Abstract
In vivo neurochemical monitoring using microdialysis sampling is important in neuroscience because it allows correlation of neurotransmission with behavior, disease state, and drug concentrations in the intact brain. A significant limitation of current practice is that different assays are utilized for measuring each class of neurotransmitter. We present a high performance liquid chromatography (HPLC) - tandem mass spectrometry method that utilizes benzoyl chloride for determination of the most common low molecular weight neurotransmitters and metabolites. In this method, 17 analytes were separated in 8 minutes. The limit of detection was 0.03–0.2 nM for monoamine neurotransmitters, 0.05–11 nM for monoamine metabolites, 2–250 nM for amino acids, 0.5 nM for acetylcholine, 2 nM for histamine, and 25 nM for adenosine at sample volume of 5 µL. Relative standard deviation for repeated analysis at concentrations expected in vivo averaged 7% (n = 3). Commercially available 13C benzoyl chloride was used to generate isotope-labeled internal standards for improved quantification. To demonstrate utility of the method for study of small brain regions, the GABAA receptor antagonist bicuculline (50 µM) was infused into rat ventral tegmental area while recording neurotransmitter concentration locally and in nucleus accumbens, revealing complex GABAergic control over mesolimbic processes. To demonstrate high temporal resolution monitoring, samples were collected every 60 s while neostigmine, an acetylcholine esterase inhibitor, was infused into the medial prefrontal cortex. This experiment revealed selective positive control of acetylcholine over cortical glutamate.
Keywords: Neurotransmitter, Microdialysis, Benzoylation, Liquid Chromatography, Mass Spectrometry
Introduction
Monitoring neurotransmitter concentration dynamics in the living brain is a critically important tool in neuroscience. In vivo measurements enable study of the relationship between neurotransmitter concentrations in relevant brain nuclei and behavior, drug effects, or disease states. Since its inception, microdialysis sampling has been the preeminent tool for making such measurements 1–3. In this approach, a semi-permeable membrane probe is inserted into the brain and perfused with artificial cerebral spinal fluid (aCSF). Molecules in the extracellular space diffuse across the membrane according to their concentration gradient and are collected into fractions which are analyzed for neurotransmitter or metabolite content. This tool has been invaluable for neuroscience, e.g. it has been used to demonstrate that all drugs of abuse activate the mesolimbic dopamine (DA) system 4, glutamate (Glu) sustains drug seeking behavior 5, 6,and adenosine (Ado) is a modulator of sleep 7. The technique is also used clinically for studying epilepsy 8 and brain trauma 9, 10 and plays a prominent role in the pharmaceutical industry when screening novel neurological and psychiatric therapeutics.
A key to using microdialysis is analysis of sample fractions 11. Many assays for neurotransmitters have been developed using high performance liquid chromatography (HPLC)-electrochemical detection 12, 13, HPLC-fluorescence detection 14, capillary electrophoresis-laser induced fluorescence 15, 16, immunoassay17 and more recently, HPLC-mass spectrometry (MS) 18–20. Despite extensive research into methods for chemical analysis of dialysate, all methods presently in use can only determine a subset of common small molecule neurotransmitters. Therefore, studies that require monitoring different types of neurotransmitters must use multiple assays which increases costs and time required for equipment maintenance, method development and analysis. Use of multiple assays also increases sample volume requirements and animal usage. Assays that measure only a single or few neurotransmitters also preclude discovering involvement of unanticipated neurotransmitter systems. A comprehensive analytical method for neurotransmitter measurements would be of great value to the neurosciences by revealing previously unknown neurotransmitter interactions. Such a method could also accelerate neurological drug development by allowing rapid evaluation of the effect of novel compounds in the brain. Any such method must be sensitive enough for dialysate samples and have sufficient throughput for the many samples generated from in vivo experiments.
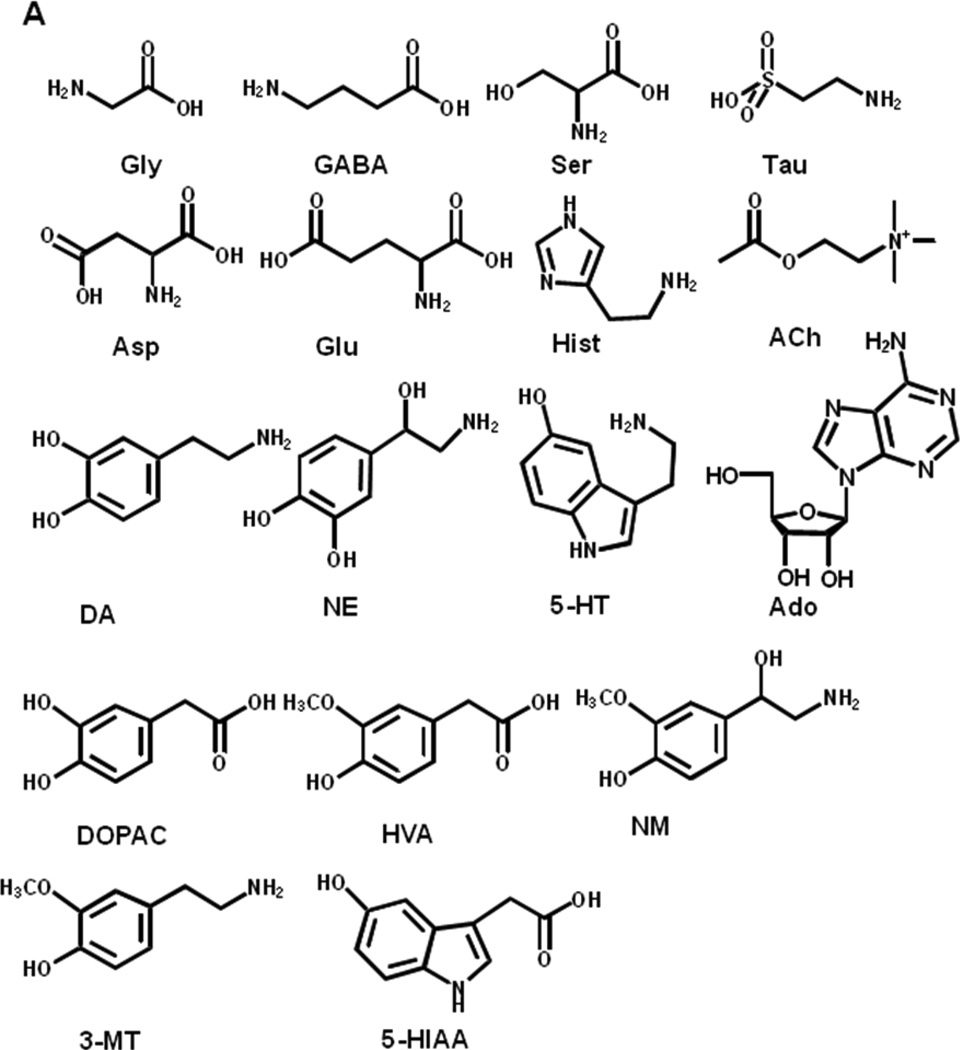
Here we report a HPLC-MS method for the measurement of 12 of the most commonly studied neurotransmitters or neuromodulators (Figure 1A) including ACh, Ado, DA, norepinephrine (NE), serotonin (5-HT), histamine (Hist), Glu, glycine (Gly), aspartate (Asp), γ-aminobutyric acid (GABA), serine (Ser), and taurine (Tau). The method also assays the metabolites homovanillic acid (HVA), 5-hydroxyindole-3-acetic acid (5-HIAA), 3,4-dihydroxyphenylacetic acid (DOPAC), normetanephrine (NM) and 3-methoxytyramine (3-MT). The method is compatible with challenging experiments which generate low concentration samples such as using small microdialysis probes for high spatial resolution and fast sampling rates (60 s/sample) for high temporal resolution in vivo monitoring.
Figure 1.
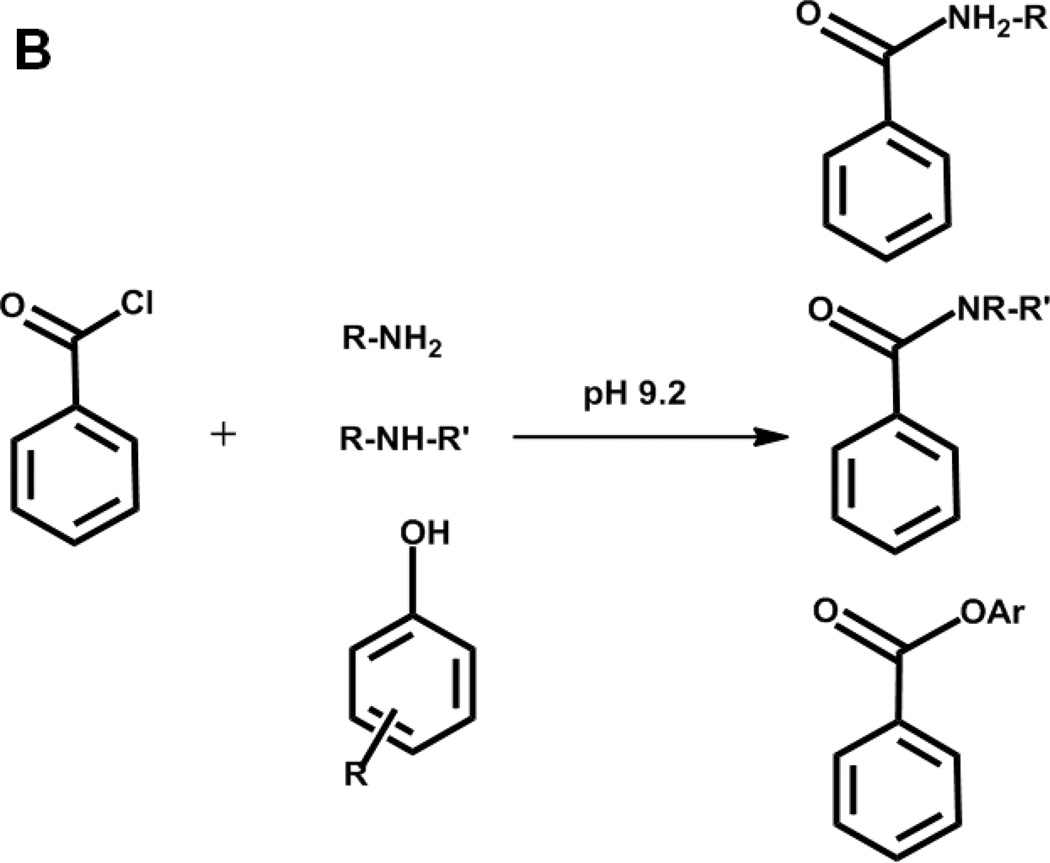
Chemical structure of targeted neurotransmitters and metabolites (A). Reaction scheme of benzoylation using benzoyl chloride (B)
A major difficulty to overcome in developing such an assay is identifying chromatographic conditions that can resolve the highly polar neurochemicals while remaining compatible with MS detection. We discovered that derivatization with benzoyl chloride renders the compounds more hydrophobic so that they can be separated by reversed phase chromatography. Derivatization also increases sensitivity and provides a convenient way to improve quantification by stable-isotope labeled internal standards generated using 13C6 benzoyl chloride. Importantly, benzoyl chloride reacts with primary and secondary amines, phenols, and ribose-hydroxyl groups (Figure 1B) allowing nearly all small organic molecule neurotransmitters to be labeled. ACh, which cannot be labeled by benzoyl chloride, is directly detected in this method. Rapid derivatization and an 8 min separation time give the method sufficient throughput for the large number of samples generated by microdialysis experiments. The method uses commercial instrumentation and readily available reagents; therefore, it can easily be adopted by other laboratories.
Methods
Chemicals and Reagents
All chemicals, drugs and reagents were purchased from Sigma Aldrich (St. Louis, MO) unless otherwise noted. Water and acetonitrile are Burdick & Jackson HPLC grade purchased from VWR (Radnor, PA). 10 mM stock solution of each analyte was made in HPLC water and kept at −80 °C. Standard mixture was diluted from stock using aCSF consisting of 145 mM NaCl, 2.68 mM KCl, 1.10 mM MgSO4, 1.22 mM CaCl2, 0.50 mM NaH2PO4, and 1.55 mM Na2HPO4, adjusted pH to 7.4 with 0.1 M NaOH. Calibration curves were made using standards at 50 nM, 500 nM, 1000 nM, 5000 nM, 10000 nM for Gly, Ser and Tau; 5 nM, 50 nM, 100 nM, 500 nM, 1000 nM for Asp, Glu, GABA, Hist, Ado, HVA, 5-HIAA and DOPAC; 0.5 nM, 5 nM, 10 nM, 50 nM, 100 nM for ACh, 5-HT, NE, DA, NM and 3-MT. Internal standard was 1 mM Gly, Ser and Tau, 100 µM Asp, Glu, GABA, Hist, Ado, HVA, 5-HIAA, and DOPAC and 10 µM 5-HT, NE, DA, NM and 3-MT derivatized with 13C6 benzoyl chloride using the same procedure as 12C reagent, then diluted 100 fold in DMSO containing 1% formic acid. d4-ACh (C/D/N isotopes, Pointe-Claire, Canada) was spiked into the reaction mixture to a final concentration of 100 nM.
Microdialysis
Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN) weighing between 295 and 355 g were used. Ketamine (65 mg/kg i.p.) and dexdormitor (0.25 mg/kg i.p.) were used for operative anesthesia. For dual probe experiments, concentric microdialysis probes (250 µm diameter) were implanted unilaterally in both the VTA (1 mm long probe) and NAc (1.5 mm long probe) according to following coordinates from bregma and dura: anterior-posterior (AP) −5.3, medial-lateral (ML) ± 0.5, dorsal-ventral (DV) − 8.0 mm and AP + 1.2, ML ± 1.4 and DV − 7.8 mm, respectively. For single probe experiments, probes (3 mm long) were implanted into the mPFC from bregma and dura: AP +3.0, ML ± 0.5, DV − 4.0 (Paxinos and Watson 2007). Probes were secured to the skull by acrylic dental cement and metallic screws. Following surgery, rats were allowed to recover and experiments were performed later. Animals were awake and freely moving with access to food and water throughout the experiment. Microdialysis probes were flushed at 1.5 µL/min with aCSF for 3 h using a Chemyx (Stafford, TX) Fusion 400 syringe pump. Perfusion flow rate was reduced to 0.6 µL/min and samples were collected every 20 min for VTA-NAc experiments. For mPFC experiments, perfusion flow rates were reduced to 1 µL/min to generate 1 µL samples. 1 µL fractions were diluted with 4 µL aCSF, then treated same way as 5 µL sample described below. Following collection of basal fractions, VTA lines were switched to aCSF containing 50 µM bicuculline for the duration (2 h) of experiments. Though 12 µL of dialysate were collected per fraction, only 5 µL of total volume was used for analysis. For mPFC, following 20 min of basal fraction collections, 1 µM neostigmine was perfused through the probe for 5 min.
Benzoylation reaction
5 µL of standard or sample was mixed with 2.5 µL borate buffer (sodium tetraborate, 100 mM) and 2.5 µL benzoyl chloride (2% in acetonitrile, v/v). The mixture was vortexed and 2.5 µL internal standard was added before LC-MS analysis.
HPLC-MS analysis
The HPLC system was a Waters (Milford, MA) nanoAcquity HPLC. A Waters BEH C18 column (1 mm × 100 mm, 1.7 µm, 130 Å pore size) was used for separation. Mobile phase A was 10 mM ammonium formate, 0.15% (v/v) formic acid in water. Mobile phase B was acetonitrile. The mobile phase gradient for all 17 analytes was: initial, 0% B; 0.1 min, 15% B; 2 min, 20% B; 2.3 min, 25% B; 2.31 min, 50% B; 5.31 min, 50% B; 5.57 min, 65 % B; 6.57 min, 65%B; 6.58 min, 0% B; 8.0 min, 0% B.
The flow rate was 100 µL/min and sample injection volume was 9 µL in partial loop injection mode. Autosampler was kept at ambient temperature and column was maintained at 27 °C. A Waters/Micromass Quattro Ultima triple quadrupole mass spectrometer was used for detection. Atmospheric pressure ionization source was operated in positive ESI mode at 3 kV. Source temperature was 140 °C and desolvation temperature was 400 °C. Cone gas and desolvation gas flowed at 150 L/h and 500 L/h, respectively.
MRM conditions are listed in Table S1. Interchannel delay and intercycle delay were both 10 ms. Automated peak integration was performed using Waters Masslynx version 4.1. All peaks were visually inspected to ensure proper integration. Calibration curves were constructed based on peak area ratio (Panalyte/PI.S.) versus concentrations of internal standard by linear regression.
Statisical analysis
Data were transformed to percent of baseline measurement to normalize pretreatment levels to 100 percent. All analyses were performed in Graphpad (La Jolla, CA) Prism 5. The measurements were all continuous variables and the Kolmogorov-Smirnov test was used to assess normality of the residuals for each individual repeated measurement and this assumption was met. A two-tailed repeated measures analysis of variance (RM ANOVA) was performed on all microdialysis data followed by a post-hoc Tukey test to test the pairwise difference between every time point and baseline (i.e. 100%). Although animal numbers were relatively small (n = 8 for the bicuculline study and n = 5 for the neostigmine study), following baseline we collected 7 (for bicuculline) and 60 (for neostigmine) measures over time on each animal, enabling analyses of variation in the outcomes both between and within animals. In addition, with 8 animals in one group and 5 in the other, and 8 repeated measures on each animal, a RM ANOVA will have more than 90% power to detect a mean difference of 50 percentage points between the two groups at a 0.05 level of significance (assuming correlations of 0.25 of the repeated measures, and standard deviations of 10 percentage points in each group). RM ANOVA is the primary statistical test used for such continuous measurements. Since the two experiments (i.e. bicuculline and neostigmine) were performed independently, and did not contain control groups since basal levels were taken as control, no randomization was necessary. However, the individual who processed the data was blind to any expected outcomes for each measure.
Results
HPLC-MS of benzoylated neurotransmitters
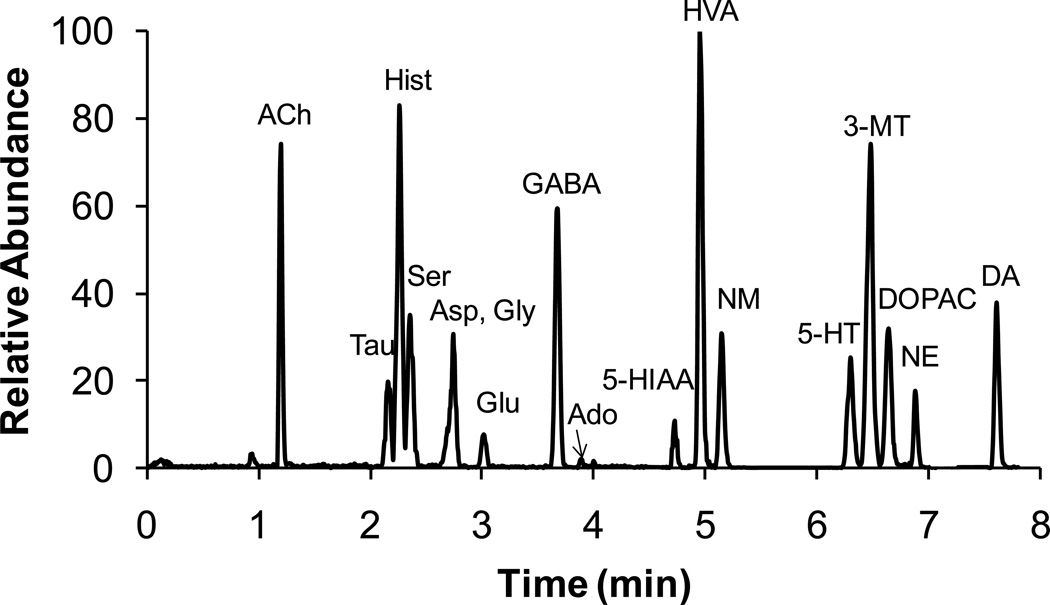
All analytes of interest were benzoylated (except ACh), resolved by reversed phase HPLC, and detected by MS/MS (Figure 2). Amino acids were singly benzoylated while monoamines containing phenolic groups were doubly or triply labeled as determined by the mass detected. Protonated benzoylation products (MW+1) were observed by positive electrospray ionization (ESI) for primary, secondary amines and ribose hydroxy groups. For analytes containing only phenol groups (HVA, DOPAC, 5-HIAA), the ammonium adduct of their products (MW+18) was detected. For ACh, the unlabeled molecular ion was used for detection.
Figure 2.
Ion chromatogram for all 17 analytes. The concentration of each analyte was: ACh (10 nM); Tau (1000 nM); Hist (100 nM); Ser (1000 nM); Asp (100 nM); Gly (1000 nM); Glu (100 nM); GABA (100 nM); Ado (100 nM); 5-HIAA (100 nM); HVA (100 nM); NM (10 nM); 5-HT (10 nM); DOPAC (100 nM); 3-MT (10 nM); NE (10 nM); and DA (10 nM).
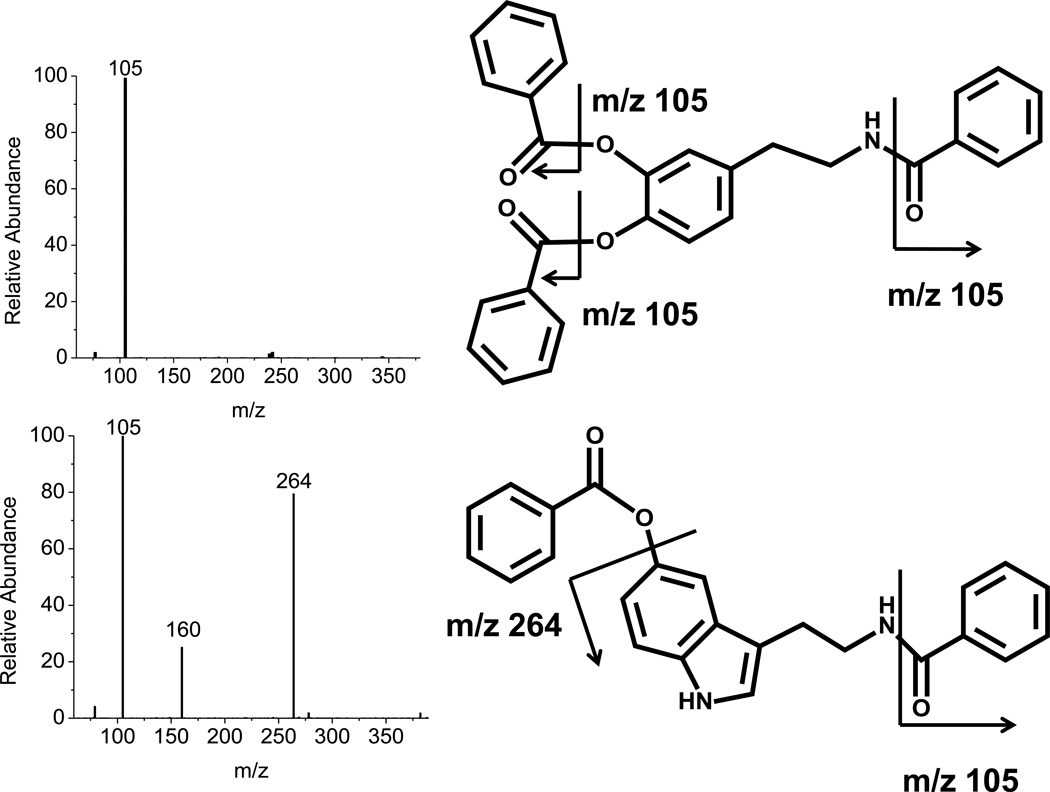
Analytes were detected by MS/MS under collision activated dissociation (CAD) conditions; therefore, the fragmentation of each analyte was examined to determine the best product ions to use for quantification (Table S1). For most benzoylated analytes, the benzoyl fragment (m/z 105) was the most abundant product ion (Figure 3, top) and was subsequently used for multiple reaction monitoring (MRM). 5-HT generated additional fragment ions (m/z 160, m/z 264) besides the benzoyl fragment (Figure 3, bottom). The unique fragment (m/z 264) was selected for MRM of 5-HT. Most analytes were fully labeled yielding a single chromatographic peak; however, benzoylated Ado produced two peaks corresponding to N6-benzoyl-Ado and 3’-O-benzoyl-Ado. The latter product is a result of activity of the 3’-hydroxy group on the ribose ring in nucleophilic substitutions21, 22. Besides m/z 105, both N6-benzoyl-Ado and 3’-O-benzoyl-Ado generated a characteristic m/z 136 fragment corresponding to an adenine moiety under MS/MS conditions. The 3’-O-benzoyl-Ado produced more abundant fragment ions and was chosen for MRM quantification. For ACh, the quanternary ammonium residue (m/z 87) after loss of acetyl ester was used for quantification.
Figure 3.
MS/MS spectra of benzoylated DA (top) and 5-HT (bottom). Benzoylated 5-HT gave a unique fragment of 264 m/z besides the characteristic 105 m/z (benzoyl group). Most other benzoylated analytes like DA produced only 105 m/z in CAD
Besides enhancing retention on reversed phase columns, benzoylation also enhanced sensitivity for ESI-MS. For example, signal (peak area) was increased 22-fold for 5-HT, 23-fold for Glu, 37-fold for DA, 460-fold for 3-MT, and 1,400-fold for GABA compared to their respective native form. Analytical performance of benzoylation HPLC-MS method was satisfactory, with average relative standard deviation (RSD) of 7% for analysis of samples made in triplicate, linear response (R2 > 0.99), biologically relevant limits of detection (LOD), and adequate analyte stablity (Table 1). Use of isotopically labeled internal standard improved measurement precision with RSD of repeated analysis of the same sample reduced from 4–6% to 2% (Figure S1).
Table 1.
Figures of merit for benzoylation-HPLC-MS method. RSDs were calculated by comparing peak area of 3 individually derivatized standards at approximate in vivo concentrations, thus evaluating both reaction and analytical variability. Dynamic range tested for linearity was 500 – 10,000 nM for Gly, Ser and Tau; 5 – 100 nM for HVA, 5-HIAA, NM, 3-MT and DOPAC; 50 – 1000 nM for GABA, Asp, Glu, Hist and Ado; 0.5 – 100 nM for ACh,5-HT, NE and DA. Percent remaining was calculated by comparing peak area of stored standard solution (room temperature for a week) to a freshly derivatized standard solution. Basal concentration of each analyte in dialysate from different brain regions is listed. These values are not corrected for microdialysis probe recovery.
| analyte | RSD (n = 3) |
LOD (nM) |
R2 | % remaining after storage (n = 3) |
basal in vivo dialysate concentration | ||
|---|---|---|---|---|---|---|---|
| VTA (n = 8) | NAc (n = 8) | mPFC (n = 5) | |||||
| Gly | 5 | 500 | 0.9933 | 80 ± 18 | 4 ± 2 µM | 7 ± 2 µM | 7 ± 1 µM |
| GABA | 5 | 2 | 0.9989 | 114 ± 10 | 0.04 ± 0.01 µM | 0.03 ± 0.01 µM | 44 ± 8 nM |
| Ser | 11 | 250 | 0.9975 | 94 ± 21 | 6.4 ± 0.8 µM | 20 ± 6 µM | 10 ± 4 µM |
| Tau | 7 | 250 | 0.9995 | 86 ± 21 | 12 ± 4 µM | 4 ± 1 µM | 4.3 ± 0.6 µM |
| Asp | 10 | 50 | 0.9987 | 109 ± 19 | 0.6 ± 0.1 µM | 0.7 ± 0.1 µM | n.d. |
| Glu | 8 | 5 | 0.9995 | 107 ± 14 | 0.9 ± 0.2 µM | 1.9 ± 0.3 µM | 0.7 ± 0.3 µM |
| DA | 4 | 0.03 | 0.9981 | 109 ± 14 | 3 ± 1 nM | 8 ± 2 nM | 2.2 ± 0.4 nM |
| NE | 10 | 0.2 | 0.9997 | 97 ± 7 | 1.4 ± 0.2 nM | 2.0 ± 0.5 nM | 2.0 ± 0.4 nM |
| 5-HT | 4 | 0.1 | 0.9998 | 118 ± 19 | 0.36 ± 0.06 nM | 0.32 ± 0.08 nM | 0.36 ± 0.08 nM |
| Hist | 13 | 2 | 0.9961 | 118 ± 14 | 1.2 ± 0.5 nM | 0.8 ± 0.2 nM | 1.0 ± 0.4 nM |
| Ado | 4 | 25 | 0.9974 | 102 ± 15 | 18 ± 3 nM | 28 ± 5 nM | n.d. |
| 3-MT | 1 | 0.05 | 0.9999 | 116 ± 18 | 2.2 ± 0.3 nM | 1.7 ± 0.4 nM | n.d. |
| DOPAC | 5 | 2 | 0.9973 | 103 ± 5 | 0.8 ± 0.1 µM | 5 ± 1 µM | 110 ± 9 nM |
| HVA | 4 | 0.5 | 0.9973 | 108 ± 3 | 0.12 ± 0.01 µM | 0.7 ± 0.2 µM | 49 ± 9 nM |
| NM | 2 | 0.1 | 0.9996 | 102 ± 2 | 0.8 ± 0.1 nM | 0.46 ± 0.03 nM | 2.7 ± 0.9 nM |
| 5-HIAA | 7 | 5 | 0.9999 | 115 ± 20 | 1.1 ± 0.1 µM | 0.67 ± 0.07 µM | 0.26 ± 0.04 µM |
| ACh | 7 | 0.5 | 0.9963 | non-labeled | 8 ± 2 nM | 15 ± 2 nM | 3.8 ± 0.6 nM |
Microdialysis
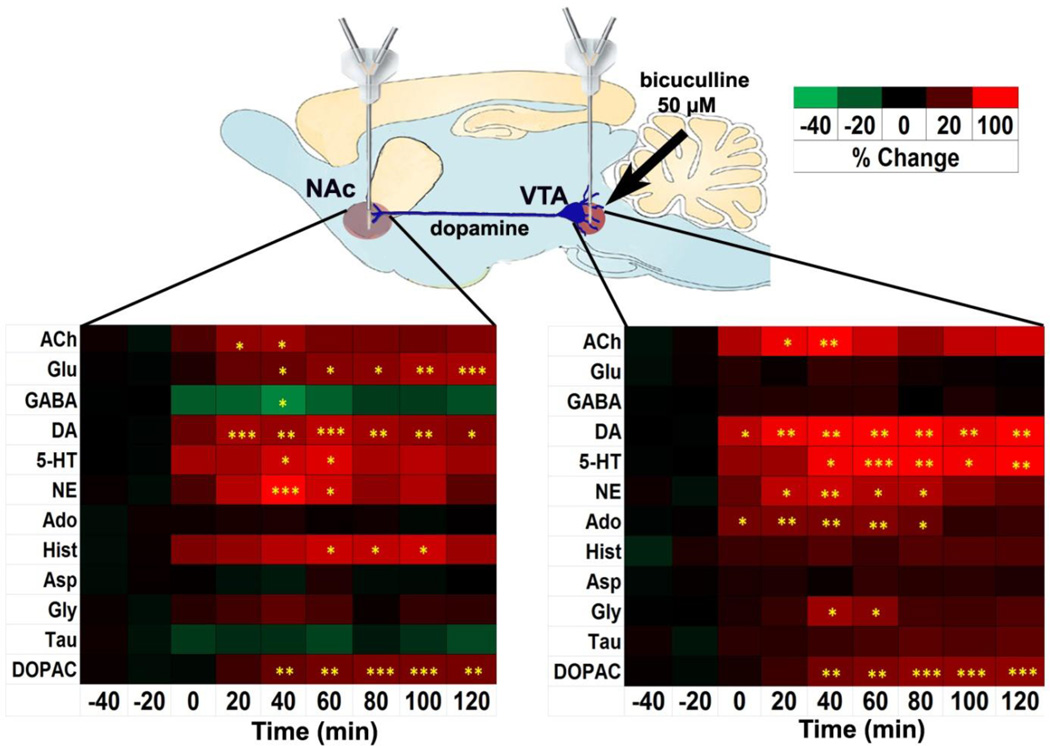
To demonstrate utility of the method under conditions requiring good spatial resolution, we performed dual probe microdialysis experiments in the mesolimbic pathway, i.e. the ventral tegmental area (VTA) and the nucleus accumbens (NAc). Though probes were only 1 mm and 1.5 mm in length, respectively, we detected all expected neurochemicals in these samples (Figure S2). Basal neurotransmitter concentrations in VTA and NAc dialysate, reported in Table 1, are within the expected range 23, 24.
Perfusion of the GABAA receptor antagonist bicuculline (50 µM) into the VTA (n = 8) yielded a complex set of changes in the neurochemicals measured (Figure 4 and Figure S3). This treatment stimulated local dendritic (153%; F(7,7) = 10.15, p < 0.0001) and limbic (106%; F(7,7) = 5.835, p < 0.0001) DA release, as anticipated 25. DA release also correlated with an increase in the DA metabolite DOPAC in the VTA (60%; F(7,5) = 9.209, p < 0.0001), and the NAc (49%; F(7,5) = 9.641, p < 0.0001). We observed unanticipated increases in VTA 5-HT (146%; F(7,7) = 5.00, p = 0.003), NE (97%; F(7,7) = 3.570, p = 0.0035), ACh (153%; F(7,5) = 3.809, p = 0.0036), Ado (53%; F(7,5) = 5.086, p = 0.0005), and Gly (66%; F(7,6) = 3.588, p = 0.0041).
Figure 4.
Dual probe microdialysis of the mesolimbic pathway. Bicuculline (50 µM) was perfused in the VTA while monitoring neurotransmitters both locally and distally in the NAc. Heat map shows all changes of neurochemicals monitored where colors correlate with changes expressed as percentage of baseline. Times in heat map are referenced to infusion of biculline. RM ANOVA and a post-hoc Tukey test were performed to compare basal levels against post drug levels. *p < 0.05, **p < 0.01, ***p < 0.001 compared to basal level in the respective brain region. A line graph of these data is presented in Figure S3.
In the NAc we observed unanticipated increases in 5-HT (106%; F(7,6) = 2.450, p = 0.0335), NE (132%; F(7,7) = 4.843, p = 0.0003), Glu (73%; F(7,6) = 6.759, p < 0.0001), ACh (66%; F(7,5) = 2.782, p = 0.0207), and Hist (95%; F(7,5) = 3.137, p = 0.0111). We also observed an unexpected reduction in GABA (34%; F(7,6) = 2.291, p = 0.0452) in the NAc (Figure 4 and Figure S3).
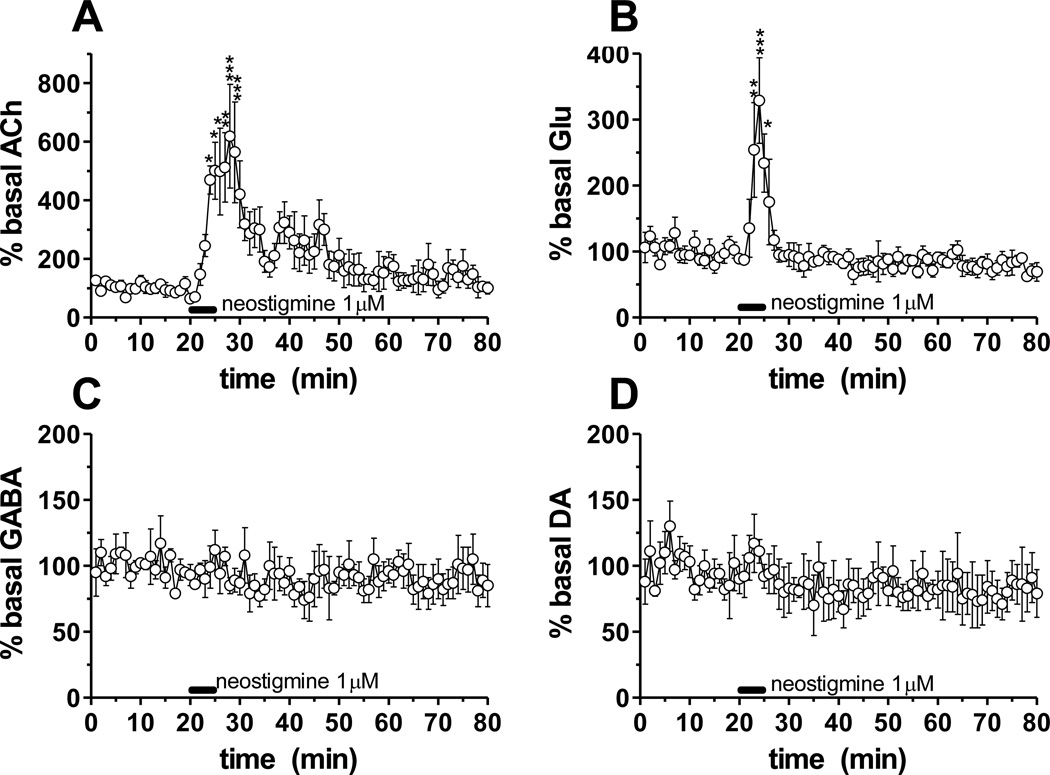
To demonstrate the utility of the method under conditions of high temporal resolution, we used 60 s sampling in the medial prefrontal cortex (mPFC) to observe changes in ACh release following a 5 min local perfusion of the ACh esterase inhibitor neostigmine (1 µM; n = 5). Neostigmine perfusion caused a prompt increase in ACh (518%; F(60,4) = 4.193, p < 0.0001) levels in the mPFC as anticipated (Figure 5A). We also observed a large (229%; F(60,4 = 4.443, p < 0.0001) transient increase in Glu concentration (Figure 5B). Neostigmine perfusion had no effect on DA, GABA (Figure 5C, D) or other measured neurotransmitters (data not shown). Basal neurotransmitter concentrations in mPFC dialysate are reported in Table 1.
Figure 5.
Microdialysis in the mPFC. Neostigmine (1 µM) was perfused (black bar) for 5 min in the mPFC while neurotransmitters were monitored locally. Dialysate was collected every 60 s. Error bar is ± 1 SEM (n = 5). Data was expressed as percent of baseline levels. RM ANOVA and a post-hoc Tukey test to compare basal levels against post drug levels. *p < 0.05, **p < 0.01, ***p < 0.001 compared to basal mPFC level.
Discussion
HPLC-MS of neurotransmitters
This study demonstrates that HPLC-MS is suitable for measuring all the most commonly studied low molecular weight neurotransmitters, neuromodulators, and metabolites in a single analysis based on both direct detection (ACh) and benzoylation. Unlike reagents that target only primary amines, benzoyl chloride also labels secondary amines, phenols, and ribose hydroxyl groups. This allows several neurochemicals which do not have primary amine groups, such as DOPAC, 5-HIAA, and HVA, to be labeled. Even though many molecules can be detected directly by MS without derivatization, labeling of polar neurotransmitters has several distinct advantages. Benzoylation enhances reversed phase LC retention, improves ESI-MS sensitivity, and allows for a low-cost stable isotope internal standard.
Although benzoyl chloride has been used for polyamine and steroid analysis 26, 27, we found that some specific conditions were necessary to achieve reproducible results for neurotransmitter detection. Borate buffer was preferred over other basic buffers for derivatization because it improved precision in catecholamine measurements. We believe that this is because borate forms a reversible complex with catechol groups 28 and protects them against oxidation under the high pH conditions used for labeling 29. Another issue is that metabolites labeled at multiple sites became hydrophobic and insoluble in 100% aqueous solution; therefore, 25% organic solvent content in the reaction mixture was used to keep these compounds in solution.
Benzoyl chloride labeling is similar to dansyl chloride labeling previously reported for metabolomics 30 but has several advantages due to its structural simplicity. A preliminary study revealed that monoamines which were multiply-labeled with the dansyl group had low CAD efficiency on our triple quadrupole mass spectrometer, rendering MRM of these analytes unsuitable for high sensitivity analysis. In contrast, multiply labeled benzoylated monoamines are easily fragmented. 13C dansyl chloride must be synthesized for differential labeling using light and heavy isotope reagent 30 while 13C6 benzoyl chloride is commercially available at relatively low cost. Dansylation requires 30 min reaction time at elevated temperatures while the benzoylation reaction was instant at room temperature. Unlike dansyl chloride, excess benzoyl chloride is completely hydrolyzed so there is no need to scavenge reagent after the reaction. Importantly, neither benzoyl chloride nor the benzoylated product is light sensitive in contrast to dansyl chloride and its derivatives.
The HPLC separation time is 8 min and total analysis time for each sample is around 12 min, taking into account autosampler injection, elution, and column re-equilibration. Over 80 samples plus standards are routinely analyzed per day in our laboratory using this method. Such throughput is important for microdialysis since hundreds of samples are typically generated per study.
Mesolimbic regulation by GABA
To demonstrate the utility of this system, we investigated GABAergic control of the mesolimbic pathway. The mesolimbic pathway is made up of DA containing cells which originate in the VTA and extend to the NAc. This pathway is intensively studied for its role in reward processing, motivation and addiction since all drugs of abuse activate this pathway 4, 31, 32. GABA, primarily from VTA interneurons, is an important regulator of the mesolimbic pathway 33, 34 and blockade of GABAA receptors stimulates mesolimbic DA release 25. Evidence suggests that the rewarding effects of opiates, cannabinoids, and benzodiazepines rely on their ability to activate the mesolimbic DA pathway by inhibiting GABAergic interneruons in the VTA 34–36. Neurotransmitters other than dopamine regulated by GABA have received less attention. Here our method has uncovered previously unknown GABAergic involvement in several neurotransmitter systems apart from DA as bicuculline perfusion into the VTA not only evoked the expected increase in NAc DA23 (and DA metabolite, DOPAC) but also 5-HT, NE, Glu, ACh, and Hist while reducing GABA levels. Locally in the VTA, bicuculline enhanced not only dendritic DA (and DOPAC), but also evoked local 5-HT, NE, ACh, Ado, and Gly release (Figure 4). These results show that GABAergic control of the VTA is not limited to limbic DA release and that a complex interplay between several neuronal substrates is involved in mesolimbic activation, and possibly reward. These measurements also demonstrate compatibility with high spatial resolution experiments. The VTA is ~1 mm in height × ~1 mm in width 37, so a 1 mm long × 250 µm diameter dialysis probe was used. Small probe produces low recovery (~10 %) requiring high sensitivity - a criterion met by the current method.
Prefrontal ACh and Glu interaction
We also measured prefrontocortical signaling to test the method under the challenging condition of a high sampling rate. The mPFC is a known center of emotional and cognitive function and is often studied in psychiatric and attentional disorders 38, 39. To uncover physiologically and behaviorally relevant neurotransmitter interactions in the mPFC, common microdialysis sampling rates (i.e. 10– 30 min/sample) remain inadequate. Here we have made such measurements every 60 s to better capture concentration dynamics on a more physiologically relevant time scale 39. Measurement at short intervals requires high sensitivity (1 µL samples are collected containing femtomoles of analyte) and good throughput because of the large number of samples generated (e.g., 80 samples per session in Figure 5).
The ACh esterase inhibitor neostigmine (5 min perfusion) elevated ACh concentrations in the mPFC as expected, but also correlated with a transient increase in mPFC Glu concentration. This result suggests that muscarinic or nicotinic ACh receptors in the mPFC are responsible for stimulating Glu release. Past studies have shown that exogenous ACh receptor agonists such as nicotine enhance cognition and stimulate Glu activity in the mPFC 40. Our data show that stimulated endogenous ACh is capable of enhancing Glu release in this brain area. This ACh-Glu interaction may play a role in cognition and the beneficial effects of drugs which stimulate ACh receptors 40. Interestingly other neurotransmitter systems, including the monoamines, were left unaffected by neostigmine perfusion. These results show how a comprehensive view of neuropharmacological effects can be assayed by the method. They also show the value of the temporal resolution as the transient rise of Glu would likely be undetected at longer sampling intervals.
Conclusion
The results show that benzoylation with HPLC-MS is a robust method for determination of small molecule neurotransmitters and metabolites. The method allows 17 neurotransmitters and metabolites to be detected with 8 min separation at concentrations from picomolar to micromolar. Internal standard is conveniently generated using commercial 13C6 reagent, enabling improved quantification. The comprehensiveness, low sample volume requirement, sensitivity, and throughput make this method valuable for neurochemical monitoring under challenging conditions such as small brain regions (VTA) and at short intervals. These properties will make the method widely applicable for pharmacological and brain disorder studies. The good temporal resolution will be important for correlating neurotransmitter concentrations to behaviors which change on similar time scales.
Supplementary Material
Acknowledgements
We thank the Center for Statistical Consultation and Research (CSCAR) at the University of Michigan for assistance with statistical analyses. This work was supported by NIH grant R37 EB003320 (R.T.K.) National Institute on Drug Abuse T32 training grant DA07268 (O.S.M.).
References
- 1.Tossman U, Ungerstedt U. Acta Physiol. Scand. 1986;128:9–14. doi: 10.1111/j.1748-1716.1986.tb07943.x. [DOI] [PubMed] [Google Scholar]
- 2.Robinson TE, Justice JBJ. Techniques in the behavioral and neural sciences. Amsterdam, Netherlands: Elsevier; 1991. [Google Scholar]
- 3.Westerink BHC, Cremers TIFH. Handbook of microdialysis. Amsterdam, Netherlands: Elsevier; 2007. [Google Scholar]
- 4.Di Chiara G, Imperato A. Proc. Natl. Acad. Sci. U. S. A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornish JL, Kalivas PW. J. Neurosci. 2000;20:RC89. doi: 10.1523/JNEUROSCI.20-15-j0006.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.LaLumiere RT, Kalivas PW. J. Neurosci. 2008;28:3170–3177. doi: 10.1523/JNEUROSCI.5129-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porkka-Heiskanen T, Strecker RE, Thakkar M, Bjorkum AA, Greene RW, McCarley RW. Science. 1997;276:1265–1268. doi: 10.1126/science.276.5316.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.During MJ, Ryder KM, Spencer DD. Nature. 1995;376:174–177. doi: 10.1038/376174a0. [DOI] [PubMed] [Google Scholar]
- 9.Feuerstein D, Manning A, Hashemi P, Bhatia R, Fabricius M, Tolias C, Pahl C, Ervine M, Strong AJ, Boutelle MG. J. Cereb. Blood Flow Metab. 2010;30:1343–1355. doi: 10.1038/jcbfm.2010.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bullock R, Zauner A, Woodward J, Young HF. Stroke. 1995;26:2187–2189. doi: 10.1161/01.str.26.11.2187. [DOI] [PubMed] [Google Scholar]
- 11.Perry M, Li Q, Kennedy RT. Anal. Chim. Acta. 2009;653:1–22. doi: 10.1016/j.aca.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Felice LJ, Felice JD, Kissinger PT. J. Neurochem. 1978;31:1461–1465. doi: 10.1111/j.1471-4159.1978.tb06573.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y, Zhang J, Xu X, Zhao MK, Andrews AM, Weber SG. Anal. Chem. 2010;82:9611–9616. doi: 10.1021/ac102200q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshitake T, Yoshitake S, Fujino K, Nohta H, Yamaguchi M, Kehr J. J. Neurosci. Methods. 2004;140:163–168. doi: 10.1016/j.jneumeth.2004.04.041. [DOI] [PubMed] [Google Scholar]
- 15.Zhou SY, Zuo H, Stobaugh JF, Lunte CE, Lunte SM. Anal. Chem. 1995;67:594–599. doi: 10.1021/ac00099a017. [DOI] [PubMed] [Google Scholar]
- 16.Robert F, Bert L, Denoroy L, Renaud B. Anal. Chem. 1995;67:1838–1844. doi: 10.1021/ac00107a013. [DOI] [PubMed] [Google Scholar]
- 17.Maidment NT, Brumbaugh DR, Rudolph VD, Erdelyi E, Evans CJ. Neuroscience. 1989;33:549–557. doi: 10.1016/0306-4522(89)90407-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhang XZ, Rauch A, Lee H, Xiao HB, Rainer G, Logothetis NK. Rapid Commun. Mass Spectrom. 2007;21:3621–3628. doi: 10.1002/rcm.3251. [DOI] [PubMed] [Google Scholar]
- 19.Eckstein JA, Ammerman GM, Reveles JM, Ackermann BL. J. Neurosci. Methods. 2008;171:190–196. doi: 10.1016/j.jneumeth.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Ji CJ, Li WL, Ren XD, El-Kattan AF, Kozak R, Fountain S, Lepsy C. Anal. Chem. 2008;80:9195–9203. doi: 10.1021/ac801339z. [DOI] [PubMed] [Google Scholar]
- 21.Hong S, Pedersen PL. Microbiol. Mol. Biol. Rev. 2008;72:590–641. doi: 10.1128/MMBR.00016-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Skorka G, Shuker P, Gill D, Zabicky J, Parola AH. Biochemistry. 1981;20:3103–3109. doi: 10.1021/bi00514a018. [DOI] [PubMed] [Google Scholar]
- 23.You ZB, Wang B, Zitzman D, Azari S, Wise RA. J. Neurosci. 2007;27:10546–10555. doi: 10.1523/JNEUROSCI.2967-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Parsons LH, Justice JB. J. Neurochem. 1993;61:1611–1619. doi: 10.1111/j.1471-4159.1993.tb09794.x. [DOI] [PubMed] [Google Scholar]
- 25.Westerink BHC, Kwint HF, deVries JB. J. Neurosci. 1996;16:2605–2611. doi: 10.1523/JNEUROSCI.16-08-02605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Redmond JW, Tseng A. J. Chromatogr. 1979;170:479–481. [Google Scholar]
- 27.Novotny M, Alasandro M, Konishi M. Anal. Chem. 1983;55:2375–2377. doi: 10.1021/ac00264a038. [DOI] [PubMed] [Google Scholar]
- 28.Higa S, Suzuki T, Hayashi A, Tsuge I, Yamamura Y. Anal. Biochem. 1977;77:18–24. doi: 10.1016/0003-2697(77)90285-8. [DOI] [PubMed] [Google Scholar]
- 29.Miki K, Sudo A. Clin. Chem. 1998;44:1759–1762. [PubMed] [Google Scholar]
- 30.Guo K, Li L. Anal. Chem. 2009;81:3919–3932. doi: 10.1021/ac900166a. [DOI] [PubMed] [Google Scholar]
- 31.Nestler EJ. Nat. Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 32.Koob GF, Le Moal M. Neurobiology of addiction. Amsterdam, Netherlands: Elsevier; 2006. [Google Scholar]
- 33.Dobi A, Margolis EB, Wang HL, Harvey BK, Morales M. J. Neurosci. 2010;30:218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson SW, North RA. J. Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szabo B, Siemes S, Wallmichrath I. Eur. J. Neurosci. 2002;15:2057–2061. doi: 10.1046/j.1460-9568.2002.02041.x. [DOI] [PubMed] [Google Scholar]
- 36.Tan KR, Brown M, Labouebe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Luscher C. Nature. 2010;463:769–U778. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6 ed. Amsterdam, Netherlands: Elsevier; 2007. [Google Scholar]
- 38.Lipska BK, Weinberger DR. Neuropsychopharmacology. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- 39.Parikh V, Kozak R, Martinez V, Sarter M. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hasselmo ME, Sarter M. Neuropsychopharmacology. 2010;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.