Abstract
Curcumin, a natural diphenolic compound derived from turmeric Curcuma longa, has proven to be a modulator of intracellular signaling pathways that control cancer cell growth, inflammation, invasion, apoptosis and cell death, revealing its anticancer potential. In this review, we focus on the design and development of nanoparticles, self-assemblies, nanogels, liposomes and complex fabrication for sustained and efficient curcumin delivery. We also discuss the anticancer applications and clinical benefits of nanocurcumin formulations. Only a few novel multifunctional and composite nanosystem strategies offer simultaneous therapy as well as imaging characteristics. We also summarize the challenges to developing curcumin delivery platforms and up-to-date solutions for improving curcumin bioavailability and anticancer potential for therapy.
Cancer continues to be the second leading cause of death in the USA. Current chemotherapeutic agents, such as alkylating agents, mustards, anti-metabolites, spindle poisons, and DNA binders and cutters, target a specific pathway, which ultimately shrinks tumor size but often fails to eradicate tumors or prevent their recurrence. Repeated treatment with these agents eventually results in tumors that become resistant to the chemotherapies. Therefore, it is crucial to identify ‘natural products’ that have growth inhibitory and apoptosis induction properties on human cancer cells and that target multiple cellular signaling pathways without resulting in toxicity issues in normal cells. Curcumin, a diphenolic compound extracted from the rhizome of turmeric (Curcuma longa), is a prominent candidate for treating anti-inflammatory, cystic fibrosis, Alzheimer’s and malarial diseases in addition to cancer [1].
A comprehensive review of the literature characterized curcumin as an excellent molecule among many naturally occurring compounds for cancer therapeutics [2]. Pleiotropic properties of the curcumin molecule enable it to target the genome (DNA), messengers (RNA) and enzymes (proteins) within cells, actions that can be sequential or simultaneous. Specifically, unlike other chemotherapeutic agents, curcumin exhibits pleiotropic properties that involve the modulation of nuclear factor-kappaB (NF-κB), transcription factor activator protein-1 (AP-1), mitogen-activated protein kinase (MAPK), tumor protein 53 (p53), nuclear β-catenin signaling, and serine/threonine protein kinase (AKT) signaling pathways [3]. Curcumin has been shown to suppress the expression of epidermal growth receptor and estrogen receptors, which are cancer-associated growth factors [4]. Some proof-of-concept studies have demonstrated that curcumin efficiently sensitizes tumor cells to first-line chemotherapies and radiation [5–7]. The development of multidrug resistance (MDR) in cancer therapeutics results in a minimal response to conventional cytotoxic agents and targeted biological therapies [8–10]. This occurs as a result of the overexpression of ATP-binding cassette transporters, which act as drug–efflux pumps involved in the aggressive removal of drug molecules from the cells, which therefore reduces the intracellular levels of these therapeutic molecules. This phenomenon can be overcome by curcumin treatment, which downregulates P-glycoprotein (P-gp), breast cancer resistance protein (ABCG2) and multidrug resistance protein (MRP-1) expression [11]. Owing to its valuable properties, almost 100 pharmaceutical and chemical companies are producing various curcumin products in the form of tablets, capsules, coloring agents, creams, dairy, drinks, extracts, gels, nasal sprays, and so on. for daily and medical needs [12]. Various clinical trials are underway or have been completed to judge the efficacy of curcumin as a therapeutic molecule in medicine (http://clinicaltrials.gov/ct2/results?term=curcumin).
A major limiting factor of curcumin is its low solubility in water (i.e. 0.0004 mg/ml at pH 7.3) and soluble curcumin molecules are extremely sensitive at physiological pH [13–17]. Many preclinical and clinical studies in mice, rats and humans revealed a low bioavailability of curcumin [2,18]. Although 10 or 12 g/ml of curcumin administered orally in humans showed curcumin levels in serum to be approximately 50 ng/ml, this resulted in a minimum availability of curcumin in the blood circulation to achieve its therapeutic effects [19]. Proven explorations suggest that improved curcumin properties; targeted delivery, tissue distribution and bioavailability in tumors can be efficiently achieved in the presence of an adjuvant, piperine [20,21].
A simple way of solving the limiting factors of curcumin is to improve its bioavailability, protect it from degradation and metabolism, and increase its targeting capacity toward cancer tumor(s). Various types of nanoparticle (NPs), such as polymer NPs, polymeric micelles, liposome/phospholipid, nano-/microemulsions, nanogels, solid lipid NPs, polymer conjugates, self-assemblies, and so on, are suitable for the delivery of an active form of curcumin to tumors (Figure 1a) [22]. Research efforts during the past 8–10 years have been devoted to this direction. A year-by-year diagram describing its increasing use by the medical community is shown in Figure 1b. Our group has developed a series of curcumin or anticancer nanoformulations for effective anticancer, hyperthermia and imaging applications in cancer therapy (Figure 1c) [7,23–28]; a short description on available curcumin nanoformulations is provided in Table 1. The improved solubility, bioavailability and pharmacokinetic properties of curcumin through various micro- and/or nanoformulations have been reviewed systematically elsewhere [29,30]. Therefore, the focus of this review is to discuss the design and development of NPs, self-assemblies, nanogels, liposome and complex fabrication, and their important contributions to cancer therapeutics.
Figure 1.
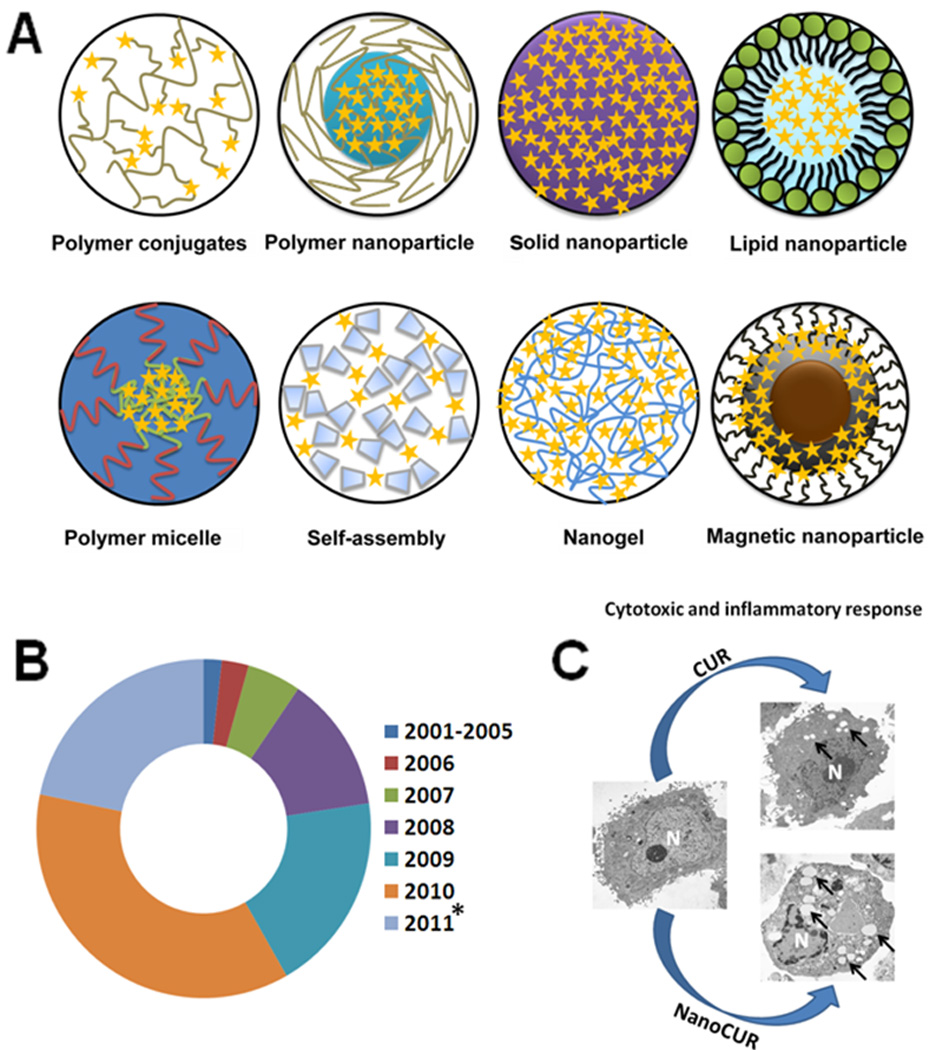
Types of curcumin nanoformulation used in cancer therapeutics. (a) Various types of curcumin nanoformulation developed during the past 10 years. (b) Increased use of curcumin nanoformulations during the past decade. Data were collected from journals published by Elsevier, Wiley Interscience, American Chemical Society, Springer, BMC Central and PubMed. * indicates data collected from Jan 2011 to July 2011. (c) Chemotherapeutic effects in PC-3 cancer cells of poly(lactic-co-glycolic acid) (PLGA) nanoparticles (nanoCUR) over free curcumin (CUR) through the formation of vacuoles involving the entire cell structure, as observed under a transmission electron microscope. Abbreviation: N, nucleus. Black arrows indicate vacuoles.
Table 1.
A comprehensive list of commercially available curcumin NP formulations
| Curcumin nanoformulation | Composition | Company name and website |
|---|---|---|
| Liposomal curcumin | Curcumin, phospholipids from lecithin, natural flavors, stevia, ethyl alcohol and potassium sorbate | NanoLiposomal Nutritionals, USA; http://www.NanoLiposomals.com |
| NanoBioSphere™ complex (solid lipid nanocurcumin) | Vitamin E (d,l-alpha tocopherol, sunflower oil, phospholipids, saffloweroil, ethanol, medium-chain triglycerides, glycerin, glyceryl stearate, ascorbyl palmitate with curcumin C3 complex, solid triglyceride and phosphatidylcholine | Life Enhancement Products, Inc., USA; http://www.life-enhancement.com |
| Curcumin C3 complex® vegetarian capsules | Curcumin C3 complex, curcuminods and black pepper extract (Piper nigrum; from fruit) | BestVite, Inc., USA; http://www.bestvite.com |
| Nanocurcumin (N-curcusorb) | Curcumin in nanosized particles | Konark Herbals & Health Care, India; http://www.curcuminindia.com |
| CurcuPlus D Ultra™ | Curcumin (Longvida®), vitamin D3, ascorbyl palmitate, microcrystalline cellulose, soy lecithin, stearic acid, maltodextrin and silicon dioxide | Advanced Orthomolecular Research, Inc., Canada; http://www.aor.ca/ |
| Nanocurcumin (in the scale-up process) | Liposomal curcumin formulation; nanocurcumin formulation; PLGA-curcumin formulation | SignPath Pharmaceuticals, Inc., USA; http://www.signpathpharma.com/ |
| Nutrivene Longvida™ curcumin (cutting-edge solid-lipid curcumin particle [SLCP™] technology) | Curcumin particles, vegetable-derived stearic acid dextrin, hydroxypropyl methylcellulose (vegetarian capsule), soy lecithin, ascorbyl palmitate and silicon dioxide | International Nutrition, Inc., USA; http://www.nutrivene.com/ |
| Enhansa (enhanced absorption curcumin) | A special curcumin compound | Lee Silsby Compounding Pharmacy, USA; http://www.leesilsby.com |
| Nano curcumin/nano curcuma | Nanocurcumin solution (colloid) products | Nano Tech Miso-N, Korea; http://www.miso-n.co.kr |
Curcumin nanocrystals and conjugates
Nano drug-crystals have a greater dissolution rate owing to a larger specific surface area (Figure 2a). Development of successful nanocrystal formulation by any of these bottom-up approaches depends on the stabilization process. Curcumin crystal formation is a time-dependant process, in that it takes 90 min in a solution of alcohol and water [31]. After 90 min, curcumin crystals start to aggregate and form as a precipitate (Figure 2b). The aggregation of surfactant molecules, including sodium dodecyl sulfate, cetyltrimethylammonium bromide, Tween 80, Triton X-100 and pluronic polymers, form micelles at a critical micelle concentration and can provide the required influence to stabilize curcumin molecules [32]. However, cationic micelles provide greater stability to curcumin even at elevated pH and are preferred for their medicinal implications. Similarly, plasma proteins have also been recognized as carriers for curcumin because of their ability to stabilize curcumin molecules [33]. High-pressure homogenization (HPH) is a suitable method for reducing bulk curcumin into NPs. The optimized condition for producing curcumin crystals, as defined by increased dispersions, is at a temperature of 2°C and with ten cycles of HPH with an applied pressure of 150 MPa [34]. Stable self-emulsifying liquid formulations of curcumin with particles size of approximately 30 nm and approximately 99% curcumin loading have successfully been developed [35]. These formulations have a 10–14-fold greater absorption rate in male Wistar-strain rats given an oral treatment of 50 mg/kg of curcumin, compared with the same oral dose of free curcumin. D-α tocopheryl polyethylene glycol 1000 succinate (TPGS) stabilized curcumin nanosuspension (CUR-NS) crystal formulation has been evaluated for the pharmacokinetics and biodistribution of curcumin after intravenous administration in rabbits and mice [36]. The area under the plasma concentration (AUC; 0–∞) of CUR-NS (700.43±281.53 µg/ml, min) was 3.8-fold greater than for a curcumin solution (145.4 ±9.29 µg/ml, min). The mean residence time was 11.2-fold longer with CUR-NS compared with a curcumin solution (194.57±32.18 min vs 15.88±3.56 min).
Figure 2.
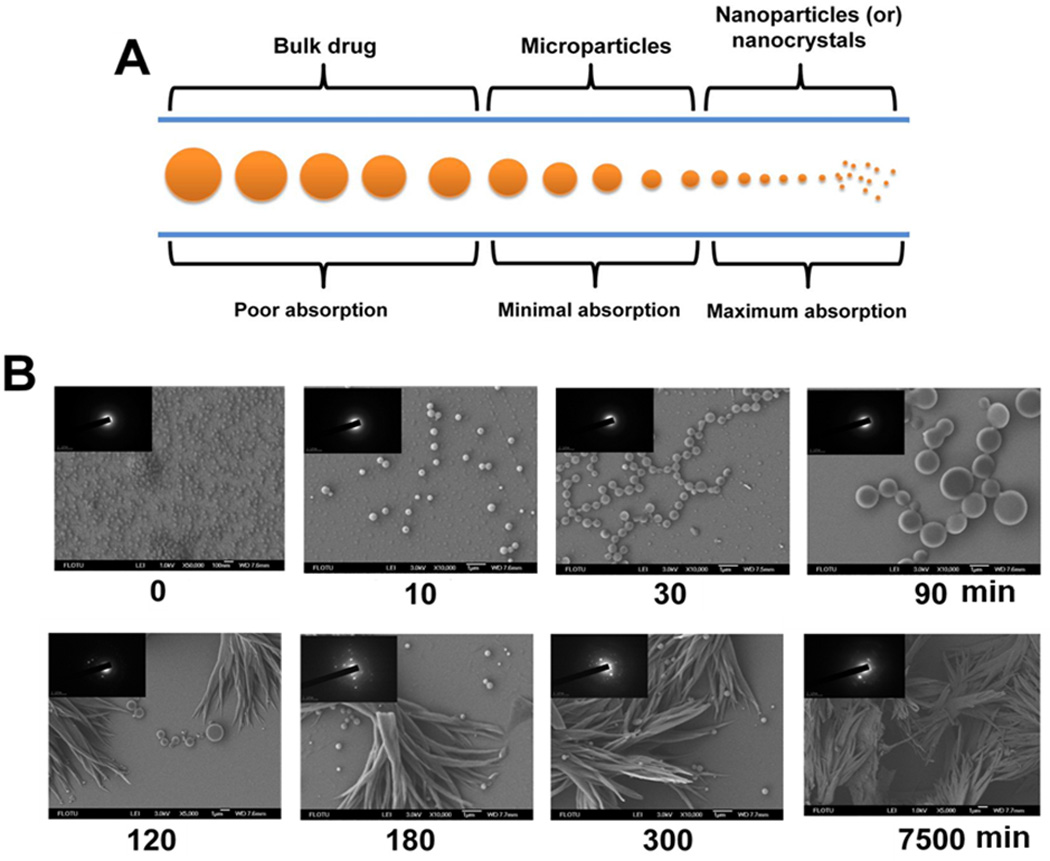
Size-dependant absorption characteristics of drug crystals and curcumin crystal formation. (a) Large drug crystals have poor absorption properties, whereas smaller particles (micro–nanometer) result in rapid dissolution and bioavailability. (b) Curcumin drug particle formation and conversion into precipitation in a bottom-up approach in an alcohol and water mixture with time. Reprinted, with permission, from [31].
Polymer–drug conjugates are considered to be alternative therapeutics from the nanoscale family. Two phenolic rings and active methylene groups are potential sites to conjugate any biomacromolecules onto curcumin. Kumar et al. [37] designed nucleoside–curcumin bioconjugates to obtain high levels of glucuronide and sulfate curcumin conjugates in healthy human volunteers [38]. A recent patent technology based on a luteinizing hormone-releasing hormone (LHRH)–curcumin conjugate synthesized by fluorenylmethoxycarbonyl (Fmoc) solid phase has exhibited an improved antitumor effect in xenograft models of pancreatic cancer [39]. Fmoc is a base label protecting group for LHRH or amine groups. Mono- and diester bioconjugates of curcumin were prepared by solution phase synthesis using urethane chemistry [40]. Methyl jasmonate and salicylic acid in cell culture media enhanced the glucoside formation with curcumin (curcumin-4′,4′-O-beta-D-digentiobioside) and showed higher solubility of curcumin (0.65 mmol/ml) [41]. Similarly, monoglutathionyl curcumin conjugates can be achieved in biological systems when catalyzed in the presence of human glutathione S-transferase [42]. Improved bioavailability and biological effects of these curcumin conjugates were achieved as a result of excretion to the intestinal lumen.
Panday et al. [43] proposed novel polyethylene glycosylated (PEGylated) curcumin analogs for potent nuclear factor erythroid-2 related factor 2 (Nrf2) activators, which regulate the antioxidant defense system and act as modifiers for inflammatory diseases. These analogs improved curcumin solubility from 0.6 × 10−6 to 0.98 × 10−6 g/ml. PEGylated curcumin conjugates had a key role in growth inhibitory effects on a panel of human pancreatic cancer cell lines [44]. The improved effects were achieved by greater suppression of Jun activation domain-binding protein-1 (Jab1) activity by curcumin conjugates. This was confirmed by a significant elevation of the protein levels of p27, Smad2, Smad4 and reduction of c-Jun by 5 µM conjugate. Emerging products of PEG–curcumin (PEG–CUR) nanoconjugates are useful for obtaining a higher cytotoxicity in cancer cells [45]. The synergist activity is dependent on the type of linker chain terminal functionality and molecular weight. A cationic poly(vinyl pyrrolidone)–curcumin (PVP–CUR) conjugate formulation with stable particle size and ζ potential with a pH range of 3–9 has been judged by MTT assay to be more potent in L929 fibroblast cells over free curcumin [46].
Polycatocol–curcumin conjugates were synthesized by condensation polymerization of curcumin and anhydrides [47]. An optimized polycatocol-curcumin conjugate (PCurc 8) is highly cytotoxic to SKOV-3, OVCAR-3 ovarian cancers, and MCF-7 breast cancer cell lines (Figure 3a). These conjugates were taken up efficiently by cancer cells, hydrolyzed and released in an active form of curcumin in lysosomes (Figure 3b). The arrest of cancer cell growth was found in G(0)/G(1) phase and induced apoptosis through the caspase-3 dependent pathway (Figure 3c). A 68% decrease in tumor growth was observed with intravenous injection of PCurc 8 in the SKOV-3 intraperitoneal tumor xenograft mouse model compared with the control group [47]. Polyamine conjugates of curcumin analogs were efficiently transported to mitochondria by intracellular uptake [48]. A direct conjugation of curcumin molecules to carboxylic acid groups of a hyaluronic acid (HA) polymer was performed to obtain nanosize micelle in aqueous solution through hydrophobic interactions [49]. The equivalent of approximately 13 µg of curcumin in conjugate was able to kill 80% of the L929 cells, indicating its potential in therapeutics. HA–curcumin conjugates can be specifically targeted to cell-specific surface markers, such as CD44.
Figure 3.
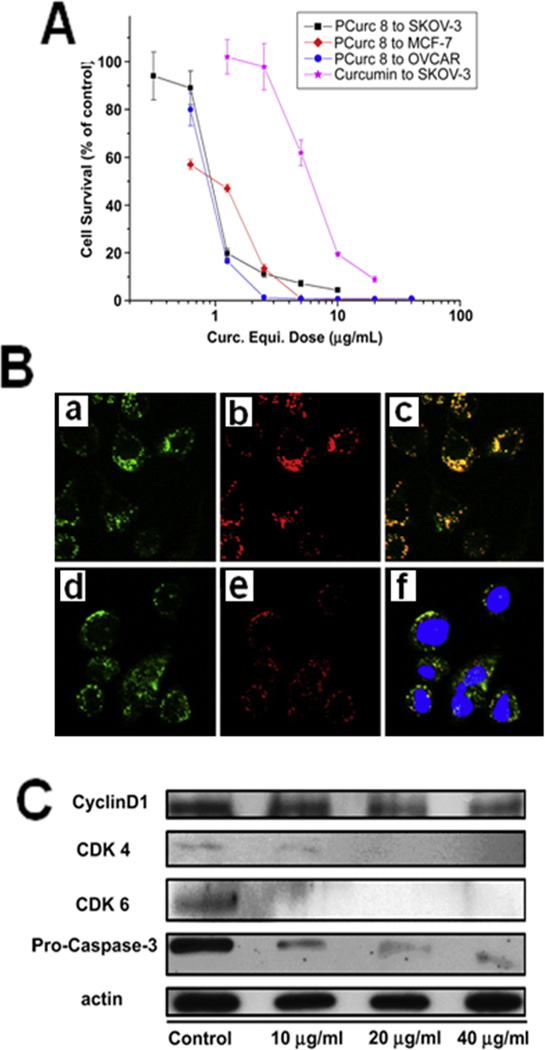
Improved therapeutic effects of curcumin by curcumin conjugate (PCurc8) formulation in cancer cells. (a) Cytotoxicity effect of PCurc 8 formulation in SKOV-3, OVCAR-3 and MCF-7 cancer cell lines after 72-h incubation followed by 24-h incubation in free medium. (b) Intracellular localization of curcumin conjugate observed by confocal microscopy. SKOV-3 ovarian cancer cells incubated with PCurc 8 at 25 µg/ml for 2 h (i–iii) and 24 h (iv–vi). PCurc 8 channel (green, left column) and the LysoTracker® channel (red, middle column), and overlapped channels (right column). (vi) also indicates the overlap of the nuclear dye channel with a blue color. (c) Immunoblotting with anti-cyclin D1, CDK4, CDK6 and procaspase-3 antibodies after treating cells with 10, 20 and 40 µg/ml of PCurc 8. Downregulation of CDK4 and CDK6 indicates that the SKOV-3 cells were hindered at the G0/G1 or G1 phases before going into S phase. Reprinted, with permission, from [47].
Curcumin emulsions, liposome and phospholipid formulations
Microemulsions are isotropic nanostructural, stable solutions comprising surfactant(s), oil and water. Curcumin-based microemulsions are expected to improve curcumin delivery via local and transdermal routes for scleroderma, psoriasis and skin cancer. Eucalyptol-based curcumin microemulsions have very high permeability and flux with moderate solubility of curcumin compared with many oleic acid- and esteem oil-based microemulsions [50]. The enhanced penetration capacity of this applied curcumin microemulsion formulation, as well as its effect on the cellular structure of skin, have been observed. Furthermore, a new microemulsion (approximately 10 nm) composition of limonene, polysorbate 80, ethanol and water promotes curcumin presence in skin (9.29–29.99 µg in an area of 0.785 cm2) [51]. Myristic, palmitic, stearic and behenic acids (i.e., fatty acids; FA)-based solid lipid NPs were prepared by a coacervation technique in the presence of polymeric non-ionic surfactants [52]. This method initially loaded up to 28–81% with a 500-nm particle size. After hydrolysis, this diameter size reduced to 300 nm. A stable self-microemulsifying drug delivery system (SMEDDS) comprising 20% ethanol, 60% Cremophor RH40® and 20% isopropyl myristate was able to encapsulate 50 mg/ml of curcumin and to release it completely in 10 min [53].
Curcumin microemulsions prepared in the presence of ethyl oleate and isopropyl myristate result in homogeneous, yellow, transparent solutions, whereas soybean oil and peppermint oil are turbid in nature [54]. Highly smooth surface gelatin microspheres with 75.5% curcumin encapsulation efficiency were formulated by the emulsion crosslinking method suitable for lung targeting [55]. These spheres are 5–30 µm in diameter, with 50% curcumin release within 22 h for immediate therapeutic effects. Co-administration of curcumin and paclitaxel nanoemulsion formulations are capable of overcoming multidrug resistance in SKOV3 (TR) human ovarian adenocarcinoma cells by inhibiting NF-κB activity, downregulating P-gp and promoting apoptotic responses [56]. Additionally, curcumin nanoemulsion increases the bioavailability of paclitaxel up to 5.2-fold, and there is a 3.2-fold increase in its accumulation at the tumor site in an oral administration to SKOV3 tumor-bearing xenograft mice models. This resulted from downregulation of intestinal P-gp and cytochrome P450 3A2 (CYP3A2) protein levels [57].
Liposomes comprise artificial phospholipid vesicles, considered to be biologically safe, biocompatible and protect drugs from external stimuli. It has been known that an extended absorption capacity of curcumin can be attained by dissolving, mixing or complexing it with different types of phospholipid. Sou et al. [58] have successfully prepared a curcumin lipid formulation using 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) and an anionic amphiphile, l-glutamic acid, N-(3-carboxy-1-oxopropyl)-, 1,5-dihexadecyl ester (SA). Intravenous administration of this formulation in rats showed no acute response in circulating blood cells and much of the curcumin accumulated in bone marrow and spleen tissues. A recent pharmacokinetic study of solid lipid curcumin NPs in patients with osteosarcoma reported up to 31.42–41.15 ng/ml of curcumin within 4 h of 2000–4000 mg oral dose treatment [59]. More importantly, the patients did not experience any adverse effects. In another study, solid lipid NPs with 134 nm and 84% encapsulation of curcumin exhibited slow release of curcumin over a week [60]. This formulation resulted in high oral bioavailability in plasma of male Wistar rats. Curcumin solid lipid NPs at a single oral dose of 1, 12.5, 25 and 50 mg/kg pharmacokinetics in rats achieved a maximum concentration (Cmax) of bioavailability in serum 1.00±0.01, 7.87±3.02, 8.00±1.87, and 14.29±0.15 µg/ml, respectively, whereas free curcumin was only present at 0.292±0.06 µg/ml, even at a dose of 50 mg/kg. This enhancement in bioavailability is to the result of increased curcumin absorption.
A comparative study revealed the uptake of liposome- and serum albumin-loaded curcumin formations in normal spleen lymphocytes and EL4 lymphoma cells via fluid phase pinocytosis and membrane fusion, respectively [61]. Curcumin-loaded liposomal formulation was a better delivery vehicle and further significant absorption and fluorescence levels in lymphoma cells compared with normal cells were observed. Li et al. [62] evaluated the lipid curcumin ratio (10:1 wt./wt.) on various pancreatic carcinoma cell lines, including ASPC-1, BxPC-3, Capan-1, Capan-2, HS766-T and MiapaCa2, and demonstrated an inhibition concentration of IC50 at 2.0–37.8 µM, whereas IC90 was 6.75–94.5 µM, as assessed by cytotoxicity. In a recent study, curcumin was successfully incorporated in egg-phosphatidylcholine (EPC) liposomes at a 1:14 molar ratio (curcumin:liposome). A twofold increase in concentration of curcumin was present in rat plasma with the lecithin curcumin formulation compared with a curcumin and curcumin-lecithin mixture after administration of a single oral dose of 100 mg curcumin/kg body weight [63]. Narayanan et al. [64] provided an interesting insight into the use of curcumin and resveratrol in liposome to evaluate their combined effects on: (i) cell growth, apoptosis and the cell cycle; and (ii) on activated p-Akt, cyclin D1, mammalian target of rapamycin (m-TOR) and androgen receptor (AR) proteins involved in tumor progression of PTEM-CaP8 prostate cancer cells. Overall, this combined formulation significantly decreased prostatic adenocarcinoma in vivo (P <0.001) and incidence. Transferrin-solid lipid NPs retained up to 83% of curcumin for 6 months and specifically targeted MCF-7 breast cancer cells four- and fivefold in 24- and 48-h treatments, respectively [65]. Transferrin-mediated endocytosis was confirmed, which promotes reactive oxygen species (ROS) and time-dependant apoptosis by transferrin-solid lipid curcumin NPs. Curcumin structural features facilitated nanodisk NPs comprising a disk-shaped lipid bilayer formation usually stabilized by an apolipoprotein scaffold [66]. The efficacy of anticancer properties of this lipid curcumin formulation in human hepatocellular carcinoma (HepG2) was significant over that of free curcumin.
Curcumin encapsulated polymer NPs
Poly(lactic-co-glycolic acid) (PLGA) is a widespread choice in the production of a variety of biomedical devices owing to its biodegradability and biocompatibility. In an effort to create a safe carrier, several different types of PLGA NP for curcumin encapsulation have been explored. A simple solid–oil–water solvent evaporation method has been used to prepare curcumin-encapsulated PLGA NPs [67]. The particle size can be controlled by the surfactant concentration and sonication time. Subsequently, our solvent evaporation method is designed to regulate the curcumin-encapsulated PLGA NPs through a lower particle size, enhanced intracellular uptake and antibody conjugation features [23]. The development of surface-modified PLGA NPs with a thiolated chitosan was first studied by Grabovac and Bernkop [68]. Surface functionalization of curcumin-loaded PLGA NPs by a bis(sulfosuccinimidyl)suberate (BS3) facilitates conjugation of annexin A2 and results in an efficient target therapy of curcumin to annexin A2-positive MDA-MB-231 cancer cells [69]. Shahani and Panyam [70] designed a sustained and injectable microparticle formulation with higher curcumin loading capacity (i.e. 38.1 mg/100 mg of particles; 76.2% encapsulation efficiency) compared with many formulations. An improved glutathione-s-transferase (GST) activity in liver was noticed with injectable microparticles and this observed phenomenon was consistent over 4 weeks. GST activity represents a potent endogenous defense mechanism against carcinogens. In vivo mice studies using this formulation resulted in a tenfold increase in the concentration of curcumin blood, lungs and brain compared with curcumin dissolved in PEG 400 formulation [71]. Tsai et al. [72] designed curcumin-loaded PLGA and demonstrated the half-life of curcumin in the cerebral cortex and hippocampus to be significantly increased from 2.32 to 19.9 and 7.56 to 16.7 min, respectively. Additionally, retention time values of the cerebral cortex and the hippocampus were increased approximately 2.0- and 1.8-fold, respectively. The curcumin plasma levels were slightly higher with this nanoformulation.
Nanoformulations based on dextran sulfate-chitosan are considered to be biocompatible materials that can be used for oral, intravenous and controlled delivery purposes. Another study quantified the cellular uptake of curcumin encapsulated in dextran sulfate-chitosan NPs using a spectrophotometric method in L929, MCF-7, PC3 and MG 63 cells [73]. Furthermore, a cytotoxicity assay and fluorescence-activated cell sorting (FACS) study suggested that anticancer activity of this formulation is high in MCF-7 compared with other cancer cells. A curcumin analog encapsulated in polycaprolactone (PCL) NPs (approximately 650 nm) administered intravenously to male Wistar rats demonstrated higher intracellular levels in liver cells, which suggests that this formulation can be used to target the liver [74]. Co-encapsulation of curcumin and doxorubicin in polymer NPs enabled multi-drug resistance cancer cells (K562 cells) to be treated more effectively [75]. Initial curcumin release from NPs downregulated MDR1 and BCL-2 expression and inhibited the nuclear efflux mechanism; the subsequent release of doxorubicin stimulated cancer cell death. A patented technology described by synthesis of curcumin-encapsulated chitosan NPs proved to be safe in mice and rat studies at a dose of 4 mg and 40 mg of formulations, respectively, for 14 days [76].
Curcumin self-assemblies and nanogel
A few different methodologies have been developed for curcumin complexation or self-assembly formation with β-cyclodextrin and their derivative(s). In addition, a few possible host–guest complexations of β-cyclodextrin and curcumin have been reported recently [24]. This type of inclusion process offers suppression of curcumin degradation, stability, dispersibility and bioavailability [13–17,77]. US patents were recently approved for cyclodextrin–curcumin complexes to improve water solubility, stability and their effective use in medical, pharmaceutical and nutraceutical applications [78,79].
A few complexes of diblock copolymer, modified starch and cyclodextrins have also been successful in increasing the solubility of curcumin 1670–131 000 times [80–82]. Curcumin–rubusoside (CUR–RUB) complexes with 8-nm particles efficiently increased curcumin solubility from 61 µg/ml to 2.318 mg/ml depending upon the RUB range (1% to 10% (w/v) used in the complexation [83]. This CUR–RUB complex demonstrated stability under physiological conditions, did not form clusters or precipitation, and no degradation was observed. It also showed anticancer efficacy against human colon, breast, and pancreatic cancer cell lines. β-casein (B-CN) is an amphiphilic self-assembling protein that helps to solubilize curcumin more efficiently in aqueous solution [84]. This micellar curcumin solubility is 7.7 × 10−5 mol/l, whereas that of curcumin is only 2.99 × 10−8 mol/l. This suggests at least a 2500-fold enhancement in the solubility of curcumin.
A cyclodextrin–curcumin self-assembly has shown greater effects over curcumin in inhibiting tumor necrosis factor (TNF)-induced expression of NF-kB regulated genes (VEGF, MMP-9 and cyclin D1) and upregulation of death receptors (DR4 and DR5) in KBM-5 cancer cells [85]. A similar pattern of encapsulation of curcumin into cyclodextrin and poly(cyclodextrin) led to a self-assembly formation that promoted its anticancer potential by downregulating pro-survival Bcl2 family genes, Bax and Bcl-xL, and induction of apoptosis (cleaved poly[ADP-ribose]polymerase, [PARP]) [25]. Hydration of curcumin in the presence of poly(ethylene)–cholesteryl ether (PEG–Chol) to produce uniform NPs (10 nm) showed an synergistic effect on myeloma (RPMI 8226, U266 and 5TGM1) cell lines [86]. An exploratory study designed intracellular-labile amphiphilic surfactant-like curcumin with short oligo(ethylene glycol) chains via β-thioester bonds that are easily labile in the presence of glutathione and esterase [87]. This formulation achieved higher inhibition potential in cancer cell lines as well as in athymic mice xenograft SKOV-3 tumors and MDA-MB-468 tumors. A complex of liposome, PEG and polyethylene glycol was used to encapsulate curcumin [88]. The complex exhibited 5- and 20-fold higher inhibitory effects on HepG2, HT-29, HeLa, A549, CT26/cur-r and B16F10/cur-r adenocarcinoma cells. A 60–90% inhibition of tumor growth was observed in mice bearing CT-26 or B16F10 cells with this curcumin nanoformulation.
Crosslinked copolymer nanogels were synthesized through redox-free radical polymerization of N-isopropylacrylamide, N-vinyl-2-pyrrolidone in the presence of PEG monoacrylate [28]. The free network structures throughout the hydrogel NPs offer the opportunity to load various types of lipophilic drug molecule. Bisht et al. [89] tested the effects of hydrogel nanocurcumin formulation on pancreatic cancer cell lines. Nanocurcumin efficiently blocked the activation of NF-κB, downregulated steady-state transcripts of multiple pro-inflammatory cytokines and inhibited interleukin (IL)-6 synthesis. The parenteral administration of the hydrogel nanocurcumin formulation significantly inhibited tumor growth in both subcutaneous and orthotopic settings in xenograft models of human pancreatic cancer in athymic mice [90]. Furthermore, hydrogel nanocurcumin and gemcitabine treatments promoted a tumor growth inhibition capacity, a result that supports an additive therapeutic strategy. This new formulation has the potential to overcome clinical translation of curcumin in cancer treatment as a result of its improved systemic bioavailability as well as therapeutic efficacy. The nanocurcumin formulation has achieved a dose-dependant anti-proliferation effect in embryonal tumor-derived lines DAOY and D283Med, and the glioblastoma neurosphere lines HSR-GBM1 and JHH-GBM14 [91]. A series of curcumin-loaded dextran-modified hydrogel NPs were studied by Goncalves et al. [92]. Another group described fabrication of curcumin-encapsulated chitosan-PVA silver nanocomposite, poly(acrylamide)-poly(vinyl sulfonic acid) silver nanocomposite, and poly(acrylamide)-carboxymethyl cellulose magnetic nanocomposites to improve the therapeutic effects of curcumin [93]. Curcumin-loaded hydrogel NPs using PVP and hydroxyl propyl methyl cellulose in the presence of pluronic® F68 have been formulated using a solvent emulsion-evaporation technique [94]. The developed hydrogel NPs (approximately 100 nm) have proven oral and cellular safety in a study using mice.
Novel curcumin nanoformulations
Kurien and Scofield pointed out that heat-stabilized curcumin in water increased the water solubility of curcumin from 0.6 µg/ml to 7.4 µg/ml (an approximately 12-fold increase) without altering its biological activity [95]. Curcumin-loaded monopolymer (ethylcellulose, EC) and dipolymer (a blend of ethylcellulcose-methylcellulose, ECMC) nanoformulations fabricated through a self-assembly process exhibited dose-dependant free radical scavenging and cytotoxicity effects in MCF-7 and HepG2 cancer cells [96]. Through scanning electron microscopy, these NPs have shown an improved adherence to stomach mucosa and sustained release of curcumin into the circulation. A combined glycerol monooleate and pluronic F-127 polymer nanocarrier is also well suited to the delivery of curcumin to various types of cancer cell (i.e. PANC-1, MiaPaCa-2, K-562, MCF-7, A549 and HCT-16) [97]. This formulation has shown significant anticancer properties at all concentrations (5–30 µM) compared with free curcumin. These improved effects are correlated with the inhibition of phosphorylation and activation of the Akt pathway, which inactivates NF-κB and inhibits the proliferation and induction of apoptosis. Silk fibroin NPs less than 100 nm entrap more than 96% of curcumin molecules within their nanostructures, compared with all other silk fibroin chitosan blend NPs [98]. Modified curcuminoid (i.e., 4-[3,5-bis(2-chlorobenzylidene-4-oxo-piperidine-1-yl)-4-oxo-2-butenoic acid]; CLEFMA) was successfully entrapped within a CD-liposome [99]. In nude rats bearing a H441xenograft, a 94% reduction in tumor volume was noticed after intravenous treatment with CLEFMA liposomes (Figure 4). No apparent toxicity issues in liver, lung and kidney were observed upon treatment, as confirmed by histopathological examination.
Figure 4.
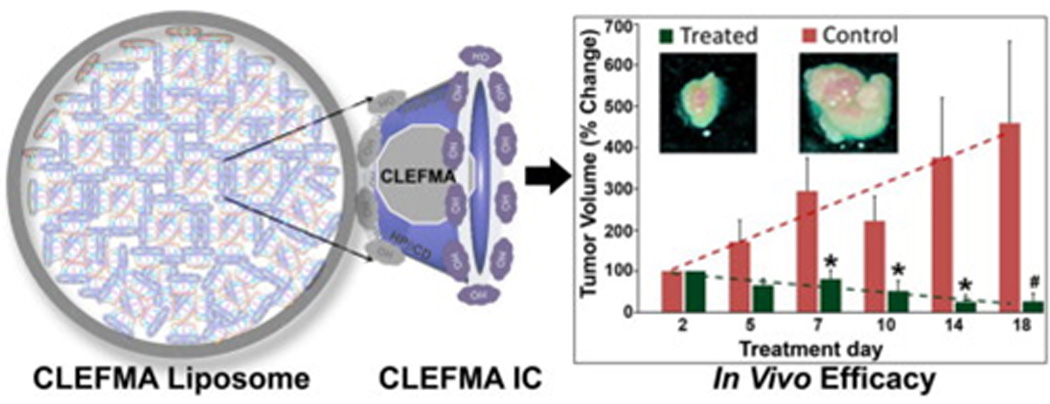
Schematic representation of 4-[3,5-bis(2-chlorobenzylidene-4-oxo-piperidine-1-yl)-4-oxo-2-butenoic acid] (CLEFMA) liposomes obtained by a drug-in CD-in liposome method, detailing tumor volume control with liposomal CLEFMA treatment. Insets show representative pictures of excised tumors upon necropsy. The dashed lines are the trend lines of the plotted data. The data are presented as the mean ± SEM of results from experiments on n = 4 (treatment) and n = 3 (control) rats.Reprinted, with permission, from [99].
These curcumin NPs, formed by surfactant micelles of cetyltrimethylammonium bromide (CTAB) into micelle rod shapes, facilitate the uptake of curcumin compared with the traditional spherical micelle arrangement [100]. A similar approach of encapsulating curcumin in silica magnetic NP reservoirs without altering the magnetization properties, has been used for prototype material for targeted drug delivery [101]. A comparative pharmacokinetic and bio-distribution study in mice administered curcumin encapsulated poly(n-butylcyanoacrylate) NPs (5 mg/kg) and curcumin (10 mg/kg) via the tail vein [102]. The mean residence time and area of concentration of curcumin NPs were increased 14- and 2.53-fold, respectively, compared with that of curcumin solution. This increased distribution was the result of receptor-mediated uptake.
Our group has recently developed a curcumin nanoformulation with multiple properties, such as anticancer potential, hyperthermia and imaging [27]. The combining of therapeutic and diagnostic (theranostic) properties into a single nanoplatform might result in a significantly improved approach over conventional medicine. NPs comprising Eudragit® S100 (a copolymer of methacrylic acid and methyl acrylate) and curcumin demonstrated no acute, subacute or genotoxicity issues in a study of mice [103]. Furthermore, this formulation was highly hemocompatible with human red blood cells. Theracurcumin (curcumin NPs) was studied for the first time in six human volunteers at a single oral dose of 150 mg followed by a 210 mg dose after 2 weeks. No toxicities were found other than diarrhea. The AUC was 2649 ± 350 and 3649 ± 430 ng/ml × h; and the t (1/2) was 9.7 ± 2.1 h 13.0 ± 3.3 h for 150 mg and 210 mg doses, respectively [104].
Prospects and conclusions
Several types of NP have been found to be suitable for the encapsulation or loading of curcumin to improve its effects in cancer therapeutics. The characteristics of these curcumin nanoformulations can be tailored according to the specific requirement for inducing cellular death by various mechanisms. Overall, our understanding from the available literature is that the use of curcumin nanoformulations in chemotherapy for cancer treatment is a facile modality that improves existing curcumin therapies by targeting tumors and by reducing the dose required. Safe toxicological profiles of the various curcumin nanoformulations and their efficacy in the cell-line models highlight their potential for evaluation in in vivo models. Human trials need to be conducted to establish their effectiveness in clinical applications as an improved therapeutic modality for cancer treatment.
Although curcumin NPs have many advantages for chemotherapy, the main obstacle is lack of tissue specificity. Thus, in addition to delivering the drug to the cancer cells, it also spreads to surrounding healthy tissues. Therefore, future studies that combine curcumin delivery with other first-line anticancer chemotherapeutic agents or imaging-, contrast-, antibody- or peptide-targeted delivery are needed to achieve better-targeted therapeutic modalities. Additionally, studies are needed that evaluate the efficacy and toxicity of curcumin NP formulations in both small and large cohorts, as well as in patients with cancer in phase I/II clinical trials. These studies would compare the anticancer efficiency of curcumin NP formulations and free curcumin and reveal whether curcumin NP formulations can be developed as a useful modality for cancer treatment.
Various curcumin nanoformulations developed at the laboratory scale are based on dispersed or precipitation processes. It is important to regulate the size of curcumin NPs to between 10 nm and 200 nm for drug delivery applications. Overall, these developed processes must be simple, efficient, continuous and suitable for large-scale convertible production that is acceptable by regulatory authorities. In addition, a process with flexible amounts of curcumin loading or encapsulation is also desirable. Adopting commercialized approaches for curcumin nanoformulations similar to nanocrystal, nanomorph, nanoedge, nanopure, crititech and nanocochleate technology would aid immediate implementation in the drug market. In conclusion, the overall experimental evidence suggests that curcumin-based NP formulations hold great promise in cancer therapeutics as anticancer chemotherapeutic agents that target and sustain curcumin delivery in tumors.
Acknowledgements
The authors acknowledge Cathy Christopherson for editorial assistance. This work was partially supported by grants from Sanford Research/USD, PC073887, Governor’s Cancer 2010, and NIH RO1 CA142736.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maheshwari RK, et al. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78:2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 2.Goel A, et al. Curcumin as ‘curecumin’: from kitchen to clinic. Biochem. Pharmacol. 2008;75:787–809. doi: 10.1016/j.bcp.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 3.Hatcher H, et al. Curcumin: from ancient medicine to current clinical trials. Cell. Mol. Life Sci. 2008;65:1631–1652. doi: 10.1007/s00018-008-7452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kunnumakkara AB, et al. Curcumin inhibits proliferation, invasion, angiogenesis and metastasis of different cancers through interaction with multiple cell signaling proteins. Cancer Lett. 2008;269:199–225. doi: 10.1016/j.canlet.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Landis-Piwowar KR, et al. The proteasome as a potential target for novel anticancer drugs and chemosensitizers. Drug Resist. Update. 2006;9:263–273. doi: 10.1016/j.drup.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Page P, Yang LX. Novel chemoradiosensitizers for cancer therapy. Anticancer Res. 2010;30:3675–3682. [PubMed] [Google Scholar]
- 7.Yallapu MM, et al. Curcumin induces chemo/radio-sensitization in ovarian cancer cells and curcumin nanoparticles inhibit ovarian cancer cell growth. J. Ovarian Res. 2010;3:11. doi: 10.1186/1757-2215-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ejendal KF, Hrycyna CA. Multidrug resistance and cancer: the role of the human ABC transporter ABCG. Curr. Protein Pept. Sci. 2002;3:503–511. doi: 10.2174/1389203023380521. [DOI] [PubMed] [Google Scholar]
- 9.Loo TW, Clarke DM. The human multidrug resistance P-glycoprotein is inactive when its maturation is inhibited: potential for a role in cancer chemotherapy. FASEB J. 1999;13:1724–1732. doi: 10.1096/fasebj.13.13.1724. [DOI] [PubMed] [Google Scholar]
- 10.Szakacs G, et al. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006;5:219–234. doi: 10.1038/nrd1984. [DOI] [PubMed] [Google Scholar]
- 11.Um Y, et al. Synthesis of curcumin mimics with multidrug resistance reversal activities. Bioorg. Med. Chem. 2008;16:3608–3615. doi: 10.1016/j.bmc.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 12.Goel A, et al. Multi-targeted therapy by curcumin: how spicy is it? Mol. Nutr. Food Res. 2008;52:1010–1030. doi: 10.1002/mnfr.200700354. [DOI] [PubMed] [Google Scholar]
- 13.Tonnesen H. Studies on curcumin and curcuminoids. XVI. Effect of curcumin analogs on hyaluronic acid degradation in vitro. Inter. J. Pharm. 1989;51:259–261. [Google Scholar]
- 14.Tonnesen HH, et al. Studies on curcumin and curcuminoids. IX: investigation of the photobiological activity of curcumin using bacterial indicator systems. J. Pharm. Sci. 1987;76:371–373. doi: 10.1002/jps.2600760506. [DOI] [PubMed] [Google Scholar]
- 15.Tonnesen HH, Karlsen J. Studies on curcumin and curcuminoids. VI. Kinetics of curcumin degradation in aqueous solution. Z. Lebensm. Unters. Forsch. 1985;180:402–404. doi: 10.1007/BF01027775. [DOI] [PubMed] [Google Scholar]
- 16.Tonnesen HH, et al. Studies on curcumin and curcuminoids. VIII. Photochemical stability of curcumin. Z. Lebensm. Unters. Forsch. 1986;183:116–122. doi: 10.1007/BF01041928. [DOI] [PubMed] [Google Scholar]
- 17.Tonnesen HH. Solubility, chemical and photochemical stability of curcumin in surfactant solutions. Studies of curcumin and curcuminoids, XXVIII. Pharmazie. 2002;57:820–824. [PubMed] [Google Scholar]
- 18.Aggarwal BB, et al. Curcumin: the Indian solid gold. Adv. Exp. Med. Biol. 2007;595:1–75. doi: 10.1007/978-0-387-46401-5_1. [DOI] [PubMed] [Google Scholar]
- 19.Lao CD, et al. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006;6:10. doi: 10.1186/1472-6882-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kakarala M, et al. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res. Treat. 2009;122:777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pathak N, Khandelwal S. Comparative efficacy of piperine, curcumin and picroliv against Cd immunotoxicity in mice. Biometals. 2008;21:649–661. doi: 10.1007/s10534-008-9150-y. [DOI] [PubMed] [Google Scholar]
- 22.Muqbil I, et al. Progress in nanotechnology based approaches to enhance the potential of chemopreventive agents. Cancer. 2011;3:428–445. doi: 10.3390/cancers3010428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yallapu MM, et al. Fabrication of curcumin encapsulated PLGA nanoparticles for improved therapeutic effects in metastatic cancer cells. J. Colloid Interface Sci. 2010;351:19–29. doi: 10.1016/j.jcis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 24.Yallapu MM, et al. beta-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf. B. 2010;79:113–125. doi: 10.1016/j.colsurfb.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 25.Yallapu MM, et al. Poly(beta-cyclodextrin)/curcumin self-assembly: a novel approach to improve curcumin delivery and its therapeutic efficacy in prostate cancer cells. Macromol. Biosci. 2010;10:1141–1151. doi: 10.1002/mabi.201000084. [DOI] [PubMed] [Google Scholar]
- 26.Yallapu MM, et al. Scope of nanotechnology in ovarian cancer therapeutics. J. Ovarian Res. 2010;3:19. doi: 10.1186/1757-2215-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yallapu MM, et al. Multi-functional magnetic nanoparticles for magnetic resonance imaging and cancer therapy. Biomaterials. 2011;32:1890–1905. doi: 10.1016/j.biomaterials.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yallapu MM, et al. Synthesis, characterization and antiproliferative activity of rapamycin-loaded poly(N-isopropylacrylamide)- based nanogels in vascular smooth muscle cells. J. Biomed. Nanotech. 2008;4:16–24. [Google Scholar]
- 29.Anand P, et al. Bioavailability of curcumin: problems and promises. Mol. Pharm. 2007;4:807–818. doi: 10.1021/mp700113r. [DOI] [PubMed] [Google Scholar]
- 30.Bansal SS, et al. Advanced drug-delivery systems of curcumin for cancer chemoprevention. Cancer Prev. Res. 2011 doi: 10.1158/1940-6207.CAPR-10-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He Y, et al. Structure evolution of curcumin nanoprecipitation from a micromixer. Cryst. Growth Des. 2010;10:1021–1024. [Google Scholar]
- 32.Wang Z, et al. The role of charge in the surfactant-assisted stabilization of the natural product curcumin. Langmuir. 2010;26:5520–5526. doi: 10.1021/la903772e. [DOI] [PubMed] [Google Scholar]
- 33.Leung MH, Kee TW. Effective stabilization of curcumin by association to plasma proteins: human serum albumin and fibrinogen. Langmuir. 2009;25:5773–5777. doi: 10.1021/la804215v. [DOI] [PubMed] [Google Scholar]
- 34.Donsi F, et al. Preparation of curcumin sub-micrometer dispersions by high-pressure homogenization. J. Agric. Food Chem. 2010;58:2848–2853. doi: 10.1021/jf903968x. [DOI] [PubMed] [Google Scholar]
- 35.Setthacheewakul S, et al. Development and evaluation of self-microemulsifying liquid and pellet formulations of curcumin, and absorption studies in rats. Eur. J. Pharm. Biopharm. 2010;76:475–485. doi: 10.1016/j.ejpb.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 36.Gao Y, et al. Preparation, characterization, pharmacokinetics, and tissue distribution of curcumin nanosuspension with TPGS as stabilizer. Drug Dev. Ind. Pharm. 2010;36:1225–1234. doi: 10.3109/03639041003695139. [DOI] [PubMed] [Google Scholar]
- 37.Kumar S, et al. Design and synthesis of curcumin-bioconjugates to improve systemic delivery. Nucleic Acids Symp. Ser. 2000;44:75–76. doi: 10.1093/nass/44.1.75. [DOI] [PubMed] [Google Scholar]
- 38.Vareed SK, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomarkers Prev. 2008;17:1411–1417. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hansel W, et al. Curcumin conjugates for treating and preventing cancers, WO/2010/033580. XXXX. [Google Scholar]
- 40.Dubey SK, et al. Design, synthesis and characterization of some bioactive conjugates of curcumin with glycine, glutamic acid, valine and demethylenated piperic acid and study of their antimicrobial and antiproliferative properties. Eur. J. Med. Chem. 2008;43:1837–1846. doi: 10.1016/j.ejmech.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 41.Kaminaga Y, et al. Production of unnatural glucosides of curcumin with drastically enhanced water solubility by cell suspension cultures of Catharanthus roseus. FEBS Lett. 2003;555:311–316. doi: 10.1016/s0014-5793(03)01265-1. [DOI] [PubMed] [Google Scholar]
- 42.Usta M, et al. Human glutathione S-transferase-mediated glutathione conjugation of curcumin and efflux of these conjugates in Caco-2 cells. Chem. Res. Toxicol. 2007;20:1895–1902. doi: 10.1021/tx7002245. [DOI] [PubMed] [Google Scholar]
- 43.Pandey MK, et al. Design, synthesis and evaluation of novel PEGylated curcumin analogs as potent Nrf2 activators in human bronchial epithelial cells. Eur J. Pharm. Sci. 2011;43:16–24. doi: 10.1016/j.ejps.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Li J, et al. Polyethylene glycosylated curcumin conjugate inhibits pancreatic cancer cell growth through inactivation of Jab1. Mol. Pharmacol. 2009:81–90. doi: 10.1124/mol.109.054551. [DOI] [PubMed] [Google Scholar]
- 45.Safavy A, et al. Design and development of water-soluble curcumin conjugates as potential anticancer agents. J. Med. Chem. 2007;50:6284–6288. doi: 10.1021/jm700988f. [DOI] [PubMed] [Google Scholar]
- 46.Manju S, Sreenivasan K. Synthesis and characterization of a cytotoxic cationic polyvinylpyrrolidone-curcumin conjugate. J. Pharm. Sci. 2011;100:504–511. doi: 10.1002/jps.22278. [DOI] [PubMed] [Google Scholar]
- 47.Tang H, et al. Curcumin polymers as anticancer conjugates. Biomaterials. 2010;31:7139–7149. doi: 10.1016/j.biomaterials.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 48.Simoni E, et al. Polyamine conjugation of curcumin analogues toward the discovery of mitochondria-directed neuroprotective agents. J. Med. Chem. 2010;53:7264–7268. doi: 10.1021/jm100637k. [DOI] [PubMed] [Google Scholar]
- 49.Manju S, Sreenivasan K. Conjugation of curcumin onto hyaluronic acid enhances its aqueous solubility and stability. J. Colloid Interface Sci. 2011;359:318–325. doi: 10.1016/j.jcis.2011.03.071. [DOI] [PubMed] [Google Scholar]
- 50.Liu CH, Chang FY. Development and characterization of eucalyptol microemulsions for topic delivery of curcumin. Chem. Pharm. Bull. 2011;59:172–178. doi: 10.1248/cpb.59.172. [DOI] [PubMed] [Google Scholar]
- 51.Liu CH, et al. Terpene microemulsions for transdermal curcumin delivery: effects of terpenes and cosurfactants. Colloids. Surf. B. 2011;82:63–70. doi: 10.1016/j.colsurfb.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 52.Chirio D, et al. Formulation of curcumin-loaded solid lipid nanoparticles produced by fatty acids coacervation technique. J. Microencapsul. 2011 doi: 10.3109/02652048.2011.590615. [DOI] [PubMed] [Google Scholar]
- 53.Wu X, et al. Self-microemulsifying drug delivery system improves curcumin dissolution and bioavailability. Drug Dev. Ind. Pharm. 2011;37:15–23. doi: 10.3109/03639045.2010.489560. [DOI] [PubMed] [Google Scholar]
- 54.Lin CC, et al. stability and characterization of phospholipid-based curcumin-encapsulated microemulsions. Food Chem. 2009;116:923–928. [Google Scholar]
- 55.Cao FL, et al. Preparation and characterization of curcumin loaded gelatin microspheres for lung targeting. Zhong Yao Cai. 2009;32:423–426. [PubMed] [Google Scholar]
- 56.Ganta S, Amiji M. Coadministration of Paclitaxel and curcumin in nanoemulsion formulations to overcome multidrug resistance in tumor cells. Mol. Pharm. 2009;6:928–939. doi: 10.1021/mp800240j. [DOI] [PubMed] [Google Scholar]
- 57.Ganta S, et al. Curcumin enhances oral bioavailability and anti-tumor therapeutic efficacy of paclitaxel upon administration in nanoemulsion formulation. J. Pharm. Sci. 2010;99:4630–4641. doi: 10.1002/jps.22157. [DOI] [PubMed] [Google Scholar]
- 58.Sou K, et al. Loading of curcumin into macrophages using lipid-based nanoparticles. Int. J. Pharm. 2008;352:287–293. doi: 10.1016/j.ijpharm.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 59.Gota VS, et al. Safety and pharmacokinetics of a solid lipid curcumin particle formulation in osteosarcoma patients and healthy volunteers. J. Agric. Food Chem. 2010;58:2095–2099. doi: 10.1021/jf9024807. [DOI] [PubMed] [Google Scholar]
- 60.Kakkar V, et al. Exploring solid lipid nanoparticles to enhance the oral bioavailability of curcumin. Mol. Nutr. Food Res. 2011;55:495–503. doi: 10.1002/mnfr.201000310. [DOI] [PubMed] [Google Scholar]
- 61.Kunwar A, et al. Transport of liposomal and albumin loaded curcumin to living cells: an absorption and fluorescence spectroscopic study. Biochim. Biophys. Acta. 2006;1760:1513–1520. doi: 10.1016/j.bbagen.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 62.Li L, et al. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104:1322–1331. doi: 10.1002/cncr.21300. [DOI] [PubMed] [Google Scholar]
- 63.Pandelidou M, et al. Preparation and characterization of lyophilised EGG PC liposomes incorporating curcumin and evaluation of its activity against colorectal cancer cell lines. J. Nanosci. Nanotechnol. 2011;11:1259–1266. doi: 10.1166/jnn.2011.3093. [DOI] [PubMed] [Google Scholar]
- 64.Narayanan NK, et al. Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int. J. Cancer. 2009;125:1–8. doi: 10.1002/ijc.24336. [DOI] [PubMed] [Google Scholar]
- 65.Mulik RS, et al. Transferrin mediated solid lipid nanoparticles containing curcumin: enhanced in vitro anticancer activity by induction of apoptosis. Int, J. Pharm. 2010;398:190–203. doi: 10.1016/j.ijpharm.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 66.Ghosh M, et al. Curcumin nanodisks: formulation and characterization. Nanomedicine. 2011;7:162–167. doi: 10.1016/j.nano.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mukerjee A, Vishwanatha JK. Formulation, characterization and evaluation of curcumin-loaded PLGA nanospheres for cancer therapy. Anticancer Res. 2009;29:3867–3875. [PubMed] [Google Scholar]
- 68.Grabovac V, Bernkop-Schnurch A. Development and in vitro evaluation of surface modified poly(lactide-co-glycolide) nanoparticles with chitosan-4-thiobutylamidine. Drug Dev. Ind. Pharm. 2007;33:767–774. doi: 10.1080/03639040601050163. [DOI] [PubMed] [Google Scholar]
- 69.Thamake SI, et al. Surface functionalization of PLGA nanoparticles by non-covalent insertion of a homo-bifunctional spacer for active targeting in cancer therapy. Nanotechnology. 2011;22:035101. doi: 10.1088/0957-4484/22/3/035101. [DOI] [PubMed] [Google Scholar]
- 70.Shahani K, Panyam J. Highly loaded, sustained-release microparticles of curcumin for chemoprevention. J. Pharm. Sci. 2011;100:2599–2609. doi: 10.1002/jps.22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shahani K, et al. Injectable sustained release microparticles of curcumin: a new concept for cancer chemoprevention. Cancer Res. 2010;70:4443–4452. doi: 10.1158/0008-5472.CAN-09-4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsai YM, et al. Curcumin and its nano-formulation: the kinetics of tissue distribution and blood–brain barrier penetration. Int. J. Pharm. 2011 doi: 10.1016/j.ijpharm.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 73.Anitha A, et al. Preparation, characterization in vitro drug release and biological studies of curcumin loaded dextran sulphate–chitosan nanoparticles. Carbohyd. Polym. 2011;84:1158–1164. [Google Scholar]
- 74.Anuradha CA, Aukunuru J. Preparation, characterisation and in vivo evaluation of bis-demethoxy curcumin analogue (BDMCA) nanoparticles. Trop. J. Pharma. Res. 2010;9:51–58. [Google Scholar]
- 75.Misra R, Sahoo SK. Co-formulation of doxorubicin and curcumin in poly (D, L-lactide-co-glycolide) nanoparticles suppress the development of multi drug resistance in K562 cells. Mol. Pharm. 2011;8:852–866. doi: 10.1021/mp100455h. [DOI] [PubMed] [Google Scholar]
- 76.Kar SK, et al. Curcumin nanoparticles and methods of producing the same, WO2010013224. XXXX. [Google Scholar]
- 77.Tomren MA, et al. Studies on curcumin and curcuminoids XXXI. Symmetric and asymmetric curcuminoids: stability, activity and complexation with cyclodextrin. Int. J. Pharm. 2007;338:27–34. doi: 10.1016/j.ijpharm.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 78.Desai K. Curcumin cyclodextrin combination for preventing or treating various diseases, 20100179103. XXXX. [Google Scholar]
- 79.Parkkinen J. Novobion. Soluble complexes of curcumin, 20110034564 [Google Scholar]
- 80.Letchford K, et al. Solubilization of hydrophobic drugs by methoxy poly(ethylene glycol)-block-polycaprolactone diblock copolymer micelles: theoretical and experimental data and correlations. J. Pharm. Sci. 2008;97:1179–1190. doi: 10.1002/jps.21037. [DOI] [PubMed] [Google Scholar]
- 81.Yu HL, Huang QR. Enhanced in vitro anti-cancer activity of curcumin encapsulated in hydrophobically modified starch. Food Chem. 2010;119:669–674. [Google Scholar]
- 82.Baglole K, et al. Fluorescence enhancement of curcumin upon inclusion into parent and modified cyclodextrins. J. Photochem. Photobiol. A. 2005;173:230–237. [Google Scholar]
- 83.Zhang F, et al. A novel solubility-enhanced curcumin formulation showing stability and maintenance of anticancer activity. J. Pharm. Sci. 2011;100:2778–2789. doi: 10.1002/jps.22512. [DOI] [PubMed] [Google Scholar]
- 84.Esmaili M, et al. Beta casein-micelle as a nano vehicle for solubility enhancement of curcumin; food industry application. LWT Food Sci. Tech. 2011 [Google Scholar]
- 85.Yadav VR, et al. Cyclodextrin-complexed curcumin exhibits anti-inflammatory and antiproliferative activities superior to those of curcumin through higher cellular uptake. Biochem. Pharmacol. 2010;80:1021–1032. doi: 10.1016/j.bcp.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Sou K, et al. Characterization and cytotoxicity of self-organized assemblies of curcumin and amphiphatic poly(ethylene glycol) J. Biomed. Nanotechnol. 2009;5:202–208. doi: 10.1166/jbn.2009.1025. [DOI] [PubMed] [Google Scholar]
- 87.Tang H, et al. Amphiphilic curcumin conjugate-forming nanoparticles as anticancer prodrug and drug carriers in vitro and in vivo effects. Nanomedicine. 2010;5:855–865. doi: 10.2217/nnm.10.67. [DOI] [PubMed] [Google Scholar]
- 88.Lin YL, et al. A lipo-PEG-PEI complex for encapsulating curcumin that enhances its antitumor effects on curcumin-sensitive and curcumin-resistance cells. Nanomedicine. 2011 doi: 10.1016/j.nano.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 89.Bisht S, et al. Polymeric nanoparticle-encapsulated curcumin (‘nanocurcumin’): a novel strategy for human cancer therapy. J. Nanobiotechnol. 2007;5:3. doi: 10.1186/1477-3155-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bisht S, et al. Systemic administration of polymeric nanoparticle-encapsulated curcumin (NanoCurc) blocks tumor growth and metastases in preclinical models of pancreatic cancer. Mol. Cancer Ther. 2010;9:2255–2264. doi: 10.1158/1535-7163.MCT-10-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lim KJ, et al. A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biol. Ther. 2011;11:464–473. doi: 10.4161/cbt.11.5.14410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gonçalves C. IBB-Institute for Biotechnology and Bioengineering, Centre for Biological Engineering. Minho University; 2010. Development of self-assembled dextrin nanogels; pp. 53–155. (XXXX, eds) [Google Scholar]
- 93.Varaprasad K, et al. Synthesis and characterization of hydrogel-silver nanoparticle-curcumin composites for wound dressing and antibacterial application. J. Appl. Polym. Sci. 2011;121:784–796. [Google Scholar]
- 94.Dandekar PP, et al. Curcumin-loaded hydrogel nanoparticles: application in anti-malarial therapy and toxicological evaluation. J. Pharm. Sci. 2010;99:4992–5010. doi: 10.1002/jps.22191. [DOI] [PubMed] [Google Scholar]
- 95.Kurien BT, et al. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev. Technol. 2007;5:567–576. doi: 10.1089/adt.2007.064. [DOI] [PubMed] [Google Scholar]
- 96.Suwannateep N, et al. Mucoadhesive curcumin nanospheres: biological activity, adhesion to stomach mucosa and release of curcumin into the circulation. J. Control. Release. 2011;151:176–182. doi: 10.1016/j.jconrel.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 97.Mohanty C, Sahoo SK. The in vitro stability and in vivo pharmacokinetics of curcumin prepared as an aqueous nanoparticulate formulation. Biomaterials. 2010;31:6597–6611. doi: 10.1016/j.biomaterials.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 98.Gupta V, et al. Fabrication and characterization of silk fibroin-derived curcumin nanoparticles for cancer therapy. Int. J. Nanomed. 2009;4:115–122. doi: 10.2147/ijn.s5581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agashe H, et al. Cyclodextrin-mediated entrapment of curcuminoid 4-[3,5-bis(2-chlorobenzylidene 4-oxo-piperidine-1-yl) 4- oxo 2-butenoic acid] or CLEFMA in liposomes for treatment of xenograft lung tumor in rats. Colloids Surf. B. 2011;84:329–337. doi: 10.1016/j.colsurfb.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Clifford NW, et al. Encapsulation and controlled release of nutraceuticals using mesoporous silica capsules. J. Mater. Chem. 2008;18:162–165. [Google Scholar]
- 101.Chin SF, et al. Encapsulation and sustained release of curcumin using superparamagnetic silica reservoirs. Chemistry. 2009;15:5661–5665. doi: 10.1002/chem.200802747. [DOI] [PubMed] [Google Scholar]
- 102.Sun M, et al. Enhancement of transport of curcumin to brain in mice by poly(n-butylcyanoacrylate) nanoparticle. J. Nanopart. Res. 2010;12:3111–3122. [Google Scholar]
- 103.Dandekar P, et al. Toxicological evaluation of pH-sensitive nanoparticles of curcumin: acute, sub-acute and genotoxicity studies. Food. Chem. Toxicol. 2010;48:2073–2089. doi: 10.1016/j.fct.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 104.Kanai M, et al. Dose-escalation and pharmacokinetic study of nanoparticle curcumin, a potential anticancer agent with improved bioavailability, in healthy human volunteers. Cancer Chemother. Pharmacol. 2011 doi: 10.1007/s00280-011-1673-1. [DOI] [PubMed] [Google Scholar]


