homomeric counterparts. Am J Physiol Cell Physiol. 2005;288:C613–C623. doi: 10.1152/ajpcell.00341.2004. [DOI] [PubMed] [Google Scholar]
- 18.Sun Y, Tang W, Chang Q, Wang YF, Kong YY, Lin X. Connexin30 null and conditional connexin26 null mice display distinct pattern and time course of cellular degeneration in the cochlea. J. Comp. Neurol. 2009;516:569–579. doi: 10.1002/cne.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilch E, Zhu M, Burkhart KB, Regier M, Elfenbein JL, Fisher RA, Friderici KH. Expression of GJB2 and GJB6 is reduced in a novel DFNB1 allele. Am J Hum Genet. 2006;79:174–179. doi: 10.1086/505333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pujol R, Lavigne-Rebillard M, Lenoir M. development of sensory and neural structures in the mammalian cochlea. In: Rubel EW, Popper AN, Fay RR, editors. Development of the auditory system. New York, Berlin: Springer; 1998. pp. 146–192. [Google Scholar]
- 21.Wangemann P. K+ cycling and the endocochlear potential. Hear Res. 2002;165:1–9. doi: 10.1016/s0378-5955(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 22.Kikuchi T, Adams J, Miyabe Y, So E, Kobayashi T. Potassium ion recycling pathway via gap junction systems in the mammalian cochlea and its interruption in hereditary nonsyndromic deafness. Med Electron Microsc. 2000;33:p51–p56. doi: 10.1007/s007950070001. [DOI] [PubMed] [Google Scholar]
- 23.Chang Q, Tang W, Ahmad S, Zhou B, Lin X. Gap junction mediated intercellular metabolite transfer in the cochlea is compromised in connexin30 null mice. PLoS ONE. 2008;3:e4088. doi: 10.1371/journal.pone.0004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.