Abstract
Sphingosine-1-phosphate (S1P) is a bioactive lipid with diverse functions including the promotion of cell survival, proliferation, and migration, as well as the regulation of angiogenesis, inflammation, immunity, vascular permeability and nuclear mechanisms that control gene transcription. S1P is derived from metabolism of ceramide, which itself has diverse and generally growth-inhibitory effects through its impact on downstream targets involved in regulation of apoptosis, senescence and cell cycle progression. Regulation of ceramide, S1P and the biochemical steps that modulate the balance and interconversion of these two lipids are major determinants of cell fate, a concept referred to as the “sphingolipid rheostat.” There is abundant evidence that the sphingolipid rheostat plays a role in the origination, progression and drug resistance patterns of hematopoietic malignancies. The pathway has also been exploited to circumvent the problem of chemotherapy resistance in leukemia and lymphoma. Given the broad effects of sphingolipids, targeting multiple steps in the metabolic pathway may provide possible therapeutic avenues. However, new observations have revealed that sphingolipid signaling effects are more complex than previously recognized, requiring a revision of the sphingolipid rheostat model. Here, we summarize recent insights regarding the sphingolipid metabolic pathway and its role in hematopoietic malignancies.
Keywords: cancer, ceramide, chemotherapy, leukemia, lymphoma, multiple myeloma, sphingolipid, sphingosine-1-phosphate
Introduction
Sphingosine-1-phosphate (S1P) is a lipid signaling molecule generated by sphingosine kinase (SK)-mediated phosphorylation of the long chain base sphingosine. Sphingosine is itself a degradation product of the central molecule of sphingolipid metabolism, ceramide. Between 1990 and 1992, several research groups reported that S1P exerts biological effects in mammalian cells, including the ability to stimulate calcium release from intracellular stores and to promote cell proliferation, migration and tumor cell invasiveness 1–3. In 1993 a landmark study by Olivera and Spiegel demonstrated that SK activation and S1P formation contributed to the effects of growth factor tyrosine kinase-mediated mitogenic signaling pathways 4. In 1989, ceramide generated from sphingomyelin breakdown through the actions of a neutral sphingomyelinase was shown to promote differentiation of HL60 leukemia cells 5. In 1993 ceramide was shown to elicit apoptosis in leukemia cells, and in 1996–8 S1P was shown to counteract the pro-apoptotic effects attributed to ceramide 6–8. The notion that these two inter-related metabolites (i.e., S1P and ceramide) exert opposing effects on cell fate became recognized as the “sphingolipid rheostat”, adopted for many years as one of the central tenets of sphingolipid-mediated biology (Figure 1). The rheostat was a conceptually tidy notion that suggested ceramide-induced cell death is counteracted by the actions of its downstream metabolite S1P, thereby presenting a self-limiting biochemical model of internal feedback that has been useful for explaining many observed phenomena in the field. The possible role of S1P signaling and the sphingolipid rheostat in carcinogenesis and the potential of modulating this delicate balance for therapeutic purposes in cancer was recognized early and has been the subject of prolonged and intense investigation.
Figure 1. Sphingolipid rheostat.
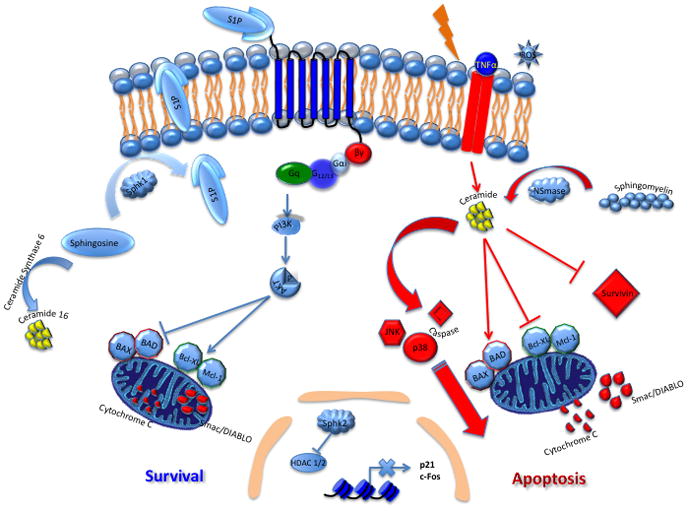
S1P is produced from the conversion of sphingosine to S1P via SK enzymes. S1P is then shuttled to the extracellular space, where it can activate five known GPCRs. Upon activation, downstream signaling events occur to produce myriad effects. Ceramide can be produced via sphingomyelinases (SMase) by conversion of sphingomyelin to ceramide. Radiation, ROS, ischemia and TNFα can also contribute to ceramide generation. The traditional ‘rheostat’ model suggests a balance of forces between S1P and ceramide, with S1P promoting survival and ceramide promoting apoptosis. Downstream mediators affected by the rheostat include Bad, Bax, Bcl-XL and Mcl-1. Ceramide is also shown here to affect signaling via JNK and p38. Ceramide can also affect caspase activation and survivin suppression. Contrary to the ‘rheostat’ model, ceramide 16 promotes growth and inhibits the ER stress response in some cancers. SphK2 is also shown here to repress HDAC1/2 with subsequent upregulation of p21 and c-fos transcription.
Between 1997–2000, the individual genetic components of S1P metabolism and signaling were identified, including genes encoding two SKs (SphK1 and SphK2) responsible for S1P biosynthesis, S1P lyase and S1P phosphatases responsible for its degradation and recycling, respectively, and five G protein coupled receptors that mediate S1P signaling events originating at the plasma membrane 9. S1P was also shown to be a substrate for dephosphorylation by nonspecific lipid phosphatases, and more recently S1P cell export functions of ATP-binding cassette (ABC) and Spns2 transporter proteins have been revealed 10–13. The elucidation of these critical regulators of S1P levels, transport and action has stimulated an explosion of research into S1P’s biological functions and regulation. Many studies have examined the potential oncogenic role of S1P signaling. In fact, as annual publications related to S1P have steadily increased (from 2 in 1991 to 380 in 2010, totaling 2800 as of this writing), between 10–29% of these publications in every year since 1994 have focused on the observed or potential role of S1P signaling in cancer.
Over the past two decades, S1P signaling has become increasingly recognized for its diverse roles in embryology and physiology, including essential functions in developmental angiogenesis and cardiac morphogenesis, and its influence over post-natal vascular homeostasis, lymphocyte trafficking and inflammatory pathways 9. S1P acts as an intracellular signaling molecule that influences calcium mobilization, nuclear functions, gene expression and proteosomal degradation. It also serves as a ligand for a family of functionally distinct G protein coupled receptors (GPCRs) that act in autocrine and paracrine fashion to modulate cell migration, cell-cell interactions and generally promote cell survival in response to stressful conditions including ischemia, hypoxia, nutrient deprivation, inflammation, oxidant stress, oncogenic stress and DNA damage from radiation and chemotherapy agents. Many of these processes are related to carcinogenesis, cancer progression and therapeutic resistance.
In 2000, SphK1 was shown to behave as an oncogene in cell and animal models of tumorigenesis wherein it appeared to mediate the mitogenic effects of oncogenic Ras 14. In 2003, SphK1 upregulation was confirmed in a variety of human tumors, whereas its pharmacological inhibition was cytotoxic to tumor cells 15. Since that time, specific alterations in the metabolic and signaling components responsible for maintaining S1P homeostasis have been implicated in various cancers of adults and children and in cell and animal models of cancer. These molecular changes affect patient outcome and prognosis, tumor progression, and chemotherapy resistance 16. New findings suggest that the rheostat model may no longer suffice to explain the effects of ceramide and S1P in all contexts of cancer, including the requirements of some tumors for C16 ceramides for survival, as well as examples of S1P-mediated apoptotic and autophagic cell death 17–19.
Multiple reviews have described the role of ceramide, S1P and the sphingolipid metabolic pathway in cancer 16, 20–24. This article will review basic concepts pertaining to sphingolipid structure, S1P signaling and S1P metabolism, and will then focus on current understanding of the role of S1P signaling specifically in the context of hematological malignancies.
Components of S1P Metabolism and Signaling
A sphingolipid consists of a long chain amino base (sphingoid base), most commonly sphingosine in mammalian cells, which forms the structural anchor of the molecule. This base can be modified by the addition of fatty acids of specific chain lengths at the free amino group, thereby generating a variety of distinct ceramide molecular species. In addition, the hydroxyl group at the C1 position of the long chain base can be modified by the addition of a polar head group or sugar residue, singly or in sequential reactions, thereby generating myriad complex sphingolipids found in the lipid rafts of the plasma membrane 25. De novo sphingolipid biosynthesis involves the formation of a 3-ketosphinganine that is subsequently converted to dihydrosphingosine through the actions of a 3-ketosphinganine reductase. Dihydrosphingosine can be acylated by a family of ceramide synthases with specific fatty acid substrate preferences, thereby giving rise to the formation of various dihydroceramides. The dihydroceramides are then converted to ceramides by dihydroceramide desaturase, which introduces a double bond into the long chain base backbone, converting it from dihydrosphingosine to sphingosine. In contrast, sphingolipid recycling, which occurs by hydrolysis of the polar head group of membrane sphingolipids in which the long chain base has already been desaturated, directly produces ceramides. The most prominent example of this is the generation of ceramide from sphingomyelin by stress-activated sphingomyelinases.
The sphingolipid degradation pathway is initiated by the deacylation of ceramides by a family of pH-dependent ceramidases, thereby releasing the free long chain base. SphK1 or SphK2 can then phosphorylate free sphingosine, thereby yielding S1P or, in the case of other long chain bases, producing the corresponding long chain base phosphate 26. SphK1 is primarily cytosolic. However, mitogenic signals including phorbol esters, tumor necrosis factor-α (TNFα), growth factor receptors, estrogens, cytokines, calcium and phospholipase D induce the phosphorylation of SphK1 on Ser225 by extracellular signal-regulated kinases (ERK)1/2, leading to its membrane translocation. This event, which is also facilitated by the calcium and integrin binding protein (CIB1), substantially increases SphK1 activity 27. In contrast, SphK2 is primarily nuclear in its subcellular localization and has unique functions as a member and negative regulator of a histone deacetylase (HDAC) 1/2 complex that represses the expression of p21, c-Fos and potentially other targets 28. There is also evidence that SphK2 can function as a pro-apoptotic Bcl-2 homology 3 (BH3)-only protein 29. Once formed, S1P can be dephosphorylated by the actions of S1P phosphohydrolases and lipid phosphatases or irreversibly degraded by S1P lyase to yield a long chain aldehyde and ethanolamine phosphate 30, 31. These enzymes are implicated in the regulation of cell fate through their impact on intracellular levels of S1P, sphingosine and ceramide 32–36. An alternative pathway of ceramide metabolism involves its direct phosphorylation by the actions of ceramide kinase, thereby producing ceramide-1-phosphate, which has itself turned out to be an interesting signaling molecule involved in inflammatory signaling 37, 38.
Whereas ceramide appears to mediate its effects intracellularly, S1P has both intracellular functions and extracellular functions mediated through GPCRs. Currently, there are five known GPCRs belonging to the S1P group of receptors, formerly known as Endothelial Differentiation Gene (EDG) receptors and now designated S1P1–5. Nearly every human cell type examined expresses one or more S1P receptor, and many cells express a combination of these. Ligand binding and activation of these receptors initiates multiple signaling pathways, including ERKs, phosphoinositide-3-kinase (PI3K), and cyclic AMP downstream mediators 39, 40. Further, S1P receptors interact and exhibit cross-talk with other growth factor receptors including those activated by vascular endothelial growth factor and platelet derived growth factor (PDGF), thereby increasing the complexity of S1P signaling and its ramifications for cell biology 41.
The specific functions and regulation of the S1P receptors and the enzymes affecting the sphingolipid rheostat have been described in detail elsewhere, as cited above. In the following sections, we will focus on describing the evidence supporting a role for S1P signaling and metabolism in the development, progression and acquisition of drug resistance of hematopoietic malignancies, including leukemia, lymphoma and multiple myeloma.
The Sphingolipid Rheostat in Leukemia Cell Lines
In the 1980s, HL60 leukemia cells were shown to generate ceramide by activating neutral sphingomyelinase in response to vitamin D3 treatment, thereby leading to cell differentiation. This was the first demonstration that intracellular ceramide generated by a “sphingomyelin cycle” could function as a lipid mediator 5. Subsequently, ceramide was found to activate stress-activated protein kinases in HL60 cells, and to induce apoptosis via Bax translocation and inhibition of the antiapoptotic protein Bcl-xL 42, 43. Cellular ceramide levels were shown to increase in leukemia cells in response to many cytotoxic factors including TNFα, dexamethasone, activators of Fas, chemotherapeutic agents, reactive oxygen species (ROS) and ionizing radiation (see Figure 1) 44–51.
Endogenous ceramide accumulation has been shown to originate from many sources in leukemic cells, including sphingomyelin breakdown (via the activation of sphingomyelinases) 5, de novo biosynthesis and the direct action of ceramide synthases 52, inhibition of ceramidase 53, 54, and most recently by inhibition of its incorporation into sphingomyelin in Jurkat cells 55. Ceramide-mediated cell death has been attributed to its impact on numerous downstream targets in leukemia cells. Ceramide can induce apoptosis through activation of caspases in MOLT4 leukemia cells 56. It activates a protein phosphatase 2A (PP2A)-type phosphatase in HL60 cells, leading to a block in transcriptional elongation of the TNF-α downstream target c-myc and resulting in inhibition of cell growth 57, 58. Activation of stress-induced protein kinases including p38 and c-Jun N-terminal kinase (JNK) contribute to apoptosis in response to ceramide generation or exogenous treatment with ceramide analogs 59, 60. Ceramide reduces survivin expression in human and rat natural killer large granular lymphocyte (LGL) cells 61. In addition, the ability of ceramide to represses human telomerase (hTERT) promoter activity by HDAC1-mediated deacetylation of Sp3 was paralleled by findings of ceramide-mediated repression of hTERT in KG1 leukemia cells (see Figure 2) 62, 63.
Figure 2. S1P signaling in hematopoeitic malignancies.
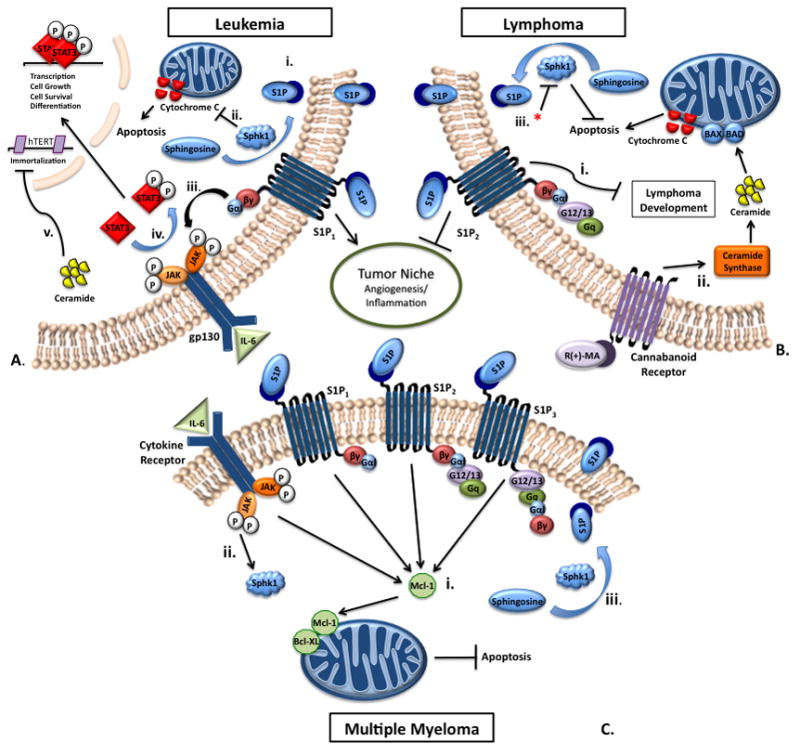
A.) The role of S1P in leukemia. i.) Sphingosine is converted by SphK1 into S1P and is exported to the extracellular space, where it can then activate S1P receptors. ii.) Upregulation of SphK1 inhibits cytochrome c transport to the cytoplasm. iii.) S1P activates S1P1, which persistently activates the STAT3 pathway. iv.) STAT3 is transiently activated by IL-6 and travels to the nucleus for transcription. v.) Ceramide represses human telomerase (hTERT) promoter activity through HDAC-1-mediated deacetylation of sp3. B.) S1P signaling and apoptosis in lymphomas. i.) S1P2 exerts a negative effect on B-cell lymphoma development, as S1P2 knockout mice exhibit enhanced lymphoma formation. ii.) R(+)-methanandamide treatment results in decreased cell viability through increased ceramide synthase activity in mantle cell lymphoma. iii.) When SK is inhibited by DMS or SphK1 is inhibited by siRNA (*) in the presence of R(+)-methanandamide, cytochrome c induced apoptosis is potentiated in mantle cell lymphoma. C.) S1P signaling and apoptosis in Multiple Myeloma. i.) Mcl-2 anti-apoptotic effect is activated by S1P through S1P1, S1P2, and S1P3. ii.) IL-6 confers an anti-apoptotic effect through activation of SphK1. iii.) IL-6 activation of SphK1 mediates conversion of sphingosine into S1P to prevent apoptosis.
In the early 1990s, the mitogenic effects of S1P and SK activation as downstream events in mitogenic signaling pathways were demonstrated, thereby revealing an interesting contrast between the pro- and anti-proliferative effects of these two sphingolipid metabolites 4. In 1996, exogenous S1P was shown to induce calcium mobilization and activation of phospholipase C (PLC) in HL60 leukemia cells, and a pertussis-toxin sensitive receptor was shown to be involved 64, 65. That same year it was shown that human erythroleukemia cells activate SK in response to phorbol esters in a protein kinase C (PKC)-dependent manner 66. Later studies in the human acute leukemia Jurkat, U937 and HL60 cell lines showed that S1P has an inhibitory effect on apoptosis 67. The effects of S1P were attributed to the inhibition of cytochrome c and Smac/DIABLO translocation to the cytoplasm from the mitochondria in the presence of S1P. Treatment of HL60 cells with vitamin D3 was found to activate SK in a PKC-dependent manner, and treatment with S1P protected HL60 cells from ceramide-induced apoptosis, whereas SK inhibition augmented it 68. These studies indicated the existence of a delicate balance in sphingolipid metabolism that can affect cell survival pathways in leukemia as well as other cell types (Figure 1).
Anoikis, which is a form of apoptotic cell death mediated by detachment from the substratum, was also shown to be prevented by SK activation in U937 cells, whereas SK inhibition induced by detachment promoted anoikis in HL60 cells 69, 70. SK activation and S1P production was also implicated in chemoattractant signaling in differentiated HL60 cells 71. Many subsequently observed effects of S1P and ceramide in the context of leukemia are consistent with the sphingolipid rheostat model 72. Upon recognition that S1P serves as a ligand for EDG/S1P receptors, some of S1P’s effects on leukemia cells were attributed to receptor signaling 73. For example, it was soon established that HL60 cells preferentially express S1P3, and that S1P3 is downregulated during differentiation induced by dibutyryl cAMP, retinoic acid, and vitamin D3 74.
Mast cells are bone marrow derived cells that mediate allergic responses, and rat basophilic leukemia (RBL)-2H3 cells serve as a model of mast cell function. Cross-linking of the high-affinity immunoglobulin E receptor on (RBL)-2H3 cells results in activation of SphK1, export of S1P through ABC transporters and activation of S1P1 and S1P2, leading to degranulation and chemotaxis 75. These findings raise the possibility that S1P signaling could be involved in proliferative disorders of the mast cell including mast-cell leukemia and/or mastocytosis.
S1P Signaling in Leukemia
The previous studies using leukemia-derived transformed cell lines revealed many of the basic effectors of ceramide and S1P signaling in leukemia cells and established their generally opposing actions in the context of leukemia. More recent studies have examined the effects of sphingolipid signaling in human primary leukemia cells and animal models of the disease. Findings in these systems have provided more substantial evidence strengthening the physiological relevance of sphingolipid signaling in hematopoietic malignancies.
For example, microarray analysis demonstrated that an S1P5 receptor is upregulated in human T-cell LGL leukemia 76. LGL leukemia cells appear to derive from a clonal expansion of terminally differentiated, antigen-primed, competent cytotoxic T lymphocytes. As such, patients with LGL leukemia have autoimmune conditions such as rheumatoid arthritis 77. Recent studies have implicated PDGF and IL-15 as key signaling factors responsible for the pathogenesis and activation of LGL 78. Interestingly, multiple genes involved in the sphingolipid metabolic pathway were found to be differentially expressed in these tumors 79. Among the genes identified in this study were SphK1, acid ceramidase, and neutral sphingomyelinases. Inhibition of acid ceramidase or SK, or treatment with FTY720, a sphingosine analog that functions as an immunomodulatory drug by binding to and promoting internalization of S1P1, induced apoptosis or sensitized the cells to Fas-induced apoptosis. Thus, dysregulation of the S1P signaling may contribute to the effects of upstream growth factor activation in LGL, such as by facilitating the escape of activated T cells from Fas-mediated cell death.
A central role for SphK1 in the development of erythroleukemia was revealed when microarray analysis was used to compare gene expression in nontumorigenic and tumorigenic proerythroblasts from a transgenic mouse model of erythroleukemia 80. Using this approach, SphK1 upregulation was found to be a recurrent oncogenic event underlying the tumorigenic phenotype in proerythroblasts. When erythroleukemic cells were forced to overexpress SphK1, they exhibited a proliferative advantage, increased clonogenicity, tumorigenicity when implanted in nude mice, and resistance to apoptosis in response to serum-deprivation. This protection from stress was mediated through an ERK- and PI3K-dependent pathway. In vitro suppression of SK activity either by expressing a dominant negative SphK1 or through pharmacological inhibition blocked the proliferation and stress resistance of erythroleukemia cells.
In chronic myelogenous leukemia (CML), the tyrosine kinase ABL is in a constitutively active state, resulting in unrelenting growth signaling. The drug imatinib, which inhibits ABL tyrosine kinase activity, is the first example of a new class of chemotherapy agents that target a mutant enzyme that is characteristic of a particular cancer cell. This distinguishes imatinib from the many nonspecific agents that target DNA replication and cell cycle progression and thereby affect all or most rapidly dividing cell populations. The development of imatinib mesylate for CML has revolutionized CML treatment. However, resistance has been a problem in achieving complete killing of tumor cells 81. A recent study examined the mechanisms for resistance to imatinib-induced apoptosis using a pair of isogenic human K562 CML cell lines, one of which is resistant to imatinib and the other sensitive. In these cells, ceramide and S1P were found to have opposing effects on imatinib-induced apoptosis 82. Imatinib treatment induced the generation of endogenous C18 ceramide, resulting in enhanced apoptosis in the sensitive cell line, whereas decreased apoptosis was observed in imatinib-resistant cells that failed to generate C18 ceramide, exhibited elevated SphK1 expression and had an increased ratio of S1P/C18 ceramide. Importantly, partial knockdown of SphK1 in the resistant cells increased their sensitivity to imatinib. In a separate study, cell lines derived from CML patients in blast crisis were used to investigate the role of SphK1 in imatinib resistance 83. Overexpression of SphK1 resulted in resistance to imatanib-induced cell death, concomitant with decreased levels of cytochrome c and Smac/DIABLO expression. Further, SphK1 activity was significantly decreased with imatanib treatment. Recently, continuous exposure of K562 cells to gradually increasing concentrations of imatinib was used to select an imatinib-resistant cell line. This line exhibited upregulation of both BCR-ABL and SphK1. SphK1 was found to be upregulated in an AKT2-dependent manner that promoted imatinib resistance. Both BCR-ABL upregulation and imatinib resistance could be reversed by SphK1 knockdown and by pharmacological inhibition of SK activity using the sphingosine analog N,N-dimethylsphingosine (DMS) 84. These studies suggest that modulating the sphingolipid rheostat could be exploited to address the problem of drug resistance in long-term treatment of CML.
In an effort to investigate mechanisms of chemotherapy resistance in acute myeloid leukemia (AML) cells, the role of SphK1 was explored in AML cells demonstrating resistant to the chemotherapeutic agents doxorubicin or etoposide 83. As in CML, elevated SphK1 expression levels correlated with survival and inhibition of apoptosis in AML, and these findings were associated with a block in mitochondrial cytochrome c translocation to the cytoplasm (see Figure 2).
The predominance of published studies analyzing S1P and ceramide signaling in cancer have focused on the intracellular events controlling their biosynthesis and degradation. However, recent reports have begun to establish a role for S1P receptors in hematopoietic malignancy as well. For example, the S1P1 receptor has recently been implicated in myeloid tumor progression via a mechanism involving signal transducer and activator of transcription 3 (STAT3) signaling 85. The STAT3 protein is a transcription factor that is activated by many cytokines and plays a central role in inflammation, cell growth and apoptosis 86. The binding of IL-6 cytokine family members to the gp130 receptor results in STAT3 phosphorylation by Janus kinase 2 (JAK2), and constitutive activation of this signaling pathway has been implicated as a causative factor in cancer. STAT3 signaling has been shown to be important in determining sensitivity of leukemia cells to apoptosis 87. Recently, STAT3 was shown to be involved in a feedback loop with S1P1 85. S1P1 was found to be a transcriptional target of STAT3, and STAT3 positive tumor cells exhibited elevated S1P1 expression. In addition, S1P1 signaling led to activation of STAT3 and increased IL-6 via upregulation of JAK2 activity, thereby resulting in an auto-stimulatory loop. Unlike transient activation of STAT3 by IL-6, S1P1 signaling was shown to persistently activate this pathway through its influence on tumor cells and the tumor microenvironment (see Figure 2). Importantly, the effects of this auto-activating signaling loop promoted tumor growth and metastatic spread, whereas inhibition of S1P1 signaling prevented these effects. These results provide new insight into how S1P signaling may contribute fundamentally to leukemogenesis. They simultaneously raise the possibility that S1P signaling may be an Achilles heel for some leukemias in which the pathway is dysregulated.
S1P Signaling in Lymphomas
In one of the earliest studies exploring how T lymphoma cells invade their microenvironment, investigators found that their ability to invade a fibroblast monolayer required the actions of Tiam1 and its downstream mediator Rac 88. Serum lysophospholipid mediators S1P and lysophosphatidic acid (LPA) were shown to act on cell surface GPCRs, thereby mediating RhoA and PLC signaling pathways that stimulated the formation of pseudopodia and enhanced infiltration. These seminal findings were subsequently followed by many other published reports showing the migration of tumor cells, endothelial cells, neurons and bone marrow derived cells were orchestrated by similar mechanisms linking S1P and LPA signaling to cytoskeletal changes that promote cell movement 89.
Runx genes play roles in development, including the generation of hematopoietic stem cells 90. Overexpression of Runx genes in transgenic mice can promote the development of leukemia and lymphoma in collaboration with c-myc. Further, lymphoid cells and fibroblasts overexpressing Runx genes exhibit reduced apoptosis and enhanced survival under the stress. Recently, investigators seeking to understand how Runx genes influence cell fate under stress performed gene array comparisons that identified significant differences in three key enzymes in sphingolipid metabolism in Runx-overexpressing cells 91. Genes encoding two enzymes of sphingolipid biosynthesis whose actions reduce free ceramides (UDP-glucose ceramide glycosyltransferase and beta-galactoside alpha-2,3-sialyltransferase 5) were upregulated by Runx gene expression, whereas the S1P phosphatase 1 (Sgpp1) gene was down-regulated. These changes correlated with Runx-binding sites found in the promoters of the genes, and reduced ceramides and elevated S1P levels were observed in Runx expressing cells compared to control cells. Addition of exogenous S1P conferred improved survival in control cells, suggesting that some of the Runx growth advantage is imparted by elevated S1P.
In large B-cell lymphoma development, S1P2 signaling appears to serve an anti-oncogenic role. It was observed that 54% of S1P2 receptor null mice monitored over a two year time span developed germinal center derived DLBCL, compared to 3.6 % incidence in littermate control mice 94. This finding is compatible with that of another study demonstrating that the tumor environment of S1P2 knockout mice supported Lewis lung carcinoma or B16 melanoma cell tumor growth and angiogenesis better than control mice 95. These studies illustrate that S1P signaling through S1P2 is inhibitory to lymphoma and other cancer development and progression. S1P2 has been shown to mediate the G12/13 and Rho-dependent inhibitory effects of S1P on AKT, Rac, and cell migration, thereby negatively regulating angiogenesis 92, 93. Whether the effect of S1P2 on lymphomagenesis is an effect intrinsic to bone marrow-derived progenitors of lymphoma or rather due to an effect on cells within the tumor niche involved in angiogenesis, inflammation and cancer surveillance remains to be determined (see Figure 2).
The sphingolipid rheostat has also been implicated in mantle cell lymphomas (MCL). The endocannabinoid analogue R(+)-methanandamide binding to the endocannabinoid receptor CB1 leads to increased ceramide synthase 3 and 6 transcription and a subsequent increase in synthesis of multiple forms of ceramide including C16, 18, 24 an 24:1 ceramides, leading to decreased cell viability 96. Moreover, inhibition of the enzymes SphK1 and glucosylceramide synthase using specific inhibitors or siRNA prevented the further catabolism of ceramides and potentiated cell death in the presence of the R(+)-methanandamide. Interestingly, MCLs have been shown to express significant levels of S1P1 as a distinguishing feature compared to other lymphomas (see Figure 2). 97
Additional clinical studies are beginning to report interesting findings related to S1P signaling in human lymphomas. One study comparing Sphk1 expression in clinical tissue samples from 44 patients with non-Hodgkin’s lymphoma (NHL) versus 25 patients with reactive lymphoid hyperplasias (nonmalignant conditions) demonstrated significantly higher levels of SphK1 mRNA and protein in varying grades of NHL compared to nonmalignant lymphoid hyperplasia, with a clear trend toward increasing expression levels correlating with higher grade lymphomas 98. Although in some cases immunohistochemistry (IHC) showed SphK1 expression in nonmalignant tissues within the biopsy, in most cases the IHC confirmed expression within malignant cells.
The follicular lymphoma variant translocation 1 (FVT1) gene, which was identified through its involvement in an atypical follicular lymphoma translocation, turns out to be the principal 3-ketosphinganine reductase in mammalian cells 99. This enzyme is required for synthesis of long chain bases through the de novo sphingolipid biosynthetic pathway. The expression of FVT1 was investigated in B-cell non-Hodgkin lymphoma biopsy material 100. FVT1 expression was shown to be significantly reduced by germinal center-type diffuse large B-cell lymphoma (DLBCL) when compared with non-germinal center-type DLBCL, follicular lymphoma, and normal tonsil control samples. These findings suggest that bioactive sphingolipids may be important in the pathogenesis and treatment of some types of DLBCL, although it is not clear how flux through the de novo pathway impacts this process.
S1P Signaling in Multiple Myeloma
S1P has been shown to be protective against apoptosis in multiple myeloma cells, which were found to express S1P1–3. Addition of exogenous S1P to these cells was shown to upregulate the anti-apoptotic protein Mcl-1, whereas pan-inhibition of the S1P receptors led to an attenuation of Mcl-1 expression 101. Other studies in multiple myeloma cells have focused on the relationship between IL-6 and SphK1. IL-6 is an important target in multiple myeloma, since it confers an anti-apoptotic effect. It is now known that IL-6 partially mediates its anti-apoptotic effects via activation of SphK1 (see Figure 2)102.
In 1994, 2-amino-2- 2- 4-octylphenyl ethyl - 1,3-propanediol hydrochloride (FTY720) from the fungus Isaria sinclairii was discovered to have potent immunosuppressive properties by preventing lymphocyte trafficking 103. In 2003, FTY20 was found to act as a sphingosine analog that becomes phosphorylated in vivo and binds to S1P receptors, thereby linking S1P signaling to lymphocyte trafficking 104–106. FTY720-P preferentially targets S1P1 and S1P3–5, leading to receptor activation and subsequent downregulation 107. However, FTY720 has also been shown to exert anti-tumor effects and to promote apoptosis in cancer cells 108–110. FTY720 has been shown to have growth inhibitory effects on chemotherapy-resistant multiple myeloma cells 111. Similar to the action of ceramide on cytochrome c and Smac/DIABLO, FTY720 was able to induce translocation of these mediators of apoptosis. FTY720 was effective in inducing apoptosis in both sensitive and resistant cells, and its function could be augmented by combination treatment with dexamethasone and anti-Fas antibodies 111.
However, it should be noted that FTY720 has pro-apoptotic effects on some leukemia and lymphoma cells that appear to be completely independent of FTY720’s effect on S1P receptors and S1P-dependent signaling. These effects include its ability to activate PP2A and thereby promote the dephosphorylation of oncogenic c-KIT on tumor cells, resulting in reduced signaling through downstream targets such as AKT, STAT5 and ERK and causing apoptosis 112. This example points to the importance of recognizing that sphingolipid analogs may have off-target pharmacological effects that can contribute significantly to their impact on tumorigenicity113.
Targeting the Sphingolipid Pathway
Based on the fundamental role that sphingolipids play in regulating hematopoietic cell proliferation, conserved death pathways and drug resistance patterns, it is understandable that significant effort is being expended to harness the potential of this pathway for therapeutic benefit in cancer. Strategies have included development of small molecule inhibitors of SK, ceramidase and glucosylceramide synthase, as well as ceramide and long chain base analogs, S1P receptor antagonists and monoclonal antibodies against S1P. Testing of these therapeutic strategies is underway in clinical trials in cancer and other diseases, and reports are beginning to emerge in the literature 22, 114.
Studies targeting SK were among the earliest therapeutic strategies to be employed and have produced some promising results. As expected, inhibition of SK with DMS led to apoptosis of HL60 human leukemia cells 115. A recent study on L-threo-dihydrosphingosine (Safingol), which acts an inhibitor of SphK1 and PKC, has shown promising results with advanced solid tumors 116, 117. Safingol enhanced cellular toxicity in drug resistant cells when given in combination with doxorubicin 118. In the most recent study using Safingol, a dose-dependent reduction in plasma S1P levels was achieved, with only reversible hepatotoxicity as a side effect. Although a Phase I clinical trial, stable disease and partial response was observed in several patients receiving safingol in combination with cisplatin 117. Given a cohort of patients with advanced disease and documented resistance to cisplatin, these results are encouraging and reinforce the notion of manipulating the sphingolipid rheostat to overcome drug resistance.
Studies with a selective SphK1 inhibitor, BML-258, have shown promising results as well 119. BML-258 decreased growth in human leukemia U937 and Jurkat cells with increased apoptosis in leukemic blasts. Similar results were seen in AML allografts.
Recent studies have shown that inhibition of SphK2 by 3-(4-chlorophenyl)-adamantane-1-carboxylic acid (pyridin-4-ylmethyl)amide (ABC294640) is able to induce cell death in kidney carcinoma, prostate, and breast adenocarcinoma cell lines 120. Interestingly, in this report the major mechanism for tumor cell killing was found to be autophagy rather than apoptosis.
Although FTY720 has been considered primarily an immune modulator, multiple studies have also provided evidence that FTY720 can function as an anti-tumor agent. Effects on tumor growth, apoptosis and angiogenesis have been demonstrated in prostate, breast, hepatocellular, pancreatic, gastric, and hematopoietic cancers 121, 122. The effects of FTY720 and FTY720P reveal the complexity inherent in targeting the sphingolipid metabolic pathway. Recent work by Yasui et. al., has shown that FTY720 treatment resulted in ~40% inhibition of purified SphK1 111. Interestingly, vinylphosphonate, the S enantiomer of FTY720, produced an 80% reduction in SphK1 activity. In addition to catalytic inhibition, SphK1 underwent proteosomal degradation in the presence of FTY720 or (S)-FTY720 vinylphosphonate in hPASMC and MCF-7 cell lines. The proteosomal degradation of SphK1 led to apoptosis, as evidenced by caspase-3 activation.
The use of sphingosine has also been shown to have an effect on human leukemia cell lines. Using sphingosine in micromolar concentrations, CMK-7, HL60 and U937 leukemic cell lines were induced to undergo apoptosis with high frequencies 123. Various long chain base analogs have been exploited to emulate the effects of sphingosine. For example, phytosphingosine derivatives that serve as SK inhibitors were recently described to promote apoptotic cell death in HL60 cells 124. In addition, naturally occurring sphingadiene compounds found in soy were shown to induce apoptotic and autophagic cell death in colon cancer cells through an AKT-dependent mechanism and to suppress tumor formation in animal models 125. Whether these strategies will be relevant to the treatment of hematopoietic malignancies remains to be tested.
The S1P monoclonal antibody, Sphingomab, has shown very promising results as a novel strategy for removing S1P and thereby preventing pro-angiogenic signaling and tumor-promoting effects associated with S1P signaling. Initial results have demonstrated excellent results against murine xenografts and allografts 114, 126.
Several recent studies using ceramidase inhibitors show promise as a strategy for raising ceramide levels and thereby inducing cytotoxicity and reducing tumor growth 127–132.
As interest in the field continues to grow, critical information including identification of the crystal structures of key enzymes and targets in the pathway should become available. Combined with advanced drug screening efforts, this information should facilitate the development of more specific small molecule modulators of the sphingolipid rheostat. The strategies that are now being tested, as well as more challenging ones such as achieving targeted S1P catabolism and ceramide synthesis within tumors and inhibiting S1P export to prevent autocrine and paracrine S1P signaling within tumors and their microenvironment, have not been fully explored and remain intriguing possibilities for therapeutic intervention.
Beyond the Rheostat
As the examples above have illustrated, the rheostat model appears to have substantial merit as a general framework in which to understand the mechanisms of sphingolipid signaling in cancer. The identification of molecular targets at the membrane, in the cytosol, in the nucleus and in the extracellular space that mediate the specific effects of S1P and ceramide on tumor cell fate have gradually populated the picture of sphingolipid activities in the malignant cell and its niche with details, in most cases without refuting the rheostat model.
However, some recent findings indicate that a rigid rheostat model is insufficient to explain the pleiotropic effects of sphingolipids in the malignant cell and its environment. For example, C16 ceramides generated by ceramide synthase 6 appear to potentiate the growth of some tumors, and inhibition of the biosynthesis of these lipids can promote the ER stress response 18. In addition, the development of B cell lymphomas in S1P2 knockout mice and the enhanced growth of tumor xenografts placed in the context of a S1P2 knockout mouse niche as described above demonstrate that S1P signaling is not all tumor-promoting. The finding that SphK2 can function as a pro-apoptotic protein is also not consistent with the original rheostat model. Furthermore, autophagy, a conserved catabolic process that can facilitate tumor cell survival in the absence of nutrients but also can lead to cell death, is activated by both ceramide and S1P signaling 17. In addition, S1P has been shown to promote apoptosis in certain contexts 19.
Other sphingolipid intermediates besides S1P and ceramide may have an impact on cell fate and carcinogenesis, including dihydrosphingosines, dihydroceramides, sphingosine and the long chain aldehyde product generated by S1P lyase, hexadecenal. Early studies showed that sphingosine induces dephosphorylation of the retinoblastoma gene product (pRb), thereby inducing G1 cell cycle arrest in the lymphoblastic leukemia cell line MOLT-4 133, 134, whereas dihydrosphingosine and dihydryoceramide have been implicated in the pro-apoptotic effects of tocopherol gamma, a form of vitamin E that has chemopreventive activity 135. Fenretinide, a retinoid chemotherapeutic agent was found to function as an inhibitor of dihydroceramide desaturase, resulting in dephosphorylation of pRb and G0/1 cell cycle arrest 136. These findings raise the possibility that dihydroceramide, previously considered an inert sphingolipid intermediate, may function as a growth inhibitory molecule. We recently showed that hexadecenal, the long chain aldehyde produced by S1P lyase-mediated S1P cleavage, induces apoptosis and cell detachment through a JNK-dependent pathway that involves ROS generation 137. JNK activation by hexadecenal resulted in c-Jun phosphorylation, cytochrome c release, Bax activation, Bid cleavage and increased translocation of Bim into mitochondria, thereby promoting apoptosis. Inhibition of JNK abrogated the cytoskeletal changes and apoptosis caused by trans-2-hexadecenal, whereas Rac1 and RhoA were not involved. Aldehydes are also well known to induce DNA damage, and we have observed that hexadecenal can indeed form adducts with DNA (our unpublished observations). Whether hexadecenal formation plays a role in genetic instability in cancer is not known, but this could potentially account for the unexpected finding that S1P lyase is upregulated in many cancers. We have also recently shown S1P lyase to be a regulator of acid sphingomyelinase, the G2 checkpoint and DNA repair, demonstrating the complex feedback mechanisms within the sphingolipid biochemical pathways that have ramifications with regard to modulation of specific targets 138.
While some of these new findings are not inconsistent with a rheostat model, they do significantly alter the number of factors that may weigh in on the balance of positive and negative forces exerted by sphingolipids on cell fate. Gene expression patterns and biochemical parameters of some tumors are not easily accommodated by a rheostat model. Some of these observations, such as upregulation of genes involved in S1P catabolism in cancer, may be explained by a reaction of the tumor microenvironment to S1P production by the tumor or by the niche in response to tumor signals. However, many instances cannot be explained conveniently by the conventional model and suggest that a revision of the rheostat will be required to encompass the full dynamic range of sphingolipid signaling in hematopoietic malignancies and other cancer contexts.
S1P Signaling in the Supportive Management of Hematological Malignancies
In treating patients with hematological malignancies, many of whom are children and young adults, there are other factors in addition to achieving cure that are important components of clinical management. As acute lymphocytic lymphoblastic leukemia cure rates in children reach 80% or greater and patients are expected to enjoy a long life expectancy, long-term side-effects such as late cardiotoxic effects of anthracyclines, chronic graft-versus-host disease (GVHD) and sterility become less tolerable sequelae of treament 139. In this context, S1P signaling and inhibiting ceramide formation may be relevant, as they have been shown to play a protective role in the maintenance of viability of nonmalignant cells including protection of oocytes from chemotherapy- and radiation-induced apoptosis 140. The protection of stem cells and gut endothelial cells from radiation has also been achieved by increasing S1P levels and inhibiting ceramide formation 141–143. Studies have suggested that FTY720 may reduce GVHD without compromising malignant cell killing in the setting of bone marrow transplantation for leukemia 144. S1P lyase activation sensitizes cells to radiation and chemotherapy-induced apoptosis through a variety of mechanisms, whereas its inhibition appears to protect cells from DNA damage, ischemia, and other forms of injury 138, 145, 146. Therefore, S1P lyase suppression could potentially be used in strategies to protect normal tissues and thereby improve the therapeutic index of chemotherapy agents and radiation.
Concluding Remarks
The sphingolipid metabolic pathway is essential to normal cellular function. However, hematopoietic malignancies and the tumor microenvironment exhibit changes in sphingolipid signaling and metabolism that affect cell growth, apoptosis, invasion, and resistance to chemotherapy. The complexity of the pathway, which includes multiple enzymes, receptors and downstream effectors, presents a challenge to the researcher and clinician. Many questions still remain to be answered. For example, why have mutations directly affecting the expression or function of genes involved in sphingolipid metabolism and signaling only rarely been identified in cancer, whereas expression changes in SphK1, S1P lyase and other components of S1P signaling appear to be common manifestations of cancer? Many of our model systems cannot distinguish between effects on tumor promotion secondary to environmental cues versus effects that are intrinsic to the tumor cell. The effects of modulating the sphingolipid pathway on critical malignant and nonmalignant cell populations such as cancer stem cells and tissue stem cells remain unknown. As each specific target of S1P or ceramide is revealed, it also begs the question whether other sphingolipids directly or indirectly exert opposing effects on that target. As we gain new information, including the crystal structures of key targets in the pathway, rational drug design and targeted manipulation of this pathway for therapeutic benefit will become ever more feasible. New cancer profiling approaches are likely to reveal many more specific instances in which molecular events affecting sphingolipid signaling and metabolism contribute to carcinogenesis, cancer progression and the development of drug resistance. As we develop more sophisticated and individualized tumor profiling technologies that can be applied in advance of and throughout the course of treatment, this information may be coupled to new treatment options targeting the components of the sphingolipid pathway for hematological malignancies as well as other forms of cancer.
Acknowledgments
We apologize to the many investigators whose work could not be cited in this review due to limitations of space. This work was supported by the Swim Across America Foundation and National Institutes of Health Grants CA77528, CA129438 and RAT005336 (JDS), CIRM clinical fellowship (KCL), and CIRM (DB). The authors would like to thank Babak Oskouian and Morgan Carlson for critical review of the manuscript.
LIST OF ABBREVIATIONS
- AML
Acute myelogenous leukemia
- ABC
ATP-binding cassette
- BH3
Bcl-2 homology 3
- CIB1
calcium and integrin binding protein-1
- CML
chronic myelogenous leukemia
- JNK
c-Jun N-terminal kinase
- DLBCL
diffuse large B-cell lymphoma
- EDG
endothelial differentiation gene
- ERK
extracellular signal-regulated kinase
- FVT1
follicular lymphoma variant translocation 1
- GPCR
G protein coupled receptor
- GVHD
graft versus host disease
- HDAC
histone deacetylase
- hTERT
human telomerase
- IHC
immunohistochemistry
- JAK2
janus kinase 2
- LGL
large granulocytic lymphoma
- LPA
lysophosphatidic acid
- MCL
mantle cell lymphoma
- DMS
N,N-dimethylsphingosine
- NHL
nonhodgkins lymphoma
- PI3K
phosphoinositide-3-kinase
- PLC
phospholipase C
- PDGF
platelet derived growth factor
- PKC
protein kinase C
- PP2A
protein phosphatase 2A
- ROS
reactive oxygen species
- pRb
retinoblastoma gene product
- STAT3
signal transducer and activator of transcription 3
- SK
sphingosine kinase
- S1P
sphingosine-1-phosphate
- TNFα
tumor necrosis factor α
References
- 1.Ghosh T, Bian J, Gill D. Intracellular calcium release by sphingosine derivatives generated in cells. Science. 1990;248:1653–1656. doi: 10.1126/science.2163543. [DOI] [PubMed] [Google Scholar]
- 2.Sadahira Y, Ruan F, Hakomori S, Igarashi Y. Sphingosine 1-phosphate, a specific endogenous signaling molecule controlling cell motility and tumor cell invasiveness. Proc Natl Acad Sci US A. 1992;89:9686–9690. doi: 10.1073/pnas.89.20.9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang H, Desai N, Olivera A, Seki T, Brooker G, Spiegel S. Sphingosine-1-Phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991;114(1):155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olivera A, Spiegel S. Sphingosine 1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–559. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- 5.Okazaki T, Bell R, Hannun Y. Sphingomyelin turnover induced by vitamin D3 in HL-60 cells. J Biol Chem. 1989;264(32):19076–19080. [PubMed] [Google Scholar]
- 6.Obeid L, Linardic C, Karolak L, Hannun Y. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 7.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381(6585):800–3. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 8.Cuvillier O, Rosenthal DS, Smulson ME, Spiegel S. Sphingosine 1-phosphate inhibits activation of caspases that cleave poly(ADP-ribose) polymerase and lamins during Fas- and ceramide- mediated apoptosis in Jurkat T lymphocytes. J Biol Chem. 1998;273(5):2910–6. doi: 10.1074/jbc.273.5.2910. [DOI] [PubMed] [Google Scholar]
- 9.Fyrst H, Saba J. An update on sphingosine-1-phosphate and other sphingolipid mediators. Nat Chem Biol. 2010;6(7):489–497. doi: 10.1038/nchembio.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi N, Yamaguchi A, Nishi T. Characterization of the ATP-dependent sphingosine 1-phosphate transporter in rat erythrocytes. J Biol Chem. 2009;284(32):21192–200. doi: 10.1074/jbc.M109.006163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A. 2006;103(44):16394–9. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawahara A, Nishi T, Hisano Y, Fukui H, Yamaguchi A, Mochizuki N. The sphingolipid transporter spns2 functions in migration of zebrafish myocardial precursors. Science. 2009;323:524–527. doi: 10.1126/science.1167449. [DOI] [PubMed] [Google Scholar]
- 13.Kim RH, Takabe K, Milstien S, Spiegel S. Export and functions of sphingosine-1-phosphate. Biochim Biophys Acta. 2009 doi: 10.1016/j.bbalip.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia P, Gamble JR, Wang L, Pitson SM, Moretti PA, Wattenberg BW, D’Andrea RJ, Vadas MA. An oncogenic role of sphingosine kinase. Curr Biol. 2000;10(23):1527–30. doi: 10.1016/s0960-9822(00)00834-4. [DOI] [PubMed] [Google Scholar]
- 15.French K, Schrecengost R, Lee B, Zhuang Y, Smith S, Eberly J, Yun J, Smith C. Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res. 2003;63:5962–5969. [PubMed] [Google Scholar]
- 16.Ponnusamy S, Meyers-Needham M, Senkal CE, Saddoughi SA, Sentelle D, Selvam SP, Salas A, Ogretmen B. Sphingolipids and cancer: ceramide and sphingosine-1-phosphate in the regulation of cell death and drug resistance. Future Oncol. 2010;6(10):1603–24. doi: 10.2217/fon.10.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lavieu G, Scarlatti F, Sala G, Carpentier S, Levade T, Ghidoni R, Botti J, Codogno P. Regulation of autophagy by sphingosine kinase 1 and its role in cell survival during nutrient starvation. J Biol Chem. 2006;281:8518–8527. doi: 10.1074/jbc.M506182200. [DOI] [PubMed] [Google Scholar]
- 18.Senkal CE, Ponnusamy S, Bielawski J, Hannun YA, Ogretmen B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2010;24(1):296–308. doi: 10.1096/fj.09-135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagen N, Van Veldhoven PP, Proia RL, Park H, Merrill AH, Jr, Van Echten-Deckert G. Subcellular origin of sphingosine-1-phosphate is essental for its toxic effect in lyase deficient neurons. J Biol Chem. 2009 doi: 10.1074/jbc.M807336200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiegel S, Kolesnick R. Sphingosine-1-phosphate as a therapeutic agent. Leukemia. 2002;16:1596–602. doi: 10.1038/sj.leu.2402611. [DOI] [PubMed] [Google Scholar]
- 21.Oskouian B, Saba J. Cancer treatment strategies targeting sphingolipid metabolism. In: Chalfant CE, Del Poeta M, editors. Sphingolipids as Signaling and Regulatory Molecules. Landes Bioscience; Austin, TX: 2009. (in press) [Google Scholar]
- 22.Shida D, Takabe K, Kapitonov D, Milstien S, Spiegel S. Targeting SphK1 as a new strategy against cancer. Curr Drug Targets. 2008;9(8):662–73. doi: 10.2174/138945008785132402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pyne NJ, Pyne S. Sphingosine 1-phosphate and cancer. Nature Reviews Cancer. 2010;10(7):489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 24.Patwardhan GA, Liu YY. Sphingolipids and expression regulation of genes in cancer. Prog Lipid Res. 2011;50(1):104–114. doi: 10.1016/j.plipres.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spiegel S, Milstien S. Functions of the multifaceted family of sphingosine kinases and some close relatives. J Biol Chem. 2006 doi: 10.1074/jbc.R600028200. [DOI] [PubMed] [Google Scholar]
- 27.Jarman KE, Moretti PA, Zebol JR, Pitson SM. Translocation of sphingosine kinase 1 to the plasma membrane is mediated by calcium- and integrin-binding protein 1. J Biol Chem. 2010;285(1):483–92. doi: 10.1074/jbc.M109.068395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hait NC, Allegood J, Maceyka M, Strub GM, Harikumar KB, Singh SK, Luo C, Marmorstein R, Kordula T, Milstien S, Spiegel S. Regulation of histone acetylation in the nucleus by sphingosine-1-phosphate. Science. 2009;325(5945):1254–7. doi: 10.1126/science.1176709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Toman RE, Goparaju S, Maceyka M, Nava VE, Sankala H, Payne SG, Bektas M, Ishii I, Chun J, Milstien S, Spiegel S. Sphingosine kinase type 2 is a putative BH3-Only protein that induces apoptosis. J Biol Chem. 2003;278:40330–6. doi: 10.1074/jbc.M304455200. [DOI] [PubMed] [Google Scholar]
- 30.Mandala SM. Sphingosine-1-phosphate phosphatases. Prostaglandins Other Lipid Mediat. 2001;64(1–4):143–56. doi: 10.1016/s0090-6980(01)00111-3. [DOI] [PubMed] [Google Scholar]
- 31.Serra M, Saba JD. Sphingosine 1-phosphate lyase, a key regulator of sphingosine 1-phosphate signaling and function. Adv Enzyme Regul. 2010;50(1):349–62. doi: 10.1016/j.advenzreg.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colie S, Van Veldhoven PP, Kedjouar B, Bedia C, Albinet V, Sorli SC, Garcia V, Djavaheri-Mergny M, Bauvy C, Codogno P, Levade T, Andrieu-Abadie N. Disruption of sphingosine 1-phosphate lyase confers resistance to chemotherapy and promotes oncogenesis through Bcl-2/Bcl-xL upregulation. Cancer Res. 2009;69(24):9346–53. doi: 10.1158/0008-5472.CAN-09-2198. [DOI] [PubMed] [Google Scholar]
- 33.Min J, Stegner A, Alexander H, Alexander S. Overexpression of sphingosine-1-phosphate lyase or inhibition of sphingosine kinase in Dictyostelium discoideum results in a selective increase in sensitivity to platinum-based chemotherapy drugs. Eukaryotic Cell. 2004;3 doi: 10.1128/EC.3.3.795-805.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oskouian B, Sooriyakumaran P, Borowsky A, Crans A, Dillard-Telm L, Tam Y, Bandhuvula P, Saba J. Sphingosine-1-phosphate lyase potentiates apoptosis via p53- and p38-dependent pathways and is downregulated in colon cancer. Proc Natl Acad Sci U S A. 2006;103:17384–17389. doi: 10.1073/pnas.0600050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson KR, Johnson KY, Becker KP, Bielawski J, Mao C, Obeid LM. Role of human sphingosine-1-phosphate phosphatase 1 in the regulation of intra- and extracellular sphingosine-1-phosphate levels and cell viability. J Biol Chem. 2003;278(36):34541–34547. doi: 10.1074/jbc.M301741200. [DOI] [PubMed] [Google Scholar]
- 36.Le Stunff H, Galve-Roperh I, Peterson C, Milstien S, Spiegel S. Sphingosine-1-phosphate phosphohydrolase in regulation of sphingolipid metabolism and apoptosis. J Cell Biol. 2002;158(6):1039–49. doi: 10.1083/jcb.200203123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gomez-Munoz A, Duffy P, Martin A, O’Brien L, Byun H, Bittman R, Brindley D. Short-chain ceramide-1-phosphates are novel stimulators of DNA synthesis and cell division: antagonism by cell-permeable ceramides. Mol Pharmacol. 1995;47(5):833–839. [PubMed] [Google Scholar]
- 38.Lamour NF, Chalfant CE. Ceramide kinase and the ceramide-1-phosphate/cPLA2alpha interaction as a therapeutic target. Curr Drug Targets. 2008;9(8):674–82. doi: 10.2174/138945008785132349. [DOI] [PubMed] [Google Scholar]
- 39.Skoura A, Hla T. Lysophospholipid receptors in vertebrate development, physiology, and pathology. J Lipid Res. 2009;50(Suppl):S293–8. doi: 10.1194/jlr.R800047-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosen H, Goetzl EJ. Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol. 2005;5(7):560–70. doi: 10.1038/nri1650. [DOI] [PubMed] [Google Scholar]
- 41.Hobson JP, Rosenfeldt HM, Barak LS, Olivera A, Poulton S, Caron MG, Milstien S, Spiegel S. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 2001;291(5509):1800–1803. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- 42.Westwick JK, Bielawska AE, Dbaibo G, Hannun YA, Brenner DA. Ceramide activates the stress-activated protein kinases. J Biol Chem. 1995;270(39):22689–92. doi: 10.1074/jbc.270.39.22689. [DOI] [PubMed] [Google Scholar]
- 43.Kim HJ, Mun JY, Chun YJ, Choi KH, Kim MY. Bax-dependent apoptosis induced by ceramide in HL-60 cells. FEBS Lett. 2001;505(2):264–8. doi: 10.1016/s0014-5793(01)02836-8. [DOI] [PubMed] [Google Scholar]
- 44.Laurent G, Jaffrezou JP. Signaling pathways activated by daunorubicin. Blood. 2001;98(4):913–24. doi: 10.1182/blood.v98.4.913. [DOI] [PubMed] [Google Scholar]
- 45.Haimovitz-Friedman A, Kan C, Ehleiter D, Persaud R, McLoughlin M, Fuks Z, Kolesnick R. Ionizing radiation acts on cellular membranes to generate ceramide and initiate apoptosis. J Exp Med. 1994;180:525–535. doi: 10.1084/jem.180.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dbaibo G, Obeid L, Hannun Y. Tumor necrosis factor-α (TNF-α) signal transduction through ceramide. J Biol Chem. 1993;268(24):17762–17766. [PubMed] [Google Scholar]
- 47.Schütze S, Machleidt T, Krönke M. The role of diacylglycerol and ceramide in tumor necrosis factor and interleukin-1 signal transduction. J Leukoc Biol. 1994;56:533–541. doi: 10.1002/jlb.56.5.533. [DOI] [PubMed] [Google Scholar]
- 48.Takeda Y, Tashima M, Takahashi A, Uchiyama T, Okazaki T. Ceramide generation in nitric oxide-induced apoptosis. Activation of magnesium-dependent neutral sphingomyelinase via caspase-3. J Biol Chem. 1999;274(15):10654–60. doi: 10.1074/jbc.274.15.10654. [DOI] [PubMed] [Google Scholar]
- 49.Cremesti A, Paris F, Grassme H, Holler N, Tschopp J, Fuks Z, Gulbins E, Kolesnick R. Ceramide enables fas to cap and kill. J Biol Chem. 2001;276(26):23954–61. doi: 10.1074/jbc.M101866200. [DOI] [PubMed] [Google Scholar]
- 50.Hannun YA. Functions of ceramide in coordinating cellular responses to stress. Science. 1996;274(5294):1855–9. doi: 10.1126/science.274.5294.1855. [DOI] [PubMed] [Google Scholar]
- 51.Perez GI, Knudson CM, Leykin L, Korsmeyer SJ, Tilly JL. Apoptosis-associated signaling pathways are required for chemotherapy- mediated female germ cell destruction [see comments] Nat Med. 1997;3(11):1228–32. doi: 10.1038/nm1197-1228. [DOI] [PubMed] [Google Scholar]
- 52.Bose R, Verheij M, Haimovitz-Friedman A, Scotto K, Fuks Z, Kolesnick R. Ceramide synthase mediates daunorubicin-induced apoptosis: an alternative mechanism for generating death signals. Cell. 1995;82(3):405–14. doi: 10.1016/0092-8674(95)90429-8. [DOI] [PubMed] [Google Scholar]
- 53.Franzen R, Pfeilschifter J, Huwiler A. Nitric oxide induces neutral ceramidase degradation by the ubiquitin/proteasome complex in renal mesangial cell cultures. FEBS Lett. 2002;532(3):441–4. doi: 10.1016/s0014-5793(02)03727-4. [DOI] [PubMed] [Google Scholar]
- 54.Choi MS, Anderson MA, Zhang Z, Zimonjic DB, Popescu N, Mukherjee AB. Neutral ceramidase gene: role in regulating ceramide-induced apoptosis. Gene. 2003;315:113–22. doi: 10.1016/s0378-1119(03)00721-2. [DOI] [PubMed] [Google Scholar]
- 55.Lafont E, Milhas D, Carpentier S, Garcia V, Jin ZX, Umehara H, Okazaki T, Schulze-Osthoff K, Levade T, Benoist H, Segui B. Caspase-mediated inhibition of sphingomyelin synthesis is involved in FasL-triggered cell death. Cell Death Differ. 2010;17(4):642–54. doi: 10.1038/cdd.2009.130. [DOI] [PubMed] [Google Scholar]
- 56.Smyth MJ, Perry DK, Zhang J, Poirier GG, Hannun YA, Obeid LM. prICE: a downstream target for ceramide-induced apoptosis and for the inhibitory action of Bcl-2. Biochem J. 1996;316(Pt 1):25–8. doi: 10.1042/bj3160025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolff R, Dobrowsky R, Bielawska A, Obeid L, Hannun Y. Role of ceramide-activated protein phosphatase in ceramide-mediated signal transduction. J Biol Chem. 1994;269(30):19605–19609. [PubMed] [Google Scholar]
- 58.Dobrowsky R, Kamibayashi C, Mumby M, Hannun Y. Ceramide activates a heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268(21):15523–15530. [PubMed] [Google Scholar]
- 59.Mondal S, Mandal C, Sangwan R, Chandra S. Withanolide D induces apoptosis in leukemia by targeting the activation of neutral sphingomyelinase-ceramide cascade mediated by synergistic activation of c-Jun N-terminal kinase and p38 mitogen-activated protein kinase. Mol Cancer. 2010;9:239. doi: 10.1186/1476-4598-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jarvis WD, Fornari FA, Jr, Auer KL, Freemerman AJ, Szabo E, Birrer MJ, Johnson CR, Barbour SE, Dent P, Grant S. Coordinate regulation of stress- and mitogen-activated protein kinases in the apoptotic actions of ceramide and sphingosine. Mol Pharmacol. 1997;52(6):935–47. doi: 10.1124/mol.52.6.935. [DOI] [PubMed] [Google Scholar]
- 61.Liu X, Ryland L, Yang J, Liao A, Aliaga C, Watts R, Tan SF, Kaiser J, Shanmugavelandy SS, Rogers A, Loughran K, Petersen B, Yuen J, Meng F, Baab KT, Jarbadan NR, Broeg K, Zhang R, Liao J, Sayers TJ, Kester M, Loughran TP., Jr Targeting of survivin by nanoliposomal ceramide induces complete remission in a rat model of NK-LGL leukemia. Blood. 2010;116(20):4192–201. doi: 10.1182/blood-2010-02-271080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wooten-Blanks LG, Song P, Senkal CE, Ogretmen B. Mechanisms of ceramide-mediated repression of the human telomerase reverse transcriptase promoter via deacetylation of Sp3 by histone deacetylase 1. FASEB J. 2007;21(12):3386–97. doi: 10.1096/fj.07-8621com. [DOI] [PubMed] [Google Scholar]
- 63.Beyne-Rauzy O, Prade-Houdellier N, Demur C, Recher C, Ayel J, Laurent G, Mansat-De Mas V. Tumor necrosis factor-alpha inhibits hTERT gene expression in human myeloid normal and leukemic cells. Blood. 2005;106(9):3200–5. doi: 10.1182/blood-2005-04-1386. [DOI] [PubMed] [Google Scholar]
- 64.Okajima F, Tomura H, Sho K, Nochi H, Tamoto K, Kondo Y. Involvement of pertussis toxin-sensitive GTP-binding proteins in sphingosine 1-phosphate-induced activation of phospholipase C-Ca2+ system in HL60 leukemia cells. FEBS. 1996;379:260–264. doi: 10.1016/0014-5793(95)01526-4. [DOI] [PubMed] [Google Scholar]
- 65.Van Koppen C, Meyer zHM, Laser KT, Zhang C, Jakobs KH, Bunemann M, Pott L. Activation of a high affinity Gi protein-coupled plasma membrane receptor by sphingosine-1-phosphate. J Biol Chem. 1996;271(4):2082–2087. doi: 10.1074/jbc.271.4.2082. [DOI] [PubMed] [Google Scholar]
- 66.Buehrer B, Bardes E, Bell R. Protein kinase C-dependent regulation of human erythroleukemia (HEL) cell sphingosine kinase activity. Biochim Biophys Acta. 1996;1303:233–242. doi: 10.1016/0005-2760(96)00092-6. [DOI] [PubMed] [Google Scholar]
- 67.Cuvillier O, Levade T. Sphingosine 1-phosphate antagonizes apoptosis of human leukemia cells by inhibiting release of cytochrome c and Smac/DIABLO from mitochondria. Blood. 2001;98:2828–2836. doi: 10.1182/blood.v98.9.2828. [DOI] [PubMed] [Google Scholar]
- 68.Kleuser B, Cuvillier O, Spiegel S. 1Alpha, 25-dihydroxyvitamin D3 inhibits programmed cell death in HL60 cells by activation of sphingosine kinase. Cancer Res. 1998;58:1817–1824. [PubMed] [Google Scholar]
- 69.Nakamura H, Oda T, Hamada K, Hirano T, Shimizu N, Utiyama H. Survival by Mac-1-mediated adherence and anoikis in phorbol ester-treated HL-60 cells. J Biol Chem. 1998;273(25):15345–51. doi: 10.1074/jbc.273.25.15345. [DOI] [PubMed] [Google Scholar]
- 70.Hamada K, Nakamura H, Oda T, Hirano T, Shimizu N, Utiyama H. Involvement of Mac-1-mediated adherence and sphingosine-1-phosphate in survival of phorbol ester-treated U937 cells. Biochem Biophys Res Commun. 1998;244:745–750. doi: 10.1006/bbrc.1998.8328. [DOI] [PubMed] [Google Scholar]
- 71.Alemany R, Meyer zu Heringdorf D, van Koppen C, Jakobs K. Formyl peptide receptor signaling in HL-60 cells through sphingosine kinase. J Biol Chem. 1999;274:3994–3999. doi: 10.1074/jbc.274.7.3994. [DOI] [PubMed] [Google Scholar]
- 72.Sobue S, Nemoto S, Murakami M, Ito H, Kimura A, Gao S, Furuhata A, Takagi A, Kojima T, Nakamura M, Ito Y, Suzuki M, Banno Y, Nozawa Y, Murate T. Implications of sphingosine kinase 1 expression level for the cellular sphingolipid rheostat: relevance as a marker for daunorubicin sensitivity of leukemia cells. Int J Hematol. 2008;87(3):266–75. doi: 10.1007/s12185-008-0052-0. [DOI] [PubMed] [Google Scholar]
- 73.Lee MJ, Van Brocklyn JR, Thangada S, Liu CH, Hand AR, Menzeleev R, Spiegel S, Hla T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279(5356):1552–5. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- 74.Sato K, Murata N, Kon J, Tomura H, Nochi H, Tamoto K, Osada M, Ohta H, Tokumitsu Y, Ui M, Okajima F. Downregulation of mRNA expression of Edg-3, a putative sphingosine-1-phosphate receptor coupled to Ca2+ signaling, during differentiation of HL-60 leukemia cells. Biochem Biophys Res Commun. 1998;253:253–256. doi: 10.1006/bbrc.1998.9745. [DOI] [PubMed] [Google Scholar]
- 75.Jolly PS, Bektas M, Watterson KR, Sankala H, Payne SG, Milstien S, Spiegel S. Expression of SphK1 impairs degranulation and motility of RBL-2H3 mast cells by desensitizing S1P receptors. Blood. 2005;105(12):4736–42. doi: 10.1182/blood-2004-12-4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kothapalli R, Kusmartseva I, Loughran TP. Characterization of a human sphingosine-1-phosphate receptor gene (S1P5) and its differential expression in LGL leukemia. Biochim Biophys Acta. 2002;1579(2–3):117–23. doi: 10.1016/s0167-4781(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 77.Sokol L, Loughran TP., Jr Large granular lymphocyte leukemia. Oncologist. 2006;11(3):263–73. doi: 10.1634/theoncologist.11-3-263. [DOI] [PubMed] [Google Scholar]
- 78.Zhang R, Shah MV, Yang J, Nyland SB, Liu X, Yun JK, Albert R, Loughran TP., Jr Network model of survival signaling in large granular lymphocyte leukemia. Proc Natl Acad Sci U S A. 2008;105(42):16308–13. doi: 10.1073/pnas.0806447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shah MV, Zhang R, Irby R, Kothapalli R, Liu X, Arrington T, Frank B, Lee NH, Loughran TP., Jr Molecular profiling of LGL leukemia reveals role of sphingolipid signaling in survival of cytotoxic lymphocytes. Blood. 2008;112(3):770–81. doi: 10.1182/blood-2007-11-121871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Le Scolan E, Pchejetski D, Banno Y, Denis N, Mayeux P, Vainchenker W, Levade T, Moreau-Gachelin F. Overexpression of sphingosine kinase 1 is an oncogenic event in erythroleukemic progression. Blood. 2005;106(5):1808–16. doi: 10.1182/blood-2004-12-4832. [DOI] [PubMed] [Google Scholar]
- 81.Chen Y, Peng C, Li D, Li S. Molecular and cellular bases of chronic myeloid leukemia. Protein & Cell. 2010;1(2):124–32. doi: 10.1007/s13238-010-0016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baran Y, Salas A, Senkal CE, Gunduz U, Bielawski J, Obeid LM, Ogretmen B. Alterations of ceramide/sphingosine 1-phosphate rheostat involved in the regulation of resistance to imatinib-induced apoptosis in K562 human chronic myeloid leukemia cells. J Biol Chem. 2007;282(15):10922–34. doi: 10.1074/jbc.M610157200. [DOI] [PubMed] [Google Scholar]
- 83.Bonhoure E, Pchejetski D, Aouali N, Morjani H, Levade T, Kohama T, Cuvillier O. Overcoming MDR-associated chemoresistance in HL-60 acute myeloid leukemia cells by targeting sphingosine kinase-1. Leukemia. 2006;20(1):95–102. doi: 10.1038/sj.leu.2404023. [DOI] [PubMed] [Google Scholar]
- 84.Marfe G, Di Stefano C, Gambacurta A, Ottone T, Martini V, Abruzzese E, Mologni L, Sinibaldi-Salimei P, de Fabritis P, Gambacorti-Passerini C, Amadori S, Birge RB. Sphingosine kinase 1 overexpression is regulated by signaling through PI3K, AKT2, and mTOR in imatinib-resistant chronic myeloid leukemia (CML) cells. Exp Hematol. 2011 doi: 10.1016/j.exphem.2011.02.013. accepted manuscript. [DOI] [PubMed] [Google Scholar]
- 85.Lee H, Deng J, Kujawski M, Yang C, Liu Y, Herrmann A, Kortylewski M, Horne D, Somlo G, Forman S, Jove R, Yu H. STAT3-induced S1PR1 expression is crucial for persistent STAT3 activation in tumors. Nat Med. 2010;16(12):1421–8. doi: 10.1038/nm.2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41(16):2502–12. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 87.Benekli M, Baumann H, Wetzler M. Targeting signal transducer and activator of transcription signaling pathway in leukemias. J Clin Oncol. 2009;27(26):4422–32. doi: 10.1200/JCO.2008.21.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stam J, Michiels F, van der Kammen R, Moolenaar W, Collard J. Invasion of T-lymphoma cells: cooperation between Rho family GTPases and lysophospholipid receptor signaling. EMBO J. 1998;17:4066–4074. doi: 10.1093/emboj/17.14.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Takuwa Y, Takuwa N, Sugimoto N. The Edg family G protein-coupled receptors for lysophospholipids: their signaling properties and biological activities. J Biochem. 2002;131(6):767–71. doi: 10.1093/oxfordjournals.jbchem.a003163. [DOI] [PubMed] [Google Scholar]
- 90.Ito Y. RUNX genes in development and cancer: regulation of viral gene expression and the discovery of RUNX family genes. Adv Cancer Res. 2008;99:33–76. doi: 10.1016/S0065-230X(07)99002-8. [DOI] [PubMed] [Google Scholar]
- 91.Kilbey A, Terry A, Jenkins A, Borland G, Zhang Q, Wakelam MJ, Cameron ER, Neil JC. Runx regulation of sphingolipid metabolism and survival signaling. Cancer Res. 2010;70(14):5860–9. doi: 10.1158/0008-5472.CAN-10-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li MH, Sanchez T, Pappalardo A, Lynch KR, Hla T, Ferrer F. Induction of antiproliferative connective tissue growth factor expression in Wilms’ tumor cells by sphingosine-1-phosphate receptor 2. Mol Cancer Res. 2008;6(10):1649–56. doi: 10.1158/1541-7786.MCR-07-2048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Skoura A, Sanchez T, Claffey K, Mandala SM, Proia RL, Hla T. Essential role of sphingosine 1-phosphate receptor 2 in pathological angiogenesis of the mouse retina. J Clin Invest. 2007;117(9):2506–16. doi: 10.1172/JCI31123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cattoretti G, Mandelbaum J, Lee N, Chaves AH, Mahler AM, Chadburn A, Dalla-Favera R, Pasqualucci L, MacLennan AJ. Targeted disruption of the S1P2 sphingosine 1-phosphate receptor gene leads to diffuse large B-cell lymphoma formation. Cancer Res. 2009;69(22):8686–92. doi: 10.1158/0008-5472.CAN-09-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Du W, Takuwa N, Yoshioka K, Okamoto Y, Gonda K, Sugihara K, Fukamizu A, Asano M, Takuwa Y. S1P(2), the G protein-coupled receptor for sphingosine-1-phosphate, negatively regulates tumor angiogenesis and tumor growth in vivo in mice. Cancer Res. 2010;70(2):772–81. doi: 10.1158/0008-5472.CAN-09-2722. [DOI] [PubMed] [Google Scholar]
- 96.Gustafsson K, Sander B, Bielawski J, Hannun YA, Flygare J. Potentiation of cannabinoid-induced cytotoxicity in mantle cell lymphoma through modulation of ceramide metabolism. Mol Cancer Res. 2009;7(7):1086–98. doi: 10.1158/1541-7786.MCR-08-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nishimura H, Akiyama T, Monobe Y, Matsubara K, Igarashi Y, Abe M, Sugihara T, Sadahira Y. Expression of sphingosine-1-phosphate receptor 1 in mantle cell lymphoma. Mod Pathol. 2010;23(3):439–49. doi: 10.1038/modpathol.2009.194. [DOI] [PubMed] [Google Scholar]
- 98.Bayerl MG, Bruggeman RD, Conroy EJ, Hengst JA, King TS, Jimenez M, Claxton DF, Yun JK. Sphingosine kinase 1 protein and mRNA are overexpressed in non-Hodgkin lymphomas and are attractive targets for novel pharmacological interventions. Leuk Lymphoma. 2008;49(5):948–54. doi: 10.1080/10428190801911654. [DOI] [PubMed] [Google Scholar]
- 99.Gupta SD, Gable K, Han G, Borovitskaya A, Selby L, Dunn TM, Harmon JM. Tsc10p and FVT1: topologically distinct short-chain reductases required for long-chain base synthesis in yeast and mammals. J Lipid Res. 2009;50(8):1630–40. doi: 10.1194/jlr.M800580-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Czuchlewski DR, Csernus B, Bubman D, Hyjek E, Martin P, Chadburn A, Knowles DM, Cesarman E. Expression of the follicular lymphoma variant translocation 1 gene in diffuse large B-cell lymphoma correlates with subtype and clinical outcome. Am J Clin Pathol. 2008;130(6):957–62. doi: 10.1309/AJCP12HIRWSRQLAN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li QF, Wu CT, Guo Q, Wang H, Wang LS. Sphingosine 1-phosphate induces Mcl-1 upregulation and protects multiple myeloma cells against apoptosis. Biochemical and biophysical research communications. 2008;371:159–62. doi: 10.1016/j.bbrc.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 102.Li QF, Wu CT, Duan HF, Sun HY, Wang H, Lu ZZ, Zhang QW, Liu HJ, Wang LS. Activation of sphingosine kinase mediates suppressive effect of interleukin-6 on human multiple myeloma cell apoptosis. British journal of haematology. 2007;138:632–9. doi: 10.1111/j.1365-2141.2007.06711.x. [DOI] [PubMed] [Google Scholar]
- 103.Fujita T, Inoue K, Yamamoto S, Ikumoto T, Sasaki S, Toyama R, Chiba K, Hoshino Y, TO Fungal metabolites. Part 11. A potent immunosuppressive activity found in Isaria sinclairii metabolite. J Antibiot (Tokyo) 1994;47:208–215. doi: 10.7164/antibiotics.47.208. [DOI] [PubMed] [Google Scholar]
- 104.Mandala S, Hajdu R, Bergstrom J, Quackenbush E, Xie J, Milligan J, Thornton R, Shei GJ, Card D, Keohane C, Rosenbach M, Hale J, Lynch CL, Rupprecht K, Parsons W, Rosen H. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 2002;296(5566):346–9. doi: 10.1126/science.1070238. [DOI] [PubMed] [Google Scholar]
- 105.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277(24):21453–7. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 106.Zemann B, Kinzel B, Muller M, Reuschel R, Mechtcheriakova D, Urtz N, Bornancin F, Baumruker T, Billich A. Sphingosine kinase type 2 is essential for lymphopenia induced by the immunomodulatory drug FTY720. Blood. 2006;107(4):1454–8. doi: 10.1182/blood-2005-07-2628. [DOI] [PubMed] [Google Scholar]
- 107.Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G protein-coupled receptors. FASEB J. 2004;18(3):551–3. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- 108.Neviani P, Santhanam R, Oaks JJ, Eiring AM, Notari M, Blaser BW, Liu S, Trotta R, Muthusamy N, Gambacorti-Passerini C, Druker BJ, Cortes J, Marcucci G, Chen CS, Verrills NM, Roy DC, Caligiuri MA, Bloomfield CD, Byrd JC, Perrotti D. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest. 2007;117(9):2408–21. doi: 10.1172/JCI31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fujino M, Li XK, Kitazawa Y, Guo L, Kawasaki M, Funeshima N, Amano T, Suzuki S. Distinct pathways of apoptosis triggered by FTY720, etoposide, and anti-Fas antibody in human T-lymphoma cell line (Jurkat cells) J Pharmacol Exp Ther. 2002;300(3):939–45. doi: 10.1124/jpet.300.3.939. [DOI] [PubMed] [Google Scholar]
- 110.Nagahara Y, Matsuoka Y, Saito K, Ikekita M, Higuchi S, Shinomiya T. Coordinate involvement of cell cycle arrest and apoptosis strengthen the effect of FTY720. Jpn J Cancer Res. 2001;92(6):680–7. doi: 10.1111/j.1349-7006.2001.tb01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yasui H, Hideshima T, Raje N, Roccaro AM, Shiraishi N, Kumar S, Hamasaki M, Ishitsuka K, Tai YT, Podar K, Catley L, Mitsiades CS, Richardson PG, Albert R, Brinkmann V, Chauhan D, Anderson KC. FTY720 induces apoptosis in multiple myeloma cells and overcomes drug resistance. Cancer Res. 2005;65(16):7478–84. doi: 10.1158/0008-5472.CAN-05-0850. [DOI] [PubMed] [Google Scholar]
- 112.Roberts KG, Smith AM, McDougall F, Carpenter H, Horan M, Neviani P, Powell JA, Thomas D, Guthridge MA, Perrotti D, Sim AT, Ashman LK, Verrills NM. Essential requirement for PP2A inhibition by the oncogenic receptor c-KIT suggests PP2A reactivation as a strategy to treat c-KIT+ cancers. Cancer Res. 2010;70(13):5438–47. doi: 10.1158/0008-5472.CAN-09-2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cristobal I, Garcia-Orti L, Cirauqui C, Alonso MM, Calasanz MJ, Odero MD. PP2A impaired activity is a common event in acute myeloid leukemia and its activation by forskolin has a potent anti-leukemic effect. Leukemia. 2011 doi: 10.1038/leu.2010.294. [DOI] [PubMed] [Google Scholar]
- 114.Sabbadini RA. Sphingosine-1-phosphate antibodies as potential agents in the treatment of cancer and age-related macular degeneration. Br J Pharmacol. 2011;162(6):1225–38. doi: 10.1111/j.1476-5381.2010.01118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ohta H, Sweeney E, Masamune A, Yatomi Y, Hakomori S, Igarashi Y. Induction of apoptosis by sphingosine in human leukemic HL-60 cells: a possible endogenous modulator of apoptotic DNA fragmentation occurring during phorbol ester-induced differentiation. Cancer Res. 1995;55(3):691–697. [PubMed] [Google Scholar]
- 116.Coward J, Ambrosini G, Musi E, Truman JP, Haimovitz-Friedman A, Allegood JC, Wang E, Merrill AH, Jr, Schwartz GK. Safingol (L-threo-sphinganine) induces autophagy in solid tumor cells through inhibition of PKC and the PI3-kinase pathway. Autophagy. 2009;5(2):184–93. doi: 10.4161/auto.5.2.7361. [DOI] [PubMed] [Google Scholar]
- 117.Dickson MA, Carvajal RD, Merrill AH, Gonen M, Cane LM, Schwartz GK. A phase I clinical trial of safingol in combination with cisplatin in advanced solid tumors. Clin Cancer Res. 2011 doi: 10.1158/1078-0432.CCR-10-2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schwartz GK, Ward D, Saltz L, Casper ES, Spiess T, Mullen E, Woodworth J, Venuti R, Zervos P, Storniolo AM, Kelsen DP. A pilot clinical/pharmacological study of the protein kinase C-specific inhibitor safingol alone and in combination with doxorubicin. Clin Cancer Res. 1997;3(4):537–43. [PubMed] [Google Scholar]
- 119.Paugh SW, Paugh BS, Rahmani M, Kapitonov D, Almenara JA, Kordula T, Milstien S, Adams JK, Zipkin RE, Grant S, Spiegel S. A selective sphingosine kinase 1 inhibitor integrates multiple molecular therapeutic targets in human leukemia. Blood. 2008;112(4):1382–91. doi: 10.1182/blood-2008-02-138958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Beljanski V, Knaak C, Smith CD. A novel sphingosine kinase inhibitor induces autophagy in tumor cells. J Pharmacol Exp Ther. 2010;333(2):454–64. doi: 10.1124/jpet.109.163337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Azuma H, Takahara S, Ichimaru N, Wang JD, Itoh Y, Otsuki Y, Morimoto J, Fukui R, Hoshiga M, Ishihara T, Nonomura N, Suzuki S, Okuyama A, Katsuoka Y. Marked prevention of tumor growth and metastasis by a novel immunosuppressive agent, FTY720, in mouse breast cancer models. Cancer Res. 2002;62(5):1410–9. [PubMed] [Google Scholar]
- 122.Azuma H, Takahara S, Horie S, Muto S, Otsuki Y, Katsuoka Y. Induction of apoptosis in human bladder cancer cells in vitro and in vivo caused by FTY720 treatment. J Urol. 2003;169(6):2372–7. doi: 10.1097/01.ju.0000064938.32318.91. [DOI] [PubMed] [Google Scholar]
- 123.Sweeney EA, Sakakura C, Shirahama T, Masamune A, Ohta H, Hakomori S, Igarashi Y. Sphingosine and its methylated derivative N,N-dimethylsphingosine (DMS) induce apoptosis in a variety of human cancer cell lines. Int J Cancer. 1996;66(3):358–366. doi: 10.1002/(SICI)1097-0215(19960503)66:3<358::AID-IJC16>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 124.Park SR, Cho HJ, Moon KJ, Chun KH, Kong SY, Yoon SS, Lee JS, Park S. Cytotoxic effects of novel phytosphingosine derivatives, including N,N-dimethylphytosphingosine and N-monomethylphytosphingosine, in human leukemia cell line HL60. Leuk Lymphoma. 2010;51(1):132–45. doi: 10.3109/10428190903383419. [DOI] [PubMed] [Google Scholar]
- 125.Fyrst H, Oskouian B, Bandhuvula P, Gong Y, Byun HS, Bittman R, Lee AR, Saba JD. Natural sphingadienes inhibit Akt-dependent signaling and prevent intestinal tumorigenesis. Cancer Res. 2009;69(24):9457–64. doi: 10.1158/0008-5472.CAN-09-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Visentin B, Vekich J, Sibbald B, Cavalli A, Moreno K, Matteo R, Garland W, Lu Y, Yu S, Hall H, Kundra V, Mills G, Sabbadini R. Validation of an anti-sphingosine-1-phosphate antibody as a potential therapeutic in reducing growth, invasion, and angiogenesis in multiple tumor lineages. Cancer Cell. 2006;9:225–238. doi: 10.1016/j.ccr.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 127.Elojeimy S, Liu X, McKillop JC, El-Zawahry AM, Holman DH, Cheng JY, Meacham WD, Mahdy AE, Saad AF, Turner LS, Cheng J, TAD, Dong JY, Bielawska A, Hannun YA, Norris JS. Role of acid ceramidase in resistance to FasL: therapeutic approaches based on acid ceramidase inhibitors and FasL gene therapy. Mol Ther. 2007;15(7):1259–63. doi: 10.1038/sj.mt.6300167. [DOI] [PubMed] [Google Scholar]
- 128.Saad AF, Meacham WD, Bai A, Anelli V, Elojeimy S, Mahdy AE, Turner LS, Cheng J, Bielawska A, Bielawski J, Keane TE, Obeid LM, Hannun YA, Norris JS, Liu X. The functional effects of acid ceramidase overexpression in prostate cancer progression and resistance to chemotherapy. Cancer Biol Ther. 2007;6(9):1455–60. doi: 10.4161/cbt.6.9.4623. [DOI] [PubMed] [Google Scholar]
- 129.Liu X, Elojeimy S, Turner LS, Mahdy AE, Zeidan YH, Bielawska A, Bielawski J, Dong JY, El-Zawahry AM, Guo GW, Hannun YA, Holman DH, Rubinchik S, Szulc Z, Keane TE, Tavassoli M, Norris JS. Acid ceramidase inhibition: a novel target for cancer therapy. Front Biosci. 2008;13:2293–8. doi: 10.2741/2843. [DOI] [PubMed] [Google Scholar]
- 130.Mahdy AE, Cheng JC, Li J, Elojeimy S, Meacham WD, Turner LS, Bai A, Gault CR, McPherson AS, Garcia N, Beckham TH, Saad A, Bielawska A, Bielawski J, Hannun YA, Keane TE, Taha MI, Hammouda HM, Norris JS, Liu X. Acid ceramidase upregulation in prostate cancer cells confers resistance to radiation: AC inhibition, a potential radiosensitizer. Mol Ther. 2009;17(3):430–8. doi: 10.1038/mt.2008.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim YJ, Kim EA, Sohn UD, Yim CB, Im C. Cytotoxic Activity and Structure Activity Relationship of Ceramide Analogues in Caki-2 and HL-60 Cells. Korean J Physiol Pharmacol. 2010;14(6):441–7. doi: 10.4196/kjpp.2010.14.6.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gouaze-Andersson V, Flowers M, Karimi R, Fabrias G, Delgado A, Casas J, Cabot MC. Inhibition of acid ceramidase by a 2-substituted aminoethanol amide synergistically sensitizes prostate cancer cells to N-(4-hydroxyphenyl) retinamide. Prostate. 2010 doi: 10.1002/pros.21321. [DOI] [PubMed] [Google Scholar]
- 133.Dobrowsky R, Werner M, Castellino A, Chao M, Hannun Y. Activation of the sphingomyelin cycle through the low-affinity neurotrophin receptor. Science. 1994;265:1596–1599. doi: 10.1126/science.8079174. [DOI] [PubMed] [Google Scholar]
- 134.Chao R, Kahn W, Hannun Y. Retinoblastoma protein dephosphorylation induced by D-erythro-sphingosine. J Biol Chem. 1992;267(33):23459–23462. [PubMed] [Google Scholar]
- 135.Jiang Q, Wong J, Fyrst H, Saba JD, Ames BN. gamma-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc Natl Acad Sci U S A. 2004;101(51):17825–30. doi: 10.1073/pnas.0408340102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kraveka J, Li L, Szulc Z, Bielawski J, Ogretmen B, Hannun Y, Obeid L, Bielawska A. Involvement of dihydroceramide desaturase in cell cycle progression in human neuroblastoma cells. J Biol Chem. 2007;282:16718–16728. doi: 10.1074/jbc.M700647200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kumar A, Byun HS, Bittman R, Saba JD. The sphingolipid degradation product trans-2-hexadecenal induces cytoskeletal reorganization and apoptosis in a JNK-dependent manner. Cell Signal. 2011 doi: 10.1016/j.cellsig.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kumar A, Oskouian B, Fyrst H, Zhang M, Paris F, Saba JD. S1P lyase regulates DNA damage responses through a novel sphingolipid feedback mechanism. Cell Death Dis. 2011;2(1):e119. doi: 10.1038/cddis.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.O’Leary M, Krailo M, Anderson JR, Reaman GH. Progress in childhood cancer: 50 years of research collaboration, a report from the Children’s Oncology Group. Semin Oncol. 2008;35(5):484–93. doi: 10.1053/j.seminoncol.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Paris F, Perez G, Fuks Z, Haimovitz-Friedman A, Nguyen H, Bose M, Ilagan A, Hunt P, Morgan W, Tilly J, Kolesnick R. Sphingosine-1-phosphate preserves fertility in irradiated female mice without propagating genomic damage in offspring. Nat Med. 2002;8:901–902. doi: 10.1038/nm0902-901. [DOI] [PubMed] [Google Scholar]
- 141.Bonnaud S, Niaudet C, Legoux F, Corre I, Delpon G, Saulquin X, Fuks Z, Gaugler MH, Kolesnick R, Paris F. Sphingosine-1-phosphate activates the AKT pathway to protect small intestines from radiation-induced endothelial apoptosis. Cancer Res. 2010;70(23):9905–15. doi: 10.1158/0008-5472.CAN-10-2043. [DOI] [PubMed] [Google Scholar]
- 142.Maj J, Paris F, Haimovitz-Friedman A, Venkatraman E, Kolesnick R, Fuks Z. Microvascular function regulates intestinal crypt response to radiation. Cancer Res. 2003;63(15):4338–4341. [PubMed] [Google Scholar]
- 143.Rotolo JA, Mesicek J, Maj J, Truman JP, Haimovitz-Friedman A, Kolesnick R, Fuks Z. Regulation of ceramide synthase-mediated crypt epithelium apoptosis by DNA damage repair enzymes. Cancer Res. 2010;70(3):957–67. doi: 10.1158/0008-5472.CAN-09-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kim YM, Sachs T, Asavaroengchai W, Bronson R, Sykes M. Graft-versus-host disease can be separated from graft-versus-lymphoma effects by control of lymphocyte trafficking with FTY720. J Clin Invest. 2003;111(5):659–69. doi: 10.1172/JCI16950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bandhuvula P, Honbo N, Wang GY, Jin ZQ, Fyrst H, Zhang M, Borowsky AD, Dillard L, Karliner JS, Saba JD. S1P lyase (SPL): a novel therapeutic target for ischemia/reperfusion injury of the heart. Am J Physiol Heart Circ Physiol. 2011 doi: 10.1152/ajpheart.00946.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhao Y, Gorshkova I, Berdyshev E, He D, Fu P, Ma W, Su Y, Usatyuk P, Pendyala S, Oskouian B, Saba J, Garcia J, Natarajan V. Protection of LPS-induced murine acute lung injury by sphingosine-1-phosphate lyase suppression. Am J Respir Cell Mol Biol. 2011 doi: 10.1165/rcmb.2010-0422OC. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]


