Abstract
In this study, 1R,2R-dicamphanoyl-3,3-dimethydihydropyrano[2,3-c]xanthen-7(1H)-one (DCX) derivatives were designed and synthesized as novel anti-HIV agents against both wild-type and nonnucleoside reverse transcriptase (RT) inhibitor-resistant HIV-1 (RTMDR-1) strains. Twenty-four DCX analogs (6-29) were synthesized and evaluated against the non-drug-resistant HIV-1 NL4-3 strain, and selected analogs were also screened for their ability to inhibit the RTMDR-1 strain. Compared with the control 2-ethyl-3′,4′-di-O-(-)-camphanoyl-2′,2′-dimethyldihydropyrano[2,3-f]chromone (2-EDCP, 2), one of the best anti-HIV coumarin derivatives in our prior study, three DCX compounds (7, 12, and 22) showed better activity against both HIV strains with an EC50 range of 0.062 – 0.081 μM, and five additional compounds (8, 11, 16, 18, and 21) exhibited comparable anti-HIV potency. Six DCX analogs (7, 11-12, 18, and 21-22) also showed enhanced selectivity index (SI) values in comparison to the control. Structure-activity relationship (SAR) information suggested that the extended conjugated system of the pyranoxanthone skeleton facilitates the interaction of the small DCX molecule within the viral binding pocket, consequently leading to enhanced anti-HIV activity and selectivity. Compared to DCP compounds, DCX analogs are a more promising new class of anti-HIV agents.
Keywords: 1R,2R-dicamphanoyl-3,3-dimethydihydropyrano[2,3-c]xanthen-7(1H)-one (DCX); Anti-HIV activity; Structure-activity relationship (SAR)
1. Introduction
AIDS has been epidemic for over 30 years, but still no cure has been identified. Although over 30 formulations are now approved by the US FDA to treat AIDS, drug resistance problems have dramatically reduced the efficacy of current anti-HIV agents. Therefore, research to find new anti-HIV agents with either higher potency or novel mechanism has attracted great attention to overcome this problem.
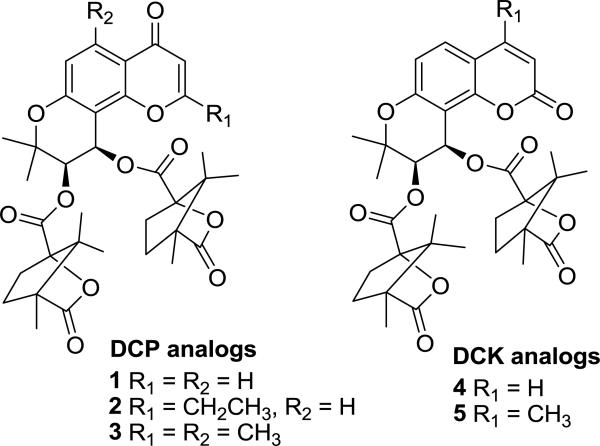
In our prior studies, 3'R,4'R-di-O-(-)-camphanoyl-(+)-cis-khellactones (DCKs) and their positional isomers, 4H-chrom-4-one derivatives (DCPs), showed high potency against wild-type HIV-1 replication (Fig. 1). DCP analogs also showed promising anti-HIV potency against drug-resistant HIV strains. Among previously reported DCP analogs, 2-ethyl DCP (2-EDCP, 2) and 2,5-dimethyl DCP (3) exhibited the best anti-HIV activity against both wild-type and drug-resistant strains with remarkable EC50 values of 0.07 and 0.11 μM for 2 and 0.036 and 0.49 μM for 3 (Fig. 1) [1, 2]. More specifically, preliminary mechanism of action-related studies indicated that a DCK analog (4-methyl DCK, 5) inhibited the activity of HIV-RT through inhibition of DNA-dependent DNA polymerase activity [3], in contrast to currently available non-nucleoside reverse transcriptase inhibitors (NNRTIs) that block HIVRT by inhibiting RNA-dependent DNA polymerization [4, 5]. The mechanistic and structural uniqueness of DCK and DCP analogs opened a new avenue for us to discover more potent, more effective novel anti-HIV drugs for AIDS therapy.
Fig. 1.
Structures of DCP (1–3) and DCK (4–5) analogs.
As previously described, structure-activity relationship (SAR) study and pharmacophore analysis based on DCP suggested that the planar ring system is an important pharmacophore to maintain anti-HIV activity against both wild-type and multi-drug resistant HIV strains [6]. We speculated that adding an aromatic ring onto the 4-pyrone ring of DCPs could produce a more rigid planar system, and that the extended conjugation might sustain or even increase the π-π stacking interaction between the ligand and the target protein.
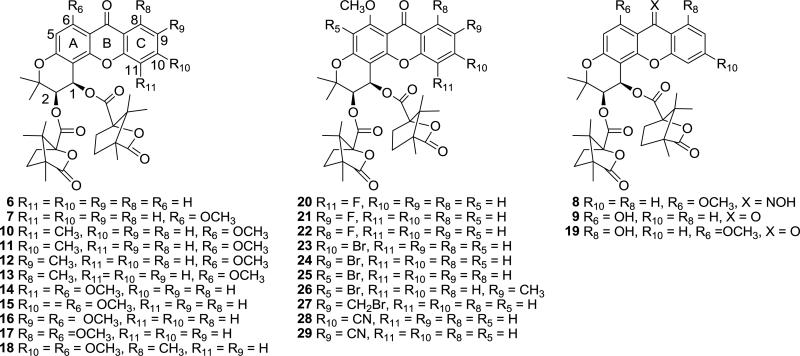
Therefore, in order to further understand the interaction between the compounds’ planar ring and the binding pocket, we designed a series of tri-aryl conjugated compounds, with a xanthen-9-one moiety replacing the chromone ring in the DCP series (Fig. 2). We postulated that this modification could enhance the interaction between the drug molecules and the target proteins, and therefore, improve the anti-HIV activity and selectivity profiles.
Fig. 2.
Structures of novel DCX analogs.
2. Design
In previous studies, introducing appropriate alkyl or O-alkyl groups on the planar ring system dramatically improved the anti-HIV activity. For example, 2-EDCP (2) and 4-methyl DCK (5) exhibited four- and five-fold better anti-HIV activity than the parent DCP (1) and DCK (4), respectively. Thus, in our current study, we first designed DCX analogs with a series of small alkyl and O-alkyl groups substituted on the C ring (R8 – R11) and A ring (R6) to evaluate the effects of alkyl substitutions (Fig. 2, compounds 6, 7, and 10-18). We then introduced other functional moieties, such as halogen, cyano, and hydroxy groups, with different physicochemical properties, as well as prepared a DCX oxime analog, to explore how these functionalities affect the anti-HIV activity (EC50) and selectivity index (SI, CC50 ÷ EC50) (Fig. 2, compounds 8, 9, and 19-29). In addition to its inductive electron withdrawing effect, a cyano group could further expand the conjugation of the xanthenone ring, and perhaps result in potent anti-HIV activity. A hydroxy group could not only increase polarity, but also could be derivatized to obtain water soluble salts.
3. Chemistry
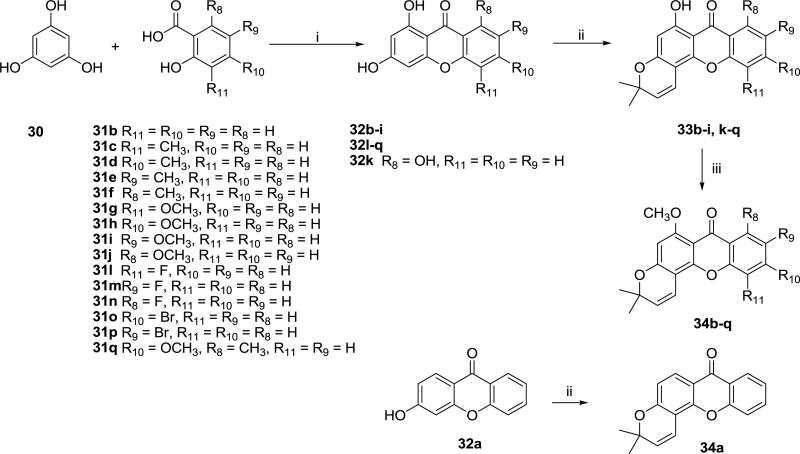
The general synthetic route to obtain pyrano-xanthones is shown in Scheme 1. Hydroxylated xanthones (32b-i, 32k-q) were produced through a cyclization reaction between phloroglucinol (30) and appropriate substituted salicylic acids (31b-j, 31l-q), in the presence of Eaton's reagent (phosphorus pentoxide solution in methanesulfonic acid) as a catalytic condensation agent [7]. A slight excess of phloroglucinol was used to complete the desired reaction and avoid the formation of side products (hydroxychromeno[3,2-b]xanthenediones). The desired products (32b-i, 32k-q) were obtained as brown solids after precipitation in ice-water, and were collected by filtration. They were used in the next reaction step without further purification. 6-Hydroxy-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-ones (33b-i, 33k-q and 34a) were synthesized from the xanthones (32, 32a is commercially available) in anhydrous pyridine by microwave-assisted alkylation and cyclization at 220 °C for 4 h [8]. Methylation of the resulting 6-hydroxy-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-ones (33) with methyl iodide and potassium carbonate yielded 6-methoxy-3,3-dimethylpyranoxanthones (34b-q) [9].
Scheme 1.
Synthesis of compounds 34a-q. Reagents and conditions: (i) Eaton's reagent, reflux; (ii) 4,4-dimethoxy-2-methyl-2-butanol, pyridine, microwave, 220 °C/4h; (iii) MeI, K2CO3, acetone, reflux.
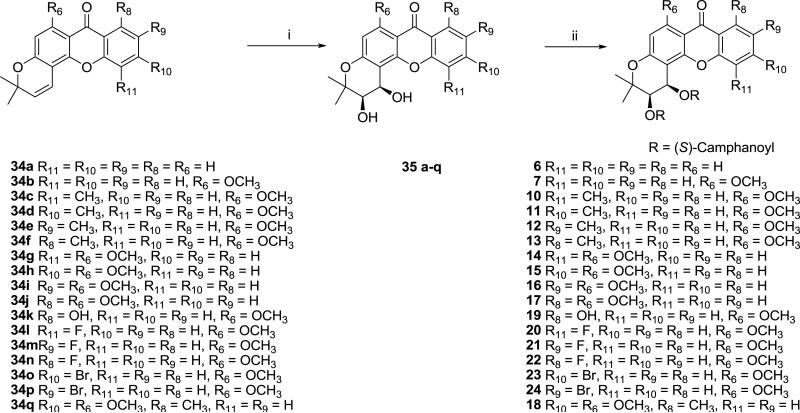
DCX analogs 6, 7, and 10-24 were synthesized from 34 by following the established literature procedures [5] for Sharpless asymmetric dihydroxylation [10. 11], which gave intermediate diols (35), followed by esterification [11, 12] with excess (S)-camphanoyl chloride (Scheme 2).
Scheme 2.
Synthesis of compounds 6, 7, 10–24. Reagents and conditions. (i) K3Fe(CN)6, (DHQ)2PYR, K2OsO2(OH)4, K2CO3, methanesulfonamide, t-butanol/H2O, 0 °C; (ii) (S)-camphanoyl chloride, DMAP, CH2Cl2, rt.
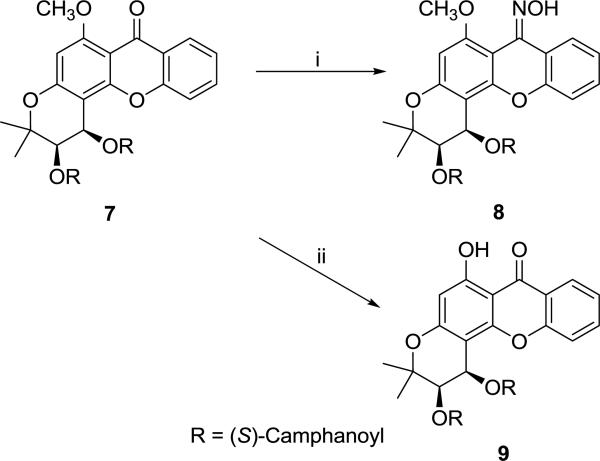
Reaction of 7 with NH2OH·HCl in anhydrous pyridine afforded DCX oxime analog 8. Demethylation of 7 with 48% hydrobromic acid solution yielded 6-hydroxy-DCX (9) in 63% yield [9]. (Scheme 3)
Scheme 3.
Synthesis of compounds 8 and 9. Reagents and conditions: (i) NH2OH·HCl, pyridine, reflux; (ii) HBr/CH3COOH, reflux.
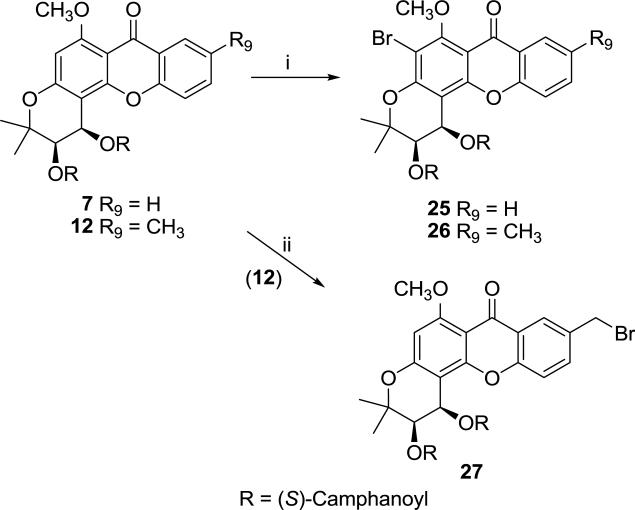
Brominated DCXs (25-27) were synthesized by reaction of 7 and 12 with N-bromosuccinimide (NBS) under appropriate conditions. Bromination of 7 and 12 at the 5-position was accomplished in dichloromethane under microwave conditions to generate 25 and 26, respectively [13], while benzylic bromination of 12 with NBS in anhydrous carbon tetrachloride in the presence of m-chloroperoxybenzoic acid (mCPBA) as a radical initiator led to 27 (Scheme 4) [6].
Scheme 4.
Synthesis of compounds 25–27. Reagents and conditions: (i) NBS, CH2Cl2, microwave 100 °C/2h; (ii) mCPBA, NBS, CCl4, reflux.
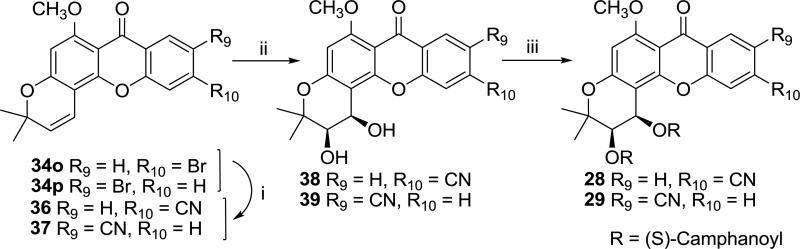
Scheme 5 illustrates the synthetic pathway to 6-methoxy-10-cyano-DCX (28) and 6-methoxy-9-cyano-DCX (29). Compounds 34o and 34p were treated with zinc cyanide in the presence of tetrakis(triphenylphosphine)palladium(0) [Pd(PPh3)4] as a catalyst to afford 36 and 37 [14]. After dihydroxylation and esterification following the existing procedures mentioned above [6-8], compounds 28 and 29 were obtained.
Scheme 5.
Synthesis of compounds 28 and 29. Reagents and conditions: (i) Zn(CN)2, tetrakis (triphenylphosphine)palladium(0), DMF, 160 °C; (ii) K3Fe(CN)6, K2CO3, (DHQ)2PYR, K2OsO2(OH)4, methanesulfonamide, t-butanol/H2O, 0 °C; (iii) (S)-camphanoyl chloride, DMAP, CH2Cl2, rt.
4. Results and discussion
All synthesized DCX analogs were screened against the wild-type HIV-1 NL4-3 strain in a single cycle infection assay using TZM-bl cells. The results are shown in Table 1.
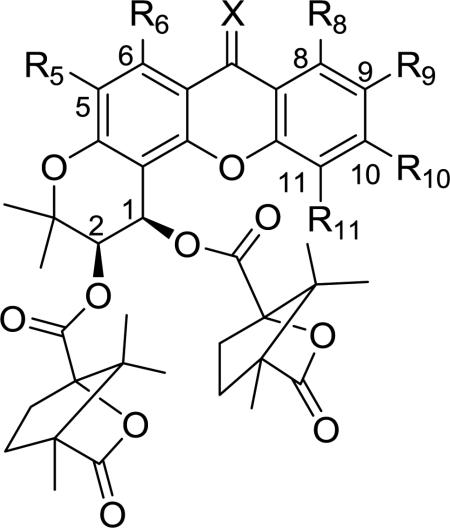
Table 1.
Anti-HIV activity of DCX analogs (6–29) against HIV-1 NL4-3 strain.a
 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Compound | R5 | R6 | R8 | R9 | R10 | R11 | X | CC50bμM | EC50cμM | SId |
| 6 | H | H | H | H | H | H | O | >29.8 | 0.308 | >96.8 |
| 7 | H | OCH3 | H | H | H | H | O | >14.3 | 0.063 | >227.0 |
| 8 | H | OCH3 | H | H | H | H | NOH | >13.9 | 0.12 | >118.2 |
| 9 | H | OH | H | H | H | H | O | N/A | N/A | ---e |
| 10 | H | OCH3 | H | H | H | CH3 | O | 4.32 | 1.52 | 2.84 |
| 11 | H | OCH3 | H | H | CH3 | H | O | >14.0 | 0.095 | >147.4 |
| 12 | H | OCH3 | H | CH3 | H | H | O | 11.4 | 0.065 | 175.4 |
| 13 | H | OCH3 | CH3 | H | H | H | O | 11.6 | 0.15 | 77.3 |
| 14 | H | OCH3 | H | H | H | OCH3 | O | N/A | N/A | --- |
| 15 | H | OCH3 | H | H | OCH3 | H | O | >13.7 | 0.362 | >37.8 |
| 16 | H | OCH3 | H | OCH3 | H | H | O | 10.8 | 0.12 | 88.8 |
| 17 | H | OCH3 | OCH3 | H | H | H | O | 11.6 | 1.70 | 6.83 |
| 18 | H | OCH3 | CH3 | H | OCH3 | H | O | >26.8 | 0.14 | >191.5 |
| 19 | H | OCH3 | OH | H | H | H | O | 8.1 | 0.33 | 24.5 |
| 20 | H | OCH3 | H | H | H | F | O | 7.8 | 0.23 | 33.9 |
| 21 | H | OCH3 | H | F | H | H | O | 26.0 | 0.10 | 260 |
| 22 | H | OCH3 | F | H | H | H | O | >13.9 | 0.062 | >224.2 |
| 23 | H | OCH3 | H | H | Br | H | O | N/A | N/A | --- |
| 24 | H | OCH3 | H | Br | H | H | O | 9.23 | 1.47 | 6.83 |
| 25 | Br | OCH3 | H | H | H | H | O | N/A | N/A | --- |
| 26 | Br | OCH3 | H | CH3 | H | H | O | N/A | N/A | --- |
| 27 | H | OCH3 | H | CH2Br | H | H | O | >25.2 | 4.53 | >5.55 |
| 28 | H | OCH3 | H | H | CN | H | O | 9.08 | 0.20 | 45.4 |
| 29 | H | OCH3 | H | CN | H | H | O | >13.8 | 0.29 | >47.8 |
| 2-EDCP(2) | 12.1 | 0.089 | 136.0 | |||||||
All data presented in this table were averaged from at least three independent experiments.
Cytotoxic activity was determined using a Promega CytoTox-Glo™ assay kit.
This assay was performed in TZM-bl cell infected with HIV-1 NL4-3 strain.
Selectivity index = CC50 ÷ EC50.
No selective anti-HIV activity (CC50 ÷ EC50 < 4)
The structures of 6, 7, and 9 differ only by the substituent at C-6; however, this slight structural differentiation resulted in significant deviation in anti-HIV activity. Analog 7, with a methoxy group at C-6, had an EC50 value of 0.063 μM, and was five-fold more potent than the unsubstituted 6. Analog 7 was also 1.5-fold more potent than 2, the positive control, and had a two-fold higher SI value, suggesting a better selectivity between HIV inhibition and cytotoxic activity. In contrast, compound 9, a6-hydroxy DCX analog, did not show notable activity or selectivity (an average SI value less than 4 was observed). One possible explanation for the lack of anti-HIV potency is that intramolecular H-bonding (HB) between the OH at C-6 and the carbonyl oxygen at C-7 might disrupt HB interaction between this oxygen and the target protein. However, 19, with a hydroxy group at C-8 rather than C-6, exhibited some anti-HIV activity (EC50 0.33 μM) and selectivity (SI 24.5), indicative of unequal HB effects between the carbonyl oxygen and the two similar adjacent OH groups (6-OH versus 8-OH). An unequal molecular environment or point-by-point interactions of the substituents on the drug molecule with the target protein could, in turn, weaken the intramolecular interaction of the 8-OH. Overall, the data suggested that introducing a hydrophobic, rather than hydrophilic, substituent at C-6 is a good strategy to maintain or enhance anti-HIV activity and selectivity, and consequently, all other target compounds have a 6-OMe group.
The DCX oxime analog, 8, exhibited somewhat decreased anti-HIV activity against wild-type HIV strain in comparison with its oxo counterpart 7, indicating that the carbonyl group at C-7 likely interacts with the binding pocket as a HB acceptor, while the NOH group in the oxime analog attenuates such interaction.
Compounds 7, 10, 14, and 20 have different substituents at C-11 (R11). The unsubstituted 7 (R11 = H) exhibited higher potency than 20 (R11 = F), which was more potent than 10 (R11 = CH3) (EC50 0.063, 0.23 and 1.52 μM, respectively). 11-Methoxy-DCX (14) showed no detectable anti-HIV activity or selectivity against wild-type HIV-1 NL4-3 strain. The R11 substituent may influence the orientation of the 1-camphanoyl group, as was previously shown to be significant to maintain high anti-HIV potency in the DCP series [2]. Introducing R11 substitutions also led to increased cytotoxic activity (smaller CC50 value was observed), which consequently led to lower SI values.
In our previous study, the 2- and 3-positions of the chromone system in DCP analogs, displayed equivalent characteristics when substituted with the same functionalities [6]. Therefore, in this study, we compared the introduction of methyl and methoxy substituents at the 9- or 10-position of DCX compounds (analogs to the 2- and 3-position, respectively, in DCP compounds). 9- (12) and 10-Methyl- (11) DCX analogs showed similar EC50 values (0.065 vs 0.095 μM, respectively), with 12 being slightly more potent than 11, while 9-methoxy-DCX (16) was three-fold more potent than 10-methoxy-DCX (15) (EC50 0.12 and 0.362 μM, respectively). Relative to 7, methylation at C-9- or C-10 did not affect anti-HIV potency, but methoxylation at these positions decreased the potency.
The effect of substitution at C-8 on anti-HIV activity was also studied, and the rank order of potency was 8-F (22) > 8-CH3 (13) > 8-OH (19) > 8-OCH3 (17). 8-Fluoro-substiuted DCX (22) exhibited the greatest anti-HIV activity and selectivity, with an EC50 value of 0.062 μM and SI value greater than 224,which were equivalent to the values for 7. Thus, the F atom with a similar size to H led to similar activity. None or a small substituent, such as H or F, is required at C-8 to maintain or enhance anti-HIV activity. Similarly, 9-fluoro-DCX (21) also showed good anti-HIV potency and selectivity.
However, bromination of the A- or C-ring in DCX led to reduced anti-HIV activity. Except for 10-Br-DCX (24), which exhibited only weak anti-HIV efficacy (EC50 1.47 μM), all other compounds (23, 25-27) lacked significant anti-HIV potency.
Introducing a cyano group at C-10 or C-9 resulted in compounds 28 and 29, respectively. Both compounds exhibited considerable anti-HIV activity, with EC50 values of 0.20 and 0.29 μM.
Eight new DCX analogs, which showed significant anti-HIV activity against the wild-type HIV strain, were selected and screened against the drug-resistant RTMDR1 HIV strain. The data are listed in Table 2. Interestingly, all selected DCX analogs (6, 7, 11-13, 16, 21, 22) also showed activity against the drug-resistant strain. Based on both EC50 and SI values, compound 22 was the most active compound followed by 7, 12, and 21. The activity profile from the drug-resistant HIV strain was consistent with that from the wild-type HIV strain.
Table 2.
Anti-HIV activity of DCX analogs against drug-resistant RTMDR1 HIV strain.a
| Compound | CC50 (μM) | EC50b (μM) | SIc |
|---|---|---|---|
| 6 | >29.8 | 0.546 | >54.6 |
| 7 | >14.3 | 0.074 | >193.2 |
| 11 | >14.0 | 0.363 | >38.6 |
| 12 | 11.4 | 0.081 | 140.7 |
| 13 | 11.6 | 0.37 | 31.4 |
| 16 | 10.8 | 0.42 | 25.7 |
| 21 | 26.0 | 0.16 | 162.5 |
| 22 | >13.9 | 0.065 | >213.8 |
| 2 | 12.1 | 0.11 | 110 |
All data presented in this table were averaged from at least three independent experiments.
This assay was performed in TZM-bl cell infected with HIVRTMDR1 strain.
Selectivity index = CC50 ÷ EC50.
In conclusion, the bioassay data generated from this study demonstrated that the new DCX compounds are potent and promising anti-HIV agents, with some analogs being more active than the lead DCP compound 2. The following SAR conclusions were derived from this study.
The planar ring extension from a two-ring conjoined system (pyranochromone in DCP) to a three-ring conjoined system (pyranoxanthone in DCX) retained or even increased the anti-HIV activity against both wild-type and drug-resistant strains, suggesting that a conjugated planar ring system may be essential to interact with the target protein through a π-π ring stacking interaction. The larger DCX molecule with enlarged conjugating planer system perhaps, would interact more efficiently with viral amino acid residues.
Compared to DCPs, selected DCXs, such as 7 and 22, exhibited increased selectivity (SI values), suggesting DCX analogs target the virus-infected cells efficiently and are less cytotoxic to normal cells.
The positioning of the substituents in the molecule dramatically affected the anti-HIV activity against both viral strains. Adding a methyl group at C-11 generated an almost inactive compound (10), while adding the same group at C-9 or C-10 led to two potent anti-HIV compounds (12 and 11, respectively). A methoxy group was essential at C-6 for enhanced anti-HIV potency, and a second methoxy group was more efficacious at C-9 (16) than at C-10 (15), C-8 (17), or C-11 (14). Although the 6-hydroxy-DCX analog (9) did not exhibit anti-HIV activity, the 6-methoxy-8-hydroxy-DCX compound (19) was relatively active with an EC50 value of 0.3 μM.
The properties of the substitutions at the positions also played an important role. As mentioned above, a free OH group at C-6 resulted in an inactive compound (9), while changing the OH to OCH3 at this position led to one of the most active compounds (7). Generally, methyl substituted compounds showed more potent anti-HIV activity than the corresponding methoxy substituted compounds. A cyano group at C-9 or C-10 was well tolerated. An oxime at C-7 lowered the anti-HIV activity, likely by reducing the HB accepting capacity relative to a 7-carbonyl group.
5. Conclusion
Our study indentified a new entity, DCX, and a series of DCX analogs, as potent anti-HIV agents. Most of the synthesized DCX analogs were active against both wild-type and drug-resistant HIV strains. Compared to the control 2-EDCP (2), three compounds (7, 12, and 22) showed better activity against both HIV strains, and another five compounds (8, 11, 16, 18, and 21) exhibited comparable anti-HIV potency against the wild-type HIV strain. The compounds that exhibited high anti-HIV potency also had high SI values. Six analogs (7, 11, 12, 18, 21, 22) showed enhanced SI values in comparison to the control. We also established an anti-HIV SAR study based on both wild-type and drug-resistant HIV strains. Further modification is currently ongoing to further improve the anti-HIV potency and pharmacological profile of this compound type.
6. Experimental section
6.1. Chemistry
The proton nuclear magnetic resonance (1H NMR) spectra were measured on Varian Inova 400 MHz and 300 MHz Varian Gemini 2000 spectrometers using TMS as internal standard. The carbon nuclear magnetic resonance (13C NMR) spectra were measured on Varian Inova 400 MHz Varian Gemini 2000 spectrometer using TMS as internal standard. The solvent used was CDCl3 unless indicated. Microwave reactions were performed with a Biotage initiator EXP US. Mass spectra were measured on Shimadzu LCMS-2010 (ESI-MS). Biotage Flash and Isco Companion systems were used for medium-pressure column chromatography. Commercial chemicals were obtained from Aldrich.
6.1.1. Synthesis of substituted 1,3-dihydroxy-9H-xanthen-9-ones (32)
To a mixture of commercially available phloroglucinol (1.2 equiv) and an appropriate substituted salicylic acid (1 equiv), 20 mL of Eaton's reagent (P2O5-CH3SO3H) was added slowly. The mixture was stirred for 3 h at 80 °C, cooled to rt, and poured onto ice. After vigorous stirring at rt for 2 h, a brown solid was precipitated. The solid was collected by filtration, washed with water (pH ~ 6), and dried at 60 °C. In most instances, this intermediate 1,3-dihydroxy-9H-xanthen-9-one was used without further purification.
6.1.1.1. 1,3-Dihydroxy-9H-xanthen-9-one (32b)
1H NMR δ 8.15 (1H, d, J = 8.1 Hz, H-8), 7.85 (1H, t, J = 8.1, 6.9 Hz, H-6), 7.61 (1H, d, J = 8.1 Hz, H-5), 7.49 (1H, t, J = 8.1, 6.9 Hz, H-7), 6.41 (1H, s, H-4), 6.23 (1H, s, H-2).
6.1.1.2. 1,3-Dihydroxy-7-methyl-9H-xanthen-9-one (32e)
1H NMR δ 12.88 (1H, s, OH-1), 11.03 (1H, s, OH-3), 7.92 (1H, s, H-8), 7.68 (1H, d, J = 8.4 Hz, H-5), 7.52 (1H, d, J = 8.4 Hz, H-6), 6.41 (1H, s, H-4), 6.22 (1H, s, H-2), 2.44 (3H, s, CH3-7).
6.1.1.3. 1,3,8-Trihydroxy-9H-xanthen-9-one (32k)
1H NMR δ 11.86 (1H, s, OH-1), 11.80 (1H, s, OH-8), 7.68 (1H, t, J = 8.4, 8.4 Hz, H-6), 7.01 (1H, d, J = 8.4 Hz, H-5), 6.80 (1H, d, J = 8.4 Hz, H-7), 6.39 (1H, d, J = 2.1 Hz, H-4), 6.23 (1H, d, J = 2.1 Hz, H-2).
6.1.2. Synthesis of 6-hydroxy-3,3-dimethylpyrano[2,3-c]-7(3H)-ones (33)
A mixture of starting compound (32c-i, 32k-q) (1 equiv), 4,4-dimethoxy-2-methyl-2-butanol (1.5-2 equiv) and anhydrous pyridine (2-3 mL) was heated at 220 °C for 4 h under high-absorption microwave conditions. The reaction mixture was cooled to rt, diluted with EtOAc, and washed with aqueous HCl (10%), and brine. The organic layer was separated, and solvent was removed in vacuo. Crude products33b, 33d and 33e were used without further purification in the methoxylation reaction, while the remaining compounds were purified by column chromatography with hexane:EtOAc = 97:3.
6.1.2.1. 6-Hydroxy-3,3,11-trimethylpyrano[2,3-c]xanthen-7(3H)-one (33c)
43.6% yield (starting from 1.0 g of crude 32c); 1H NMR δ 13.22 (1H, s, OH-6), 8.09 (1H, d, J = 7.6 Hz, H-8), 7.54 (1H, d, J = 7.2 Hz, H-10), 7.25 (1H, t, J = 7.6, 7.2 Hz, H-9), 6.83 (1H, d, J = 9.6 Hz, H-1), 6.27 (1H, s, H-5), 5.63 (1H, d, J = 9.6 Hz, H-2), 2.58 (3H, s, CH3-11), 1.49, 1.49 (each 3H, s, CH3-3,3).
6.1.2.2. 6-Hydroxy-3,3,8-trimethylpyrano[2,3-c]xanthen-7(3H)-one (33f)
30.0% Yield (starting from 1.0 g of crude 32f); 1H NMR δ 13.32 (1H, s, OH-6), 7.53 (1H, t, J = 8.0, 8.0 Hz, H-10), 7.30 (1H, d, J = 8.0 Hz, H-9), 7.11 (1H, d, J = 8.0 Hz, H-11), 6.82 (1H, d, J = 9.6 Hz, H-1), 6.24 (1H, s, H-5), 5.50 (1H, d, J = 9.6 Hz, H-2), 2.90 (3H, s, CH3-8), 1.48, 1.48 (each 3H, s, CH3-3,3).
6.1.2.3. 6-Hydroxy-3,3-dimethyl-11-methoxy-pyrano[2,3-c]xanthen-7(3H)-one (33g)
28.0% Yield (starting from 1.5 g of crude 32g); 1H NMR δ 12.93 (1H, s, OH-6), 7.81 (1H, d, J = 8.4 Hz, H-8), 7.29 (1H, d, J = 8.0 Hz, H-10), 7.24 (1H, t, J = 8.4, 8.0 Hz, H-9), 6.92 (1H, d, J = 10.0 Hz, H-1), 6.28 (1H, s, H-5), 5.62 (1H, d, J = 10.0 Hz, H-2), 4.02 (3H, s, OCH3-11), 1.48, 1.48 (each 3H, s, CH3-3,3).
6.1.2.4. 6-Hydroxy-3,3-dimethyl-10-methoxy-pyrano[2,3-c]xanthen-7(3H)-one (33h)
12.8% Yield (starting from 1.0 g of crude 32h); 1H NMR δ 13.07 (1H, s, OH-6), 8.11 (1H, d, J = 9.3 Hz, H-8), 6.92 (1H, d, J = 9.3 Hz, H-9), 6.82 (1H, s, H-11), 6.81 (1H, d, J = 9.9 Hz, H-1), 6.24 (1H, s, H-5), 5.61 (1H, d, J = 9.9 Hz, H-2), 3.93 (3H, s, OCH3-10), 1.48, 1.48 (each 3H, s, CH3-3,3).
6.1.2.5. 6-Hydroxy-3,3-dimethyl-9-methoxy-pyrano[2,3-c]xanthen-7(3H)-one (33i)
42.0% Yield (starting from 2.0 g of crude 32i); 1H NMR δ 12.98 (1H, s, OH-6), 7.61 (1H, d, J = 3.0 Hz, H-8), 7.41 (1H, d, J = 9.3 Hz, H-11), 7.32 (1H, dd, J = 9.3, 3.0 Hz, H-10), 6.84 (1H, d, J = 9.9 Hz, H-1), 6.27 (1H, s, H-5), 5.61 (1H, d, J = 9.9 Hz, H-2), 3.91 (3H, s, OCH3-9), 1.49, 1.49 (each 3H, s, CH3-3,3).
6.1.2.6. 6,8-Dihydroxy-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (33k)
28.0% Yield (starting from 1.0 g of crude 32k); 1H NMR δ 12.07, 11.95 (each 1H, s, OH-6,8), 7.56 (1H, t, J = 8.1, 8.7 Hz, H-10), 6.90 (1H, d, J = 8.1 Hz, H-9), 6.81 (1H, d, J = 10.2 Hz, H-1), 6.79 (1H, d, J = 8.7 Hz, H-11), 6.26 (1H, s, H-5), 5.63 (1H, d, J = 10.2 Hz, H-2), 1.49, 1.49 (each 3H, s, CH3-3,3).
6.1.2.7. 6-Hydroxy-11-fluoro-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (33l)
30.0% Yield (starting from 1.0 g of crude 32l); 1H NMR δ 12.77 (1H, s, OH-6), 8.01 (1H, d, J = 8.4 Hz, H-8), 7.51 (1H, t, J = 8.4, 9.6 Hz, H-9), 7.29 (1H, d, J = 9.6 Hz, H-10), 6.86 (1H, d, J = 10.4 Hz, H-1), 6.30 (1H, s, H-5), 5.63 (1H, d, J = 10.4 Hz, H-2), 1.50, 1.50 (each 3H, s, CH3-3,3).
6.1.2.8. 6-Hydroxy-9-fluoro-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (33m)
20.0% Yield (starting from 1.0 g of crude 32m); 1H NMR δ 12.77 (1H, s, OH-6), 7.90 (1H, dd, J = 8.0, 2.8 Hz, H-10), 7.43-7.46 (2H, m, H-8,11), 6.83 (1H, d, J = 10.0 Hz, H-1), 6.29 (1H, s, H-5), 5.63 (1H, d, J = 10.0 Hz, H-2), 1.49, 1.49 (each 3H, s, CH3-3,3).
6.1.2.9. 6-Hydroxy-8-fluoro-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (33n)
46.0% Yield (starting from 1.0 g of crude 32n); 1H NMR δ 12.94 (1H, s, OH-6), 7.64 (1H, t, J = 8.1, 8.4 Hz, H-10), 7.24 (1H, d, J = 8.4 Hz, H-11), 7.04 (1H, d, J = 8.1 Hz, H-9), 6.78 (1H, d, J = 10.2 Hz, H-1), 6.25 (1H, s, H-5), 5.63 (1H, d, J = 10.2 Hz, H-2), 1.49, 1.49 (each 3H, s, CH3-3,3).
6.1.2.10. 6-Hydroxy-10-bromo-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (33o)
50.0% Yield (starting from 1.8 g of crude 32o); 1H NMR δ 12.79 (1H, s, OH-6), 8.10 (1H, d, J = 8.4 Hz, H-8), 7.67 (1H, s, H-11), 7.50 (1H, d, J = 8.4 Hz, H-9), 6.79 (1H, d, J = 10.0 Hz, H-1), 6.28 (1H, s, H-5), 5.64 (1H, d, J = 10.0 Hz, H-2), 1.49, 1.49 (each 3H, s, CH3-3,3).
6.1.2.11. 6-Hydroxy-9-bromo-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (33p)
20.0% Yield (starting from 1.0 g of crude 32p); 1H NMR δ 12.72 (1H, s, OH-6), 8.24 (1H, d, J = 2.4 Hz, H-8), 7.78 (1H, dd, J = 8.8, 2.4 Hz, H-10), 7.35 (1H, d, J = 8.8 Hz, H-11), 6.80 (1H, d, J = 6.4 Hz, H-1), 6.27 (1H, s, H-5), 5.63 (1H, d, J = 6.4 Hz, H-2), 1.49, 1.49 (each 3H, s, CH3-3,3).
6.1.2.12. 6-Hydroxy-10-methoxy-3,3,8-trimethylpyrano[2,3-c]xanthen-7(3H)-one (33q)
30.0% Yield (starting from 1.0 g of crude 32q); 1H NMR δ 13.44 (1H, s, OH-6), 6.76 (1H, d, J = 10.0 Hz, H-1), 6.67 (1H, s, H-9), 6.62 (1H, s, H-11), 6.18 (1H, s, H-5), 5.56 (1H, d, J = 10.0 Hz, H-2), 3.87 (3H, s, OCH3-10), 2.81 (3H, s, CH3-8), 1.45,1.45 (each 3H, s, CH3-3,3).
6.1.3. Synthesis of 6-methoxy-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-ones (34b-q)
A mixture of compound (33b-i, k-q) (1 equiv) and K2CO3 (3 equiv) in anhydrous acetone (20 mL) was heated to reflux temperature for 3 h, then allowed to cool to rt. Methyl iodide (2-3 equiv) was added at rt and the reaction was kept stirring overnight, monitored by TLC. At completion, the reaction mixture was filtered, and the filtrate was evaporated to dryness. The crude products 34j and 34n were carried directly to the dihydroxylation reaction without purification. The remaining crude products were purified by column chromatography using EtOAc and hexane to give compounds 34b-i, k-m and o-q.
6.1.3.1. 6-Methoxy-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (34b)
95% Yield (starting from 150 mg of 33b); ESI+ (m/z, %), 309 (M+, 100); 1H NMR δ 8.29 (1H, d, J = 8.1 Hz, H-8), 7.62 (1H, t, J = 7.2, 7.8 Hz, H-10), 7.39 (1H, d, J = 7.8 Hz, H-11), 7.32 (1H, t, J = 7.2, 8.1 Hz, H-9), 6.89 (1H, d, J = 9.9 Hz, H-1), 6.31 (1H, s, H-5), 5.62 (1H, d, J = 9.9 Hz, H-2), 3.98 (3H, s, OCH3-6), 1.50, 1.50 (each 3H, s, CH3-3,3).
6.1.3.2. 6-Methoxy-3,3,11-trimethylpyrano[2,3-c]xanthen-7(3H)-one (34c)
78% Yield (starting from 300 mg of 33c); ESI+ (m/z, %), 323 (M++1, 100); 1H NMR δ 8.14 (1H, d, J = 7.6 Hz, H-8), 7.48 (1H, d, J = 7.6 Hz, H-10), 7.22 (1H, t, J = 7.6, 7.6 Hz, H-9), 6.88 (1H, d, J = 9.6 Hz, H-1), 6.31 (1H, s, H-5), 5.62 (1H, d, J = 9.6 Hz, H-2), 3.98 (3H, s, OCH3-6), 2.54 (3H, s, CH3-11), 1.51, 1.51 (each 3H, s, CH3-3,3).
6.1.3.3. 6-Methoxy-3,3,10-trimethylpyrano[2,3-c]xanthen-7(3H)-one (34d)
100% Yield (starting from 200 mg of 33d); ESI+ (m/z, %), 323 (M++1, 100); 1H NMR δ 8.17 (1H, d, J = 8.1 Hz, H-8), 7.20 (1H, s, H-11), 7.14 (1H, d, J = 8.1 Hz, H-9), 6.89 (1H, d, J = 9.9 Hz, H-1), 6.30 (1H, s, H-5), 5.62 (1H, d, J = 9.9 Hz, H-2), 3.97 (3H, s, OCH3-6), 2.48 (3H, s, CH3-10), 1.50, 1.50 (each 3H, s, CH3-3,3).
6.1.3.4. 6-Methoxy-3,3,9-trimethylpyrano[2,3-c]xanthen-7(3H)-one (34e)
86% Yield (starting from 399 mg of 33e); ESI+ (m/z, %), 323 (M++1, 100); 1H NMR δ 8.06 (1H, s, H-8), 7.45 (1H, d, J = 8.4 Hz, H-11), 7.31 (1H, d, J = 8.4 Hz, H-10), 6.90 (1H, d, J = 9.9 Hz, H-1), 6.30 (1H, s, H-5), 5.62 (1H, d, J = 9.9 Hz, H-2), 3.98 (3H, s, OCH3-6), 2.45 (3H, s, CH3-9), 1.50, 1.50 (each 3H, s, CH3-3,3).
6.1.3.5. 6-Methoxy-3,3,8-trimethylpyrano[2,3-c]xanthen-7(3H)-one (34f)
84% Yield (starting from 700 mg of 33f); 1H NMR δ 7.45 (1H, t, J = 8.0, 7.6 Hz, H-10), 7.24 (1H, d, J = 8.0 Hz, H-9), 7.06 (1H, d, J = 7.6 Hz, H-11), 6.87 (1H, d, J = 10.0 Hz, H-1), 6.28 (1H, s, H-5), 5.60 (1H, d, J = 10.0 Hz, H-2), 3.98 (3H, s, OCH3-6), 2.90 (3H, s, CH3-8), 1.50, 1.50 (each 3H, s, CH3-3,3).
6.1.3.6. 6,11-Dimethoxy-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (34g)
40% Yield (starting from 110 mg of 33g); ESI+ (m/z, %), 339 (M++1, 100); 1H NMR δ 7.87 (1H, d, J = 8.0 Hz, H-8), 7.24 (1H, t, J = 8.0, 8.0 Hz, H-9), 7,15 (1H, d, J = 8.0 Hz, H-10), 6.97 (1H, d, J = 10.0 Hz, H-1), 6.32 (1H, s, H-5), 5.63 (1H, d, J = 10.0 Hz, H-2), 4.01, 3.98 (each 3H, s, OCH3-6,11), 1.51, 1.51 (each 3H, s, CH3-3,3).
6.1.3.7. 6,10-Dimethoxy-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (34h)
95% Yield (starting from 110 mg of 33h); 1H NMR δ 8.17 (1H, d, J = 8.1 Hz, H-8), 7.20 (1H, s, H-11), 7.15 (1H, d, J = 8.1 Hz, H-9), 6.89 (1H, d, J = 9.9 Hz, H-1), 6.30 (1H, s, H-5), 5.62 (1H, d, J = 9.9 Hz, H-2), 3.97 (3H, s, OCH3-6), 2.48 (3H, s, OCH3-10), 1.50. 1.50 (each 3H, s, CH3-3,3).
6.1.3.8. 6,9-Dimethoxy-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (34i)
72% Yield (starting from 400 mg of 33i); ESI+ (m/z, %), 377 (M++K, 100); 1H NMR δ 7.66 (1H, d, J = 3.0 Hz, H-8), 7.32 (1H, d, J = 9.0 Hz, H-11), 7.20 (1H, dd, J = 9.0, 3.0 Hz, H-10), 6.86 (1H, d, J = 9.9 Hz, H-1), 6.27 (1H, s, H-5), 5.59 (1H, d, J = 9.9 Hz, H-2), 3.95 (3H, s, OCH3-6), 3.87 (3H, s, OCH3-9), 1.47, 1.47 (each 3H, s, CH3-3,3).
6.1.3.9. 6,8-Dimethoxy-3,3-dimethyl-pyrano[2,3-c]xanthen-7(3H)-one (34j)
80 mg crude product was obtained from 100 mg of 33k.
6.1.3.10. 6-Methoxy-3,3-dimethyl-8-hydroxy-pyrano[2,3-c]xanthen-7(3H)-one (34k)
95% Yield (starting from 100 mg of 33k); 1H NMR δ 13.21 (1H, s, OH-8), 7.49 (1H, t, J = 8.4, 8.0 Hz, H-10), 6.85 (1H, d, J = 9.6 Hz, H-1), 6.83 (1H, d, J = 8.4 Hz, H-9), 6.74 (1H, d, J = 8.0 Hz, H-11), 6.31 (1H, s, H-5), 5.63 (1H, d, J = 9.6 Hz, H-2), 4.03 (3H, s, OCH3-6), 1.51, 1.51 (each 3H, s, CH3-3,3).
6.1.3.11. 6-Methoxy-3,3-dimethyl-11-fluoro-pyrano[2,3-c]xanthen-7(3H)-one (34l)
40% Yield (starting from 303 mg of 33l); 1H NMR δ 8.04 (1H, d, J = 8.4 Hz, H-8), 7.41 (1H, t, J = 8.4, 9.6 Hz, H-9), 7.24 (1H, d, J = 9.6 Hz, H-10), 6.93 (1H, d, J = 10.0 Hz, H-1), 6.33 (1H, s, H-5), 5.64 (1H, d, J = 10.0 Hz, H-2), 3.98 (3H, s, OCH3-6), 1.51, 1.51 (each 3H, s, CH3-3,3).
6.1.3.12. 6-Methoxy-3,3-dimethyl-9-fluoro-pyrano[2,3-c]xanthen-7(3H)-one (34m)
61% Yield (starting from 188 mg of 33m); 1H NMR δ 7.92 (1H, d, J = 8.4 Hz, H-10), 7.27-7.41 (2H, m, H-8,11), 6.87 (1H, d, J = 10.4 Hz, H-1), 6.31 (1H, s, H-5), 5.62 (1H, d, J = 10.4 Hz, H-2), 3.98 (3H, s, OCH3-6), 1.51, 1.51 (each 3H, s, CH3-3,3).
6.1.3.13. 6-Methoxy-10-bromo-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (34o)
95% Yield (starting from 295 mg of 33o); 1H NMR δ 8.13 (1H, d, J = 8.4 Hz, H-8), 7.60 (1H, s, H-11), 7.44 (1H, d, J = 8.4 Hz, H-9), 6.83 (1H, d, J = 10.0 Hz, H-1), 6.31 (1H, s, H-5), 5.63 (1H, d, J = 10.0 Hz, H-2), 3.97 (3H, s, OCH3-6), 1.50, 1.50(each 3H, s, CH3-3,3).
6.1.3.14. 6-Methoxy-9-bromo-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (34p)
72% Yield (starting from 70 mg of 33p); ESI+ (m/z, %), 387 (M+, 100); 1H NMR δ 8.36 (1H, d, J = 2.4 Hz, H-8), 7.69 (1H, dd, J = 8.8, 2.4 Hz, H-10), 7.28 (1H, d, J = 8.8 Hz, H-11), 6.84 (1H, d, J = 10.0 Hz, H-1), 6.30 (1H, s, H-5), 5.61 (1H, d, J = 10.0 Hz, H-2), 3.96 (3H, s, OCH3-6), 1.48, 1.48 (each 3H, s, CH3-3,3).
6.1.3.15. 6,10-Dimethoxy-3,3,8-trimethylpyrano[2,3-c]xanthen-7(3H)-one (34q)
54% Yield (starting from 295 mg of 33q); 1H NMR δ 6.85 (1H, d, J = 10.0 Hz, H-1), 6.67 (1H, s, H-9), 6.63 (1H, s, H-11), 6.28 (1H, s, H-5), 5.59 (1H, d, J = 10.0 Hz, H-2), 3.96 (3H, s, OCH3-6), 3.88 (3H, s, OCH3-10), 2.86 (3H, s, CH3-8), 1.49, 1.49 (each 3H, s, CH3-3,3).
6.1.4. General procedure for the preparation of 35a-q and 38-39
A mixture of K3Fe(CN)6 (3 equiv), K2CO3 (3 equiv), (DHQ)2-PYR (2% equiv), and K2OsO2(OH)4 (2% equiv) was dissolved in t-BuOH/H2O (v/v, 1:1) at rt. The solution was cooled to 0 °C and methanesulfonamide (1 equiv) was added with stirring. After 20 min, substituted pyranochromone (34a-q, and 36-37) was added. The mixture was stirred at 0 °C for 1-2 days, monitored by TLC. At completion, Na2S2O5 (excess), water and CH2Cl2 were added, and stirring was continued for 1 h at rt. The mixture was extracted with CH2Cl2 three times, and the combined organic layer was dried over MgSO4. The solvent was removed under reduced pressure, and the residue was purified by column chromatography with hexanes:EtOAc = 3:7 to afford the pure substituted (+)-cis-3',4'-dihydroxypyranoxanthenones (35a-b, d-f, h-n, p-q). Crude compounds 35c, 35g, 35o, and 38-39 were carried to the next step without purification.
6.1.4.1. (1R,2R)-1,2-Dihydroxy-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (35a)
22% Yield (starting from 70 mg of 34a); ESI+ (m/z, %), 313 (M++1, 100); 1H NMR δ 8.35 (1H, d, J = 7.8 Hz, H-8), 8.20 (1H, d, J = 9.3 Hz, H-6), 7.71 (1H, t, J = 8.1, 8.1 Hz, H-10), 7.49 (1H, d, J = 8.1 Hz, H-11), 7.41 (1H, t, J = 8.1, 7.8 Hz, H-9), 6.89 (1H, d, J = 9.3 Hz, H-5), 5.35 (1H, d, J = 5.4 Hz, H-1), 3.93 (1H, br, H-2), 3.34, 3.10 (each 1H, br, OH-1,2), 1.52, 1.46 (each 3H, s, CH3-3,3).
6.1.4.2. (1R,2R)-1,2-Dihydroxy-6-methoxy-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (35b)
75% Yield (starting from 188 mg of 34b); ESI+ (m/z, %), 343 (M+, 100); 1H NMR δ 8.30 (1H, d, J = 7.8 Hz, H-8), 7.63 (1H, t, J = 6.9, 8.1 Hz, H-10), 7.38 (1H, d, J = 8.1 Hz, H-11), 7.36 (1H, t, J = 7.8, 6.9 Hz, H-9), 6.30 (1H, s, H-5), 5.25 (1H, br, H-1), 3.95 (3H, s, OCH3-6), 3.89 (1H, br, H-2), 3.37, 3.24 (each 1H, br, OH-1,2), 1.52, 1.48 (each 3H, s, CH3-3,3).
6.1.4.3. (1R,2R)-1,2-Dihydroxy-6-methoxy-3,3,10-trimethylpyrano[2,3-c]xanthen-7(3H)-one (35d)
45% Yield (starting from 200 mg of 34d); 1H NMR δ 8.15 (1H, d, J = 8.4 Hz, H-8), 7.14-7.16 (2H, br, H-9,11), 6.27 (1H, s, H-5), 5.23 (1H, br, H-1), 3.94 (3H, s, OCH3-6), 3.88 (1H, s, H-2), 3.55 (H, d, J = 3.6 Hz, OH-1), 3.30 (1H, d, J = 6.9 Hz, OH-2), 2.46 (3H, s, CH3-10), 1.55, 1.49 (each 3H, s, CH3-3,3).
6.1.4.4. (1R,2R)-1,2-Dihydroxy-6-methoxy-3,3,9-trimethylpyrano[2,3-c]xanthen-7(3H)-one (35e)
59% Yield (starting from 250 mg of 34e); 1H NMR δ 7.97 (1H, s, H-8), 7.34 (1H, d, J = 8.4 Hz, H-10), 7.19 (1H, d, J = 8.4 Hz, H-11), 6.20 (1H, s, H-5), 5.20 (1H, t, J = 4.5, 4.5 Hz, H-1), 3.90 (3H, s, OCH3-6), 3.87 (1H, t, J = 4.5, 4.5 Hz, H-2), 3.84 (1H, d, J = 4.5 Hz, OH-1), 3.47 (1H, d, J = 4.5 Hz, OH-2), 2.42 (3H, s, CH3-9), 1.55, 1.48 (each 3H, s, CH3-3,3),
6.1.4.5. (1R,2R)-1,2-Dihydroxy-6-methoxy-3,3,8-trimethylpyrano[2,3-c]xanthen-7(3H)-one (35f)
40% Yield (starting from 180 mg of 34f); ESI+ (m/z, %), 357 (M++1, 100); 1H NMR δ 7.42 (1H, t, J = 8.4, 7.6 Hz, H-10), 7.20 (1H, d, J = 8.4 Hz, H-9), 7.07 (1H, d, J = 7.6 Hz, H-11), 6.26 (1H, s, H-5), 5.22 (1H, d, J = 4.8 Hz, H-1), 3.95 (3H, s, OCH3-6), 3.87 (1H, d, J = 4.8 Hz, H-2), 3.32, 3.22 (each 1H, s, OH-1,2), 2.05 (3H, s, CH3-8), 1.50, 1.46 (each 3H, s, CH3-3,3).
6.1.4.6. (1R,2R)-1,2-Dihydroxy-6,11-dimethoxy-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (35g)
Starting from 35 mg of crude 34g; ESI+ (m/z, %), 339 (M++1, 100); ESI+ (m/z, %), 373 (M++1, 100).
6.1.4.7. (1R,2R)-6,10-Methoxy-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (35h)
30% Yield (starting from 110 mg of 34h); ESI+ (m/z, %), 373 (M++1, 100); 1H NMR δ 8.18 (1H, d, J = 8.7 Hz, H-8), 6.90 (1H, d, J = 8.7 Hz, H-9), 6.71 (1H, s, H-11), 6.26 (1H, s, H-5), 5.20 (1H, br, H-1), 3.92 (3H, s, OCH3-6), 3.90 (3H, s, OCH3-10), 3.50 (1H, br, H-2), 3.30 (2H, br, OH-1,2), 1.55, 1.48 (each 3H, s, CH3-3,3),
6.1.4.8. (1R,2R)-1,2-Dihydroxy-6,9-dimethoxy-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (35i)
40% Yield (starting from 200 mg of 34i); ESI+ (m/z, %), 373 (M++1, 100); 1H NMR δ 7.65 (1H, s, H-8), 7.31 (1H, d, J = 9.0 Hz, H-11), 7.22 (1H, d, J = 9.0 Hz, H-10), 6.27 (1H, s, H-5), 5.23 (1H, br, H-1), 3.94 (3H, s, OCH3-6), 3.90 (3H, s, OCH3-9), 3.35 (1H, br, H-2), 3.23, 3,23 (each 1H, s, OH-1,2), 1.51, 1.46 (each 3H, s, CH3-3,3).
6.1.4.9. (1R,2R)-1,2-Dihydroxy-6,8-dimethoxy-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (35j)
50% Yield (starting from 60 mg of 34j); ESI+ (m/z, %), 373 (M++1, 100); 1H NMR δ 7.51 (1H, t, J = 8.7, 8.4 Hz, H-10), 6.96 (1H, d, J = 8.7 Hz, H-11), 6.79 (1H, d, J = 8.4 Hz, H-9), 6.26 (1H, s, H-5), 5.22 (1H, br, H-1), 3.97, 3.91 (each 3H, s, OCH3-6,8), 3.19 (1H, br, H-2), 1.49, 1.45 (each 3H, s, CH3-3,3).
6.1.4.10. (1R,2R)-1,2-Dihydroxy-6-methoxy-8-hydroxy-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (35k)
45% Yield (starting from 50 mg of 34k); ESI+ (m/z, %), 357 (M++1, 100); 1H NMR δ 13.14 (1H, s, OH-8), 7.52 (1H, t, J = 8.4, 8.4 Hz, H-10), 6.96 (1H, d, J = 8.4 Hz, H-9), 6.79 (1H, d, J = 8.4 Hz, H-11), 6.32 (1H, s, H-5), 5.23 (1H, d, J = 4.8 Hz, H-1), 3.99 (3H, s, OCH3-6), 3.89 (1H, d, J = 4.8 Hz, H-2), 3.11, 3.10 (each 1H, s, OH-1,2), 1.50, 1.47 (each 3H, s, CH3-3,3).
6.1.4.11. (1R,2R)-1,2-Dihydroxy-6-methoxy-11-fluoro-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (35l)
50% Yield (starting from 107 mg of 34l); ESI+ (m/z, %), 361 (M++1, 100); 1H NMR δ 8.06 (1H, d, J = 8.0 Hz, H-8), 7.44 (1H, t, J = 8.0, 9.6 Hz, H-9), 7.30 (1H, d, J = 9.6 Hz, H-10), 6.34 (1H, s, H-5), 5.29 (1H, d, J = 4.4 Hz, H-1), 3.98 (3H, s, OCH3-6), 3.91 (1H, s, OH-1), 3.39 (1H, d, J = 4.4Hz, H-2), 3.21 (1H, s, OH-2), 1.51, 1.46 (each 3H, s, CH3-3,3).
6.1.4.12. (1R,2R)-1,2-Dihydroxy-6-methoxy-9-fluoro-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (35m)
50% Yield (starting from 110 mg of 34m); ESI+ (m/z, %), 361 (M++1, 100); 1H NMR δ 7.92 (1H, d, J = 8.4 Hz, H-10), 7.56 (1H, m, H-8), 7.39 (1H, m, H-11), 6.34 (1H, d, J = 4.0 Hz, H-1), 6.33 (1H, s, H-5), 5.24 (1H, d, J = 4.0 Hz, H-2), 3.97 (2H, s, OCH3-6), 3.60, 3.40 (each 1H, s, OH-1,2), 1.49, 1.47 (each 3H, s, CH3-3,3).
6.1.4.13. (1R,2R)-1,2-Dihydroxy-6-methoxy-8-fluoro-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (35n)
60% Yield (starting from 50 mg of 34n); ESI+ (m/z, %), 361 (M++1, 100); 1H NMR δ 7.56 (1H, d, J = 9.0 Hz, H-11), 7.21 (1H, d, J = 9.0 Hz, H-9), 6.98 (1H, t, J = 9.0, 9.0 Hz, H-10), 6.28 (1H, s, H-5), 5.2 (1H, br, H-1), 3.93 (3H, s, OCH3-6), 3.90 (1H, br, H-2), 3.12, 3.11 (each 1H, s, OH-1,2), 1.50, 1.57 (each 3H, s, CH3-3,3).
6.1.4.14. (1R,2R)-1,2-Dihydroxy-6-methoxy-9-bromo-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (35p)
45% Yield (starting from 50 mg of 34p); ESI+ (m/z, %), 421 (M+, 100); 1H NMR δ 8.34 (1H, d, J = 1.6 Hz, H-8), 7.69 (1H, dd, J = 8.8, 1.6 Hz, H-10), 7.25 (1H, d, J = 8.8 Hz, H-11), 6.27 (1H, s, H-5), 5.20 (1H, d, J = 4.4 Hz, H-1), 3.92 (3H, s, OCH3-6), 3.86 (1H, d, J = 4.4 Hz, H-2), 3.20, 3.12 (each 1H, s, OH-1,2), 1.49, 1.45 (each 3H, s, CH3-3,3).
6.1.4.15. (1R,2R)-1,2-Dihydroxy-6,10-dimethoxy-3,3,8-trimethylpyrano[2,3-c]xanthen-7(3H)-one (35q)
50% Yield (starting from 50 mg of 34q); 1H NMR δ 6.67 (1H, s, H-9), 6.67 (1H, s, H-11), 6.28 (1H, s, H-5), 5.23 (1H, d, J = 4.4 Hz, H-1), 4.10 (1H, d, J = 4.4 Hz, H-2), 3.93 (3H, s, OCH3-6), 3.89 (3H, s, OCH3-10), 3.13, 3.11 (each 1H, s, OH-1,2), 2.87 (3H, s, CH3-8), 1.49, 1.46 (each 3H, s, CH3-3,3).
6.1.5. 6-Methoxy-10-cyano-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (36)
A mixture of 34o (10 mg, 0.025 mmoL), zinc cyanide (3.5 mg, 0.03 mmoL) and Pd(PPh3)4 (12 mg, 0.011 mmoL) was dissolved in anhydrous DMF (0.5 mL) and heated to 130 °C for 16 h, monitored by TLC. At completion, the reaction mixture was filtered through Celite, and the crude product was purified by PTLC with EtOAc:hexane = 1:3 to afford pure 36 as yellow solid. 90% yield; MS-ESI+ (m/z, %) 334 (M+ + 1, 100); 1H NMR δ 8.35 (1H, d, J = 8.0 Hz, H-8), 7.72 (1H, s, H-11), 7.55 (1H, d, J = 8.0 Hz, H-9), 6.82 (1H, d, J = 10.0 Hz, H-1), 6.32 (1H, s, H-5), 5.64 (1H, d, J = 10.0 Hz, H-2), 3.96 (3H, s, OCH3-6), 1.57, 1.49 (each 3H, s, CH3-2,2).
6.1.6. 6-Methoxy-9-cyano-3,3-dimethylpyrano[2,3-c]xanthen-7(3H)-one (37)
The procedure was identical to that used for the preparation of 36. The crude 37 was carried to the next step without purification. MS-ESI+ (m/z, %) 334 (M+ + 1, 100).
6.1.7. General procedure for the preparation of 6-7, 10-24, 18 and 28-29
The substituted 1R,2R-dihydroxypyranoxanthone (35a-q, 38-39) (1 equiv), (S)-(-)-camphanoyl chloride (3 equiv), and DMAP (4 equiv) were stirred in CH2Cl2 for 1-2 h at rt, monitored by TLC. At completion, the mixture was diluted with CH2Cl2 and washed separately with water and brine. The solvent was then removed under reduced pressure and the residue was purified by PTLC with hexanes:EtOAc = 3:2 to afford the appropriately substituted 1R,2R-di-O-(-)-camphanoyl-3,3-dimethyldihydroprano[2,3-c]xanthone (6-7, 10-24, and 28-29).
6.1.7.1. 1R,2R-(-)-Dicamphanoyl-3,3-dimethyl-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (6)
85% Yield (starting from 15 mg of 35a); white solid; mp 146-148 °C; 1H NMR δ 8.33 (1H, d, J = 7.8 Hz, H-8), 8.31 (1H, d, J = 9.0 Hz, H-6), 7.67 (1H, t, J = 7.2, 8.4 Hz, H-10), 7.39 (1H, t, J = 7.2, 7.8 Hz, H-9), 7.35 (1H, d, J = 8.4 Hz, H-11), 6.94 (1H, d, J = 9.0 Hz, H-5), 6.93 (1H, d, J = 4.5 Hz, H-1), 5.43 (1H, d, J = 4.5 Hz, H-2), 2.52, 2.322 1.90, 1.75 (each 2H, m, camphanoyl-CH2), 1.57, 1.50 (each 3H, s, CH3-3,3), 1.14, 1.12, 1.03, 1.00, 0.93, 0.89 (each 3H, s, camphanoyl-CH3); [α]D -22.7° (c = 0.0015, CH3Cl); HRMS for (M+ + Na): calcd m/z 695.2469, found: 695.2485.
6.1.7.2. 1R,2R-(-)-Dicamphanoyl-3,3-dimethyl-6-methoxy-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (7)
88% Yield (starting from 52 mg of 35b); white solid; mp 134-135 °C; 1H NMR δ 8.40 (1H, d, J = 7.8 Hz, H-8), 7.72 (1H, t, J = 7.5, 8.1 Hz, H-10), 7.46 (1H, t, J = 7.8, 7.5 Hz, H-9), 7.39 (1H, d, J = 8.1 Hz, H-11), 6.98 (1H, d, J = 4.5 Hz, H-1), 6.46 (1H, s, H-5), 5.53 (1H, d, J = 4.5 Hz, H-2), 4.12 (3H, s, OCH3-6), 2.60, 2.33, 2.10, 1.80 (each 2H, m, camphanoyl-CH2), 1.69, 1.61 (each 3H, s, CH3-3,3), 1.25, 1.25, 1.15, 1.12, 1.02, 1.01 (each 3H, s, camphanoyl-CH3); [α]D -40.9° (c = 0.0069, CH3Cl); HRMS for (M+ + Na): calcd m/z 725.2574, found: 725.2603; 13C NMR: δ 9.78, 9.92, 16.74, 16.85, 16.93, 17.01, 21.53, 27.12, 29.20, 29.18, 31.00, 31.54, 54.59, 54.94, 55.15, 55.20, 61.57, 72.86, 72.82, 90.95, 91.49, 96.01, 96.06, 98.66, 107.82, 117.24, 123.20, 124.76, 127.06, 134.20, 154.47, 157.60, 159.07, 163.39, 167.17, 167.65, 175.13, 178.11, 178.18.
6.1.7.3. 1R,2R-(-)-Dicamphanoyl-3,3,11-trimethyl-6-methoxy-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (10)
85% Yield (starting from 41 mg of 35c); white solid; mp 174-175 °C; 1H NMR δ 8.12 (1H, d, J = 7.6 Hz, H-8), 7.36 (1H, d, J = 7.2 Hz, H-10), 7.24 (1H, t, J = 7.6, 7.2 Hz, H-9), 6.87 (1H, d, J = 4.4 Hz, H-1), 6.34 (1H, s, H-5), 5.44 (1H, d, J = 4.4 Hz, H-2), 4.01 (3H, s, OCH3-6), 2.45, 2.25, 1.90, 1.67 (each 2H, m, camphanoyl-CH2), 2.39 (3H, s, CH3-11), 1.60, 1.49 (each 3H, s, CH3-3,3), 1.14, 1.14, 1.03, 1.00, 0.93, 0.89 (each 3H, s, camphanoyl-CH3); [α]D -40.9 ° (c = 0.0069, CH3Cl); [α]D -42.5° (c = 0.0051, CH3Cl); HRMS for (M+ + Na): calcd m/z 739.2731, found: 739.2736.
6.1.7.4. 1R,2R-(-)-Dicamphanoyl-3,3,10-trimethyl-6-methoxy-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (11)
80% Yield (starting from 50 mg of 35d); white solid; mp 174-175 °C; 1H NMR δ 8.15 (1H, d, J = 8.1 Hz, H-8), 7.13 (1H, d, J = 8.1 Hz, H-9), 7.07 (1H, s, H-11), 6.87 (1H, d, J = 4.8 Hz, H-1), 6.32 (1H, s, H-5), 5.39 (1H, d, J = 4.8 Hz, H-2), 3.99 (3H, s, OCH3-6), 2.41 (3H, s, CH3-10), 2.45, 2.20, 1.95, 1.80 (each 2H, m, camphanoyl-CH2), 1.56, 1.49 (each 3H, s, CH3-3,3), 1.14, 1.13, 1.04, 1.00, 0.90, 0.87 (each 3H, s, camphanoyl-CH3); [α]D -37.2° (c = 0.0018, CH3Cl); HRMS for (M+ + Na): calcd m/z 739.2731, found: 739.2724.
6.1.7.5. 1R,2R-(-)-Dicamphanoyl-3,3,9-trimethyl-6-methoxy-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (12)
77% Yield (starting from 100 mg of 35e); light yellow solid; mp 179-180 °C; 1H NMR δ 8.05 (1H, s, H-8), 7.41 (1H, d, J = 8.7 Hz, H-11), 7.18 (1H, d, J = 8.7 Hz, H-10), 6.86 (1H, d, J = 4.5 Hz, H-1), 6.32 (1H, s, H-5), 5.40 (1H, d, J = 4.5 Hz, H-2), 4.00 (3H, s, OCH3-6), 2.44 (3H, s, CH3-9), 2.45, 2.20, 1.90, 1.70 (each 2H, m, camphanoyl-CH2), 1.57, 1.49 (each 3H, s, CH3-3,3), 1.14, 1.13, 1.03, 1.00, 0.90, 0.88 (each 3H, s, camphanoyl-CH3); [α]D -34.6° (c = 0.0028, CH3Cl); HRMS for (M+ + Na): calcd m/z 739.2731, found: 739.2724.
6.1.7.6. 1R,2R-(-)-Dicamphanoyl-3,3,8-trimethyl-6-methoxy-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (13)
75% Yield (starting from 70 mg of 35f); white solid; mp 240-242 °C; 1H NMR δ 7.39 (1H, t, J = 8.4, 7.6 Hz, H-10), 7.09 (1H, d, J = 8.4 Hz, H-9), 7.06 (1H, d, J = 7.6 Hz, H-11), 6.82 (1H, d, J = 4.8 Hz, H-1), 6.29 (1H, s, H-5), 5.37 (1H, d, J = 4.8 Hz, H-2), 3.98 (3H, s, OCH3-6), 2,86 (3H, s, CH3-8), 2.46, 2.18, 1.90, 1.65 (each 2H, m, camphanoyl CH2), 1.56, 1.55 (each 3H, s, CH3-3,3), 1.11, 1.10, 1.01, 1.00, 0.88, 0.86 (each 3H, s, camphanoyl CH3); [α]D -29.3° (c = 0.003, CH3Cl); HRMS for (M+ + Na): calcd m/z 739.2731, found: 739.2713.
6.1.7.7. 1R,2R-(-)-Dicamphanoyl-3,3-dimethyl-6,11-dimethoxy-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (14)
53% Yield (starting from 25 mg of 35g); white solid; mp 179-180 °C; 1H NMR δ 7.82 (1H, d, J = 8.0 Hz, H-8), 7.25 (1H, t, J = 8.0, 8.0 Hz, H-9), 7.09 (1H, d, J = 8.0 Hz, H-10), 6.82 (1H, d, J = 4.4 Hz, H-1), 6.33 (1H, s, H-5), 5.44 (1H, d, J = 4.4 Hz, H-2), 4.00, 3.91 (each 3H, s, OCH3-6,11), 2.44, 2.27, 1.95, 1,70 (each 2H, m, camphanoyl-CH2), 1.57, 1.47 (each 3H, s, CH3-3,3), 1.13, 1.13, 1.03, 1.01, 0.91, 0.87 (each 3H, s, camphanoyl-CH3); [α]D -40.8° (c = 0.0013, CH3Cl); HRMS for (M+ + Na): calcd m/z 755.2680, found: 755.2660.
6.1.7.8. 1R,2R-(-)-Dicamphanoyl-3,3-dimethyl-6,10-dimethoxy-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (15)
43% Yield (starting from 10 mg of 35h); white solid; mp 177-178 °C; 1H NMR δ 8.12 (1H, d, J = 8.7 Hz, H-8), 6.84 (1H, dd, J = 8.7, 2.4 Hz, H-9), 6.79 (1H, d, J = 4.5 Hz, H-1), 6.59 (1H, d, J = 2.4 Hz, H-11), 6.26 (1H, s, H-5), 5.33 (1H, d, J = 4.5 Hz, H-2), 3.92 (3H, s, OCH3-6), 3.77 (3H, s, OCH3-10), 2.40, 2.15, 1.95, 1.65 (each 2H, m, camphanoyl-CH2), 1.50, 1.42 (each 3H, s, CH3-3,3), 1.07, 1.06, 0.97, 0.93, 0.85, 0.81 (each 3H, s, camphanoyl-CH3); [α]D -16.2° (c = 0.0052, CH3Cl); HRMS for (M+ + 1): calcd m/z 733.2860, found: 733.2874.
6.1.7.9. 1R,2R-(-)-Dicamphanoyl-3,3-dimethyl-6,9-dimethoxy-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (16)
60% Yield (starting from 20 mg of 35i); white solid; mp 168-170 °C; ESI+ (m/z, %), 732 (M+, 100); 1H NMR δ 7.66 (1H, s, H-8), 7.21, 7.21 (each 1H, d, H = 9.2 Hz, H-10,11), 6.85 (1H, d, J = 4.8 Hz, H-1), 6.32 (1H, s, H-5), 5.40 (1H, d, J = 4.8 Hz, H-2), 4.00 (3H, s, OCH3-6), 3.88 (3H, s, OCH3-9), 2.5, 2.20, 1.90, 1.70 (each 2H, m, camphanoyl-CH2), 1.57, 1.49 (each 3H, s, CH3-3,3), 1.14, 1.13, 1.03, 1.00, 0.90, 0.88 (each 3H, s, camphanoyl-CH3); [α]D -35.3° (c = 0.0024, CHCl3); HRMS for (M+ + 1): calcd m/z 733.2860, found: 733.2864.
6.1.7.10. 1R,2R-(-)-Dicamphanoyl-3,3-dimethyl-6,8-dimethoxy-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (17)
52% Yield (starting from 10 mg of 35j); white solid; mp 155-156 °C; 1H NMR δ 7.44 (1H, t, J = 8.4, 8.4 Hz, H-10), 6.82 (1H, d, J = 8.4 Hz, H-11), 6.80 (1H, d, J = 4.4 Hz, H-1), 6.75 (1H, d, J = 8.4 Hz, H-9), 6.27 (1H, s, H-5), 5.36 (1H, d, J = 4.4 Hz, H-2), 3.94, 3.93 (each 3H, s, OCH3-6.8), 2.50, 2.20, 1.90, 1.70 (each 2H, camphanoyl-CH2), 1.54, 1.46 (each 3H, s, CH3-3,3), 1.13, 1.11, 1.03, 1.00, 0.88, 0.85 (each 3H, s, camphanoyl-CH3); [α]D -131.7° (c = 0.003, CHCl3); HRMS for (M+ + Na): calcd m/z 755.2680, found: 755.2671.
6.1.7.11. 1R,2R-(-)-Dicamphanoyl-3,3,8-trimethyl-6,10-dimethoxy-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (18)
52% Yield (starting from 10 mg of 35q); white solid; mp 183-184 °C; 1H NMR δ 6.85 (1H, d, J = 4.8 Hz, H-1), 6.64 (1H, d, J = 2.4 Hz, H-9), 6.53 (1H, d, J = 2.4 Hz, H-11), 6.30 (1H, s, H-5), 5.39 (1H, d, J = 4.8 Hz, H-2), 3.99 (3H, s, OCH3-6), 3.81 (3H, s, OCH3-10), 2.84 (3H, s, CH3-8), 2.45, 2.20, 1.90, 1.70 (each 2H, m, camphanoyl CH2), 1.56, 1.48 (each 3H, s, CH3-3,3), 1.13, 1.13, 1.04, 1.00, 0.91, 0.88 (each 2H, s, camphanoyl CH3); [α]D -23.6° (c = 0.0012, CHCl3); HRMS for (M+ + Na): calcd m/z 769.2836, found: 769.2835.
6.1.7.12. 1R,2R-(-)-Dicamphanoyl-3,3-dimethyl-6-methoxy-8-hydroxy-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (19)
30% Yield (starting from 10 mg of 35k); white solid; mp 155-157 °C; 1H NMR δ 12.94 (1H, s, OH-8), 7.43 (1H, t, J = 8.0, 8.4 Hz, H-10), 6.78 (1H, d, J = 4.8 Hz, H-1), 6.74 (1H, d, J = 8.0 Hz, H-9), 6.67 (1H, d, J = 8.4 Hz, H-11), 6.31 (1H, s, H-5), 5.36 (1H, d, J= 4.8 Hz, H-2), 3.99 (3H, s, OCH3-6), 2,45, 2.18, 1.90, 1.65 (each 2H, m, camphanoyl-CH2), 1.52, 1.45 (each 3H, s, CH3-3,3), 1.10, 1.09, 1.00, 0.96, 0.91, 0.88 (each 2H, s, camphanoyl-CH3); [α]D -62.5° (c = 0.0018, CHCl3); HRMS for (M+ + Na): calcd m/z 741.2523, found: 741.2528.
6.1.7.13. 1R,2R-(-)-Dicamphanoyl-3,3-trimethyl-6-methoxy-11-fluoro-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (20)
80% Yield (starting from 45 mg of 35l); white solid; mp 183-184 °C; 1H NMR δ 8.05 (1H, d, J = 8.0 Hz, H-8), 7.39 (1H, t, J = 8.0, 8.4 Hz, H-9), 7.28 (1H, d, J = 8.4 Hz, H-10), 6.76 (1H, d, J = 4.4 Hz, H-1), 6.36 (1H, s, H-5), 5.43 (1H, d, J = 4.4 Hz, H-2), 4.01 (3H, s, OCH3-6), 2.51, 2.20, 1.92, 1.75 (each 2H, m, camphanoyl-CH2), 1.58, 1.48 (each 3H, s, CH3-3,3), 1.13, 1.13, 1.06, 1.04, 1.00, 0.99 (each 3H, s, camphanoyl-CH3); [α]D -41.2° (c = 0.0052, CHCl3); HRMS for (M+ + Na): calcd m/z 743.2480, found: 743.2483.
6.1.7.14. 1R,2R-(-)-Dicamphanoyl-3,3-trimethyl-6-methoxy-9-fluoro-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (21)
48% Yield (starting from 45 mg of 35m); white solid; mp 204-205 °C; 1H NMR δ 7.92 (1H, d, J = 8.0 Hz, H-10), 7.28-7.36 (2H, m, H-9,11), 6.85 (1H, d, J = 4.4 Hz, H-1), 6.35 (1H, s, H-5), 5.40 (1H, d, J = 4.4 Hz, H-2), 4.00 (3H, s, OCH3-6), 2.49, 2.19, 1.90, 1.68 (each 2H, m, camphanoyl-CH2), 1.56, 1.49 (each 3H, s, CH3-3,3), 1.14, 1.13, 1.04, 1.00, 0.90, 0.90 (each 3H, s, camphanoyl-CH3); [α]D -23.6° (c = 0.0025, CHCl3); HRMS for (M+ + Na): calcd m/z 743.2480, found: 743.2469.
6.1.7.15. 1R,2R-(-)-Dicamphanoyl-3,3-trimethyl-6-methoxy-8-fluoro-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (22)
80% Yield (starting from 5 mg of 35n); white solid; mp 148-150 °C; 1H NMR δ 7.44 (1H, t, J = 8.7, 8.4 Hz, H-10), 7.01 (1H, d, J = 8.4 Hz, H-11), 6.92 (1H, d, J = 8.7 Hz, H-9), 6.76 (1H, d, J = 4.5 Hz, H-1), 6.26 (1H, s, H-5), 5.32 (1H, d, J = 4.5 Hz, H-2), 3.91 (3H, s, OCH3-6), 2.40, 2.12, 1.91, 1.67 (each 2H, m, camphanoyl-CH2), 1.51, 1.42 (each 3H, s, CH3-3,3), 1.07, 1.05, 0.97, 0.93, 0.83, 0.83 (each 3H, s, camphanoyl-CH3); [α]D -17.1° (c = 0.0035, CHCl3), HRMS for (M+ + Na): calcd m/z 743.2480, found: 743.2476.
6.1.7.16. 1R,2R-(-)-Dicamphanoyl-3,3-dimethyl-6-methoxy-10-bromo-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (23)
52% Yield (starting from 35 mg of 35o); white solid; mp 159-160 °C; 1H NMR δ 8.11 (1H, d, J = 8.4 Hz, H-8), 7.48 (1H, d, J = 1.6 Hz, H-11), 7.44 (1H, dd, J = 8.4 Hz, H-9), 6.80 (1H, d, J = 4.8 Hz, H-1), 6.32 (1H, s, H-5), 5.46 (1H, d, J = 4.8 Hz, H-2), 3.97 (3H, s, OCH3-6), 2.45, 2.08, 1.90, 1.70 (each 2H, m, camphanoyl CH2), 1.61, 1.61 (each 3H, s, CH3-3,3), 1.46, 1.14, 1.12, 1.05, 1.04, 1.02 (each 3H, s, camphanoyl CH3); [α]D -25.3° (c = 0.0035, CHCl3); HRMS for (M+ + Na): calcd m/z 803.1679, found: 803.1691.
6.1.7.17. 1R,2R-(-)-Dicamphanoyl-3,3-dimethyl-9-bromo-6-methoxy-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (24)
50% Yield (starting from 10 mg of 35p); white solid; mp 159-160 °C; 1H NMR δ 8.35 (1H, d, J = 2.8 Hz, H-8), 7.67 (1H, dd, J = 8.8, 2.8 Hz, H-10), 7.20 (1H, d, J = 8.8 Hz, H-11), 6.81 (1H, d, J = 4.4 Hz, H-1), 6.32 (1H, s, H-5), 5.47 (1H, d, J = 4.4 Hz, H-2), 3.98 (3H, s, OCH3-6), 2.40, 2,10, 1.90, 1.60 (each 2H, m, camphanoyl-CH2), 1.56, 1.46 (each 3H, s, CH3-3,3), 1.14, 1.12, 1.03, 1.02, 1.01, 0.77 (each 3H, s, camphanoyl-CH3); [α]D -13.0° (c = 0.004, CHCl3); HRMS for (M+ + Na): calcd m/z 803.1679, found: 803.1684.
6.1.7.18. 1R,2R-(-)-Dicamphanoyl-3,3-dimethyl-6-methoxy-10-cyano-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (28)
80% Yield (starting from 36 mg of 36); white solid; mp 164-165 °C; 1H NMR δ 8.36 (1H, d, J = 8.0 Hz, H-8), 7.60 (1H, d, J = 8.0 Hz, H-9), 7.55 (1H, s, H-11), 6.78 (1H, d, J = 4.4 Hz, H-1), 6.35 (1H, s, H-5), 5.39 (1H, d, J = 4.4 Hz, H-2), 3.99 (3H, s, OCH3-6), 2.50, 2.20, 1.90, 1.70 (each 2H, m, camphanoyl-CH2), 1.56, 1.47 (each 3H, s, CH3-3,3), 1.12, 1.11, 1.05, 1.02, 0.98, 0.95 (each 3H, s, camphanoyl-CH3); [α]D -30.0° (c = 0.0012, CHCl3); HRMS for (M+ + Na): calcd m/z 750.2527, found: 750.2523.
6.1.7.19. 1R,2R-(-)-Dicamphanoyl-3,3-dimethyl-6-methoxy-9-cyano-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (29)
65% Yield (starting from 30 mg of 37); white solid; mp 165-167 °C; 1H NMR δ 8.59 (1H, s, H-8), 7.84 (1H, d, J = 8.8 Hz, H-10), 7.37 (1H, d, J = 8.8 Hz, H-11), 6.84 (1H, d, J = 4.8 Hz, H-1), 6.39 (1H, s, H-5), 5.40 (1H, d, J = 4.8 Hz, H-2), 4.02 (3H, s, OCH3-6), 2.50, 2.10, 1.90, 1.70 (each 2H, m, camphanoyl-CH2), 1.54, 1.50 (each 3H, s, CH3-3,3), 1.14, 1.12, 1.05, 1.00, 0.93, 0.92 (each 3H, s, camphanoyl-CH3); [α]D –51.0° (c = 0.0023, CH2Cl2); HRMS for (M+ + Na): calcd m/z 750.2527, found: 750.2526.
6.1.8. 1R,2R-(-)-Dicamphanoyl-3,3-dimethyl-6-methoxy-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one oxime (8)
Compound 7 (32 mg, 0.045 mmol) and NH2OH HCl (5 mg, 0.07 mmol) were dissolved in anhydrous pyridine (1 mL) and stirred at 100 °C, monitored by TLC. At completion, the reaction mixture was poured into water and extracted with EtOAc. The organic layer was collected and the crude product was purified by PTLC with hexane:EtOAc = 1:1 to yield 8 as a white solid. 80% yield; mp 160-162 °C; 1H NMR δ 8.99 (1H, d, J = 8.4 Hz, H-8), 7.41 (1H, t, J = 7.2, 8.4 Hz, H-10), 7.21 (1H, t, J = 7.2, 8.4 Hz, H-9), 7.15 (1H, d, J = 8.4 Hz, H-11), 6.76 (1H, d, J = 4.8 Hz, H-1), 6.33 (1H, s, H-5), 5.35 (1H, d, J = 4.8 Hz, H-2), 3.98 (3H, s, OCH3-6), 2.50, 2.20, 1.90, 1.70 (each 2H, m, camphanoyl-CH2), 1.52, 1.45 (each 3H, s, CH3-3,3), 1.13, 1.12, 1.03, 0.99, 0.91, 0.89 (each 3H, s, camphanoyl-CH3); [α]D –141.3° (c = 0.0023, CH2Cl2); HRMS for (M+ + Na): calcd m/z 740.2683, found: 740.2714.
6.1.9. 1R,2R-(-)-Dicamphanoyl-3,3-dimethyl-6-hydroxy-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (9)
Compound 7 (20 mg, 0.028 mmoL) was heated with 48% HBr (2.0 mL) at reflux temperature for 12 h. The reaction mixture was allowed to cool, diluted with water, and filtered and then the residue was washed thoroughly with water. The crude product was purified by PTLC with hexane:EtOAc = 1:1 to yield 9 as a white solid. 63% yield; mp 165-167 °C; 1H NMR δ 13.67 (1H, s, OH-6), 8.25 (1H, d, J = 7.2 Hz, H-8), 7.74 (1H, d, J = 8.8, 8.4 Hz, H-10), 7.46 (1H, d, J = 8.4 Hz, H-11), 7.41 (1H, d, J = 8.8, 7.2 Hz, H-10), 6.63 (1H, d, J = 4.8 Hz, H-1), 6.44 (1H, s, H-5), 5.36 (1H, d, J = 4.8 Hz, H-2), 2.50, 2.20, 1.90, 1.70 (each 2H, m, camphanoyl CH2), 1.50, 1.47 (each 3H, s, CH3-3,3), 1.17, 1.11, 1.08, 1.07, 0.98, 0.97 (each 3H, s, camphanoyl CH3); [α]D –70.0° (c = 0.0023, CH2Cl2); HRMS for (M+ + NH4): calcd m/z 706.2864, found: 706.2896.
6.1.10. 1R,2R-(-)-Dicamphanoyl-3,3-dimethyl-5-bromo-6-methoxy-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (25)
A mixture of 7 (50 mg, 0.071 mmoL), NBS (17.0 mg, 0.10 mmol) and CH2Cl2 (2 mL) was heated to 100 °C for 2 h under high-absorption microwave conditions. At completion, the mixture was concentrated and purified by PTLC with hexane:EtOAc = 3:2 to afford pure 25 as a white solid (43.7 mg). 78.9% yield; mp 139-140 °C; 1H NMR δ 8.29 (1H, d, J = 8.0 Hz. H-8), 7.65 (1H, t, J = 7.6, 7.6 Hz, H-10), 7.39 (1H, t, J = 8.0, 7.6 Hz, H-9), 7.29 (1H, d, J = 7.6 Hz, H-11), 6.90 (1H, d, J = 4.8 Hz, H-1), 5.44 (1H, d, J = 4.8 Hz, H-2), 4.05 (3H, s, OCH3-6), 2.53, 2.21, 1.95, 1.75 (each 2H, m, camphanoyl CH2), 1.14, 1.13, 1.05, 1.01, 0.94, 0.93 (each 3H, s, camphanoyl CH3); [α]D -51.3° (c = 0.0023, CHCl3); HRMS for (M+ + Na): calcd m/z 803.1679, found: 803.1680.
6.1.11. 1R,2R-(-)-Dicamphanoyl-3,3,9-trimethyl-5-bromo-6-methoxy-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (26)
The procedure was identical to that used for the preparation of 25. 56% yield (starting from 50 mg of 12); white solid; mp 170-171 °C; 1H NMR δ 8.06 (1H, s, H-8), 7.45 (1H, d, J = 8.4 Hz, H-10), 7.19 (1H, d, J = 8.4 Hz, H-11), 6.88 (1H, d, J = 4.4 Hz, H-1), 5.43 (1H, d, J = 4.4 Hz, H-2), 4.04 (3H, s, OCH3-6), 2.44 (3H, s, CH3-9), 2.50, 2.20, 1.90, 1.70 (each 2H, m, camphanoyl CH2), 1.58, 1.58 (each 3H, s, CH3-3,3), 1.41, 1.13, 1.04, 1.01, 0.93, 0.91 (each 3H, s, camphanoyl CH3); [α]D -10.8° (c = 0.0078, CHCl3); HRMS for (M+ + Na): calcd m/z 817.1836, found: 817.1826.
6.1.12. 1R,2R-(-)-Dicamphanoyl-3,3-dimethyl-6-hydroxy-9-bromomethyl-1,2-dihydropyrano[2,3-c]xanthen-7(1H)-one (27)
A mixture of 12 (50 mg, 0.07 mmoL), NBS (18.6 mg, 0.105 mmoL), and 3-chloroperbenzoic acid (2 mg, 0.01 mmoL), dissolved in 1 mL of anhydrous CCl4 was heated to 100 °C for 5 h under high-absorption microwave conditions. At completion, the mixture was concentrated and the residue was purified by PTLC with hexane:EtOAc = 1:1 to afford pure 27 (31.3 mg, white solid). 56.1% yield; 1H NMR δ 13.62 (1H, s, OH-6), 8.20 (1H, d, J = 2.0 Hz, H-8), 7.74 (1H, dd, J = 8.8, 2.0 Hz, H-10), 7.48 (1H, d, J = 8.8 Hz, H-11), 6.89 (1H, s, H-5), 5.30, 5.30 (each 1H, s, H-1,2), 2.44 (2H, s, CH2Br-9), 2.40, 2.15, 1.90, 1.70 (each 2H, m, camphanoyl CH2), 1.56, 1.55 (each 3H, s, CH3-3,3), 1.11, 1.10, 1.04, 1.03, 0.98, 0.85 (each 3H, s, camphanoyl CH3); HRMS for (M+ + Na): calcd m/z 817.1836, found: 817.1822.
6.2. HIV-1 infectivity assay
Anti-HIV-1 activity was measured as reduction in Luc reporter gene expression after a single round of virus infection of TZM-bl cells. HIV-1 at 200 TCID50 and various dilutions of test samples (eight dilutions, four-fold stepwise) were mixed in a total volume of 100 μL growth medium in 96-well black solid plates (Corning-Costar). After 48-h incubation, culture medium was removed from each well and 100 μL of Bright Glo luciferase reagent was added to each culture well. The luciferase activity in the assay wells was measured using a Victor 2 luminometer. The 50% inhibitory dose (IC50) was defined as the sample concentration that caused a 50% reduction in Relative Luminescence Units (RLU) compared to virus control wells after subtraction of background RLU.
6.3. Cytotoxic activity assay
The general procedure was performed according to CytoTox-Glo™ cytotoxic activity assay instructions for using product G9290, G9291 and G9292. (Promega)
Supplementary Material
Acknowledgements
This investigation was supported by grant AI033066 from National Institute of Allergy and Infectious Disease (NIAID) awarded to K. H. Lee. This study was also supported in part by the Department of Health Clinical Trial and Research Center of Excellence (DOH-100-TD-B-111-004).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix. Supplementary data
HPLC conditions and summary of HPLC purity data for final compounds. Supplementary data associated with this article can be found in the online version, at
References
- 1.Huang L, Kashiwada Y, Cosentino LM, Fan S, Chen CH, McPhail AT, Fujioka T, Mihashi K, Lee KH. J. Med. Chem. 1994;37:3947–3955. doi: 10.1021/jm00049a014. [DOI] [PubMed] [Google Scholar]
- 2.Xie L, Takeuchi Y, Cosentino LM, Lee KH. J. Med. Chem. 1999;42:2662–2672. doi: 10.1021/jm9900624. [DOI] [PubMed] [Google Scholar]
- 3.Huang L, Yuan X, Yu D, Lee KH, Chen CH. Virology. 2005;332:623–628. doi: 10.1016/j.virol.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 4.Yu D, Brossi A, Kilgore N, Wild C, Allaway G, Lee KH. Bioorg. Med. Chem. Lett. 2003;13:1575–1576. doi: 10.1016/s0960-894x(03)00201-4. [DOI] [PubMed] [Google Scholar]
- 5.Yu D, Chen CH, Brossi A, Lee KH. J. Med. Chem. 2004;47:4072–4082. doi: 10.1021/jm0400505. [DOI] [PubMed] [Google Scholar]
- 6.Zhou T, Shi Q, Chen CH, Zhu H, Huang L, Ho P, Lee KH. Bioorg. Med. Chem. 2010;18:6678–6689. doi: 10.1016/j.bmc.2010.07.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreau S, Varache-Lembege M, Larrouture S, Fall D, Neveu A, Deffieux G, Vercauteren J, Nuhrich A. Eur. J. Med. Chem. 2002;37:237–253. doi: 10.1016/s0223-5234(01)01332-0. [DOI] [PubMed] [Google Scholar]
- 8.Zhou T, Shi Q, Lee KH. Tetrahedron Lett. 51:4382–4386. doi: 10.1016/j.tetlet.2010.06.058. (201)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reisch J, Herath HMT, Kumar NS. Liebigs Ann. Chem. 1991:685–689. [Google Scholar]
- 10.Kolb HC, VanNieuwenhze MS, Sharpless KB. Chem. Rev. 1994;94:2483–2547. [Google Scholar]
- 11.Mehltretter GM, Dobler C, Sundermeier U, Beller M. Tetrahedron Lett. 2000;41:8083–8087. [Google Scholar]
- 12.Xie L, Crimmins MT, Lee KH. Tetrahedron Lett. 1995;36:4529–4532. [Google Scholar]
- 13.Dallavalle S, Gattinoni S, Mazzini S, Scaglioni L, Merlini L, Tinelli S, Beretta G, Zunino F. Bioorg. Med. Chem. Lett. 2008;18:1484–1489. doi: 10.1016/j.bmcl.2007.12.055. [DOI] [PubMed] [Google Scholar]
- 14.Lynch JK, Freeman JC, Judd AS, Iyengar R, Mulhern M, Zhao G, Napier JJ, Wodka D, Brodjian S, Dayton BD, Falls D, Ogiela C, Reilly RM, Campbell TJ, Polakowski JS, Hernandez L, Marsh KC, Shapiro R, Knourek-Segel V, Droz B, Bush E, Brune M, Preusser LC, Fryer RM, Reinhart GA, Houseman K, Diaz G, Mikhail A, Limberis JT, Sham HL, Collins CA, Kym PR. J. Med. Chem. 2006;49:6569–6584. doi: 10.1021/jm060683e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.